Abstract
Korea, located in East Asia in the northern hemisphere, is experiencing severe climate changes. Specifically, the heat stress caused by global warming is negatively affecting the dairy sector, including milk production and reproductive performance, as the major dairy cattle Holstein-Friesian is particularly susceptible to heat stress. Here, we collected artificial insemination and pregnancy data of the Holstein and the Jersey cows from a dairy farm from 2014 to 2021 and analyzed the association between the conception rate and the temperature-humidity index, calculated using the data from the closest official weather station. As the temperature-humidity index threshold increased, the conception rate gradually decreased. However, this decrease was steeper in the Holstein breed than in the Jersey one at a temperature-humidity index threshold of 75. To evaluate the effects of heat stress on the oocyte quality, we examined the nuclear and cytoplasmic maturation of Holstein (n = 158, obtained from six animals) and Jersey oocytes (n = 123, obtained from six animals), obtained by ovum pick-up. There were no differences in the nuclear maturation between the different conditions (heat stress: 40.5°C, non- heat stress: 37.5°C) or breeds, although the Holstein oocytes seemed to have a lower metaphase II development (p = 0.0521) after in vitro maturation under heat stress conditions. However, we found that the Holstein metaphase II oocytes exposed to heat stress presented more reactive oxygen species and a peripheral distribution of the mitochondria, compared to those of the Jersey cattle. Here, we show that weather information from local meteorological stations can be used to calculate the temperature-humidity index threshold at which heat stress influences the conception rate, and that the Jersey cows are more tolerant to heat stress in terms of their conception rate at a temperature-humidity index over 75. The lower fertility of the Holstein cows is likely attributed to impaired cytoplasmic maturation induced by heat stress. Thus, the Jersey cows can be a good breed for the sustainability of dairy farms for addressing climate changes in South Korea, as they are more resistant to hyperthermia.
Keywords: Dairy cattle, Heat stress, Oocytes, Reactive oxygen species, Mitochondria
INTRODUCTION
Summer heat stress (HS) is not only associated with a reduced milk production, but also with a lower fertility, including conception rate (CR), in dairy cattle [1,2]. The Korean peninsula, located in East Asia in the northern hemisphere, is experiencing severe climate changes, and in the summer of 2018 a continuously extreme heat wave with the highest temperatures (above 40°C) observed since meteorological records are available was recorded [3]. Particularly, high-producing cows such as the Holstein-Friesian breed (approximately > 99% the dairy cattle in Korea) are more susceptible to HS in terms of fertility, lactation, growth and disease [4–6]. HS can lead to reduced feed-intake and hormonal imbalance, resulting in a decrease in reproductive performance and birth weight of the calves in heat stressed pregnant cows. For instance, placental hormones especially estrone sulfate during the late gestation is decreased in heat stressed cows by placental dysfunction, leading to retarded fetal development [7]. Furthermore, survival rate before puberty and subsequent reproductive performance of these calves is reduced by impaired immunity [8,9].
From a cytological point of view, mitochondria are evenly distributed in cytoplasm of oocytes and produced adenosine triphosphate (ATP) under normal conditions. However, HS impairs the localization of mitochondria in oocytes. It affects ATP synthesis and therefore reduced ATP production is impairs oocytes maturation and, subsequently embryonic development. In additions, reactive oxygen species (ROS) is closely related to mitochondrial function. Increase of ROS levels above the required threshold by stressors are compromise the oocyte maturation [10].
In 2012, Jersey cow embryos were imported from Canada and the United States with the aim to improve the fat and protein contents of dairy products and to address climate changes, making dairy farms more sustainable. The Jersey breed was more tolerant to heat than the Holstein cattle in terms of milk production and reproductive performance [11,12]. For example, under HS conditions (temperature-humidity index [THI] > 68), the average daily milk yield significantly decreased from 35.6 to 34.2 kg/d in the Holstein cows, whereas it increased significantly in the Jersey cattle from 25.9 to 26.6 kg/d [11]. According to the reports of the U.S. Department of Agriculture, the Jersey breed had the highest reproductive performance (> 40%), including CR, compared to the Brown Swiss (~35%) and the Holstein cows (< 30%), due to their even performance throughout the year without a particular decrease in a specific season [12–14].
In this study, we first compared the CR of the Holstein and the Jersey cows in our institutional dairy farm and analyzed the association of the CR with the THI, calculated using information from a close local weather station. Subsequently, we studied oocyte maturation; oocytes were obtained from the same cows studied in the CR analysis, utilizing the ovum pick-up (OPU) technique. We focused on cytoplasmic oocyte maturation, as we had previously described several HS-related microRNA biomarkers [15] associated with the cytoskeleton, mitochondrial intracellular ROS, and ATP synthesis rate that can be used as indicators for oocyte quality [10].
