Abstract
In the present study Acacia nilotica seed derived essential oils were tested against Spodoptera litura, Tenebrio molitor, Oxycarenus hyalinipennis, and Aphis fabae, as well as their effects on non-target species Eudrilus eugeniae and Artemia salina at 24 h post treatment. The seed essential oil produced insecticidal activity against A. fabae (LC50 = 41.679, LC90 = 75.212 μl/mL), O. hyalinipennis (LC50 = 37.629, LC90 = 118.485 μl/mL), T. molitor (LC50 = 56.796, LC90 = 201.912 μl/mL), and S. litura (LC50 = 62.215, LC90 = 241.183 μl/mL). Essential oils do not cause a remarkable effect on E. eugeniae and A. salina cytotoxicity. The essential oils produced a lower effect on Artemia salina (LC50 = 384.382, LC90 = 1341.397 μl/mL) and no lethal effects were observed on E. eugeniae. The histopathological evaluation showed no sub-lethal effects of essential oils on earthworm gut tissues. GC-MS analysis results revealed that the major chemical constituent was hexadecane (19.560%) and heptacosane (17.214%) and FT-IR analysis revealed the presence of alkanes and alkyles, aromatics, and amides functional groups that may be involved in insecticidal activity. Overall, the results showed that the seed derived essential oil has excellent insecticidal action against major agricultural insect pests and may therefore offer an environmentally benign alternative to conventional insecticide.
Keywords: Acacia nilotica, Essential oil, Biocontrol, Bio-pesticide, Crop pest, Eco-friendly
1. Introduction
The tobacco caterpillar, Spodoptera litura (F.) (Lepidoptera: Noctuidae), has become a globally significant and economically dangerous pest that can damage more than 350 agricultural crops worldwide. Tenebrio molitor is a stored grain insect pest. This pest can damage Zea mays (L.), Triticum aestivum (L.), and Glycine max (L.) [[1], [2], [3]]. T. molitor has the potential to contaminate stored grains and bran with insect body parts, frass, and saprophytic microbes, lowering the food quality [[4], [5], [6]]. T. molitor is believed to be responsible for up to 15% of global grain and flour losses [[7], [8], [9]]. The dusky cotton bug, Oxycarenus hyalinipennis, is a new insect pest of cotton and other agricultural crops. It frequently causes significant crop damage during the germination stage [10]. Significant damage was caused by the severe infestation, which included decreased seed oil, seed weight, and cotton yield. The pest injects poisonous saliva into the host plant, resulting in the formation of oily patches [11]. According to reports, this widespread species has also been spotted in southern Europe [12]. Abbas et al. [13] and Khan and Ahmed [14] investigated the use of plant chemicals to eradicate this bug.
Aphis fabae (Homoptera: Aphididae) can suck various plant juices from young stems and leaves, causing shoot deformation, stunted plants, lower yields, and crop damage, all of which can result in significant crop losses. This aphid was discovered first in Europe and has since spread to almost all temperate countries [15]. Many cruciferous crops suffer significant yield losses due to their preference for young leaves. Aphids that feed on the juices of plants such as beans, peas, beets, crucifers, cucurbits, potatoes, tobacco, tomatoes, and others it is also to blame for the spread of more than 40 plant viruses [16]. Juice deprivation stunts plants, twists stem, spreads deadly infections, and contaminates produce with aphid by-products. Pest insects like S. litura, T. molitor, O. hyalinipennis, and A. fabae may be to blame for lower agricultural output in India and other developing countries. Pesticides derived from synthetic chemicals, such as carbamates, organophosphorus, organochlorines, and temephos, are currently used to control medical and agricultural pests [17]. In the pest management program, regular pesticide use has significant disadvantages and obstacles. As a result, finding low-cost pesticides that are environmentally friendly, effective, and non-chemical insecticide alternatives is critical [18]. Acacia nilotica Lam. (Acacia) is a valuable plant that has a wide range of medical and biological applications [[18], [19], [20]]. Acacia trees can be found in abundance in Saudi Arabia, India, Sudan, Egypt, and Sri Lanka [21]. Acacia plants are used in folk medicine as a nutritional and medicinal treatment to prevent, relieve, and manage a variety of diseases. The Acacia tree has a broad circular crown, black branches and stems, greyish pink slashes, cracked bark, and red gum that grows to a height of five to 20 m [18]. Acacia is rich in phenolic antioxidants such as condensed tannins and phlobatannins, gallic acid, protocatechuic acid, pyrocatechol (+) catechin (−) epigallocatechin-7-gallate, and (−) epigallocatechin-5, 7-digallate [[22], [23], [24], [25]].
This Acacia tree's leaves, bark, seeds, roots, gum, flowers, fruits, and young pods have anticancer, antispasmodic, antipyretic, antifungal, antiviral, antibacterial, antihypertensive, and antioxidant properties [25]. Plant essential oils are used in organic farming for insect control because of their exceptional ecotoxicological properties, such as low human toxicity, further degradation, and low environmental impact [26,27]. Secondary metabolites/essential oils found in plants, such as alkaloids, amides, chalcones, flavones, kawapirones, lignans, neolignans, and phenols, play an important role in insect-plant interactions [[28], [29], [30]].
Essential oils can be used to manage insect pests as repellents, oviposition and feeding deterrents, growth regulators, and insect toxicity while emitting little pollution and dissolving quickly in the environment [27,[31], [32], [33]]. Several studies have been conducted to see if plant essential oils can be used to reduce insect pests in stored grain [[34], [35], [36]]. Amines and alkaloids, cyanogenic glycosides, cyclitols, fatty acids and seed oils, fluoroacetate, gums, nonprotein amino acids, terpenes (including essential oils, diterpenes, phytosterol and triterpene genins and saponins), hydrolysable tannins, flavonoids, and condensed tannins are all found in Acacia nilotica seeds. Anti-cancer, anti-tumor, Antiscorbutic, Astringent, anti-oxidant, Natriuretic, Antispasmodic, Diuretic, Intestinal pains and diarrhoea, Nerve stimulant, Cold, Congestion, Coughs, Dysenter, Fever, Hemorrhages, Leucorrhea, Ophthalmia, and Sclerosis [18]. Botanical essential oils are easily used to control medical and agricultural insect pests, and field application of plant-derived essential oils does not pollute green environments and is simple to apply [26,27,37]. Several studies have been carried out to determine whether plant essential oils can be used to reduce medical and agricultural insect pests. The current study will look into the toxicity of essential oils derived from A. nilotica L. seed against S. litura, T. molitor, O. hyalinipennis, and A. fabae in the lab, as well as the toxicity effect on non-target species, E. eugeniae and A. salina. GC-MS and FT-IR analysis were used to identify the chemical components and functional groups of the essential oil from A. nilotica L. seeds.
2. Materials and methods
2.1. Collection of plant materials
Acacia nilotica plant seeds (Fig. 1) were collected on December 20, 2017 in Sanarappatti village, Dharmapuri, Tamil Nadu, India (11.9861° N, 77.9602° E). The taxonomic classification of plants was determined by a taxonomist. In the laboratory, an herbarium voucher specimen was deposited.
