Abstract
Protein-translated mRNA analysis has been extensively used to determine the function of various traits in animals. The non-coding RNA (ncRNA), which was known to be non-functional because it was not encoded as a protein, was re-examined as it was studied to actually function. One of the ncRNAs, long non-coding RNA (lncRNA), is known to have a function of regulating mRNA expression, and its importance is emerging. Therefore, lncRNAs are currently being used to understand the traits of various animals as well as human diseases. However, studies on lncRNA annotation and its functions are still lacking in most animals except humans and mice. lncRNAs have unique characteristics of lncRNAs and interact with mRNA through various mechanisms. In order to make lncRNA annotations in animals in the future, it is essential to understand the characteristics of lncRNAs and the mechanisms by which lncRNAs function. In addition, this will allow lncRNAs to be used for a wider variety of traits in a wider range of animals, and it is expected that integrated analysis using other biological information will be possible.
Keywords: LncRNA, Animal traits, LncRNA annotation, LncRNA-mRNA interaction, Lnc-RNA function
INTRODUCTION
Most of the genes refer to a region of a DNA sequence that functions related to animal traits or diseases. Therefore, gene expression profiling has been used to analyze biological functions [1], and analysis has been conducted by reading RNA sequences from the transcription process of DNA. However, coding RNAs that are translated into protein accounts for only about 4% of RNA, and the fact that non-coding RNAs (ncRNAs) existing in a vast region, which were treated with no role in the early days, are involved in gene regulation in various ways are being investigated [2].
Among them, long non-coding RNA (lncRNA), unlike mRNA, is not translated into a protein, despite its similar sequence structure [3]. In a small number of investigations involving animals, plants, and humans, it has been revealed that lncRNA functions in certain diseases or specific environments. It turns out that lncRNAs, previously considered to have no role, play many significant roles, the most important of which is to regulate mRNA expression [4,5]. LncRNAs regulate gene expression in a variety of ways at epigenetic, chromatin remodeling, transcriptional, and translational levels [6]. With the development of Next Generation Suquencing, lncRNA identification has been performed in humans and plants but also various species of animals. As the studies progressed, it was found that lncRNA had longitudinal, tissue-specific, and environmental-specific properties, so various case studies began to progress in various animals [4]. Prior studies and database construction are insufficient in other animals compared to humans and mice, so efforts are underway to continuously discover lncRNAs with essential functions and to be studied in many livestock animal samples [7–9]. However, even after some time since the importance of lncRNA emerged, many lncRNA transcripts have not been identified in livestock animals, or the functions of lncRNAs have not been identified properly. Therefore, lncRNA research is expected to be actively conducted for higher-dimensional bioinformatic analyses and multi-omics integration (MOI).
RNA
RNA is a polymeric genetic material that plays a vital role in various life phenomena, including control of gene expression [10,11]. Unlike DNA, RNA is not a pair of double strands but a single-stranded molecule with a short chain of nucleotides [12]. Notably, RNA can be divided into two main categories: messenger RNA (mRNA), which is coded as protein, and ncRNA, which is not coded [13].
mRNA
For DNA genetic information to be expressed as a protein, DNA must first be transcribed into RNA, and this RNA transcribed to be translated into protein is called mRNA [14]. With the development of sequencing technology, it became possible to examine the transcriptome region and to identify genes representing functions. Therefore, studies on mRNA expression levels under various conditions and bioinformation analysis-related studies using these results are being actively pursued [15–17]. In humans, mRNA is mainly used for pharmaceutical and vaccine development by enhancing the understanding of the immune system [18–24]. Additionally, various studies in mice are being conducted for use in humans because mice are also very similar in their genes to humans [25–30]. Furthermore, it is widely used for various trait studies in livestock animals. Many mRNA-related studies have been conducted mainly for the analysis of animal production traits [31–39] and quality traits [40–44], and they are also used for research in a wide range of areas, such as milk production [45–48], egg production [49–51], nutrients [52–54], stress [55–60], disease [61–64], and reproductive traits [65–71].
Non-coding RNA
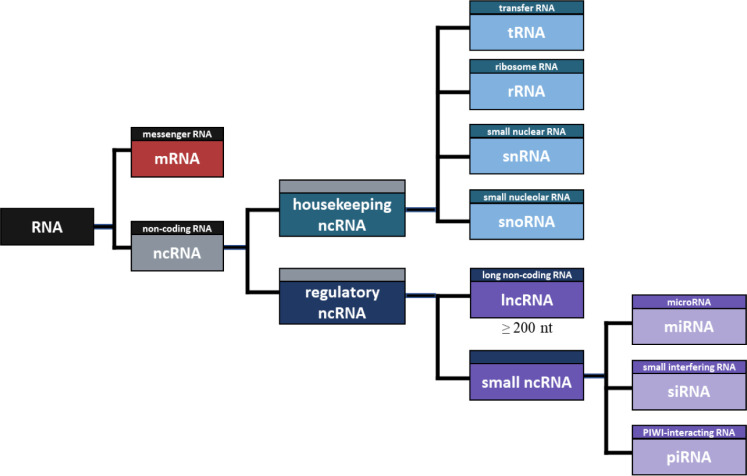
NcRNA refers to RNA that is not translated into a protein [72], and there are many different types of ncRNA. First, ncRNA can be divided into housekeeping and regulatory ncRNA (Fig. 1). Housekeeping ncRNAs include transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), and small nuclear RNA (snoRNA), while regulatory ncRNAs include microRNAs (miRNA), small interfering RNA (siRNA), piwi-interacting RNA (piRNA), and lncRNA [73]. Housekeeping ncRNAs are essentially expressed and mainly involved in rRNA modification RNA splicing control [74]. tRNA has a complementary anticodon in protein synthesis, which carries the amino acid to mRNA [75], and rRNA is an RNA that plays a structural role in ribosome formation and contributes to enzyme activity for protein synthesis [76]. Additionally, SnRNA binds with other proteins to form snRNPs, and plays a role in recognizing introns in the splicing process [77]. SnoRNA is primarily responsible for chemical transformation, such as rRNA and tRNA [78]. Notably, the main difference between the two RNAs is that snRNA influences the alternative splicing of pre-mRNA molecules to determine which sequence should be translated into proteins. In contrast, snoRNA participates in tRNA, rRNA, and mRNA editing and genome imprinting [79].
Fig. 1. A schematic diagram of RNA classification.
Regulatory ncRNAs can be divided into small RNAs and lncRNAs according to the length of RNA. The small ncRNA includes miRNA, siRNA, piRNA, and the like. MiRNA is an ncRNA composed of about 22 nt and functions in RNA silence and regulation of gene expression after transcription [80]. Likewise, SiRNA is an ncRNA composed of approximately 23 nt, which is involved in RNA interference and interferes with gene expression by inhibiting the production of specific proteins [81]. The main difference between the two RNAs is that miRNA regulates the expression of several mRNAs, and siRNA inhibits the expression of specific target mRNAs [82]. Furthermore, PiRNA consists of about 30 nt, which induces PIWI proteins to cleave the target RNA, promote heterochromatin assembly, methylate DNA, and regulate gene expression [83].