The aims of this study were to investigate the effects of HS on the CR of the Holstein and the Jersey cows using public official meteorological data, and to assess the effects of HS on the quality of the oocytes obtained from both breeds used in the CR analysis. To the best of our knowledge, this is the first study to objectively explain why the fertility of the Jersey cows is higher than that of the Holstein breed under HS conditions.
MATERIALS AND METHODS
Data source and collection
A retrospective epidemiological survey was performed using data from the CR of the Holstein and the Jersey dairy cattle in Korea. Animal data including cow identification, artificial insemination (AI) dates, parity, and pregnancy diagnosis results were collected from our dairy innovation center located in Cheonan, Korea (36°5967’ N, 127°1059’ E), from 2014 to 2021. The data were managed using Excel spreadsheets (MS Office 2021, Microsoft, Redmond, WA, USA). Ambient temperature (AT, °C) and relative humidity (RH, %) data collected from the closest official meteorological station (17.34 km; 36.7767 N, 127.1213 E; 24.89 meters above mean sea level) were used as a surrogate for on-farm environmental monitoring. The daily mean THI was calculated as follows [15–17]: THI = (0.8 × AT) + [RH × (AT – 14.4)] + 46.4.
Animals
All dairy cows were housed in a concrete floor facility, bedded with sawdust, and fed total mixed rations. Diet was formulated according to the guidelines included in “Nutrient requirements of dairy cattle (2001)” [18], and the cows were fed twice a day to meet their nutrient requirements. They were milked three times (interval 8 hr) with Lely Astronaut automatic milking system (Lely, Maassluis, Netherlands). Holstein and Jersey cows produced an average of 10,326 kg and 6,611 kg in 305 days, respectively. This study used a 2 × 2 crossover design (two dairy breed groups × two temperature values of in vitro maturation [IVM]), performing three consecutive OPU sessions, each separated by a 30-day interval, and conducting three replicates in each session. In all sessions, we evaluated the effects of HS on aspirated immature oocytes in the Holstein and the Jersey breeds by controlling the incubator temperature (38.5°C vs. 40.5°C) during the IVM.
Veterinarians at our dairy innovation center checked the medical condition of the animals before the start of each OPU session. Twelve dairy cows (Holstein-Friesian, n = 6; Jersey breed, n = 6) with ages between 2 and 4 years were deemed healthy to participate in the study. All cattle had body condition scores ranging from 3.0 to 3.25. They were managed in the same place and fed the same amount (2.5 kg) of hay twice a day, based on rice straw and tall fescue available for free to meet their nutrient requirements, as well as fresh water and minerals (mineral block) ad libitum. All experimental protocols performed on the animals were approved by the National Institute of Animal Science Animal Care and Ethics Committee in Korea (approval number: NIAS-068).
Ovum pick-up procedure
The selected animals were subjected to three consecutive OPU sessions with approximately a 30-day interval between each session, performed by the same operator in a cool season. On the day the OPU was performed, the animals were contained in a manual squeeze chute for cattle. All animals were administered a local anesthetic of 0.3 mL of 7 mg Xylazine (Rompun, Bayer Korea, Seoul, Korea) and 4 mL of 2% lidocaine HCl (Daihan Pharm, Seoul, Korea) before the procedure to ease the pain on the area and facilitate the handling of the ovaries through the rectum. The external area was cleaned using gauze soaked in 70% alcohol and dried with tissues. All visible ovarian follicles 2–5 mm in diameter were aspirated by transrectal ultrasonography using a portable scanner (4Vet Slim, Draminski, Olsztyn, Poland) with a 2–8 MHz convex array transducer, a 16-gauge needle, and a vacuum device. Aspirated immature oocytes were stored in 50 mL conical tubes containing 15 mL collection medium consisting of Hepes-buffered TCM 199 (Medium199, Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal calf serum (Gibco Life Technologies, Waltham, MA, USA), 50 ng/mL penicillin and streptomycin, and 10 IU/mL sodium heparin (Sigma-Aldrich) and were transported in a portable incubator at 37°C to be immediately moved to the laboratory for further in vitro experiments.
In vitro oocyte maturation
The retrieved cumulus oocyte complexes (COCs) were evaluated and classified into four grades according to their morphology: Good (the oocyte complex presents more than three compact and intact layers of cumulus cells and homogenous cytoplasm), regular (at least one layer of cumulus cells), denuded (partly or not covered by a cumulus cell layer), and atretic grade (dark cumulus oophorus and degenerated cytoplasm) [19]. After classification, the number of oocytes assigned to each grade was recorded, and atretic oocytes were discarded. Good, regular, and denuded oocytes were subjected to IVM. Retrieved immature oocytes were first washed three times with a commercially available washing media consisting of Hepes-buffered TCM 199 and other components (MKbiotec, Daejeon, Korea). The COCs aspirated in each OPU session were separated by breed (Holstein, Jersey) and divided into four groups according to the incubator temperature (non-HS [NHS]: 38.5°C, HS: 40.5°C) and the purpose of the staining (ROS, mitochondria). We set the NHS and HS conditions as 38.5°C and 40.5°C, respectively, based on the rectal temperature and the findings reported in our and other previous studies [15,20]. The COCs were transferred to a 4-well dish (Nunc, Roskilde, Denmark) containing 200 μL maturation medium (MKbiotec) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1 μg/mL follicle stimulating hormone (Sigma-Aldrich) and were allowed to mature for 21 h at 38.5°C or 40.5°C, with a humid atmosphere of 5% CO2.