Fig. 1.
The A. nilotica seeds derived essential oils against target and non-target species.
2.2. Hydrodistillation of essential oils
Fresh A. nilotica seed powder (1.0 kg) was hydro-distilled for 12 h in a Clevenger's apparatus (Borosil Glass Works Ltd., Mumbai, India) according to the method described by Ref. [18]. The apparatus yielded a pale yellowish-coloured EO. Furthermore, EO was partitioned with diethyl ether (3 × 50 mL) and stored after passing through anhydrous sodium sulphate (20 g) through a glass funnel. The EO yield (%) was calculated to be 1.43% (v/w).
2.3. Insect pest culture
Tenebrio molitor insect cultures were grown in the laboratory at 28 ± 2 °C, 70–80% relative humidity, and a light-dark photoperiod of 14:10. The larvae were fed with wheat bran and grown in a plastic container with adequate macro and micronutrients (55 cm long, 38 cm wide, and 10 cm high). Bioassays were performed on a healthy fourth instar in the laboratory.
The egg mass of Spodoptera litura was collected from a castor field and used to rear the insects in the laboratory. The third instar larvae were fed fresh young castor leaves obtained from the field on a daily basis to avoid microbial infection, and the larvae were allowed to grow to the third instar before being transferred to the larval bioassay.
The aphid A. fabae insect pest used in this study was raised in a laboratory setting at first. In a controlled environment with 28 ± 2 °C, 65–70% relative humidity (RH), and a photoperiod of 16:8 h (L:D), the stock culture was grown on pea seedlings (Pisum sativum L).
O. hyalinipennis was collected in large plastic jars from a cotton field by shaking the infested plant parts. In rearing cages, adult and immature (nymph) populations were separated and fed cotton bolls and leaves. Seed cotton was used in adult cages to collect eggs, which were then separated and placed on moistened filter paper until hatching. The newly hatched babies were moved to new enclosures. Adults were transferred from nymphal stage cages to adult cages to lay eggs. The eggs were collected and placed on moist filter paper inside an incubator set to 30 °C with a relative humidity of 60–65%. The newly hatched nymphs were raised in nymphal cages until they reached sexual maturity. Throughout the series of experiments, the O. hyalinipennis culture was kept alive, and adults were used for insecticidal bioassay.
2.4. Larval bioassay
The A. nilotica seed derived essential oils were tested for insecticidal activity against 3rd instar larvae of S. litura at 24 h post treatment. For the larvicidal bioassay, the leaf dipping technique was used. The seed-derived essential oils were emulsified using the emulsifier lecithin and prepared at five different concentrations (50–200 μl/mL). Then fresh castor leaves were individually dipped in various test concentrations. Released 25 third-instar larvae into the bioassay container after air-dried leaves were placed in a clean plastic bioassay container (13 × 9 × 5) with moist filter paper. The pure distilled water used as a negative control. Each concentration contains three replications, and each replicate contains 25 larvae. After 24 h of treatment, the mortality rate was calculated. The percentage of larval mortality was calculated using Abbott's formula [38].
The insecticidal effect of seed-derived essential oils was investigated 24 h after treatment. The above-mentioned essential oil test concentrations (50–200 μl/mL) were tested against 3rd instar T. molitor larvae through feeding larval diets. Each concentration was tested with three replicates; each replicate contains 25 3rd instar larvae. The larval mortality was counted 24 h after the botanically derived essential oil treatment. The pure distilled water used as negative control. Using probit analysis, the LC50 (lethal concentration that kills 50% of exposed larvae) and LC90 (lethal concentration that kills 90% of exposed larvae) values were calculated using SPSS-16.0.
The insecticidal activity (leaf dip bioassay) was measured in order to determine the direct contact effect of A. nilotica essential oils on Aphis fabae species. Each treatment oil was emulsified and prepared at five different concentrations (50–200 μg/mL) using the emulsifier lecithin. The Phaseolus vulgaris leaf was cut to size and dipped in the test essential oil solution for about 30 s. The leaves were then allowed to dry for 5–10 min to allow the volatile solvent to evaporate. The treated leaf was kept in clean petri dishes (9 cm in diameter) with wet filter paper. In such petri dishes, 25 aphids were released and allowed to settle on the treated leaf. Distilled water was used as a negative control. Each concentration has three replicates, with each replicate containing 25 adult aphids. After 24 h of treatment, the number of dead aphids was counted. Dead aphids were identified by gently striking the pest with a small brush and observing any leg or antennae movement.
The A. nilotica seed derived essential oils were tested for insecticidal activity against adult of O. hyalinipennis at 24 h post treatment. For the insecticidal bioassay, the cotton seeds dipping technique was used. The seed-derived essential oils were emulsified using the emulsifier lecithin and prepared at five different concentrations (50–200 μl/mL). Then fresh cotton seeds were individually dipped in various test concentrations mentioned above. Released 25 O. hyalinipennis adult into the bioassay container after air-dried seeds were placed in a clean plastic bioassay container (13 × 9 × 5) with moist filter paper. The pure distilled water used as a negative control. Each concentration contains three replications, and each replicate contains 25 O. hyalinipennis adult. After 24 h of treatment, the mortality rate was calculated. The percentage of adult mortality was calculated using Abbott's formula [38].
2.5. Earthworm culture
E. eugeniae is a bio-indicator of terrestrial soil pollution. The E. eugeniae was kept in a laboratory at 28 ± 1 °C.
2.6. Artificial soil assay (earthworm)
The artificial soil made up of 15% sphagnum peat, 25% kaolinite clay, and 75% fine soil. With minor modifications, this was done in accordance with [39]. A few drops of CaCO3 were added to the assay to reduce the pH to 6.0 ± 0.5. The water content of the soil was kept constant at 30%. On a dry weight basis, different concentrations of seed derived essential oils (50–200 mg/kg) were added to the artificial soil assay container. 15 adult E. eugeniae were transferred to 1000 g of test soil substrate at the appropriate concentration. Each concentration has three replicates, and each replicate has 15 adult earthworms. The distilled water is used as a negative control. To prevent E. eugeniae from escaping, the bioassay containers were sealed with lids. The dead E. eugeniae were counted seven days after treatment.
2.7. Artemia species
Artemia salina larvae were reared in 500 mL of saltwater with a salinity of 30 ppt and a pH of 8–8.5 under a 17:7 (L:D) photoperiod in the laboratory. The temperature was kept at 25 ± 3° Celsius.
2.8. Essential oils on Artemia species
A. salina was transferred to the bioassay container using a micropipette and tested for toxicity using above prepared various test doses of essential oils derived from seeds (50–200 μl/mL). 250 mL of seawater were taken in a 300 mL container, and then 25 mature A. salina were transferred into the container. Sterile seawater was used as a negative control. The LC50 and LC90 values were calculated at 24 h after treatment, the experiment was repeated three times for each concentration and each replicates contains 25 adult A. salina. Following A. salina was examined under a binocular microscope to see if its shape changed. Each concentration contains three replicates.