Among regulatory ncRNAs, RNA molecules greater than 200 nt in length are defined as lncRNA [84]. Although lncRNAs are very similar in structure to mRNAs, they are not translated into proteins and regulate gene expression through various bases, including epigenetic modification [3]. The various lists of annotated lncRNAs based on resemblance to protein-coding mRNAs account for only 0.05%–1.12% of cellular RNA, while functional intronic RNAs could constitute as much as 16% [85].
The extensive sequences that do not encode proteins (i.e., the majority of the vast regions of intronic and intergenic sequences) have been regarded as accumulated evolutionary remains arising from the early assembly of genes and/or the insertion of mobile genetic elements. However, as the aforementioned regulatory ncRNAs show, most of these supposedly inert sequences are transcribed and widely employed for gene regulation in cis and trans [86].
LONG NON-CODING RNA
Although the structure of the lncRNA seems similar to that of the mRNA, lncRNA is not coded and exists as a ncRNA rather than mRNA.
Generation of long non-coding RNA formation
LncRNAs mostly have a cap at the 5´ end and a poly(A) tail at the 3´ end, presumed to be transcribed similarly to mRNAs [87]. LncRNAs are transcribed by RNA Polymerase II (Pol II) and RNA Polymerase III at several loci of the genome, most of which are transcribed by Pol II [88]. Due to the lncRNA having a weak internal splicing signal and having a long distance between the 3’ splice site and the junction, the lncRNA is spliced more inefficiently than the mRNA [89–91]. The nuclear position and fate of lncRNAs appear to be coordinated by various causes, ranging from transcription to nuclear export via sequence motifs in cis and factors in trans [4,92]. Since the arrangement and size of lncRNAs are diverse, it is not well known precisely what biogenesis pathways they are treated. It is also challenging to understand whether ribosome-related lncRNAs are involved by ribosomes for translation, so further research is needed [4].
Genomic characteristic of long non-coding RNA
Unlike small ncRNAs such as siRNAs, miRNAs, and piRNAs, lncRNAs are relatively long and therefore have poorly conserved properties [93]. Compared to mRNA, lncRNA has a shorter transcript length and a smaller number of exons on average, and many studies have demonstrated these characteristics [94–96]. Furthermore, lncRNA has a shorter open reading frame (ORF) length than mRNA and a relatively low expression level [89,97,98].
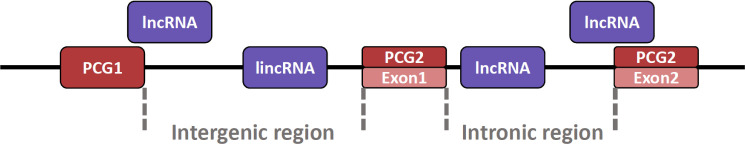
Also, lncRNA can exist at various locations in the genome (Fig. 2) [99]. The lncRNA can be present in the intron region between exon and exon and in the intergenic region between the protein-coding gene (PCG) and PCG [100]. In particular, lncRNAs present in the intergenic region are named long intergenic non-coding RNA (lincRNA). Additionally, because lincRNA does not overlap with PCG domains or other small RNA genes, it is relatively easy to conduct research such as the structure and function of lincRNA [101]. Although lincRNAs are similar in many respects to lncRNAs, they are somewhat longer than lncRNAs and are characterized by their presence in mammalian nuclei [101,102]. Furthermore, lncRNA can also exist in an exonic region where the lncRNA transcript overlaps the exon portion of the PCG [103]. Notably, there are also antisense lncRNAs characterized by transcription from opposite strands of PCG [104], which regulate the expression of their endogenous sense genes [105].
Fig. 2. Locations where lncRNA can exist in the genome.
LncRNAs present in intergenic regions are called lincRNA. PCG, protein-coding gene; lncRNA, long non-coding RNA; lincRNA, long intergenic non-coding RNA.
LncRNA has well-known tissue-specific, species-specific, and conditional-specific tendencies. Even the same individual can express lncRNA differently depending on what kind of tissue it is, and even the same tissue can express differently depending on the species [96,106–110]. Moreso, the tissue-specific characteristics of lncRNA demonstrated higher results even when compared to mRNA through the tissue specificity index calculated numerically in mammals [96,111].
Whole genomic of long non-coding RNA identification
Most animals, except humans and mice, do not yet have a well-established lncRNA database, so the process of identifying novel lncRNA identification for lncRNA analysis should be conducted. Therefore, a new Gene transfer format (GTF) file is needed to find the novel lncRNA instead of the reference gtf file of the animal containing only the information of previously known RNA. A merged GTF file is generated based on the transcripts of the samples to be analyzed and the reference GTF file of the corresponding animal [7,108,112]. Following the merged GTF file, only transcripts longer than 200 bp and an ORF transcript length shorter than 300 bp are selected. It also considers the positional relationship in the genome between lncRNA and PCG and designates transcripts consistent with the definition of lncRNA (intergenic, intronic, etc.). Subsequently, it filters only transcripts with low probability using various tools that calculate the potential for transcripts to be coded as proteins. Importantly, tools for evaluating coding potential are diverse and can be used flexibly depending on how to analyze. The transcripts filtered from the sequencing data can be selected and presented as potential novel lncRNA, and can be used for functional analysis and actual lncRNA sequence verification in the future [113–119].
INTERACTION LONG NON-CODING RNA TO mRNA
Among the many known lncRNA functions, a representative and key function is to regulate mRNA expression including , such as epigenetic modification [3,4]. Therefore, a method of conducting mRNA and lncRNA analysis is actively used as an experimental design for exploring animal traits. Importantly, it is used in a wide variety of fields, including production traits [120–123] and quality traits [124–127], milk production [128–130], egg production [9,131,132], stress [133–135], diseases [136] and reproductive traits [137–140].
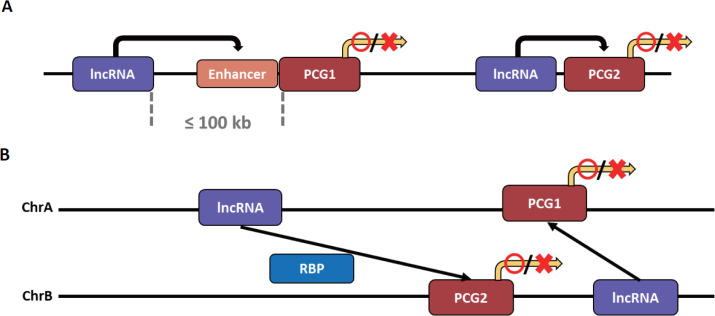
The types of lncRNA that regulate transcription can be divided into two based on the transcription site and functional location of the lncRNA. It is classified as cis-acting lncRNA if its functional location depends on the transcription site, and trans-acting lncRNA if transcribed to exert functions elsewhere without relying on the transcription site (Fig. 3) [141]. Notably, a method for obtaining a candidate target gene for cis- and trans-acting lncRNA has not yet been fully established. However, the candidate target gene interacting with cis-acting lncRNA is primarily a candidate group of PCGs within 100 kb on the same chromosome of lncRNA. In contrast, the candidate gene interacting with trans-acting lncRNA is a candidate group of PCGs on different chromosomes [141,142].