Analysis of reactive oxygen species
After IVM, the oocytes were washed three times with Hepes-buffered TCM 199 supplemented with 0.3% BSA (washing media, MKbiotec) and the cumulus cells covering the oocytes were removed by slow and gentle pipetting. Intracellular ROS in the denuded oocytes were stained with 5-(and-6-)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen, Carlsbad, CA, USA), a reliable ROS fluorogenic marker in living cells. We added 25 μL 10 mM carboxy-H2DCFDA to 10 mL IVM medium based on Hepes-buffered TCM 199 (working solution) to 4-well plates (Nunc), and the denuded oocytes were then transferred to these plates and incubated for 30 min at 38.5°C, protected from light. Stained oocytes were observed with an inverted fluorescent microscope (ECLIPSE Ti-DH, Nikon, Tokyo, Japan). The NIS-elements BR version 4.30 imaging software (Nikon) was used to capture the fluorescent images. The mean green fluorescent (ROS) intensity of each oocyte was calculated using the ImageJ software version 1.53a (National Institutes of Health, Bethesda, MD, USA) by drawing a circle around the border of the oocytes.
Mitochondrial distribution analysis
After IVM, the oocytes were washed three times with Hepes-buffered TCM 199 supplemented with 0.3% BSA (washing media, MKbiotec) and the cumulus cells covering the oocytes were removed by slow and gentle pipetting. The mitochondria of the denuded oocytes were stained with MitoTracker Red CMXRos (Invitrogen), a X-rosamine derivative. We added 1 μL of 1 mM MitoTracker supplemented with DMSO to 10 ml maturation medium (working solution), for a final concentration of 100 nM, to 4-well plates (Nunc), and the denuded oocytes were then transferred to these plates and incubated for 30 min at 38.5°C, protected from light. After incubation, the oocytes were washed three times with washing media (MKbiotec) and then fixed for 60 min at 38.5°C in 2% paraformaldehyde. Subsequently, they were transferred to a glass slide with a 1 μL-drop of Dulbecco’s phosphate-buffered saline solution supplemented with 10 μg/mL Hoechst 33342 (Invitrogen); we waited for 5 min at room temperature (25°C) in the dark before observing the nucleus of the oocytes. Four very small and thin drops of vaseline were placed on the glass slide, pressed down gently with a cover glass and sealed with clear nail varnish. The mitochondrial distribution pattern of each oocyte was observed with a confocal laser-scanning microscope (Zeiss LSM 710 META, Zeiss, Oberkochen, Germany) and the objective patterns and intensity were measured along a line drawn across MII oocytes using the Line Profile Tool (line width = 20) from the ImageJ software version 1.53a (National Institutes of Health).
Statistical analysis
The percentage of first polar body extrusion and the ROS levels in oocytes at different IVM temperatures and breeds were analyzed by ANOVA using the SPSS 26.0 statistical software (SPSS, Chicago, IL, USA) after a descriptive statistics analysis to check the normality and homogeneity of the variances. p < 0.05 was considered statistically significant. The data were expressed as mean ± SEM. The association between each THI threshold value (50 to 78) and the CR was evaluated in a general linear model (GLM) using R version 4.0.5. [21]. Significance was set at a p-value less than 0.05.
RESULTS
Effect of the different THI thresholds on the conception rate
We first checked the parities of the Holstein and the Jersey cows in our retrospective study because the parities of the cows affect the CR. Total 2541 and 474 of AI data were recorded for Holstein (n = 584) and Jersey (n = 106), respectively. The overall parity proportions were similar between the two breeds, although the Holstein cows had more primiparous animals (35%) than the Jersey cows (25%) (Fig. 1A). Moreover, the Holstein and the Jersey cows employed in this CR analysis under HS conditions (THI > 72) had a similar parity proportion (from 22.5% to 30% in heifers or in 1st and 2nd parity cows) (Fig. 1B).
Fig. 1. The parity of the Holstein and the Jersey cattle.
(A) Proportion of parities in the Holstein and the Jersey cattle. (B) Proportion of parities in the Holstein and the Jersey cattle exposed to heat stress (temperature-humidity index > 72).