2.9. Histopathological studies
Seven days after treatment, the essential oil exposed and unexposed E. eugeniae were fixed individually with 5% formalin for 4 h at 4 °C. The blocks were frozen for 2 h at −25 °C before being sliced into 4 mm-thick ribbons with 1.0 mm spacing using a microtome (Leica, Germany). Slices of E. eugeniae sections were stained with Ehrlich's haematoxylin and eosin and examined under a 40X light microscope (Olympus CH20i/India) [39].
2.10. GC–MS and FT-IR analysis
For GC-MS experiments, an Agilent 5957C, VL MS Detector with Triple-Axis Detector system (Thermo Fisher Scientific, India), and an HP 5MS 30 m 0.25 mm 0.25 mm film thickness capillary column in EI mode were used. The temperature of the column was gradually increased from 35 °C to 45 °C at a rate of 30 °C/min. By comparing their retention indices (RI) to those of various n-alkanes, the compounds were identified (C9–C24). Their EI mass spectra were compared to those in the NIST/NBS and Wiley libraries, as well as those in the literature.
The FT-IR spectrum of the essential oil was measured with an FT-IR spectrometer equipped with a DTGS detector. Pellets were created by compressing 10 mg of essential oils and 10 mg of dry potassium bromide (KBr). An FT-IR spectrophotometer was used at room temperature to analyse the pellet in the 4,000-500 cm–1 range.
2.11. Statistical analysis
To correct mortality, Abbot's formula was used [38]. The LC50 and LC90 valves, as well as their associated 95% confidence limits, were calculated using average mortality data; chi-square and degrees of freedom (df) were calculated using SPSS-16.00.
3. Results
3.1. Yield of essential oil
The essential oil of A. nilotica seeds ranged is 1.43% (v/w).
3.2. Insecticidal activity
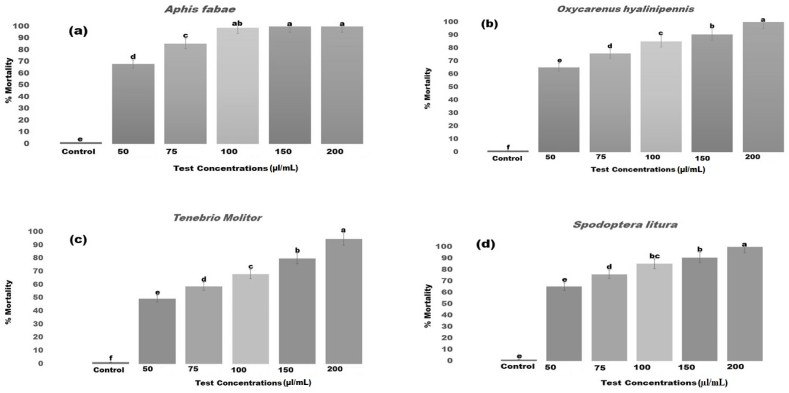
After 24 h of treatment, the results showed that the seed essential oils have remarkable insecticidal activity against insect pest. The LC50 and LC90 values for S. litura were 62.215–241.183 μl/mL (Fig. 2 D), for T. molitor they were 56.719–201.912 μl/mL (Fig. 2C), for O. hyalinipennis they were 37.629–118.485 μl/mL (Fig. 2 B), and for A. fabae they were 32.559–75.212 μl/mL (Fig. 2 A; Table 1).
Fig. 2.
The insecticidal effect of A. nilotica seeds derived essential oils against a major insect pest at 24 h post treatment. A. Aphis fabae; B. Oxycarenus hyalinipennis; C. Tenebrio molitor; D. Spodoptera litura.
Table 1.
After 24 h of treatment, the insecticidal efficacy of seed essential oil against Spodoptera litura, Tenebrio molitor, Oxycarenus hyalinipennis, and Aphis fabae was determined.
| Crop Pest | Concentration (ppm) | LC50(LCL-UCL) (ppm) | LC90(LCL-UCL) (ppm) | χ2 (Df = 3) |
|---|---|---|---|---|
| A. fabae | Control | 41.679 (32.559–47.851) | 75.212 (68.056–86.607) | 2.811 ns |
| 50 | ||||
| 75 | ||||
| 100 | ||||
| 150 | ||||
| 200 | ||||
| O. hyalinipennis | Control | 37.629 (24.606–47.529) | 118.485 (101.104–151.301) | 4.235 ns |
| 50 | ||||
| 75 | ||||
| 100 | ||||
| 150 | ||||
| 200 | ||||
| T. molitor | Control | 56.796 (43.729–67.160) | 201.912 (162.391–292.147) | 3.929 ns |
| 50 | ||||
| 75 | ||||
| 100 | ||||
| 150 | ||||
| 200 | ||||
| S. litura | Control | 62.215 (55.981–93.066) | 241.183 (202.813–260.112) | 8.844 ns |
| 50 | ||||
| 75 | ||||
| 100 | ||||
| 150 | ||||
| 200 |
3.3. Non-target bioassay
3.3.1. A. salina
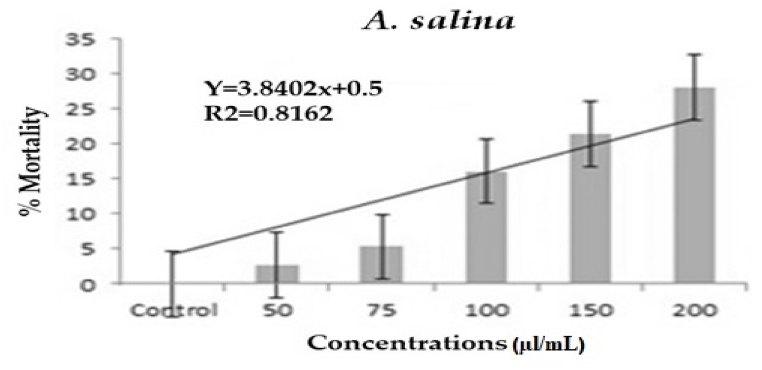
After a 24-h treatment period in the laboratory, A. nilotica seed derived essential oils were examined for non-target toxicity on A. salina. Probit analysis findings clearly demonstrated that the essential oils had a minimal toxicity effect on A. salina, with LC50 and LC90 values of 348.382–1341.397 μl/mL (Fig. 3). Essential oils derived from A. nilotica seeds exhibited minimal toxicity 24 h after treatment.
Fig. 3.
Non-target toxic efficacy of A. nilotica seeds derived essential oils on A. salina.
3.3.2. E. eugeniae
After 7 days of treatment in the laboratory, seed-derived essential oils were examined for non-target toxicity in E. eugeniae. The essential oil treatment killed no earthworm E. eugeniae, whereas the positive control (permethrin) killed 90% of them.
