Abstract
A patient presented with severe tricuspid regurgitation 20 years after dual-chamber pacing. Transesophageal echocardiography suggested ventricular pacing wire adherence to the tricuspid valve (TV) and atrial wire prolapse across the tricuspid annulus. Surgical extraction of the pacing wires revealed TV commissural fusion and subvalvular thickening causing tricuspid stenosis, requiring TV replacement. (Level of Difficulty: Intermediate.)
Key Words: pacing wires, tricuspid regurgitation, tricuspid stenosis
Central Illustration
History of Presentation
A 42-year-old woman presented with exertional breathlessness, neck and abdominal pulsations, nausea, and palpitations. Her pulse rate was 82 beats/min, blood pressure was 112/81 mm Hg, and jugular venous pulse was visible at +5 cm. Thoracic auscultation was unremarkable with no signs of fluid overload, and there was no pulsatile hepatomegaly.
Learning Objectives
-
•
In a patient with TR secondary to RV and RA pacing wires, surgical removal of pacing wires revealed underlying undiagnosed tricuspid stenosis resulting in failed TV repair, requiring TV replacement.
-
•
To recognize that the passage of passing wires is frequently associated with TR and less commonly tricuspid stenosis.
-
•
To make a differential diagnosis of TV disease using clinical history and multimodality imaging and recognize that significant TR may mask underlying tricuspid stenosis.
-
•