The maximum, minimum, and mean temperature values in this study were 36°C, −18°C, and 11.3 ± 9.8°C, respectively, and the maximum, minimum, and mean relative humidity values were 100%, 5%, and 68.8 ± 12.8%, respectively. The overall CR values were 30.0% in the Holstein cattle and 39.9% in the Jersey cows.
The CR was calculated from the herd reproductive data. We set a THI interval from 50 to 78 to determine the point where the CR decreased according to the mean THI on the day of AI. An increase in the THI threshold was associated with a gradual decline in the CR. In our GLM, the B slope started to decrease considerably at a THI threshold of 75 in both breeds. However, the decrease of the CR in the Holstein breed as the THI threshold increased was steeper than that of the Jersey breed (Table 1 and Fig. 2).
Table 1. Results of the general linear model of the mean THI thresholds associated with the CR in the Holstein (AI = 2,541) and the Jersey (AI = 474) breeds.
| THI threshold | Holstein | Jersey | ||||||
|---|---|---|---|---|---|---|---|---|
| B slope | n ≥ THI | p | CR (%) | B slope | n ≥ THI | p | CR (%) | |
| 50 | −0.90 | 1,430 | < 0.05 | 28.9 | −0.45 | 311 | < 0.05 | 38.9 |
| 51 | −0.91 | 1,390 | < 0.05 | 28.7 | −0.45 | 303 | < 0.05 | 38.9 |
| 52 | −0.91 | 1,322 | < 0.05 | 28.7 | −0.45 | 292 | < 0.05 | 39.0 |
| 53 | −0.91 | 1,266 | < 0.05 | 28.8 | −0.44 | 276 | < 0.05 | 39.1 |
| 54 | −0.90 | 1,219 | < 0.05 | 29.0 | −0.44 | 268 | < 0.05 | 39.2 |
| 55 | −0.89 | 1,180 | < 0.05 | 29.1 | −0.44 | 263 | < 0.05 | 39.2 |
| 56 | −0.87 | 1,116 | < 0.05 | 29.5 | −0.45 | 256 | < 0.05 | 39.1 |
| 57 | −0.90 | 1,038 | < 0.05 | 29.0 | −0.45 | 251 | < 0.05 | 39.0 |
| 58 | −0.89 | 1,005 | < 0.05 | 29.1 | −0.45 | 246 | < 0.05 | 39.0 |
| 59 | −0.92 | 937 | < 0.05 | 28.5 | −0.45 | 244 | < 0.05 | 38.9 |
| 60 | −0.92 | 897 | < 0.05 | 28.5 | −0.45 | 241 | < 0.05 | 39.0 |
| 61 | −0.92 | 854 | < 0.05 | 28.6 | −0.46 | 232 | < 0.05 | 38.8 |
| 62 | −0.93 | 811 | < 0.05 | 28.4 | −0.45 | 229 | < 0.05 | 38.9 |
| 63 | −0.93 | 764 | < 0.05 | 28.3 | −0.45 | 224 | < 0.05 | 38.8 |
| 64 | −0.94 | 736 | < 0.05 | 28.1 | −0.46 | 222 | < 0.05 | 38.7 |
| 65 | −0.94 | 625 | < 0.05 | 28.2 | −0.46 | 217 | < 0.05 | 38.7 |
| 66 | −0.95 | 582 | < 0.05 | 27.8 | −0.46 | 215 | < 0.05 | 38.6 |
| 67 | −0.97 | 529 | < 0.05 | 27.4 | −0.48 | 206 | < 0.05 | 38.3 |
| 68 | −0.97 | 484 | < 0.05 | 27.5 | −0.49 | 190 | < 0.05 | 37.9 |
| 69 | −1.01 | 445 | < 0.05 | 26.7 | −0.53 | 170 | < 0.05 | 37.1 |
| 70 | −1.05 | 393 | < 0.05 | 26.0 | −0.52 | 161 | < 0.05 | 37.3 |
| 71 | −1.02 | 339 | < 0.05 | 26.5 | −0.49 | 155 | < 0.05 | 38.1 |
| 72 | −1.08 | 292 | < 0.05 | 25.3 | −0.49 | 145 | < 0.05 | 37.9 |
| 73 | −1.13 | 245 | < 0.05 | 24.5 | −0.51 | 112 | < 0.05 | 37.5 |
| 74 | −1.04 | 207 | < 0.05 | 26.1 | −0.54 | 87 | < 0.05 | 36.8 |
| 75 | −1.16 | 155 | < 0.05 | 23.9 | −0.61 | 71 | < 0.05 | 35.2 |
| 76 | −1.34 | 120 | < 0.05 | 20.8 | −0.72 | 52 | < 0.05 | 32.7 |
| 77 | −1.53 | 84 | < 0.05 | 17.9 | −0.92 | 42 | < 0.05 | 28.6 |
| 78 | −1.67 | 57 | < 0.05 | 15.8 | −0.69 | 18 | = 0.05 | 33.3 |
THI, temperature-humidity index; CR, conception rate; AI, artificial insemination.