3.4. Histopathology studies
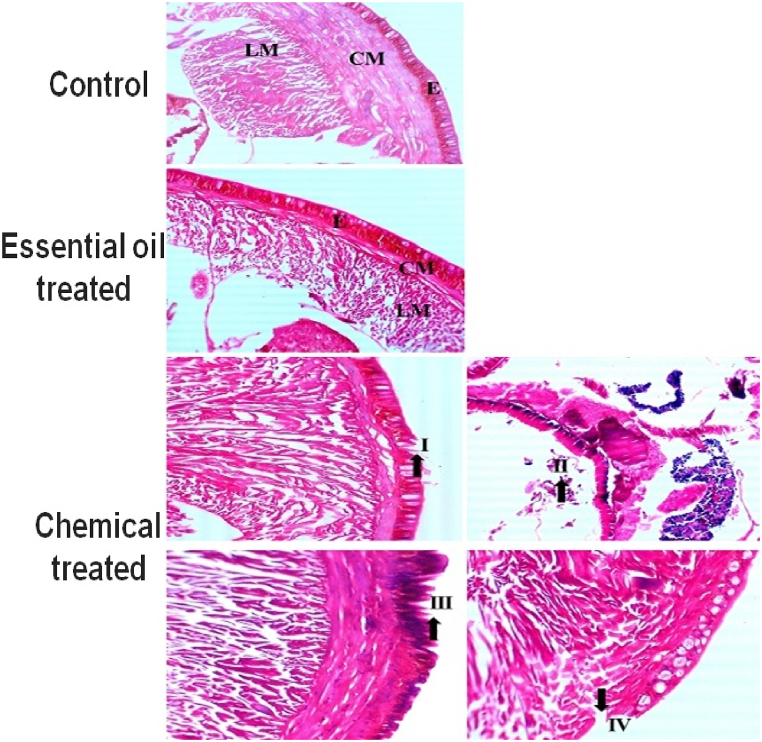
E. eugeniae is a soil-based toxicological bioindicator species. After 7 days, no mortality rates were observed with seed-derived essential oil treatment. Essential oils did not have any sublethal effects on gut tissues. As a positive control, permethrin, a chemical pesticide, caused variations in earthworm gut cells as well as an uneven epithelial surface. There was cellular debris present, and the nuclei were not spherical. Fig. 4 shows that the results of the treatment with essential oil from seeds were the same as those of the control treatment.
Fig. 4.
Histopathological studies of E. eugeniae after 7 days of essential oil treatment revealed that after the treatment there was no mortality observed in the seed essential oil treatment. No sublethal effects of oils were observed in gut tissues. The essential oil treated earthworm gut cells were significant to negative control. It was detected in histopathological studies. In the earthworm gut tissues, there is no cellular debris and the nuclei were observed to be round in shape. Similar types of results were observed in the control treatment as well. The permethrin (positive control) chemical insecticide treatment caused high damage in gut tissues. (EPI-epidermis, SE-setae, IL-intestinal lumen, LM-longitudinal muscle, CO-coelom, CM-circular muscle, MI-mitochondrion).
3.5. Characterization of essential oil
3.5.1. GC-MS analysis
A. nilotica seed derived essential oil GC-MS analysis shows the presence of ten chemical constituents. Among the chemical constituents, two major compounds are identified, such as hexadecane (19.560%) and heptacosane (17.214%), which may have insecticidal properties (Table 2). Further research into the isolation of different chemical constituents may be beneficial in determining the insecticidal potential of these compounds. Our findings suggest that the essential oil extracted from the seeds of A. nilotica can be used to control agricultural crop pests.
Table 2.
The chemical constituents of A. nilotica seed essential oils were identified using GC-MS analysis.
| S.No | RTa (min) | Area % | Compound Name | Structure | Activity |
|---|---|---|---|---|---|
| 1 | 13.539 | 0.524 | 6-hydroxymellein | 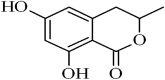 |
Inhibitors of Pollen Development in Arabidopsis thaliana |
| 2 | 15.091 | 9.157 | 1,2-Benzenedicarboxylic acid | 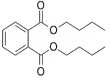 |
Antimicrobial and antifouling |
| 3 | 16.371 | 1.581 | Tridecyl ester | 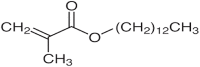 |
Antimicrobial |
| 4 | 19.200 | 17.214 | Heptacosane | 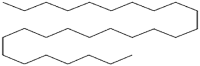 |
Insecticidal |
| 5 | 21.995 | 5.001 | Acetamide | 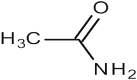 |
Penetrating agent |
| 6 | 22.551 | 19.560 | Hexadecane | 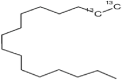 |
Antimicrobial and insecticidal |
| 7 | 23.811 | 2.226 | Eicosane | 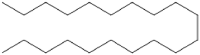 |
Wound healing |
| 8 | 24.409 | 0.65 | Pentadecane | 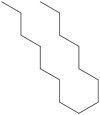 |
Antibacterial activity |
| 9 | 25.931 | 4.004 | 1,3,4-Eugenol | 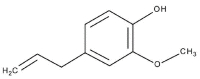 |
Insecticidal |
| 10 | 26.642 | 0.90 | Chrodrimanin A | 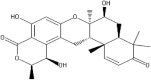 |
Antimicrobial |
3.5.2. FT-IR analysis
Seed essential oil FT-IR analysis was carried out to identify the functional groups of essential oils. The peak value in the region of IR radiation in the FT-IR analysis confirmed the presence of alcohols, alkanes and alkyles, aromatics, amides, ethers and alhyl halides. 3042.16 cm-1 corresponded to alkanes and alkyles (C–H stretch); 2932.12 cm-1 corresponded to alkanes and alkyles (C–H stretch); 1721.52 cm-1 corresponded to C C–C (O)–OH or Ar–C (O)–OH. C O stretch; 1646.32 cm-1 corresponded to amides (N–H stretch); 1456.21 cm−1 corresponds to alkanes and alkyles (CH3 C–H bond); 1327.19 cm−1 corresponded to alkyl halides (C–F stretch or ether); 1130.13 cm−1 corresponded to ethers (Ar-O-R =C–O–C); 670.30 cm−1 corresponds to alhyl halides (C–Br strech); 590.43 cm−1 corresponds to alhyl halides (C–Br strech) 500.65 cm−1 corresponded to Misc (S–S disulfide); 465.01 cm−1 corresponded to Misc (S–S disulfide).
4. Discussion
In this present study, the essential oils from the seeds of A. nilotica caused significant mortality against insect pests, with minimal concentrations producing significant insecticidal activity. Similarly, insect pests such as S. oryzae, S. zeamais, S. cerealella (Lepidoptera: Gelechiidae), and T. castaneum are highly susceptible to the garlic essential oil [[40], [41], [42], [43]]. Ogunbinu et al. [43] chose two species Acacia nilotica and Acacia albida stem bark, the Acacia albida stem bark essential oil contained 38.2% monoterpenes, 10.4% sesquiterpenoids, 25.1% aliphatic compounds, and 6.2% and 6.6% aromatic and aliphatic compounds, respectively. Among the oil constituents, a-Pinene (18.6%) was the most abundant. Furthermore, significant proportions of abietatriene (6.6%) and 6,10,14-trimethyl pentadecanone (6.1%) were identified, and A. nilotica essential oil was obtained in a yield of 0.08% v/w. Thirty-six compounds were identified, accounting for 91.3% of the total oil content. Monoterpenoid compounds (69.6%) outnumbered sesquiterpene counterparts (19.4%) in the oil. The monoterpenoids menthol (34.9%) and limonene (15.3%) were the most abundant constituents in the oil. Curcumene (6.9%) and carvacrol (4.1%) were also found in significant amounts. The chemical constituents in this study are completely different from those in this study because this study A. nilotica seed derived essential oil showed the presence of ten chemical constituents. Among the chemical constituents, two major compounds, hexadecane (19.560%) and heptacosane (17.214%), have been identified as having insecticidal properties.