Fig. 3. Two mechanisms by which lncRNAs affect PCG expression.
(A) LncRNA activates or represses the expression of PCG in the cis-acting condition. A representative method for PCG regulation of cis-acting lncRNAs is to affect enhancers. (B) LncRNA activates or represses the expression of pcg in the trans-acting condition. A representative method for pcg regulation of trans-acting lncRNAs is to affect RBPs. lncRNA, long non-coding RNA; PCG, protein-coding gene; RBP, RNA-binding proteins.
Cis-acting
As mentioned earlier, the cis-acting mechanism is preferred because lncRNA is less likely to function normally due to dilution from diffusion and transport to other cellular compartments. After all, the expression level is generally relatively low [141].
The cis-acting lncRNA can increase or inhibit the expression of target genes through various mechanisms. The mechanism by which cis-acting lncRNA increases gene expression is closely related to enhancers. These lncRNAs can be broadly divided into two categories: 1) lncRNAs derived from and transcribed from the enhancer after mutation or translocation has occurred in the gene enhancer [143,144], and 2) those transcribed from other sources that act like the enhancer of the target gene or affect the enhancer [145,146]. These both lncRNAs can activate the target gene by influencing the target gene’s enhancer or act as the enhancer and activate the target gene. As a first mechanism, lncRNA transcripts regulate enhancer activity by forming or maintaining chromatin loops with target genes [147,148]. Additionally, since the lncRNA transcript affects the nuclear localization of the enhancer, it can increase the expression of the target gene by giving strength to the enhancer as an indirect mechanism [149]. The cis-acting lncRNA can activate the expression of a target gene by influencing the enhancer through mechanisms other than spatial interaction. It is an lncRNA that attracts a protein that enhances the enhancer of the target gene [150–152]. There are also cis-acting lncRNAs that activate gene expression independent of enhancers. The lncRNA is transcribed near the target gene, or the preformed chromatin loop structure locates the lncRNA near the target gene, thereby increasing the expression of the target gene by attracting activating factors to the lncRNA [153].
The cis-acting lncRNA not only increases the expression of a target gene but also inhibits it. First, lncRNA near the target gene can suppress the expression by silencing the target gene’s promoter through the enhancer competition of the target gene [154,155]. In addition, the lncRNA is transcribed near the target gene, or the preformed chromatin loop structure places the lncRNA near the target gene so that the lncRNA attracts repressive complexes such as Polycomb repressive complex 2, resulting in the same effect as histone modification. Thus, gene expression can be inhibited [156]. Another mechanism by which cis-acting lncRNA suppresses gene expression is transcriptional interference. Through nucleosome remodeling, in which nucleosomes are rearranged, nucleosome occupancy is reduced, or multiple epigenetic modifications, lncRNA that approaches or overlaps the target gene suppresses the expression of the target gene [157,158].
Previous studies have revealed that cis-acting lncRNA does not only interact one-to-one with the target gene. One lncRNA may be involved in the transcription of several target genes, and conversely, it appears that several lncRNAs may be involved in transcribing a target gene in unison. [4,141].
Trans-acting
Unlike cis-acting lncRNAs, trans-acting lncRNAs can interact independently of complementary sequences for target gene regions [99]. Trans-acting lncRNAs function by binding to proteins, DNA, and other RNAs [159]. First, trans-acting lncRNAs can act as post-transcriptional regulatory factors by interacting with RNA-binding proteins (RBPs). These lncRNAs interact with RBPs to inhibit mRNA splicing and the stability and translation of mRNAs [160–162]. Notably, splicing regulation by lncRNA causes a mutation or transformation in the splicing regulation sequence of the target pre-mRNA, resulting in the mis-splicing of the mRNA [163].
Trans-acting lncRNAs can also promote or inhibit the stability of mRNA by interacting directly with RNA through base pairing. This is likely due to the ability to attract proteins involved in mRNA decomposition by directly base pairing with other RNAs [164,165]. While its existence has been revealed and its importance as a post-transcriptional control factor has emerged, research on trans-acting lncRNA is insufficient. Further research will be needed to clarify the apparent correlation between trans-acting lncRNA and target genes and reveal the mechanisms by which several trans-acting lncRNAs interact with RBP.
ANOTHER FUNCTION OF LONG NON-CODING RNA
Until recently, interactions between ncRNAs have rarely been studied. However, recent studies have shown that lncRNA can interact with miRNA and mRNA [166]. Importantly, the lncRNA acts as a sponge to attract miRNA and competes with PCG, which was supposed to bind to miRNA. This attraction process reduces the target gene regulation effect of miRNA [167–170]. Therefore, studies on high-dimensional access to specific biological information are being conducted by analyzing the correlation and interaction of 3 RNAs of lncRNA-miRNA-mRNA [171–173]. It has also been suggested that some lncRNAs can be preferentially post-processed into snoRNA [99,174,175]. As mentioned earlier, the possibility of interaction between lncRNAs or other RNAs is still open, such as various lncRNAs involved in regulating one mRNA expression. However, further research is needed as it is unclear. If these mechanisms are revealed, not only will we be able to understand the principles of lncRNA and mRNA interaction that have not yet been accurately identified, but we will also be able to make much more expansive use of MOI network research using lncRNA.
CONCLUSION
In the past, only studies on mRNA encoded by functional genes were conducted, but now the role of ncRNAs has been re-examined, and research on this topic is being actively conducted. Among them, lncRNA has a high probability of being present in many different places on the genome, and it is known that it has many functions. Therefore, its importance is emerging from these added investigations. As a key function of lncRNA, it can regulate gene expression through various mechanisms. In addition, since it has tissue-specific and species-specific characteristics, it is possible to analyze bioinformation using lncRNA from multiple perspectives in particular tissues of different species. This means that lncRNAs can be used as biomarkers involved in improving reproductive traits and diseases in mammals, including livestock animals. Therefore, lncRNA exploration and functional analysis are being conducted to study various animal traits. However, analysis for identifying lncRNA in animal species other than humans and mice is still lacking, and analysis of the mechanism and function of lncRNA is insufficient. If studies that can supplement these areas are conducted, it is likely that high-dimensional MOI analysis using lncRNA will be possible.
Acknowledgements
We would like to thank Joshua S. for English language review.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development, of the Rural Development Administration, Korea (PJ01620403).
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Park YB, Kim JM.
Writing - original draft: Park YB.
Writing - review & editing: Park YB, Kim JM.
Ethics approval and consent to participate
This article does not require IRB/IACUC approval because there are no human and animal experiments.