Fig. 2. Holstein and Jersey breeds CR considering temperature-humidity index thresholds from 50 to 78 on the day of AI.

CR, conception rate; AI, artificial insemination.
In vitro maturation of oocytes exposed to heat stress
Germinal vesicle (GV) stage immature oocytes were retrieved by OPU from the Holstein and the Jersey cattle and were subsequently exposed to two different temperatures (NHS: 38.5°C, HS: 40.5°C) during their IVM. After 21 h, nuclear maturation was assessed by first polar body extrusion. There were no statically significant differences in the nuclear maturation rates between the two breeds or between the HS and NHS conditions. However, the rate was slightly lower in the Holstein cows (77.5%, p = 0.0521, Table 2) under HS conditions.
Table 2. Effects of heat stress on the in vitro maturation of Holstein and Jersey oocytes.
| Breed | Temperature | No. of GV oocytes | No. of oocytes with the 1st PB (%) |
|---|---|---|---|
| Holstein | 38.5°C | 78 | 69 (88.4) |
| 40.5°C | 80 | 62 (77.5) | |
| Jersey | 38.5°C | 59 | 52 (88.1) |
| 40.5°C | 64 | 53 (82.8) |
Fisher’s exact with correction p = 0.0521 (Holstein) 0.2823 (Jersey).
GV, germinal vesicle; PB, polar body.
Effects of heat stress on oocyte cytoplasmic maturation
We selected in vitro-matured oocytes with a polar body and evaluated their intracellular ROS levels to determine the effect of HS on cytoplasmic maturation. The ROS levels differed slightly between the Holstein (19.30 ± 0.74) and the Jersey (16.46 ± 1.10) oocytes under normothermic conditions (p = 0.029). However, there was a greater difference in the ROS levels between the two breeds when their oocytes had been exposed to HS (40.5°C) during IVM, with the Holstein oocytes showing increased average ROS levels (32.20 ± 0.91) compared to the Jersey oocytes (20.34 ± 1.07) (p = 0.000). Interestingly, HS induced an increase in the intracellular ROS levels in the Jersey oocytes, but the levels were similar to those of NHS Holstein oocytes (Figs. 3A and 3B).
Fig. 3. Intracellular ROS levels in metaphase II oocytes.
(A) Representative images of stained (green) intracellular ROS levels in the Holstein and the Jersey oocytes under NHS and HS conditions. (B) Relative intensity of ROS levels in the different breeds under NHS and HS conditions. Significantly different level: *p < 0.05, **p < 0.01, ***p < 0.001. ROS, reactive oxygen species; NHS, non-heat stress; HS, heat stress.
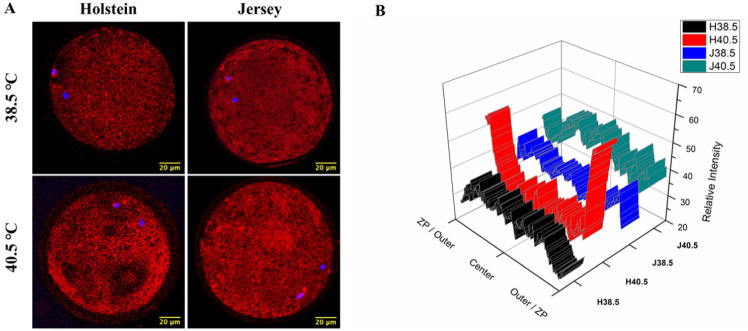
Subsequently, we investigated the effect of HS on the distribution of the mitochondria in matured oocytes via fluorescence intensity after MitoTracker Red CMXRos uptake. Overall, the intensities were not different between the breeds or the IVM conditions. Under NHS conditions, the mitochondria were homogeneously distributed in the matured oocytes, regardless of the breed. However, we observed two different patterns in the distribution of active mitochondria in the cytoplasm of the Holstein oocytes compared with the Jersey ones in the IVM under HS condition. The HS-Holstein oocytes showed a more intense peripheral distribution in the ooplasm, while the mitochondria of the Jersey oocytes were more homogenously distributed in the cytoplasm (Figs. 4A and 4B).
Fig. 4. Intracellular mitochondrial distribution in MII oocytes.
(A) Representative images of stained (MitoTracker Red) mitochondria in the Holstein and the Jersey oocytes under non-heat stress and heat stress conditions. Scale bar = 20 μm. (B) Mitochondrial distribution pattern expressed as a relative fluorescence intensity value by drawing a line across the MII oocytes. Each graph in Fig. 2 corresponded with the same position (row and column) in Fig. 2A. ZP, zona pellucida; MII, metaphase II.