Essential oils obtained from 43 plant species were tested for aphid fumigation efficacy. EOs with LC50 values less than 1 μl/L−1 (1 ppm in air) or causing aphid mortality greater than 90% upon application of 2 μl/L−1 (Calamintha umbrosa, Citrus sinensis, Cuminum cyminum, Foeniculum vulgare, Lavandula angustifolia, Majorana hortensis, Melissa ofcinalis, Mentha piperita, M. pulegium, Ocimum basilicum, Origanum syriacum, O. vulgare, Pimpinella anisum, Salvia ofcinalis, Thymus vulgaris, and Verbena ofcinalis). These EOs are promising for the development of fumigant BIs for use in enclosed spaces. Fumigation tests were used to study EOs obtained from plants from 12 families, with Lamiaceae accounting for the majority of species, followed by Apiaceae, Myrtaceae, and Rutaceae [44].
Only five plant species essential oils (Foeniculum vulgare, Mentha piperita, M. pulegium, Ocimum basilicum, and Pimpinella anisum) were found to be effective against aphids in contact and fumigation tests. By chance, these plants can be grown in monocultures and produce relatively high yields of EOs [45,46]. As a result, they can be regarded as an excellent source of active substances for the development of botanical aphidicides. The A. nilotica seed essential oil harmed S. litura, T. molitor, O. hyalinipennis, and A. fabae larvae and adults significantly 24 h after treatment. After 24 h of treatment, the essential oils caused remarkable insecticidal efficacy with low LC50 and LC90 values of 62.215–241.183 μl/mL in S. litura, 56.719–201.912 μl/mL in T. molitor, 37.629–118.485 μl/mL in O. hyalinipennis, and 32.559–75.212 μl/mL. All had high toxicity levels, with 89% mortality in S. litura, 92% mortality in T. molitor, 96% mortality in O. hyalinipennis, and 100% mortality in A. fabae. Similarly, A. nilotica seed essential oils and seed pod crude extract demonstrated remarkable toxicity in laboratory conditions against disease-causing mosquito larvae of Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus [18]. Vivekanandhan et al. [32] discovered that essential oils derived from Eucalyptus globulus leaves killed major mosquito larvae under laboratory experiments.
The seed derived essential oil has no effect on non-target species such as E. eugeniae and A. salina. No earthworms died after seven days of treatment with the seed-derived essential oil. Essential oils had no sublethal effects in the gastrointestinal or other tissues of earthworms. Permethrin, a chemical insecticide, was used as a positive control, and the results revealed abnormalities in earthworm gut cells as well as an uneven epithelial surface. The nuclei were not spherical, and there was cellular debris. The outcomes of the seed-derived essential oil treatment and the control treatment were identical. Similarly, M. anisopliae and F. oxysporum chemical components are not toxic to non-target earthworm species [33,47]. Cu NPs derived from M. robertsii have a lower cytotoxic effect on earthworm and Artemia species in the laboratory than chemical pesticides [39]. After a 24-h treatment period, essential oils extracted from A. nilotica seeds were tested on adult A. salina for non-target toxicity. The Probit analysis results clearly demonstrated that the essential oils had a minimal toxicity effect on A. salina, with LC50 and LC90 values of 348.382–1341.397 μl/mL. A. nilotica seeds were found to have 30% mortality rates 24 h after being treated with A. salina. According to Vivekanandhan et al. [33], the chemical parts of M. anisopliae are not harmful to non-target species.
The chemical constituents of essential oil derived from A. nilotica seeds revealed 15 chemical constituents, among which two have insecticidal properties [18]. The oil extracted from A. nilotica seeds may have insecticidal properties due to key chemical components such as hexadecane and heptacosane. More research into the isolation of various chemical constituents may aid in determining these substances insecticidal potential. Our findings suggest that A. nilotica essential oil can be used to treat a variety of agricultural crop pests. Similarly, Vivekanandhan et al. [18] discovered that the same chemical ingredients, hexadecane and heptacosane, have mosquito larvicidal activity in the laboratory. Essential oil FT-IR analysis was used to identify the major functional groups of essential oils extracted from A. nilotica seeds. The presence of alcohols, alkanes and alkyles, aromatics, amides, ethers, and alhyl halides was confirmed by FT-IR analysis peaks values in the IR radiation range. Similarly, the functional groups of various plant crude extracts are comparable [18,48]. Basil, peppermint, and pennyroyal essential oils have been shown to be effective against whiteflies (Trialeurodes vaporariorum), thrips (Thrips palmi), and two-spotted spider mites (Tetranychus urticae) in greenhouses [49]. Our findings support the lethal effect of A. nilotica seed-derived essential oil on a wide range of agricultural crop pests. The essential oil isolated from the seeds of A. nilotica had a broad-spectrum insecticidal profile with no effect on non-target species. Aphids from A. fabae appear to be more sensitive than those from S. litura, T. molitor, and O. hyalinipennis.
Similarly, Origanum majorana and Citrus limon essential oils were found to be the most effective against E. kuehniella, with lower LC50 and LC99 values of 3.27 and 5.13 μg/mL, respectively [50]. At 32 μg/mL concentrations, oils of O. basilicum and Z. officinale killed E. kuehniella completely within 24 h. Because the oils may be used to preserve wheat flour in a 30% aqueous solution [51]. Another study looked at the insecticidal activity of E. cardamomum essential oil against adults of E. kuehniella and discovered that the insects died the most after 12 h of exposure. The toxicity of fumigators varies with season, essential oil concentration, and exposure period, as demonstrated by five Eucalyptus species tested against E. kuehniella. Another study determined the LC50 value for E. kuehniella to be 258.95 μg/mL after 9 h. The LC50 value of Z. officinale essential oil for E. kuehniella was determined to be 0.61 L/cm2 in a contact toxicity study [52,53]. The toxicity of essential oil vapours of Ferula gummosa, Elettaria cardamomum, and Salvia officinalis on adults and larvae of E. kuehniella was studied, and the results revealed that the essential oil of Ferula gummosa had the strongest insecticidal effect [54]. After 48 h of exposure, the essential oil of Cupressus sempervirens had a 20.83% insecticidal effect [55]. Another study looked into the insect repellent properties of essential oils from L. nobilis and M. communis. The repellency rates of L. nobilis and M. commonis were found to be 20.4% and 10.6%, respectively, at the lowest concentration (0.50 g/mL). Furthermore, at the highest concentration (2.00 g/mL), L. nobilis had an 84.2% repellency rate, while M. commonis had a 61.3% repellency rate Salehi and colleagues [56].
