Abstract
Patients with tricuspid regurgitation are often referred late in their disease course and present with volume overload, which is a detrimental factor leading to right-sided chamber dilatation and dysfunction. Treatment of volume overload can 1) improve patient functional status; 2) avoid repeated invasive examinations; and 3) establish eligibility for transcatheter tricuspid intervention. (Level of Difficulty: Intermediate.)
Key Words: chronic heart failure, diuretic, tricuspid valve
Central Illustration
Severe tricuspid regurgitation (TR) is common and associated with excess mortality.1 Despite improving in-hospital mortality rates after isolated TR surgery (5%-13%), these procedures are still offered to highly selected surgical candidates.2,3 As a result, among more than 1.6 million patients with clinically relevant moderate to severe or worse TR in the United States, only about 10,000 TR procedures are performed annually.4 The unmet clinical need of TR treatment paved the way for the development of several less invasive transcatheter treatment options.5 Although randomized trials are still ongoing, multicenter real-world data suggest that transcatheter tricuspid valve interventions are safe and effective.6 Among these, tricuspid transcatheter edge-to-edge repair (T-TEER) using the MitraClip or TriClip (Abbott Vascular) or the PASCAL (Edwards Lifesciences) device is the most commonly performed treatment approach.6
Learning Objectives
-
•
To recognize the impact of volume overload on TR assessment and transcatheter tricuspid valve intervention eligibility.
-
•
To acknowledge that treatment of volume overload can reduce RA and RV volumes, TV leaflet tethering, and coaptation gaps.
-
•
To understand that diagnostic work-up and establishment for transcatheter tricuspid valve intervention candidacy must be performed after patient volume status is optimized.
Despite the availability of dedicated treatment options, referral for interventional TR treatment is still suboptimal. Historically, TR has been considered a phenomenon secondary to left-sided heart disease, resolving when such concomitant pathologies are treated and being neglected as an innocent bystander. This misconception still leads to late referral of patients with TR to dedicated heart valve centers that offer multidisciplinary assessment and screening for transcatheter treatment options.6 Such late presentation following long-standing TR is often associated with significant volume overload with associated distortion of right ventricular (RV) and right atrial (RA) chambers, further increasing annular dilatation and tricuspid valve leaflet tethering, leading to even worse TR with large coaptation gaps.7 At this advanced stage, surgical treatment options are associated with high or prohibitive risk, and patients may be even deemed ineligible for T-TEER.
The aim of this case series is to: 1) illustrate how volume overload affects TR assessment; 2) how these patients can be managed; and 3) how volume optimization can lead to (re-)establishment of T-TEER candidacy, allowing successful transcatheter TR treatment.
Case 1
An 81-year-old man with a history of hypertension, atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate <30 mL/min), and heart failure with preserved ejection fraction (HFpEF) presented with massive edema (anasarca) and was admitted to the heart failure (HF) unit. His admission weight was 118 kg (261 lb).
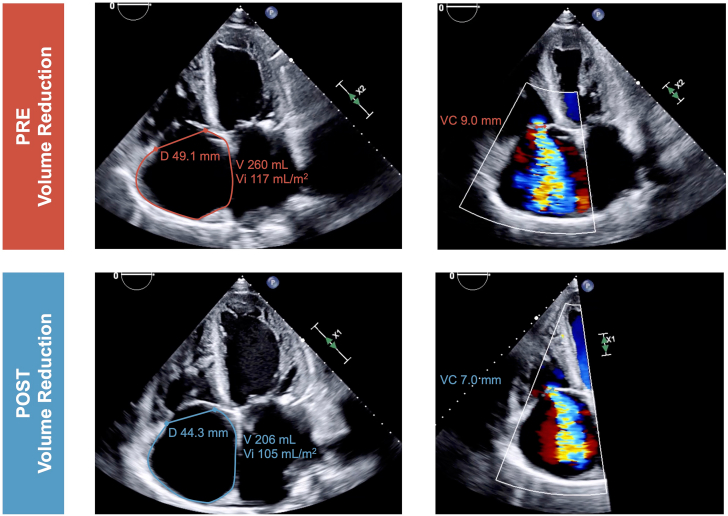
Initial diagnostic work-up by transthoracic echocardiography confirmed HFpEF, with a left ventricular ejection fraction of 50%, and revealed severe atrial functional TR with systolic flow reversal seen in the hepatic veins. The tricuspid annular diameter (4-chamber view) was 49.1 mm, with severe dilation of the RA. After heart team assessment, including interventional cardiology, interventional cardiac imaging, cardiac surgery, and advanced HF cardiology, the patient was deemed not a good surgical candidate, and transcatheter treatment was considered pending volume state optimization. The patient was managed in the HF unit with intravenous diuretic agents and 2 large-volume paracenteses of 14 L in total, leading to weight loss of 45 kg (100 lb) during the hospitalization. The prior maintenance treatment with furosemide 160 mg once daily was subsequently changed to torsemide 100 mg twice daily at hospital discharge.