DISCUSSION
We first analyzed the CR of the Holstein and the Jersey cattle in Korea from 2014 to 2021, particularly focusing on the association between HS and the CR, and showed that the Jersey cows have a greater tolerance to heat in terms of their reproductive performance than the Holstein breed. In this study, THI values were calculated based on public data available from a close weather station to estimate the effects of HS on the CR of the Holstein and the Jersey cattle. The THI thus calculated were not accurate, as the weather conditions on-farm were different from those at the weather station, and HS can be underestimated [22]. However, several studies reported that the THI calculated from the weather station data could be used as a HS indicator [23–25]. In addition, the CR and the weather data were obtained from the same experimental farm and its closest weather station, and AI was performed always following the same procedures, suggesting that the weather data can be used to determine the THI threshold at which the CR is affected, and to compare the heat tolerance between the Holstein and the Jersey cattle. In our retrospective study, we found that the overall CR was approximately 10% higher in the Jersey breed compared to the Holstein cows. Interestingly, the differences in the CR between the Holstein and the Jersey cows increased as the THI increased, indicating that the two breeds may have different responses to HS in terms of oocyte or early embryo development.
The CR of dairy cattle that has experienced HS reportedly decreases by 20% to 30% [26], due to a reduced dry matter intake and an alteration of the hormonal balance, including circulating estradiol [27,28], luteinizing hormone, and progesterone levels [29,30], which lead to a poor oocyte quality [31,32]. We were interested in the oocyte maturation process as immature oocytes must undergo a correct nuclear and cytoplasmic maturation to develop into MII oocytes. To evaluate the effects of HS on the competence of the oocytes, we first examined the nuclear maturation by the 1st polar body extrusion, and we observed no significant differences between the breeds or the IVM conditions, although HS seemed to affect the nuclear maturation of the Holstein oocytes. Subsequently, we investigated the effects of HS on cytoplasmic maturation by observing the mitochondrial distribution and ROS levels. Nuclear maturation (extrusion of the 1st polar body) does not imply that the MII oocytes will also accomplish cytoplasmic maturation [33,34], as this involves the reorganization of organelles such as the mitochondria, that play a key role in determining the quality of the oocytes [33–35].
Here, we showed that the HS-exposed Holstein oocytes produced more ROS than the Jersey oocytes, suggesting that excessive ROS may lead to cytoplasmic defects and abnormal chromosomal segregation in the oocytes in the summer, as an imbalance between ROS production and the antioxidative defenses can induce DNA damage and apoptosis [10,36,37]. In addition, we also found a more intense peripheral distribution of the mitochondria rather than a homogeneous one in the Holstein oocytes exposed to HS during IVM. Previous studies reported that bovine oocyte quality can be classified into four grades according to the mitochondrial distribution pattern in the cytoplasm of their oocytes [10,38]. Moreover, mitochondria were homogeneously distributed in the cytoplasm of good-quality oocytes, whereas they were heterogeneously distributed and clustered around the periphery of the ooplasm in poor-quality oocytes [10,36,38]. Interestingly, the proportion of poor-quality oocytes that contained unevenly distributed mitochondria in their cytoplasm increased under HS conditions in the Holstein cows [39], as cytoskeletal elements such as microtubules and actin filaments in the oocytes are closely related to the mitochondrial distribution pattern, and also are highly sensitive to HS [10,39].
Our in vitro assessment of the nuclear and cytoplasmic maturation of the oocytes obtained by OPU can explain why the Jersey cows had a higher CR under HS conditions in our retrospective study, and our findings are consistent with the results of previous studies in which more ROS were generated, and the mitochondria were not homogenously distributed in the MII oocytes of the Holstein cows and other cattle after IVM under HS conditions [10,36,39].
This is the first report comparing the fertility of the Holstein and the Jersey breeds both in vitro (oocytes) and in vivo (CR) under HS conditions. In this study, we demonstrated that weather information from a local meteorological station can be used to calculate the THI threshold for the influence of HS on the CR of cattle, and that the Jersey breed is more tolerant than the Holstein one to HS in terms of their CR under HS conditions. Moreover, the incomplete cytoplasmic maturation of the Holstein oocytes exposed to HS, including an increase in ROS production and a peripheral distribution of the mitochondria, supports our observation of a lower CR of the Holstein cows with a THI over 75.
Acknowledgements
We thank the coworker of Dairy Science Division at NIAS for helping us work on this project (PJ015006) and JS Lee and JY Ban, researchers at Chungnam National University for helping us with data analysis.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
Not applicable.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Lee J, Choi I.
Data curation: Lee J, Choi I.
Formal analysis: Lee J, Kim Doosan, Choi I.
Methodology: Lee J, Choi I.
Software: Lee J, Jeon E, Choi I.
Validation: Lee J, Choi I.
Investigation: Lee J, Son J, Kim Donghyeon, Jung D, Han M, Ha S, Hwang S, Choi I.
Writing - original draft: Lee J, Choi I.
Writing - review & editing: Lee J, Kim Doosan, Son J, Kim Donghyeon, Jeon E, Jung D, Han M, Ha S, Hwang S, Choi I.