To understand the multipathologic role of pacing wires (ventricular and atrial) in the etiology of TV disease.
Medical History
The patient had a dual-chamber pacemaker for Mobitz type 2 atrioventricular block 20 years prior to clinical presentation, with subsequent pacemaker generator box changes in 2008 and 2018. There was nothing else of note in her medical history.
Differential Diagnosis
The differential diagnosis included tricuspid regurgitation (TR) and/or stenosis (related or unrelated to pacing wires), carcinoid syndrome, infective endocarditis, and rheumatic disease.
Investigations
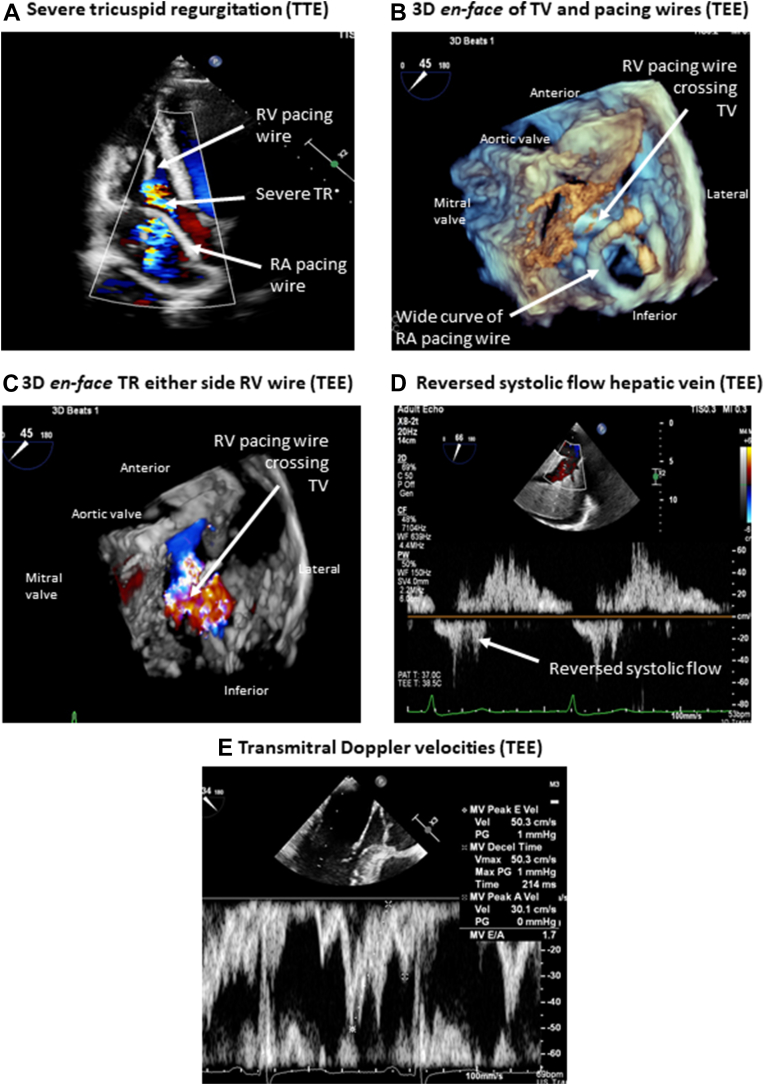
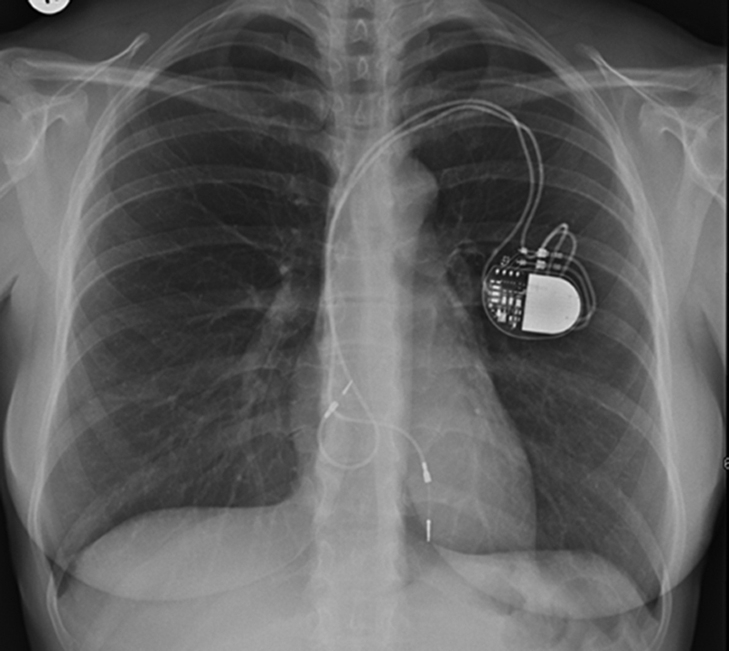
Chest radiography showed a loop of the ventricular lead at the level of the tricuspid valve (TV) (Figure 1). Transthoracic echocardiography (TTE) (Video 1) demonstrated severe TR, a dilated right atrium (RA), normal right ventricular (RV) dimensions and function (Figure 2A), and estimated pulmonary artery pressure of 33 mm Hg. Transesophageal echocardiography suggested severe TR on either side of the RV pacing lead (Video 2, Figures 2B and 2C), a curved RA wire prolapsing across the TV annulus (Video 2), reversed systolic flow in the hepatic vein (Figure 2D), and normal mitral velocities to exclude significant rheumatic mitral valve disease (Figure 2E).
Figure 1.
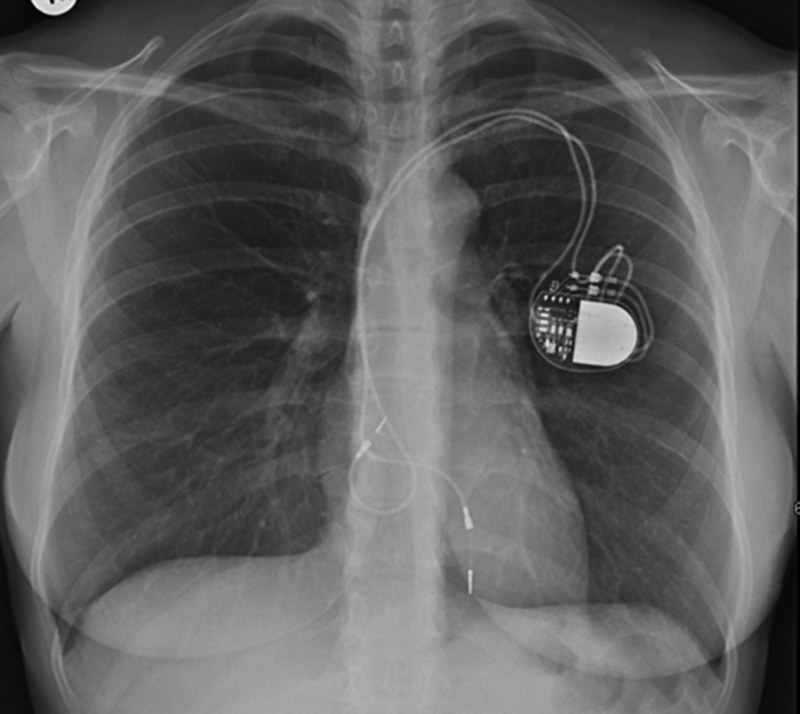
Chest Radiography
A loop of the atrial lead at the level of the tricuspid valve.