REFERENCES
- 1.Kim K, Zakharkin SO, Allison DB. Expectations, validity, and reality in gene expression profiling. J Clin Epidemiol. 2010;63:950–9. doi: 10.1016/j.jclinepi.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil VS, Zhou R, Rana TM. Gene regulation by non-coding RNAs. Crit Rev Biochem Mol Biol. 2014;49:16–32. doi: 10.3109/10409238.2013.844092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20:5573. doi: 10.3390/ijms20225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Im JH, Muschel RJ. New evidence of lncRNA role in tumor progression and metastasis. Hepatobiliary Surg Nutr. 2012;1:55–6. doi: 10.3978/j.issn.2304-3881.2012.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Min L, Qiu X, Wu X, Liu C, Ma J, et al. Biological function of long non-coding RNA (lncRNA) Xist. Front Cell Dev Biol. 2021;9:645647. doi: 10.3389/fcell.2021.645647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Shi G, Chen G, Li J, Li M, Zou C, et al. Transcriptome analysis suggests the roles of long intergenic non-coding RNAs in the growth performance of weaned piglets. Front Genet. 2019;10:196. doi: 10.3389/fgene.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia L, Wang J, Luoreng Z, Wang X, Wei D, Yang J, et al. Progress in expression pattern and molecular regulation mechanism of lncRNA in bovine mastitis. Animals. 2022;12:1059. doi: 10.3390/ani12091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adetula AA, Gu L, Nwafor CC, Du X, Zhao S, Li S. Transcriptome sequencing reveals key potential long non-coding RNAs related to duration of fertility trait in the uterovaginal junction of egg-laying hens. Sci Rep. 2018;8:13185. doi: 10.1038/s41598-018-31301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce GF. The antiquity of RNA-based evolution. Nature. 2002;418:214–21. doi: 10.1038/418214a. [DOI] [PubMed] [Google Scholar]
- 11.Arraiano CM, Andrade JM, Domingues S, Guinote IB, Malecki M, Matos RG, et al. The critical role of RNA processing and degradation in the control of gene expression. FEMS Microbiol Rev. 2010;34:883–923. doi: 10.1111/j.1574-6976.2010.00242.x. [DOI] [PubMed] [Google Scholar]
- 12.Seetin MG, Mathews DH. In: Bacterial regulatory RNA. Keiler K, editor. Totowa, NJ:: Humana Press; 2012. RNA structure prediction: an overview of methods; pp. 99–122. editor. p. [DOI] [PubMed] [Google Scholar]
- 13.Brandenburger T, Salgado Somoza A, Devaux Y, Lorenzen JM. Noncoding RNAs in acute kidney injury. Kidney Int. 2018;94:870–81. doi: 10.1016/j.kint.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Clancy S, Brown W. Translation: DNA to mRNA to protein. Nat Educ. 2008;1:101. [Google Scholar]
- 15.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Gharib TG, Huang CC, Taylor JMG, Misek DE, Kardia SLR, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–13. doi: 10.1074/mcp.M200008-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394:189–93. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 19.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Reape TJ, Li X, Rayner K, Webb CL, Burnand KG, et al. Induced expression of adipophilin mRNA in human macrophages stimulated with oxidized low-density lipoprotein and in atherosclerotic lesions. FEBS Lett. 1999;462:145–50. doi: 10.1016/S0014-5793(99)01521-5. [DOI] [PubMed] [Google Scholar]
- 21.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–24. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 22.Fujii T, Yamada S, Watanabe Y, Misawa H, Tajima S, Fujimoto K, et al. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J Neuroimmunol. 1998;82:101–7. doi: 10.1016/S0165-5728(97)00195-1. [DOI] [PubMed] [Google Scholar]
- 23.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–37. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 24.Hollams EM, Giles KM, Thomson AM, Leedman PJ. MRNA stability and the control of gene expression: implications for human disease. Neurochem Res. 2002;27:957–80. doi: 10.1023/A:1020992418511. [DOI] [PubMed] [Google Scholar]
- 25.Craig Venter J, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 26.Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur J Pharmacol. 2001;423:235–41. doi: 10.1016/S0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- 27.Hörtnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–72. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrillo-Vico A, García-Pergañeda A, Naji L, Calvo JR, Romero MP, Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci. 2003;60:2272–8. doi: 10.1007/s00018-003-3207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan ME, van Bilsen JHM, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, South T, Han M, Chen J, Wang R, Huang XF. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–9. doi: 10.1016/j.brainres.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 31.Buzala M, Janicki B. Review: effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult Sci. 2016;95:2151–9. doi: 10.3382/ps/pew173. [DOI] [PubMed] [Google Scholar]
- 32.Shen H, Zhao SH, Cao JH, Li XY, Fan B. Porcine MuRF2 and MuRF3: molecular cloning, expression and association analysis with muscle production traits. Mol Biol Rep. 2011;38:5115–23. doi: 10.1007/s11033-010-0659-0. [DOI] [PubMed] [Google Scholar]
- 33.Juszczuk-Kubiak E, Bujko K, Grześ M, Cymer M, Wicińska K, Szostak A, et al. Study of bovine Mef2B gene: the temporal-spatial expression patterns, polymorphism and association analysis with meat production traits. J Anim Sci. 2016;94:4536–48. doi: 10.2527/jas.2016-0741. [DOI] [PubMed] [Google Scholar]
- 34.Zeng F, Xie L, Pang X, Liu W, Nie Q, Zhang X. Complementary deoxyribonucleic acid cloning of avian G0/G1 switch gene 2, and its expression and association with production traits in chicken. Poult Sci. 2011;90:1548–54. doi: 10.3382/ps.2010-01204. [DOI] [PubMed] [Google Scholar]
- 35.Szreder T, Zwierzchowski L. Estrogen receptors and their genes—potential markers of functional and production traits of farm animals. Mol Biol Rep. 2007;34:207–11. doi: 10.1007/s11033-006-9030-x. [DOI] [PubMed] [Google Scholar]
- 36.Bai Y, Sun G, Kang X, Han R, Tian Y, Li H, et al. Polymorphisms of the pro-opiomelanocortin and agouti-related protein genes and their association with chicken production traits. Mol Biol Rep. 2012;39:7533–9. doi: 10.1007/s11033-012-1587-y. [DOI] [PubMed] [Google Scholar]
- 37.Cochran SD, Cole JB, Null DJ, Hansen PJ. Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and production traits in Holstein cattle. BMC Genet. 2013;14:49. doi: 10.1186/1471-2156-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu LX, Dou TF, Li QH, Rong H, Tong HQ, Xu ZQ, et al. Myostatin mRNA expression and its association with body weight and carcass traits in Yunnan Wuding chicken. Genet Mol Res. 2016;15:1–12. doi: 10.4238/gmr15048967. [DOI] [PubMed] [Google Scholar]