Ethics approval and consent to participate
All experimental protocols performed on the animals were approved by the National Institute of Animal Science Animal Care and Ethics Committee in Korea (approval number: NIAS-068).
REFERENCES
- 1.Ray DE, Halbach TJ, Armstrong DV. Season and lactation number effects on milk production and reproduction of dairy cattle in Arizona. J Dairy Sci. 1992;75:2976–83. doi: 10.3168/jds.S0022-0302(92)78061-8. [DOI] [PubMed] [Google Scholar]
- 2.Lim DH, Mayakrishnan V, Ki KS, Kim Y, Kim TI. The effect of seasonal thermal stress on milk production and milk compositions of Korean Holstein and Jersey cows. Anim Biosci. 2021;34:567–74. doi: 10.5713/ajas.19.0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek JB, Kwon Y. Analysis of domestic heatwave research trends. J Soc Disaster Inf. 2021;17:755–68. [Google Scholar]
- 4.Aguilar I, Misztal I, Tsuruta S. Short communication: genetic trends of milk yield under heat stress for US Holsteins. J Dairy Sci. 2010;93:1754–8. doi: 10.3168/jds.2009-2756. [DOI] [PubMed] [Google Scholar]
- 5.Nardone A, Ronchi B, Lacetera N, Ranieri MS, Bernabucci U. Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci. 2010;130:57–69. doi: 10.1016/j.livsci.2010.02.011. [DOI] [Google Scholar]
- 6.Biffani S, Bernabucci U, Vitali A, Lacetera N, Nardone A. Short communication: effect of heat stress on nonreturn rate of Italian Holstein cows. J Dairy Sci. 2016;99:5837–43. doi: 10.3168/jds.2015-10491. [DOI] [PubMed] [Google Scholar]
- 7.Tao S, Dahl GE, Laporta J, Bernard JK, Orellana Rivas RM, Marins TN. PHYSIOLOGY SYMPOSIUM: effects of heat stress during late gestation on the dam and its calf. J Anim Sci. 2019;97:2245–57. doi: 10.1093/jas/skz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dado-Senn B, Laporta J, Dahl GE. Carry over effects of late-gestational heat stress on dairy cattle progeny. Theriogenology. 2020;154:17–23. doi: 10.1016/j.theriogenology.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Moore RB, Fuquay JW, Drapala WJ. Effects of late gestation heat stress on postpartum milk production and reproduction in dairy cattle. J Dairy Sci. 1992;75:1877–82. doi: 10.3168/jds.S0022-0302(92)77947-8. [DOI] [PubMed] [Google Scholar]
- 10.Roth Z. Symposium review: reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J Dairy Sci. 2018;101:3642–54. doi: 10.3168/jds.2017-13389. [DOI] [PubMed] [Google Scholar]
- 11.Smith DL, Smith T, Rude BJ, Ward SH. Short communication: comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J Dairy Sci. 2013;96:3028–33. doi: 10.3168/jds.2012-5737. [DOI] [PubMed] [Google Scholar]
- 12.Badinga L, Collier RJ, Thatcher WW, Wilcox CJ. Effects of climatic and management factors on conception rate of dairy cattle in subtropical environment. J Dairy Sci. 1985;68:78–85. doi: 10.3168/jds.S0022-0302(85)80800-6. [DOI] [PubMed] [Google Scholar]
- 13.Norman HD, Wright JR, Hubbard SM, Miller RH, Hutchison JL. Reproductive status of Holstein and Jersey cows in the United States. J Dairy Sci. 2009;92:3517–28. doi: 10.3168/jds.2008-1768. [DOI] [PubMed] [Google Scholar]
- 14.Norman HD, Guinan FL, Megonigal JH, Dürr JW. Reproductive status of cows in Dairy Herd Improvement programs and bred using artificial insemination (2020) [Internet] [[cited 2022 Sep 13]];Council on Dairy Cattle Breeding. 2020 https://queries.uscdcb.com/publish/dhi/current/reproall.html . [Google Scholar]
- 15.Lee J, Lee S, Son J, Lim H, Kim E, Kim D, et al. Analysis of circulating-microRNA expression in lactating Holstein cows under summer heat stress. PLOS ONE. 2020;15:e0231125. doi: 10.1371/journal.pone.0231125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikmen S, Hansen PJ. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J Dairy Sci. 2009;92:109–16. doi: 10.3168/jds.2008-1370. [DOI] [PubMed] [Google Scholar]
- 17.Mader TL, Davis MS, Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci. 2006;84:712–9. doi: 10.2527/2006.843712x. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council . Nutrient requirements of dairy cattle. 7th rev. ed. Washington, DC: The National Academies Press; 2001. [Google Scholar]
- 19.Seneda MM, Esper CR, Garcia JM, Oliveira JA, Vantini R. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim Reprod Sci. 2001;67:37–43. doi: 10.1016/S0378-4320(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 20.Muller CJC, Botha JA. Effect of summer climatic conditions on different heat tolerance indicators in primiparous Friesian and Jersey cows. S Afr J Anim Sci. 1993;23:98–103. [Google Scholar]