Figure 2.
Preoperative Echocardiography
(A) Severe tricuspid regurgitation (TR) on transthoracic echocardiography (TTE) associated with dilated right atrium (RA) and normal right ventricular (RV) size. Transesophageal echocardiography (TEE) demonstrated a curved RA wire prolapsing across the tricuspid valve (TV), suggesting adherence of the RV pacing wire to the tricuspid valve (B), causing severe TR on either side of the RV pacing wire (C), reversed systolic flow in the hepatic vein (D), and normal flow velocities across the mitral valve (MV). Decel = deceleration; PG = pressure gradient; Vel = velocity.
Management
The multidisciplinary team (MDT) was concerned that RV lead extraction under nonsurgical conditions would be unpredictable and that TR severity could worsen with subsequent deterioration in RV function if the pacing wires were left in situ. As the patient was pacing independent, the MDT outcome was surgical TV repair with de novo pacing.
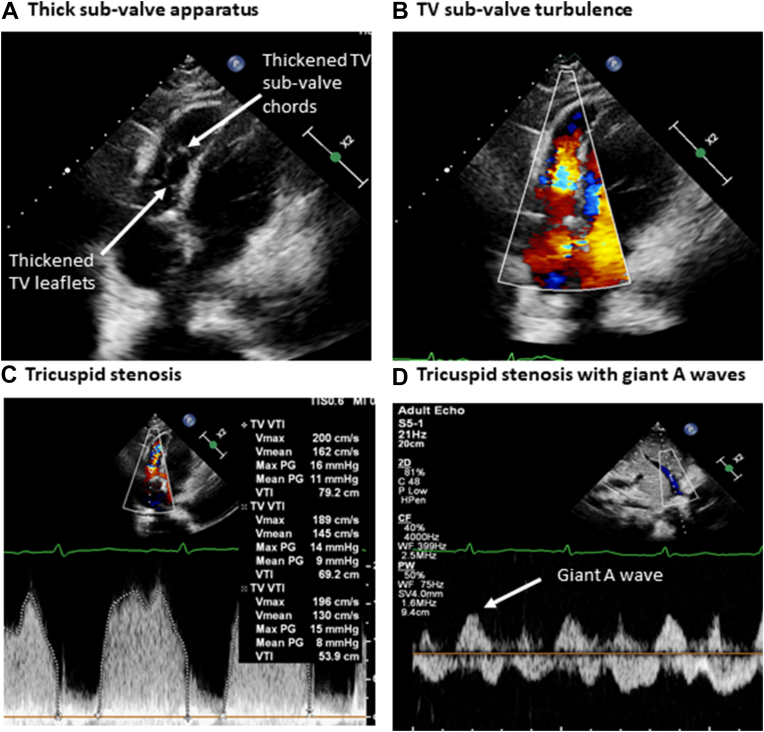
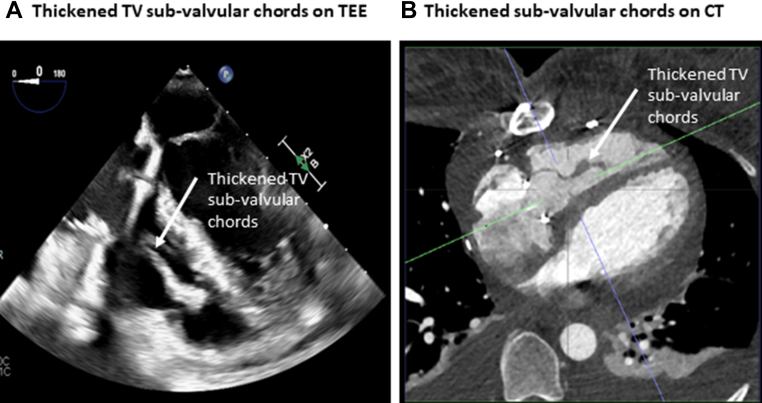
At the time of TV repair surgery, and once the endocardial pacing wires were removed, the TV was found to be fibrotic with fused commissures, and the subvalvular apparatus was thickened. The TV was surgically repaired using anterior leaflet augmentation, a 30-mm annuloplasty ring was implanted, the subvalvular apparatus was left unrepaired, and a new epicardial pacing system was implanted. Postoperative TTE demonstrated thickened, tethered, and restricted TV leaflets (Figure 3A); color Doppler velocities were increased in the subvalvular apparatus (Figure 3B), the mean TV gradient was 9 mm Hg (Figure 3C), and giant A waves were recorded in the hepatic veins (Figure 3D), all suggesting significant tricuspid stenosis. Transesophageal echocardiography and cardiac computed tomography confirmed TV subvalvular thickening (Figure 4, Video 3), increased color Doppler velocities in the subvalvular apparatus (Video 4), and subvalvular narrowing after initial TV repair (Video 5). In a reconvened MDT discussion, it was concluded that TV valvuloplasty would likely have limited benefit, and redo TV surgery was recommended. After several intraoperative attempts to re-repair and widen the TV were unsuccessful, TV replacement with a 31-mm Perimount (Edwards Lifesciences) device was performed. Histologic findings were inconclusive, revealing fibrosis without signs of active endocarditis.
Figure 3.
Postoperative Transthoracic Echocardiography
Following tricuspid valve (TV) repair, postoperative echocardiography imaging demonstrated thickened, tethered, and restricted TV leaflets (A), increased turbulence in the subvalvular apparatus (B), increased mean TV gradient (C), and giant A waves in the hepatic veins (D). Max = maximal; PG = pressure gradient; Vmax = maximal velocity; Vmean = mean velocity; VTI = velocity-time integral.