At a follow-up visit in the structural heart clinic, the patient’s volume status was significantly improved, with a dry weight of 73 kg (160 lb). A slight improvement of exertional dyspnea was reported, but the patient remained in New York Heart Association functional class II despite optimized and maximally tolerated guideline-directed medical therapy. Right heart catheterization was performed, showing RA pressure of 14 mm Hg, RV pressures of 50/14 mm Hg, and pulmonary artery pressures of 50/20/30 mm Hg. Pulmonary capillary wedge pressure was 17 mm Hg, with a transpulmonary gradient of 15 mm Hg and pulmonary vascular resistance of 2.2 WU. Despite the reversal of fluid overload, resulting in a marked reduction of RA and RV sizes, persistent severe atrial functional TR was still evident (Video 1), and a T-TEER procedure was subsequently scheduled (TRI-SCORE predicted in-hospital mortality of isolated tricuspid valve surgery 10/12, 65%).
Following right femoral venous access and with transesophageal echocardiographic guidance, the steerable guide catheter was advanced into the RA. Using 3-dimensional echocardiographic guidance, a MitraClip XT (Abbott Vascular) was positioned and implanted between the anterior and septal leaflets. A second XT clip was implanted posterior to the first clip in a posteroseptal position. At the end of the procedure, there was mild residual TR, with a mean transvalvular gradient of 1 mm Hg (Video 2). The patient was discharged 2 days after the procedure, with postprocedural transthoracic echocardiography confirming mild residual TR.
Case 2
An 84-year-old woman presented to the HF unit with severe bilateral leg edema and significant functional impairment. In addition to HFpEF (left ventricular ejection fraction 60%), the patient had atrial fibrillation, hypertension, and nonobstructive coronary artery disease. Initial transthoracic echocardiographic evaluation revealed severe atrial functional TR in a tricuspid valve with partially fused anterior and posterior leaflets, creating a near bicuspid morphology (type II morphology).8 RA and RV volumes were increased, and mild septal leaflet tethering was noted (Video 3). Her admission weight was 77 kg (169 lb). To treat the massive leg edema, escalating doses of intravenous diuretic agents were given during the hospital stay. Initial treatment with furosemide 40 mg once daily was optimized to furosemide 40 mg twice daily at discharge.
At follow-up assessment, the patient was still symptomatic (dyspnea on exertion and limited functional status), and severe TR was still present despite ongoing diuretic therapy and euvolemic volume state with a dry weight of 69 kg (153 lb). Repeat echocardiography showed improved RA and RV dimensions with normal RV systolic function and reduced coaptation gaps (Video 3). A T-TEER procedure was therefore planned (TRI-SCORE predicted in-hospital mortality of isolated tricuspid valve surgery 4/12, 8%).
The procedure was performed in a similar fashion to case 1. A MitraClip XTW was implanted in an anteroseptal position, followed by a second XTW clip placed between the posterior and septal leaflets. At the end of the procedure, there was mild residual TR, with a mean transvalvular gradient of 1 mm Hg (Video 4).
Discussion
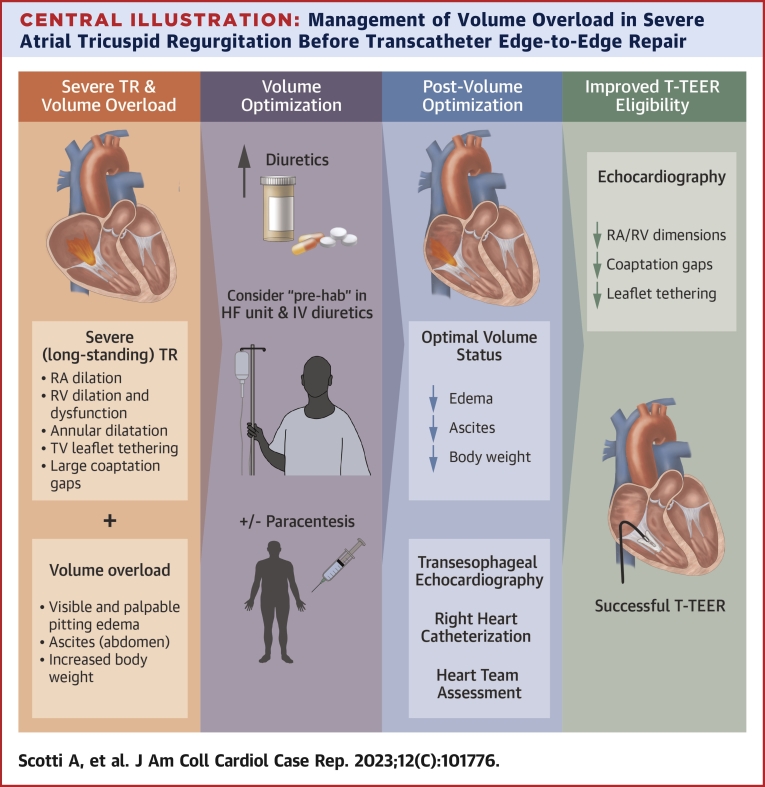
When evaluating patients with TR for transcatheter intervention (ie, edge-to-edge repair, annuloplasty, and orthotopic or heterotopic valve replacement) the following anatomical criteria play a central role in device selection: RA and RV dimensions, coaptation gaps, and leaflet tethering.9 Patients with TR are often referred late in their disease course and present with volume overload, which is a detrimental factor leading to right-sided chamber distortion. As these anatomical characteristics used in the screening algorithm are load dependent, their assessment may be inaccurate in presence of a hypervolemic state. Exaggerated RA and RV dilation can increase the sizes of coaptation gaps, regurgitant orifice, and severity of leaflet tethering (Central Illustration). Therefore, it is of utmost importance to evaluate patients with TR when loading conditions are optimized.