- 21.R Core Team R: a language and environment for statistical computing [Internet] [[cited 2022 Sep 13]];R Foundation for Statistical Computing. 2021 https://www.R-project.org/ [Google Scholar]
- 22.Ouellet V, Cabrera VE, Fadul-Pacheco L, Charbonneau É. The relationship between the number of consecutive days with heat stress and milk production of Holstein dairy cows raised in a humid continental climate. J Dairy Sci. 2019;102:8537–45. doi: 10.3168/jds.2018-16060. [DOI] [PubMed] [Google Scholar]
- 23.Ravagnolo O, Misztal I, Hoogenboom G. Genetic component of heat stress in dairy cattle, development of heat index function. J Dairy Sci. 2000;83:2120–5. doi: 10.3168/jds.S0022-0302(00)75094-6. [DOI] [PubMed] [Google Scholar]
- 24.Bernabucci U, Biffani S, Buggiotti L, Vitali A, Lacetera N, Nardone A. The effects of heat stress in Italian Holstein dairy cattle. J Dairy Sci. 2014;97:471–86. doi: 10.3168/jds.2013-6611. [DOI] [PubMed] [Google Scholar]
- 25.Carabaño MJ, Logar B, Bormann J, Minet J, Vanrobays ML, Díaz C, et al. Modeling heat stress under different environmental conditions. J Dairy Sci. 2016;99:3798–814. doi: 10.3168/jds.2015-10212. [DOI] [PubMed] [Google Scholar]
- 26.de Rensis F, Marconi P, Capelli T, Gatti F, Facciolongo F, Franzini S, et al. Fertility in postpartum dairy cows in winter or summer following estrus synchronization and fixed time AI after the induction of an LH surge with GnRH or hCG. Theriogenology. 2002;58:1675–87. doi: 10.1016/S0093-691X(02)01075-0. [DOI] [PubMed] [Google Scholar]
- 27.Wilson SJ, Kirby CJ, Koenigsfeld AT, Keisler DH, Lucy MC. Effects of controlled heat stress on ovarian function of dairy cattle.2. Heifers. J Dairy Sci. 1998;81:2132–8. doi: 10.3168/jds.S0022-0302(98)75789-3. [DOI] [PubMed] [Google Scholar]
- 28.Wilson SJ, Marion RS, Spain JN, Spiers DE, Keisler DH, Lucy MC. Effects of controlled heat stress on ovarian function of dairy cattle. 1. Lactating cows. J Dairy Sci. 1998;81:2124–31. doi: 10.3168/jds.S0022-0302(98)75788-1. [DOI] [PubMed] [Google Scholar]
- 29.Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci. 2000;60-61:535–47. doi: 10.1016/S0378-4320(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 30.Wolfenson D, Roth Z. Impact of heat stress on cow reproduction and fertility. Anim Front. 2019;9:32–8. doi: 10.1093/af/vfy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira RM, Ayres H, Chiaratti MR, Ferraz ML, Araújo AB, Rodrigues CA, et al. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J Dairy Sci. 2011;94:2383–92. doi: 10.3168/jds.2010-3904. [DOI] [PubMed] [Google Scholar]
- 32.Gendelman M, Roth Z. Seasonal effect on germinal vesicle-stage bovine oocytes is further expressed by alterations in transcript levels in the developing embryos associated with reduced developmental competence. Biol Reprod. 2012;8:1–9. doi: 10.1095/biolreprod.111.092882. [DOI] [PubMed] [Google Scholar]
- 33.Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82:E14–23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- 34.Mao L, Lou H, Lou Y, Wang N, Jin F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod Biomed Online. 2014;28:284–99. doi: 10.1016/j.rbmo.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Reader KL, Stanton JAL, Juengel JL. The role of oocyte organelles in determining developmental competence. Biology. 2017;6:35. doi: 10.3390/biology6030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sa SJ, Jeong J, Cho J, Lee SH, Choi I. Heat waves impair cytoplasmic maturation of oocytes and preimplantation development in Korean native cattle (Hanwoo) Korean J Agric Sci. 2018;45:493–8. [Google Scholar]
- 37.Yaacobi-Artzi S, Shimoni C, Kalo D, Hansen PJ, Roth Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology. 2020;158:477–89. doi: 10.1016/j.theriogenology.2020.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P. Gonçalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–9. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 39.Gendelman M, Roth Z. Incorporation of coenzyme q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol Reprod. 2012;87:118. doi: 10.1095/biolreprod.112.101881. [DOI] [PubMed] [Google Scholar]