Figure 4.
Postoperative Imaging of TV Subvalvular Apparatus
Thickened subvalvular apparatus noted on transesophageal echocardiography (TEE) and cardiac computed tomography (CT). TV = tricuspid valve.
Discussion
Implantation of permanent pacing leads is associated with significant TR in 20% to 30% of patients,1 and the pathophysiology of pacemaker-related TR includes leaflet and chordal entanglement, impingement, adherence, laceration, or avulsion (following lead extraction).2 However, right-sided permanent pacing may also result in tricuspid stenosis, and there are case reports of iatrogenic stenosis caused by endocarditis,3 septal perforation,4 leaflet fusion,5,6 or the space-occupying effects of multiple pacing leads.7
In our case study, preoperative imaging suggested predominately TR assumed to be due to adherence of the RV lead to the large anterior TV leaflet. However, our patient also had tricuspid stenosis: the presence of pacing artifacts may have resulted in failure to recognize additional subvalvular thickening, which might have been caused by long-standing direct mechanical irritation by the RV wire, resulting in subvalvular fibrosis.8,9 We also hypothesize that the large looping and mobile atrial lead that prolapsed through the TV annulus may have resulted in additional reactive fibrosis at annular level,8,10 causing the pathologic commissural fusion noted at initial TV surgery.
Follow-Up
The patient reported symptomatic improvement at clinical review 6 weeks after TV replacement. Concurrent TTE demonstrated a well-functioning TV replacement with a mean gradient of 3 mm Hg, normal RV function, and pulmonary artery pressure < 20 mm Hg.
Conclusions
The passage of RV and RA permanent pacing wires can be associated with significant morbidity, with pacing-induced TR present in approximately one-quarter of cases. Tricuspid stenosis is less common and may be caused by direct mechanical trauma to the subvalvular apparatus from the RV lead, resulting in fibrosis. In addition, the additional large looping and mobile RA lead that prolapsed through the TV annulus may have caused additional reactive fibrosis at the annular level, resulting in commissural fusion. The options for management of tricuspid stenosis secondary to a permanent pacemaker lead include surgical management, but repair may be complex and TV replacement may be necessary.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Preoperative Transthoracic Echocardiography. Severe tricuspid regurgitation on either side of the right ventricular pacing lead and prolapse of the curved right atrial wire across the tricuspid valve annulus.