S. zeamais and T. castaneum are extremely susceptable to garlic essential oil and its constituents [57]. T. molitor was found to be more sensitive to diallyl sulphide and diallyl disulfide during the larval stage, then larvae and adults. One possible explanation for the developmental stage differences is that efficacy is determined by the penetration of garlic compounds into the body and the insect's ability to metabolise them. Chemical compounds derived from plant essential oils exhibited significant insecticidal activity.
Acacia nilotica essential oil contains a high percentage of diallyl disulfide as the main volatile chemical component and has insecticidal properties against mosquitos such as A. aegypti, A. stephensi, and C. quinquefasciatus [18]. Similarly toxic chemicals in A. nilotica essential oil killed S. litura, T. molitor, O. hyalinipennis, and A. fabae. Under laboratory conditions, A. nilotica essential oil and its primary chemical constituents have strong insecticidal activity against three mosquito species [18]. The essential oil its chemical constituents, according to our findings, reduced insect species respiration rates for up to 3 h after treatment, which is likely reflected in their behavioural response. The total energy demand of the physiological processes required to establish defence systems against essential oils and toxic compounds can be calculated using insect respiration rate and body mass. As a result, a low rate of respiration indicates physiological stress, and essential oils can affect insect respiration by lowering muscle function, resulting in paralysis. Plant volatiles and their constituents were found to successfully disrupt the host substrate recognition process and influence insect walking behaviour in several studies [58,59]. The seed pod powder extract of A. nilotica had a 90% mortality rate on disease spreading mosquitoes. A. hispidum leaf crude extract has been shown to have significant insecticidal activity, which is consistent with our findings [37]. Ipomoea cairica plant oil was also shown to have a significant larvicidal effect on cockroaches in the laboratory [60]. Botanical oils from a variety of taxa demonstrated good larvicidal activity against Anopheles stephensi (LC50 values ranging from 60 to 538 mg/L), with Lippie sidoides leaf extract performing particularly well against A. aegypti (LC50: 67 mg/L) [61].
Cordia leucomalloides oil is extremely effective against the pathogen transmitting mosquito vector C. quinquefasciatus [62]. Botanically derived essential oils and their constituents are highly toxic insect pest [63]. However, our findings show that all three major insect pests highly susceptible to the seed derived essential oils. Plant-derived essential oils have high insecticidal activity against key mosquito species, according to Ajaegbu et al. [64] Lantana camara essential oil was tested for larvicidal and adult knockdown activity against medical insect pest [65,66]. Plant-based phytochemicals and plant products are increasingly being used to control insect pests [67,68].
5. Conclusion
The present study concludes that the toxic efficacy of A. nilotica seed-derived essential oil produced strong insecticidal efficacy against S. litura, T. molitor, O. hyalinipennis, and A. fabae, with only a minor effect on non-target species E. eugeniae, and A. salina, indicating that they can be used individually to manage insect populations in an environmentally friendly manner. Our findings show that A. nilotica seed essential oil has superior insecticidal activity against S. litura, T. molitor, O. hyalinipennis, and A. fabae, with only a minor effect on non-target species E. eugeniae and A. salina. Two bioactive chemical constituents of A. nilotica seed essential oil, hexadecane and heptacosane, have strong insecticidal activity. A thorough investigation into how to separate the chemicals that kill insects can aid in the development of botanical insecticides that are safe for the environment and can be used to control crop-damaging insects.
Ethics approval and consent to participate
This article contains no studies with human or animal participants conducted by any of the authors.
Consent for publication
The manuscript has not been published elsewhere in whole or in part, and it is not currently being considered for publication in another journal. The final version of the manuscript has been reviewed by all of the authors.
Availability of data and material
This is the author's original work, which has never been published before and has no conflict of interest. The data will be made available to the corresponding author upon request.
Author contribution statement
Vivekanandhan Perumal, Swathy Kannan, and Patcharin Krutmuang: Conceived and designed the experiments.
Vivekanandhan Perumal, Swathy Kannan: Performed the experiments.
Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, Ragavendran Chinnasamy, and Patcharin Krutmuang: Analyzed and interpreted the data;], Contributed reagents, materials, analysis tools or data, Wrote the Paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgments
The authors gratefully acknowledge the laboratory facilities provided by the Society for Research and Initiatives for Sustainable Technologies and Institutions, Grambharti, Gujarat-382735, India, and the Department of Physiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai-77, Tamil Nadu, India.
Contributor Information
Vivekanandhan Perumal, Email: mosqvk@gmail.com.
Patcharin Krutmuang, Email: patcharin.k@cmu.ac.th.
References
- 1.Punzo F., Mutchmor J.A. Effects of temperature, relative humidity and period of exposure on the survival capacity of Tenebrio molitor (Coleoptera: tenebrionidae) J. Kans. Entomol. Soc. 1980;1:260–270. [Google Scholar]
- 2.Fazolin M., Estrela J.L., Catani V., Alécio Lima M.R. Insecticidal properties of essential oils of Piper hispidinervum C. DC.; Piper aduncum L. and Tanaecium nocturnum (Barb. Rodr.) Bur. & K. Shum against Tenebrio molitor L. 1758. Cienc. E Agrotecnol. 2007;31:113–120. [Google Scholar]
- 3.Cosimi S., Rossi E., Cioni P.L., Canale A. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: evaluation of repellency against Sitophiluszeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.) J. Stored Prod. Res. 2009;45:125–132. [Google Scholar]
- 4.Loudon C. Development of Tenebrio molitor in low oxygen levels. J. Insect Physiol. 1988;34:97–103. [Google Scholar]
- 5.Schroeckenstein D.C., Meier-Davis S., Bush R.K. Occupational sensitivity to Tenebrio molitor Linnaeus (yellow mealworm) J. All. Clin. Immunol. 1990;86:182–188. doi: 10.1016/s0091-6749(05)80064-8. [DOI] [PubMed] [Google Scholar]
- 6.Barnes A.I., Siva-Jothy M.T. Density–dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc. RoyalSoc.Lon. Series B:Biol Sci. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkel F.V. The stored grain ecosystem: a global perspective. J. Stored Prod. Res. 1992;28:73–87. [Google Scholar]