- 39.Ramayo-Caldas Y, Ballester M, Sánchez JP, González-Rodríguez O, Revilla M, Reyer H, et al. Integrative approach using liver and duodenum RNA-Seq data identifies candidate genes and pathways associated with feed efficiency in pigs. Sci Rep. 2018;8:558. doi: 10.1038/s41598-017-19072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee EA, Kim JM, Lim KS, Ryu YC, Jeon WM, Hong KC. Effects of variation in porcine MYOD1 gene on muscle fiber characteristics, lean meat production, and meat quality traits. Meat Sci. 2012;92:36–43. doi: 10.1016/j.meatsci.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Çinar MU, Fan H, Neuhoff C, Große-Brinkhaus C. eQTL analysis and association of MYF6 mRNA expression with meat quality traits in pigs. Kafkas Univ Vet Fak Derg. 2012;18:235–42. doi: 10.9775/kvfd.2011.5359. [DOI] [Google Scholar]
- 42.Nkrumah JD, Li C, Basarab JB, Guercio S, Meng Y, Murdoch B, et al. Association of a single nucleotide polymorphism in the bovine leptin gene with feed intake, feed efficiency, growth, feeding behaviour, carcass quality and body composition. Can J Anim Sci. 2004;84:211–9. doi: 10.4141/A03-033. [DOI] [Google Scholar]
- 43.Li X, Kim SW, Choi JS, Lee YM, Lee CK, Choi BH, et al. Investigation of porcine FABP3 and LEPR gene polymorphisms and mRNA expression for variation in intramuscular fat content. Mol Biol Rep. 2010;37:3931–9. doi: 10.1007/s11033-010-0050-1. [DOI] [PubMed] [Google Scholar]
- 44.Seo YJ, Lim B, Kim DY, Lim KS, Kim JM. Regulation of swine growth by backfat tissue during growing and finishing stages. Animals. 2021;11:3511. doi: 10.3390/ani11123511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raliou M, Dembélé D, Düvel A, Bolifraud P, Aubert J, Mary-Huard T, et al. Subclinical endometritis in dairy cattle is associated with distinct mRNA expression patterns in blood and endometrium. PLOS ONE. 2019;14:e0220244. doi: 10.1371/journal.pone.0220244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weikard R, Goldammer T, Brunner RM, Kuehn C. Tissue-specific mRNA expression patterns reveal a coordinated metabolic response associated with genetic selection for milk production in cows. Physiol Genomics. 2012;44:728–39. doi: 10.1152/physiolgenomics.00007.2012. [DOI] [PubMed] [Google Scholar]
- 47.Szewczuk M, Zych S, Czerniawska-Piątkowska E, Wójcik J. Association between IGF1R/i16/TaqI and IGF1/SnaBI polymorphisms and milk production traits in Polish Holstein-Friesian cows. Anim Sci Pap Rep. 2012;30:13–24. doi: 10.5194/aab-54-10-2011. [DOI] [Google Scholar]
- 48.Yang SH, Bi XJ, Xie Y, Li C, Zhang SL, Zhang Q, et al. Validation of PDE9A gene identified in GWAS showing strong association with milk production traits in Chinese Holstein. Int J Mol Sci. 2015;16:26530–42. doi: 10.3390/ijms161125976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo S, Bai Y, Zhang Q, Zhang H, Fan Y, Han H, et al. Associations of CALM1 and DRD1 polymorphisms, and their expression levels, with Taihang chicken egg-production traits. Anim Biotechnol. 2021 doi: 10.1080/10495398.2021.2008948. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Z, Chen Y, Chen Q, Guo M, Kang L, Zhu G, et al. Characterization of chicken MMP13 expression and genetic effect on egg production traits of its promoter polymorphisms. G3. 2016;6:1305–12. doi: 10.1534/g3.116.027755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Shen X, Zhou M, Fang M, Zeng H, Nie Q, et al. The genetic effects of the dopamine D1 receptor gene on chicken egg production and broodiness traits. BMC Genet. 2010;11:17. doi: 10.1186/1471-2156-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vonnahme KA, Zhu MJ, Borowicz PP, Geary TW, Hess BW, Reynolds LP, et al. Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J Anim Sci. 2007;85:2464–72. doi: 10.2527/jas.2006-805. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Zhu F, Yang H, Li J, Li Y, Ding X, et al. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J Anim Physiol Anim Nutr. 2019;103:618–25. doi: 10.1111/jpn.13059. [DOI] [PubMed] [Google Scholar]
- 54.Weller MMDCA, Albino RL, Marcondes MI, Silva W, Daniels KM, Campos MM, et al. Effects of nutrient intake level on mammary parenchyma growth and gene expression in crossbred (Holstein × Gyr) prepubertal heifers. J Dairy Sci. 2016;99:9962–73. doi: 10.3168/jds.2016-11532. [DOI] [PubMed] [Google Scholar]
- 55.Rhoads ML, Kim JW, Collier RJ, Crooker BA, Boisclair YR, Baumgard LH, et al. Effects of heat stress and nutrition on lactating Holstein cows: II. aspects of hepatic growth hormone responsiveness. J Dairy Sci. 2010;93:170–9. doi: 10.3168/jds.2009-2469. [DOI] [PubMed] [Google Scholar]
- 56.Deb R, Sajjanar B, Singh U, Kumar S, Brahmane MP, Singh R, et al. Promoter variants at AP2 box region of Hsp70.1 affect thermal stress response and milk production traits in Frieswal cross bred cattle. Gene. 2013;532:230–5. doi: 10.1016/j.gene.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol. 1995;269:C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 58.do Amaral BC, Connor EE, Tao S, Hayen J, Bubolz J, Dahl GE. Heat stress abatement during the dry period influences prolactin signaling in lymphocytes. Domest Anim Endocrinol. 2010;38:38–45. doi: 10.1016/j.domaniend.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Sejian V, Bhatta R, Gaughan JB, Dunshea FR, Lacetera N. Review: adaptation of animals to heat stress. Animal. 2018;12:s431–44. doi: 10.1017/S1751731118001945. [DOI] [PubMed] [Google Scholar]
- 60.Lim C, Lim B, Kil DY, Kim JM. Hepatic transcriptome profiling according to growth rate reveals acclimation in metabolic regulatory mechanisms to cyclic heat stress in broiler chickens. Poult Sci. 2022;101:102167. doi: 10.1016/j.psj.2022.102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J, Kim M, Na G, Jeon I, Kwon YK, Kim JH, et al. Glucocorticoids modulate NF-κB-dependent gene expression by up-regulating FKBP51 expression in Newcastle disease virus-infected chickens. Mol Cell Endocrinol. 2007;278:7–17. doi: 10.1016/j.mce.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa H, Rahman MM, Yamauchi M, Takashima S, Wakihara Y, Kamatari YO, et al. mRNA profile in milk extracellular vesicles from bovine leukemia virus-infected cattle. Viruses. 2020;12:669. doi: 10.3390/v12060669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann B, Beer M, Reid SM, Mertens P, Oura CAL, van Rijn PA, et al. A review of RT-PCR technologies used in veterinary virology and disease control: sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health. Vet Microbiol. 2009;139:1–23. doi: 10.1016/j.vetmic.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 64.Lim B, Kim S, Lim KS, Jeong CG, Kim SC, Lee SM, et al. Integrated time-serial transcriptome networks reveal common innate and tissue-specific adaptive immune responses to PRRSV infection. Vet Res. 2020;51:128. doi: 10.1186/s13567-020-00850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu Y, Fu J, Wang A. Association of EphA4 polymorphism with swine reproductive traits and mRNA expression of EphA4 during embryo implantation. Mol Biol Rep. 2012;39:2689–96. doi: 10.1007/s11033-011-1023-8. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Yin H, Li C, Qin C, Cai W, Cao M, et al. Genetic effects of PDGFRB and MARCH1 identified in GWAS revealing strong associations with semen production traits in Chinese Holstein bulls. BMC Genet. 