Central Illustration.
Management of Volume Overload in Severe Atrial Tricuspid Regurgitation Before Transcatheter Edge-to-Edge Repair
Patients with volume overload and severe functional tricuspid regurgitation require volume optimization before screening for transcatheter edge-to-edge repair. The echocardiographic effects of volume reduction might improve transcatheter edge-to-edge feasibility. LV = left ventricle; RA = right atrium; RV = right ventricle; TR = tricuspid regurgitation; T-TEER = tricuspid transcatheter edge-to-edge repair; TV = tricuspid valve.
Patients with severe TR should be evaluated for signs of volume overload: visible and palpable pitting edema, ascites, and increased body weight (Central Illustration). Before proceeding further with diagnostic work-up, correction of the hypervolemic state should be done first. This includes aggressive diuretic therapy and, if appropriate, paracentesis. For patients with advanced-stage TR and significant frailty, a short hospitalization stay (“prehab”) in an HF unit for intravenous diuretic therapy and clinical monitoring can be considered. When volume status is optimized, further diagnostic testing, including transesophageal echocardiography and right heart catheterization, is recommended. Besides acute interventions to treat volume overload, adoption of optimized guideline-directed medical therapy is fundamental as maintenance treatment for patients with TR and should be continued after the procedure.
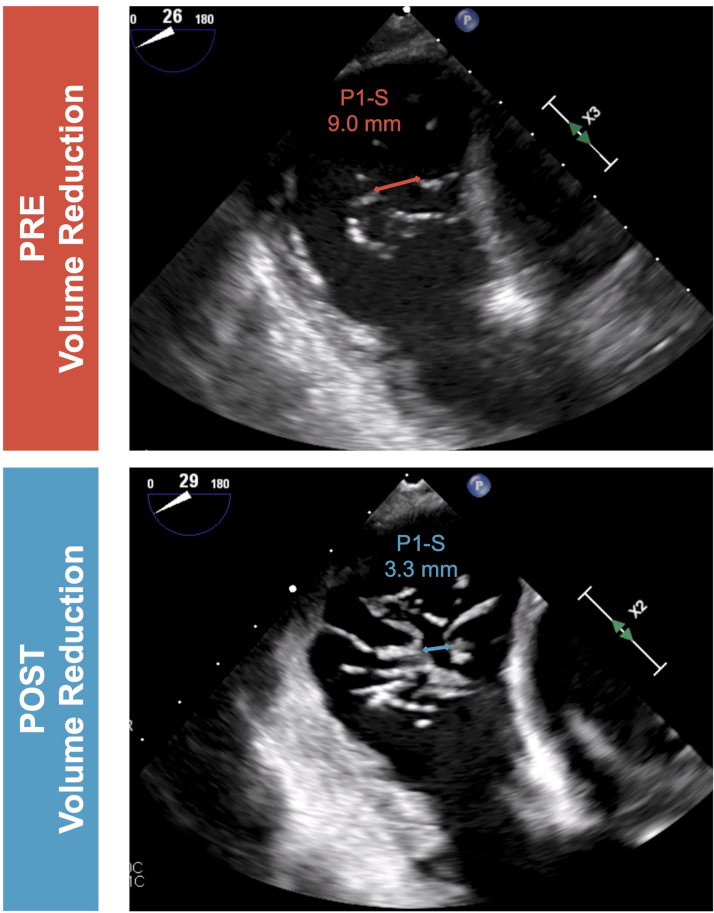
As demonstrated by the presented clinical cases, a multidisciplinary management strategy including advanced HF specialists may have a significant impact on final device and procedure selection. Improvement in RV and RA volume overload can result in reduced coaptation gaps and leaflet tethering, potentially allowing patients to be eligible for procedures previously deemed technically not feasible (Figures 1 and 2). A recent study investigating a standardized table-tilt maneuver showed that lowering the feet by 10° facilitated T-TEER by reducing leaflet gaps and allowing easier grasping.10 This technique, using venous pooling by gravity to temporarily reduce RA and RV loading, could also be performed during diagnostic work-up to demonstrate load dependency and support the potential benefit of an increase of the diuretic regimen in patients with TR.