- 8.Flinn P.W., Hagstrum D.W., Reed C., Phillips T.W. United States department of agriculture–agricultural research service stored‐grain area wide integrated pest management program. Pest Manag. Sci. 2003;59:614–618. doi: 10.1002/ps.695. [DOI] [PubMed] [Google Scholar]
- 9.Neethirajan S., Karunakaran C., Jayas D.S., White N.D. Detection techniques for stored-product insects in grain. Food Con. 2007;18:157–162. [Google Scholar]
- 10.Smith T.R., Brambila J.A. Major pest of cotton, Oxycarenus hyalinipennis (Heteroptera: Oxycarenidae) in the Bahamas. Fla. Entomol. 2008;91:479–482. [Google Scholar]
- 11.Schaefer C.W., Panizzi A.R. Economic importance of Heteroptera: a general view. Heteroptera Econo Impor. 2000;28:25–30. [Google Scholar]
- 12.De Jong Y., Verbeek M., Michelsen V., de Place Bjørn P., Los W., Steeman F., Bailly N., Basire C., Chylarecki P., Stloukal E., Hagedorn G. Fauna Europaea–all European animal species on the web. Biodivers. Data J. 2014;2 doi: 10.3897/BDJ.2.e4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas M., Hafeez F., Farooq M., Ali A. Dusky cotton bug Oxycarenus spp. (Hemiptera: Lygaeidae): hibernating sites and management by using plant extracts under laboratory conditions. Polish J. Entomol. 2015;84:127. [Google Scholar]
- 14.Khan M.F., Ahmed S.M. Toxicity of neem fruit extract and seed oil against Oxycarenus [Heteroptera] of cotton crop. Acta Biol. Cracov. Ser. Zool. 2000;42 [Google Scholar]
- 15.Ellis P.R., Singh R. A review of the host plants of the cabbage aphid. Brevicoryne brassicae (Homoptera, Aphididae) 1993;16:192–201. [Google Scholar]
- 16.Kessing J.L., Mau R.F. Department of Entomology; Honolulu, Hawaii: 1991. Cabbage Aphid, Brevicoryne brassicae (Linnaeus). Crop Knowledge Master. 2 October 2013. [Google Scholar]
- 17.Zhang H.M., Guo S.S., Fan B., Du S.S., Wang Y.Y., Deng Z.W. Evaluation of efficacy of the essential oil from Ostericum viridiflorum (Turcz.) Kitagawa in control of stored product insects. Environ. Sci. Pollut. Res. 2019;26:1406–1413. doi: 10.1007/s11356-018-3728-x. [DOI] [PubMed] [Google Scholar]
- 18.Vivekanandhan P., Venkatesan R., Ramkumar G., Karthi S., Senthil-Nathan S., Shivakumar M.S. Comparative analysis of major mosquito vectors response to seed-derived essential oil and seed pod-derived extract from Acacia nilotica. Int. J. Environ. Res. Publ. Health. 2018;15:388. doi: 10.3390/ijerph15020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali A., Akhtar N., Khan B.A., Khan M.S., Rasul A., Khalid N., Waseem K., Mahmood T., Ali L. Acacia nilotica: a plant of multipurpose medicinal uses. J. Med. Plants Res. 2012;9:1492–1496. [Google Scholar]
- 20.Singh B.N., Singh B.R., Sarma B.K., Singh H.B. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem. Biol.Intera. 2009;14:20–28. doi: 10.1016/j.cbi.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Jame R. Phytochemical and pharmacological uses of Acacia nilotica- Review. Seeds. 2018;1:15–21. [Google Scholar]
- 22.El-Toumy S.A., Omara E.A., Carlos J., Bermejo J. Phenolic metabolites from Acacia nilotica flowers and evaluation of antihyperglycaemic effect of aqueous extract. Planta Med. 2009;75:PJ143. [Google Scholar]
- 23.Omara E.A., Nada S.A., Farrag A.R., Sharaf W.M., El-Toumy S.A. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine. 2012;15:1059–1067. doi: 10.1016/j.phymed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Rather L.J., Mohammad F. Acacia nilotica (L.): a review of its traditional uses, phytochemistry, and pharmacology. Sust. Chem. Pharm. 2015;2:12–30. [Google Scholar]
- 25.Kamil M. Wound healing effect of Acacia nilotica and Curcuma longa mixture. ModeApp. Pharm Pharmacol. 2018;2:3–5. [Google Scholar]
- 26.Chermenskaya T.D., Stepanycheva E.A., Shchenikova A.V., Chakaeva A.S. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crops Prod. 2010;32(2):157–163. [Google Scholar]
- 27.Zanuncio J.C., Mourão S.A., Martínez L.C., Wilcken C.F., Ramalho F.S., Plata-Rueda A., Soares M.A., Serrão J.E. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae) Sci. Rep. 2016;6:1–8. doi: 10.1038/srep30261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parmar V.S., Jain S.C., Bisht K.S., Jain R., Taneja P., Jha A., Tyagi O.D., Prasad A.K., Wengel J., Olsen C.E., Boll P.M. Phytochemistry of the genus piper. Phytochemistry (Oxf.) 1997;1:597–673. [Google Scholar]
- 29.Isman M.B. Plant essential oils for pest and disease management. Crop Protect. 2000;19:603–608. [Google Scholar]
- 30.Martínez L.C., Plata-Rueda A., Zanuncio J.C., Serrao J.E. Bioactivity of six plant extracts on adults of Demotis paneivai (Coleoptera: chrysomelidae) J. Insect Sci. 2015;15:34. doi: 10.1093/jisesa/iev021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chermenskaya T.D., Stepanycheva E.A., Shchenikova A.V., Chakaeva A.S. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crop. Prod. 2010;1:157–163. [Google Scholar]
- 32.Vivekanandhan P., Usha-Raja-Nanthini A., Valli G., Subramanian Shivakumar M. Comparative efficacy of Eucalyptus globulus (Labill) hydrodistilled essential oil and temephos as mosquito larvicide. Nat. Prod. Res. 2020;16:2626–2629. doi: 10.1080/14786419.2018.1547290. [DOI] [PubMed] [Google Scholar]
- 33.Vivekanandhan P., Swathy K., Kalaimurugan D., Ramachandran M., Yuvaraj A., Kumar A.N., Manikandan A.T., Poovarasan N., Shivakumar M.S., Kweka E.J. Larvicidal toxicity of Metarhizium anisopliae metabolites against three mosquito species and non-targeting organisms. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zapata N., Smagghe G. Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind. Crop. Prod. 2010;32:405–410. doi: 10.1002/ps.2018. [DOI] [PubMed] [Google Scholar]
- 35.Stefanazzi N., Stadler T., Ferrero A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored‐grain pests Tribolium castaneum (Coleoptera: tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae) Pest Manag. Sci. 2011;67:639–646. doi: 10.1002/ps.2102. [DOI] [PubMed] [Google Scholar]
- 36.Jemâa J.M., Tersim N., Toudert K.T., Khouja M.L. Insecticidal activities of essential oils from leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and comparative chemical composition. J. Stored Prod. Res. 2012;48:97–104. [Google Scholar]
- 37.Vivekanandhan P., Senthil-Nathan S., Shivakumar M.S. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol. Mol. Plant Pathol. 2018;101:156–162. [Google Scholar]
- 38.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- 39.Vivekanandhan P., Swathy K., Thomas A., Kweka E.J., Rahman A., Pittarate S., Krutmuang P. Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph181910536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y., Lam S.L., Ho S.H. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton. To Sitophilus zeamais Motschulsky and: Tribolium castaneum (Herbst) J. Stored Prod. Res. 2000;36:107–117. [Google Scholar]