2017;18:63. doi: 10.1186/s12863-017-0527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hui Y, Zhang Y, Wang K, Pan C, Chen H, Qu L, et al. Goat DNMT3B: an indel mutation detection, association analysis with litter size and mRNA expression in gonads. Theriogenology. 2020;147:108–15. doi: 10.1016/j.theriogenology.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 68.Kwon SG, Hwang JH, Park DH, Kim TW, Kang DG, Kang KH, et al. Identification of differentially expressed genes associated with litter size in Berkshire pig placenta. PLOS ONE. 2016;11:e0153311. doi: 10.1371/journal.pone.0153311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez-Rodriguez A, Munoz M, Fernandez A, Pena RN, Tomas A, Noguera JL, et al. Differential gene expression in ovaries of pregnant pigs with high and low prolificacy levels and identification of candidate genes for litter size. Biol Reprod. 2011;84:299–307. doi: 10.1095/biolreprod.110.085589. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Li A, Chen W, Wei J, Fu J, Wang A. Differential gene expression in uterine endometrium during implantation in pigs. Biol Reprod. 2015;92:52. doi: 10.1095/biolreprod.114.123075. [DOI] [PubMed] [Google Scholar]
- 71.Kim JM, Park JE, Yoo I, Han J, Kim N, Lim WJ, et al. Integrated transcriptomes throughout swine oestrous cycle reveal dynamic changes in reproductive tissues interacting networks. Sci Rep. 2018;8:5436. doi: 10.1038/s41598-018-23655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hüttenhofer A, Vogel J. Experimental approaches to identify non-coding RNAs. Nucleic Acids Res. 2006;34:635–46. doi: 10.1093/nar/gkj469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang P, Wu W, Chen Q, Chen M. Non-coding RNAs and their integrated networks. J Integr Bioinform. 2019;16:1–12. doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morey C, Avner P. Employment opportunities for non-coding RNAs. FEBS Lett. 2004;567:27–34. doi: 10.1016/j.febslet.2004.03.117. [DOI] [PubMed] [Google Scholar]
- 75.McClain WH. Rules that govern tRNA identity in protein synthesis. J Mol Biol. 1993;234:257–80. doi: 10.1006/jmbi.1993.1582. [DOI] [PubMed] [Google Scholar]
- 76.Weiner AM, Maizels N. tRNA-like structures tag the 3’ ends of genomic RNA molecules for replication: implications for the origin of protein synthesis. Proc Natl Acad Sci USA. 1987;84:7383–7. doi: 10.1073/pnas.84.21.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valadkhan S, Gunawardane LS. Role of small nuclear RNAs in eukaryotic gene expression. Essays Biochem. 2013;54:79–90. doi: 10.1042/bse0540079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dieci G, Preti M, Montanini B. Eukaryotic snoRNAs: a paradigm for gene expression flexibility. Genomics. 2009;94:83–8. doi: 10.1016/j.ygeno.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Ghoshal K, Jacob ST. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem Pharmacol. 1997;53:1569–75. doi: 10.1016/S0006-2952(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 80.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu W, Jiang X, Huang L. In: Comprehensive biotechnology. 3th ed. Moo-Young M, editor. Vol. 5. Oxford: Elsevier; 2019. RNA interference technology; pp. 560–75. editor. p. [DOI] [Google Scholar]
- 82.Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:E252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2019;20:89–108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- 84.Zheng H, Brennan K, Hernaez M, Gevaert O. Benchmark of long non-coding RNA quantification for RNA sequencing of cancer samples. GigaScience. 2019;8:giz145. doi: 10.1093/gigascience/giz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.St. Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–51. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 87.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 88.Schlackow M, Nojima T, Gomes T, Dhir A, Carmo-Fonseca M, Proudfoot NJ. Distinctive patterns of transcription and RNA processing for human lincRNAs. Mol Cell. 2017;65:25–38. doi: 10.1016/j.molcel.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo CJ, Ma XK, Xing YH, Zheng CC, Xu YF, Shan L, et al. Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. Cell. 2020;181:621–36.:E22. doi: 10.1016/j.cell.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Melé M, Mattioli K, Mallard W, Shechner DM, Gerhardinger C, Rinn JL. Chromatin environment, transcriptional regulation, and splicing distinguish lincRNAs and mRNAs. Genome Res. 2017;27:27–37. doi: 10.1101/gr.214205.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuckerman B, Ulitsky I. Predictive models of subcellular localization of long RNAs. RNA. 2019;25:557–72. doi: 10.1261/rna.068288.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kung JTY, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–69. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong Y, He J, Li B, Xiao Y, Zeng Q, Xu K, et al. Integrated analysis of lncRNA and mRNA in subcutaneous adipose tissue of Ningxiang pig. Biology. 2021;10:726. doi: 10.3390/biology10080726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song F, Wang L, Zhu W, Dong Z. Long noncoding RNA and mRNA expression profiles following igf3 knockdown in common carp, Cyprinus carpio. Sci Data. 2019;6:190024. doi: 10.1038/sdata.2019.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang L, Yang Q, Yu J, Liu X, Cai Y, Niu L, et al. Identification and expression analysis of lncRNA in seven organs of Rhinopithecus roxellana. Funct Integr Genom. 2021;21:543–55. doi: 10.1007/s10142-021-00797-6. [DOI] [PubMed] [Google Scholar]
- 97.Quinn JJ, Zhang QC, Georgiev P, Ilik IA, Akhtar A, Chang HY. Rapid evolutionary turnover underlies conserved lncRNA–genome interactions. Genes Dev. 2016;30:191–207. doi: 10.1101/gad.272187.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark MB, Amaral PP, Schlesinger FJ, Dinger ME, Taft RJ, Rinn JL, et al. The reality of pervasive transcription. PLOS Biol. 2011;9:e1000625. doi: 10.1371/journal.pbio.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:924–33. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suarez B, Prats-Mari L, Unfried JP, Fortes P. LncRNAs in the type I interferon antiviral response. Int J Mol Sci. 2020;21:6447. doi: 10.3390/ijms21176447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinform. 2016;14:42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Budak H, Kaya SB, Cagirici HB. Long non-coding RNA in plants in the era of reference sequences. Front Plant Sci. 2020;11:276. doi: 10.3389/fpls.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ros G, Pegoraro S, de Angelis P, Sgarra R, Zucchelli S, Gustincich S, et al. HMGA2 antisense long non-coding RNAs as new players in the regulation of HMGA2 expression and pancreatic cancer promotion. Front Oncol. 2020;9:1526. doi: 10.3389/fonc.2019.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mo S, Zhang L, Dai W, Han L, Wang R, Xiang W, et al. Antisense lncRNA LDLRAD4-AS1 promotes metastasis by decreasing the expression of LDLRAD4 and predicts a poor prognosis in colorectal cancer. Cell Death Dis. 2020;11:155. doi: 10.1038/s41419-020-2338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–14. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Huang X, Liu J, Chen G, Liu C, Zhang S, et al. New insight into long non-coding RNAs associated with bone metastasis of breast cancer based on an integrated analysis. Cancer Cell Int. 2021;21:372. doi: 10.1186/s12935-021-02068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang P, Hou Y, Fu W, Tao X, Luo J, Lu H, et al. Characterization of lncRNAs involved in cold acclimation of zebrafish ZF4 cells. PLOS ONE. 2018;13:e0195468. doi: 10.1371/journal.pone.0195468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simion V, Zhou H, Haemmig S, Pierce JB, Mendes S, Tesmenitsky Y, et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat Commun. 2020;11:6135. doi: 10.1038/s41467-020-19664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang YY, Zhu H, Zhang H. Analysis of orthologous lncRNAs in humans and mice and their species-specific epigenetic target genes. J South Med Univ. 2018;38:731–5. doi: 10.3969/j.issn.1673-4254.2018.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21:650–9. doi: 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]
- 112.Azlan A, Obeidat SM, Yunus MA, Azzam G. Systematic identification and characterization of Aedes aegypti long noncoding RNAs (lncRNAs) Sci Rep. 2019;9:12147. doi: 10.1038/s41598-019-47506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–9. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang L, Park HJ, Dasari S, Wang S, Kocher JP, Li W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013;41:e74. doi: 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li A, Zhang J, Zhou Z. PLEK: a tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014;15:311. doi: 10.1186/1471-2105-15-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun L, Luo H, Bu D, Zhao G, Yu K, Zhang C, et al. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013;41:e166. doi: 10.1093/nar/gkt646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tong X, Liu S. CPPred: coding potential prediction based on the global description of RNA sequence. Nucleic Acids Res. 2019;47:e43. doi: 10.1093/nar/gkz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo JC, Fang SS, Wu Y, Zhang JH, Chen Y, Liu J, et al. CNIT: a fast and accurate web tool for identifying protein-coding and long non-coding transcripts based on intrinsic sequence composition. Nucleic Acids Res. 2019;47:W516–22. doi: 10.1093/nar/gkz400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong Y, Huang HT, Liang Y, Trimarchi T, Aifantis I, Tsirigos A. lncRNA-screen: an interactive platform for computationally screening long non-coding RNAs in large genomics datasets. BMC Genom. 2017;18:434. doi: 10.1186/s12864-017-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun J, Xie M, Huang Z, Li H, Chen T, Sun R, et al. Integrated analysis of non-coding RNA and mRNA expression profiles of 2 pig breeds differing in muscle traits. J Anim Sci. 2017;95:1092–103. doi: 10.2527/jas.2016.0867. [DOI] [PubMed] [Google Scholar]
- 121.Mohamadipoor Saadatabadi L, Mohammadabadi M, Amiri Ghanatsaman Z, Babenko O, Stavetska R, Kalashnik O, et al. Signature selection analysis reveals candidate genes associated with production traits in Iranian sheep breeds. BMC Vet Res. 2021;17:369. doi: 10.1186/s12917-021-03077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li W, Zheng M, Zhao G, Wang J, Liu J, Wang S, et al. Identification of QTL regions and candidate genes for growth and feed efficiency in broilers. Genet Sel Evol. 2021;53:13. doi: 10.1186/s12711-021-00608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alexandre PA, Reverter A, Berezin RB, Porto-Neto LR, Ribeiro G, Santana MHA, et al. Exploring the regulatory potential of long non-coding RNA in feed efficiency of indicine cattle. Genes. 2020;11:997. doi: 10.3390/genes11090997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ali A, Al-Tobasei R, Kenney B, Leeds TD, Salem M. Integrated analysis of lncRNA and mRNA expression in rainbow trout families showing variation in muscle growth and fillet quality traits. Sci Rep. 2018;8:12111. doi: 10.1038/s41598-018-30655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li H, Huang K, Wang P, Feng T, Shi D, Cui K, et al. Comparison of long non-coding RNA expression profiles of cattle and buffalo differing in muscle characteristics. Front Genet. 2020;11:98. doi: 10.3389/fgene.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muniz MMM, Simielli Fonseca LF, Scalez DCB, Vega AS, Silva DBS, Ferro JA, et al. Characterization of novel lncRNA muscle expression profiles associated with meat quality in beef cattle. Evol Appl. 2022;15:706–18. doi: 10.1111/eva.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li R, Li B, Jiang A, Cao Y, Hou L, Zhang Z, et al. Exploring the lncRNAs related to skeletal muscle fiber types and meat quality traits in pigs. Genes. 2020;11:883. doi: 10.3390/genes11080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Özdemi̇r S, Eltas Ö, Çulha MH. Expression profiles of lincRNA and mRNA related to milk yield and milk composition traits in the milk-derived exosomes of Holstein and Doğu Anadolu Kırmızısı cows. Turk J Vet Anim Sci. 2020;44:227–34. doi: 10.3906/vet-1911-20. [DOI] [Google Scholar]
- 129.Jing H, Chen Y, Qiu C, Guo MY. LncRNAs transcriptome analysis revealed potential mechanisms of selenium to mastitis in dairy cows. Biol Trace Elem Res. 2022;200:4316–24. doi: 10.1007/s12011-021-03042-0. [DOI] [PubMed] [Google Scholar]
- 130.Mu T, Hu H, Feng X, Ma Y, Wang Y, Liu J, et al. Screening and conjoint analysis of key lncRNAs for milk fat metabolism in dairy cows. Front Genet. 2022;13:772115. doi: 10.3389/fgene.2022.772115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ouyang Q, Hu S, Wang G, Hu J, Zhang J, Li L, et al. Comparative transcriptome analysis suggests key roles for 5-hydroxytryptamlne receptors in control of goose egg production. Genes. 2020;11:455. doi: 10.3390/genes11040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ning C, Ma T, Hu S, Xu Z, Zhang P, Zhao X, et al. Long non-coding RNA and mRNA profile of liver tissue during four developmental stages in the chicken. Front Genet. 2020;11:574. doi: 10.3389/fgene.2020.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Q, Qiao J, Zhang Z, Shang X, Chu Z, Fu Y, et al. Identification and analysis of differentially expressed long non-coding RNAs of Chinese Holstein cattle responses to heat stress. Anim Biotechnol. 2020;31:9–16. doi: 10.1080/10495398.2018.1521337. [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Kong L, Deng M, Lian Z, Han Y, Sun B, et al. Heat stress-responsive transcriptome analysis in the liver tissue of Hu sheep. Genes. 2019;10:395. doi: 10.3390/genes10050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ni Y, Wu F, Chen Q, Cai J, Hu J, Shen J, et al. Long noncoding RNA and mRNA profiling of hypothalamic-pituitary-mammary gland axis in lactating sows under heat stress. Genomics. 2020;112:3668–76. doi: 10.1016/j.ygeno.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 136.Mumtaz PT, Taban Q, Bhat B, Ahmad SM, Dar MA, Kashoo ZA, et al. Expression of lncRNAs in response to bacterial infections of goat mammary epithelial cells reveals insights into mammary gland diseases. Microb Pathog. 2022;162:105367. doi: 10.1016/j.micpath.2021.105367. [DOI] [PubMed] [Google Scholar]