Figure 1.
Effect of Volume Optimization on Atrial and Annular Dimensions and Tricuspid Regurgitation Severity as Assessed by Transthoracic Echocardiography
Figure 2.
Effect of Volume Optimization on Tricuspid Leaflet Coaptation Gaps as Assessed by Transesophageal Echocardiography
Patients with severe TR should be referred early to heart valve centers with expertise in tricuspid valve treatment. Assessment for volume overload is of primary importance and should be performed in all patients being evaluated for TR therapies. This may avoid the need for repeat invasive procedures, such as transesophageal echocardiography and right heart catheterization, or inaccurate decisions regarding TR intervention eligibility. Reversing volume overload can improve patient functional status and improve eligibility for transcatheter tricuspid intervention.
Funding Support and Author Disclosures
Dr Scotti has served as a consultant for NeoChord. Dr Ho has served as a consultant for NeoChord and GE. Dr Latib has served on advisory boards for Medtronic, Abbott Vascular, Boston Scientific, Edwards Lifesciences, NeoChord, V-Dyne, GE, and Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos and an interactive Central Illustration video, please see the online version of this paper.
Appendix
Echocardiographic Assessment in Patient #1: Before and After Volume Optimization
Procedural Results After Transcatheter Tricuspid Edge-to-Edge Repair in Patient #1
Echocardiographic Assessment in Patient #2: Before and After Volume Optimization
Procedural Results After Transcatheter Tricuspid Edge-to-edge Repair in Patient #2
References
- 1.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Scotti A., Sturla M., Granada J.F., et al. Outcomes of isolated tricuspid valve replacement: a systematic review and meta-analysis of 5,316 patients from 35 studies. EuroIntervention. 2022;18:840–851. doi: 10.4244/EIJ-D-22-00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarris-Michopoulos P., Macias A.E., Sarris-Michopoulos C., et al. Isolated tricuspid valve surgery—repair versus replacement: a meta-analysis. J Card Surg. 2022;37:329–333. doi: 10.1111/jocs.16131. [DOI] [PubMed] [Google Scholar]
- 4.Zack C.J., Fender E.A., Chandrashekar P., et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–2960. doi: 10.1016/j.jacc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Latib A., Scotti A. Transfemoral transcatheter tricuspid valve replacement: will TV repair be replaced? J Am Coll Cardiol Intv. 2022;15:492–495. doi: 10.1016/j.jcin.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Scotti A., Coisne A., Taramasso M., et al. Sex-related characteristics and short-term outcomes of patients undergoing transcatheter tricuspid valve intervention for tricuspid regurgitation. Eur Heart J. 2023;44(10):822–832. doi: 10.1093/eurheartj/ehac735. [DOI] [PubMed] [Google Scholar]
- 7.Latib A., Grigioni F., Hahn R.T. Tricuspid regurgitation: what is the real clinical impact and how often should it be treated? EuroIntervention. 2018;14:AB101–AB111. doi: 10.4244/EIJ-D-18-00533. [DOI] [PubMed] [Google Scholar]
- 8.Hahn R.T., Weckbach L.T., Noack T., et al. Proposal for a standard echocardiographic tricuspid valve nomenclature. J Am Coll Cardiol Img. 2021;14:1299–1305. doi: 10.1016/j.jcmg.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Praz F., Muraru D., Kreidel F., et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17:791–808. doi: 10.4244/EIJ-D-21-00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wild M.G., Weckbach L., Braun D., et al. Mind the gap: facilitating tricuspid transcatheter edge-to-edge repair procedures by standardized table tilt. J Am Coll Cardiol Intv. 2022;15:1004–1006. doi: 10.1016/j.jcin.2022.02.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic Assessment in Patient #1: Before and After Volume Optimization
Procedural Results After Transcatheter Tricuspid Edge-to-Edge Repair in Patient #1
Echocardiographic Assessment in Patient #2: Before and After Volume Optimization
Procedural Results After Transcatheter Tricuspid Edge-to-edge Repair in Patient #2