- 41.Yang F.L., Li X.G., Zhu F., Lei C.L. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst)(Coleoptera: tenebrionidae) J. Agric. Food Chem. 2009;57:10156–10162. doi: 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- 42.Yang F.L., Zhu F., Lei C.L. Insecticidal activities of garlic substances against adults of grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae) Insect Sci. 2012;19:205–212. [Google Scholar]
- 43.O A., Ogunbinu S., Okeniyi G., Flamini P.L., Cioni I., Ogunwande A., T I., Babalola Essential oil composition of Acacia nilotica Linn., and Acacia albida Delile (Leguminosae) from Nigeria. J. Essent. Oil Res. 2010;22(6):540–542. [Google Scholar]
- 44.Ikbal C., Pavela R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest. Sci. 2019;92:971–986. [Google Scholar]
- 45.Pavela R., Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21(12):1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Machiani M.A., Javanmard A., Morshedloo M.R., Maggi F. Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.) J. Clean. Prod. 2018;171:529–537. [Google Scholar]
- 47.Vivekanandhan P., Karthi S., Shivakumar M.S., Benelli G. Synergistic effect of entomopathogenic fungus F. oxysporum extract in combination with temephos against three major mosquito vectors. Pathog. Glob. Health. 2018;2:37–46. doi: 10.1080/20477724.2018.1438228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratheeba T., Vivekanandhan P., Faeza A.N., Natarajan D. Chemical constituents and larvicidal efficacy of Naringicrenulata (Rutaceae) plant extracts and bioassay guided fractions against Culex quinquefasciatus mosquito (Diptera: Culicidae) Biocatal. Agric. Biotechnol. 2019;19 [Google Scholar]
- 49.Choi W.I., Lee S.G., Park H.M., Ahn Y.J. Toxicity of plant essential oils to Tetranychus urticae (Acari: tetranychidae) and Phytoseiuluspersimilis (Acari: Phytoseiidae) J. Econ. Entomol. 2004;97:553–558. doi: 10.1093/jee/97.2.553. [DOI] [PubMed] [Google Scholar]
- 50.Karabörklü S., Ayvaz A., Yilmaz S., Akbulut M. Chemical composition and fumigant toxicity of some essential oils against Ephestia kuehniella. J. Econ. Entomol. 2011;104:1212–1219. doi: 10.1603/ec10284. [DOI] [PubMed] [Google Scholar]
- 51.Mikhaiel A.A. Potential of some volatile oils in protecting packages of irradiated wheat flour against Ephestia kuehniella and Tribolium castaneum. J. Stored Prod. Res. 2011;47:357–364. [Google Scholar]
- 52.Abbasipour H., Mahmoudvand M., Rastegar F., Hosseinpour M.H. Fumigant toxicity and oviposition detergency of the essential oil from cardamom, Elettaria cardamomum, against three stored–product insects. J. Insect Sci. 2011;1(1):11. doi: 10.1673/031.011.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maedeh M., Hamzeh I., Hossein D., Majid A., Reza R.K. Bioactivity of essential oil from Zingiber officinale (Zingiberaceae) against three stored-product insect species. J. Ess. Oil. Bear. Plants. 2012;15:122–133. [Google Scholar]
- 54.Mahmoudvand M., Abbasipour H., Rastegar F., Hosseinpour M.H., Basij M. Efficacy of some plants as a post-harvest protectant against some major stored pests. Arch. Phytopathol. Plant Protect. 2012;45:806–811. [Google Scholar]
- 55.Ulukanli Z., Karabörklü S., Bozok F., Burhan A.T., Erdogan S., Cenet M., Karaaslan M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinusbrutia and Pinuspinea resin essential oils. Chin. J. Nat. Med. 2014;12:901–910. doi: 10.1016/S1875-5364(14)60133-3. [DOI] [PubMed] [Google Scholar]
- 56.Salehi T., Karimi J., Hasanshahi G., Askarianzadeh A., Abbasipour H., Garjan A.S. The effect of essential oils from Laurusnobilis and Myrtuscommonis on the adults of mediterranean flour moth, Ephestia kuehniella zeller (Lep.: Pyralidae) J. Ess. Oil Bear Plants. 2014;17:553–561. [Google Scholar]
- 57.Liu Z.L., Ho S.H. Bioactivity of the essential oil extracted from Evodiarutaecarpa Hook f. et Thomas against the grain storage insects, SitophiluszeamaisMotsch. andTriboliumcastaneum (Herbst) J. Stored Prod. Res. 1999;35:317–328. [Google Scholar]
- 58.Verheggen F.J., Fagel Q., Heuskin S., Lognay G., Francis F., Haubruge E. Electrophysiological and behavioral responses of the multicolored Asian lady beetle, Harmoniaaxyridis Pallas, to sesquiterpene semiochemicals. J. Chem. Ecol. 2007;33:2148–2155. doi: 10.1007/s10886-007-9370-6. [DOI] [PubMed] [Google Scholar]
- 59.Germinara G.S., De Cristofaro A., Rotundo G. Repellents effectively disrupt the olfactory orientation of Sitophilusgranarius to wheat kernels. J. Pest. Sci. 2015;88:675–684. [Google Scholar]
- 60.Thavara U., Tawatsin A., Chansang C., Asavadachanukorn P., Zaim M., Mulla M.S. Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti (L.) (Diptera: Culicidae) in water-storage containers. Southeast Asian J. Trop. Med. Publ. Health. 2007;38:269. [PubMed] [Google Scholar]
- 61.Berenbaum M.R., Johnson R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin Insect Sci. 2015;10:51–58. doi: 10.1016/j.cois.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Santos A.A., de Oliveira B.M., Melo C.R., Lima A.P., Santana E.D., Blank A.F., Picanço M.C., Araújo A.P., Cristaldo P.F., Bacci L. Sub-lethal effects of essential oil of Lippia sidoides on drywood termite Cryptotermes brevis (Blattodea: termitoidea) Ecotoxicol. Environ. Saf. 2017;1:436–441. doi: 10.1016/j.ecoenv.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 63.Pavela R. Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind. Crop. Prod. 2015;76:174–187. [Google Scholar]
- 64.Ajaegbu E.E., Danga S.P., Chijoke I.U., Okoye F.B. Mosquito adulticidal activity of the leaf extracts of Spondias mombin L. against Aedes aegypti L. and isolation of active principles. J.VecBorneDis. 2016;1(1):17. 53. [PubMed] [Google Scholar]
- 65.Dua V., Surwade S.P., Ammu S., Agnihotra S.R., Jain S., Roberts K.E., Park S., Ruoff R.S., Manohar S.K. All‐organic vapor sensor using inkjet‐printed reduced graphene oxide. Angew. Chem., Int. Ed. 2010;49:2154–2157. doi: 10.1002/anie.200905089. [DOI] [PubMed] [Google Scholar]
- 66.Mansour S.A., Foda M.S., Aly A.R. Mosquitocidal activity of two Bacillus bacterial endotoxins combined with plant oils and conventional insecticides. Ind. Crop. Prod. 2012;35:44–52. [Google Scholar]
- 67.Murugan K., Babu R., Jeyabalan D., Kumar N.S., Sivaramakrishnan S. Antipupational effect of neem oil and neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston) J. Entomol. Res. 1996;20:137–139. [Google Scholar]
- 68.OECD T.G. OECD Publishing; Paris: 2004. Earthworm Reproduction Test (Eiseniafetida/Eisenia andrei). OECD Guidelines for the Testing of Chemicals, Section 2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is the author's original work, which has never been published before and has no conflict of interest. The data will be made available to the corresponding author upon request.
.