- 137.Feng X, Li F, Wang F, Zhang G, Pang J, Ren C, et al. Genome-wide differential expression profiling of mRNAs and lncRNAs associated with prolificacy in Hu sheep. Biosci Rep. 2018;38:BSR20171350. doi: 10.1042/BSR20171350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lian Z, Zou X, Han Y, Deng M, Sun B, Guo Y, et al. Role of mRNAs and long non-coding RNAs in regulating the litter size trait in Chuanzhong black goats. Reprod Domest Anim. 2020;55:486–95. doi: 10.1111/rda.13642. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Hua R, Xue S, Li W, Wu L, Kang T, et al. mRNA/lncRNA expression patterns and the function of fibrinogen-like protein 2 in Meishan pig endometrium during the preimplantation phases. Mol Reprod Dev. 2019;86:354–69. doi: 10.1002/mrd.23109. [DOI] [PubMed] [Google Scholar]
- 140.Wichman L, Somasundaram S, Breindel C, Valerio DM, McCarrey JR, Hodges CA, et al. Dynamic expression of long noncoding RNAs reveals their potential roles in spermatogenesis and fertility. Biol Reprod. 2017;97:313–23. doi: 10.1093/biolre/iox084. [DOI] [PubMed] [Google Scholar]
- 141.Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020;21:102–17. doi: 10.1038/s41576-019-0184-5. [DOI] [PubMed] [Google Scholar]
- 142.Marques AC, Ponting CP. Intergenic lncRNAs and the evolution of gene expression. Curr Opin Genet Dev. 2014;27:48–53. doi: 10.1016/j.gde.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 143.Carelli FN, Liechti A, Halbert J, Warnefors M, Kaessmann H. Repurposing of promoters and enhancers during mammalian evolution. Nat Commun. 2018;9:4066. doi: 10.1038/s41467-018-06544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–31. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ulitsky I. Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat Rev Genet. 2016;17:601–14. doi: 10.1038/nrg.2016.85. [DOI] [PubMed] [Google Scholar]
- 146.Hezroni H, Ben-Tov Perry R, Meir Z, Housman G, Lubelsky Y, Ulitsky I. A subset of conserved mammalian long non-coding RNAs are fossils of ancestral protein-coding genes. Genome Biol. 2017;18:162. doi: 10.1186/s13059-017-1293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, et al. Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Werner MS, Sullivan MA, Shah RN, Nadadur RD, Grzybowski AT, Galat V, et al. Chromatin-enriched lncRNAs can act as cell-type specific activators of proximal gene transcription. Nat Struct Mol Biol. 2017;24:596–603. doi: 10.1038/nsmb.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Isoda T, Moore AJ, He Z, Chandra V, Aida M, Denholtz M, et al. Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer-promoter communication and T cell fate. Cell. 2017;171:103–19.:E18. doi: 10.1016/j.cell.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han X, Zhang J, Liu Y, Fan X, Ai S, Luo Y, et al. The lncRNA Hand2os1/Uph locus orchestrates heart development through regulation of precise expression of Hand2. Development. 2019;146:dev176198. doi: 10.1242/dev.176198. [DOI] [PubMed] [Google Scholar]
- 151.Ntini E, Louloupi A, Liz J, Muino JM, Marsico A, Ørom UAV. Long ncRNA A-ROD activates its target gene DKK1 at its release from chromatin. Nat Commun. 2018;9:1636. doi: 10.1038/s41467-018-04100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fanucchi S, Fok ET, Dalla E, Shibayama Y, Börner K, Chang EY, et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet. 2019;51:138–50. doi: 10.1038/s41588-018-0298-2. [DOI] [PubMed] [Google Scholar]
- 154.Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Grace Chen Y, et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 2018;173:1398–412.:E22. doi: 10.1016/j.cell.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang F, Yuan JH, Wang SB, Yang F, Yuan SX, Ye C, et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60:1278–90. doi: 10.1002/hep.27239. [DOI] [PubMed] [Google Scholar]
- 156.Kotzin JJ, Spencer SP, McCright SJ, Uthaya Kumar DB, Collet MA, Mowel WK, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–43. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Latos PA, Pauler FM, Koerner MV, Şenergin HB, Hudson QJ, Stocsits RR, et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–72. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- 158.Thebault P, Boutin G, Bhat W, Rufiange A, Martens J, Nourani A. Transcription regulation by the noncoding RNA SRG1 requires Spt2-dependent chromatin deposition in the wake of RNA polymerase II. Mol Cell Biol. 2011;31:1288–300. doi: 10.1128/MCB.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Elcheva IA, Spiegelman VS. The role of cis- and trans-acting RNA regulatory elements in leukemia. Cancers. 2020;12:3854. doi: 10.3390/cancers12123854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yap K, Mukhina S, Zhang G, Tan JSC, Ong HS, Makeyev EV. A short tandem repeat-enriched RNA assembles a nuclear compartment to control alternative splicing and promote cell survival. Mol Cell. 2018;72:525–40.:E13. doi: 10.1016/j.molcel.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee S, Kopp F, Chang TC, Sataluri A, Chen B, Sivakumar S, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tichon A, Perry RBT, Stojic L, Ulitsky I. SAM68 is required for regulation of Pumilio by the NORAD long noncoding RNA. Genes Dev. 2018;32:70–8. doi: 10.1101/gad.309138.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amodio N, Raimondi L, Juli G, Stamato MA, Caracciolo D, Tagliaferri P, et al. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol. 2018;11:63. doi: 10.1186/s13045-018-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–5. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–8. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLOS ONE. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Paraskevopoulou MD, Hatzigeorgiou AG. In: Long non-coding RNAs: methods and protocols. Feng Y, Zhang L, editors. New York: NY: Humana Press; 2016. Analyzing miRNA–lncRNA interactions; pp. 271–86. p. [DOI] [PubMed] [Google Scholar]
- 168.Wang JY, Liu X, Wu H, Ni P, Gu Z, Qiao Y, et al. CREB up-regulates long non-coding RNA. HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–83. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 170.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–6. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang J, Yang Y, Ma Y, Wang F, Xue A, Zhu J, et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. 2020;121:109627. doi: 10.1016/j.biopha.2019.109627. [DOI] [PubMed] [Google Scholar]
- 172.Zhou RS, Zhang EX, Sun QF, Ye ZJ, Liu JW, Zhou DH, et al. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. BMC Cancer. 2019;19:779. doi: 10.1186/s12885-019-5983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fan CN, Ma L, Liu N. Systematic analysis of lncRNA–miRNA–mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J Transl Med. 2018;16:264. doi: 10.1186/s12967-018-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest– and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]