Preoperative Transesophageal Echocardiography. Severe tricuspid regurgitation on either side of the right ventricular wire; the curved right atrial wire prolapses across the tricuspid valve annulus.
Postoperative Transesophageal Echocardiography. Thickened subvalvular apparatus.
Postoperative Transesophageal Echocardiography. Turbulent flow on color Doppler in the subvalvular apparatus.
Postoperative Transesophageal Echocardiography. Three-dimensional tricuspid valve en face echocardiography window demonstrating tricuspid annuloplasty and subvalvular tricuspid narrowing.
References
- 1.Anvardeen K., Rao R., Hazra S., et al. Prevalence and significance of tricuspid regurgitation post-endocardial lead placement. J Am Coll Cardiol Img. 2019;12:562–564. doi: 10.1016/j.jcmg.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Praz F., Muraru D., Kreidel F., et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17:791–808. doi: 10.4244/EIJ-D-21-00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger P., Clevenbergh P., Crasset V., Selway P., Le Clerc J.L. Pacemaker-related endocarditis inducing tricuspid stenosis. Am Heart J. 1997;133(5):605–607. doi: 10.1016/s0002-8703(97)70159-5. [DOI] [PubMed] [Google Scholar]
- 4.Hussain T., Knight W.B., McLeod K.A. Lead-induced tricuspid stenosis-successful management by balloon angioplasty. Pacing Clin Electrophysiol. 2009;32(1):140–142. doi: 10.1111/j.1540-8159.2009.02189.x. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan A., Moulick A., Sinha P., et al. Severe tricuspid valve stenosis secondary to pacemaker leads presenting as ascites and liver dysfunction: a complex problem requiring a multidisciplinary therapeutic approach. J Interv Card Electrophysiol. 2009;24(1):71–75. doi: 10.1007/s10840-008-9309-z. [DOI] [PubMed] [Google Scholar]
- 6.Khan A., Mustafa A., Ling J., Lafferty J. Severe tricuspid stenosis secondary to permanent pacemaker lead. J Med Cases. 2022;13(8):365–368. doi: 10.14740/jmc3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg Y., Myatt J.P., Feldman M., et al. Down to the wire: tricuspid stenosis in the setting of multiple pacing leads. Pacing Clin Electrophysiol. 2010;33(5):e49–e52. doi: 10.1111/j.1540-8159.2009.02633.x. [DOI] [PubMed] [Google Scholar]
- 8.Taira K., Suzuki A., Fujino A., Watanabe T., Ogyu A., Ashikawa K. Tricuspid valve stenosis related to subvalvular adhesion of pacemaker lead: a case report. J Cardiol. 2006;47(6):301–306. [PubMed] [Google Scholar]
- 9.Uijlings R., Kluin J., Salomonsz R., Burgmans M., Cramer M.J. Pacemaker lead-induced severe tricuspid valve stenosis. Circ Heart Fail. 2010;3(3):465–467. doi: 10.1161/CIRCHEARTFAILURE.109.928168. [DOI] [PubMed] [Google Scholar]
- 10.Skoric B., Baricevic Z., Brida M., Samardzic J., Jurin H., Milicic D. Dynamic tricuspid valve stenosis induced with a pacemaker lead: a case report. J Heart Valve Dis. 2014;23(1):142–144. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative Transthoracic Echocardiography. Severe tricuspid regurgitation on either side of the right ventricular pacing lead and prolapse of the curved right atrial wire across the tricuspid valve annulus.
Preoperative Transesophageal Echocardiography. Severe tricuspid regurgitation on either side of the right ventricular wire; the curved right atrial wire prolapses across the tricuspid valve annulus.
Postoperative Transesophageal Echocardiography. Thickened subvalvular apparatus.
Postoperative Transesophageal Echocardiography. Turbulent flow on color Doppler in the subvalvular apparatus.
Postoperative Transesophageal Echocardiography. Three-dimensional tricuspid valve en face echocardiography window demonstrating tricuspid annuloplasty and subvalvular tricuspid narrowing.