Abstract
Transplantation-associated thrombotic microangiopathy (TA-TMA) is an increasingly recognized complication of hematopoietic cell transplantation (HCT) associated with significant morbidity and mortality. However, TA-TMA is a clinical diagnosis, and multiple criteria have been proposed without universal application. Although some patients have a self-resolving disease, others progress to multiorgan failure and/or death. Poor prognostic features also are not uniformly accepted. The lack of harmonization of diagnostic and prognostic markers has precluded multi-institutional studies to better understand incidence and outcomes. Even current interventional trials use different criteria, making it challenging to interpret the data. To address this urgent need, the American Society for Transplantation and Cellular Therapy, Center for International Bone Marrow Transplant Research, Asia-Pacific Blood and Marrow Transplantation, and European Society for Blood and Marrow Transplantation nominated representatives for an expert panel tasked with reaching consensus on diagnostic and prognostic criteria. The panel reviewed literature, generated consensus statements regarding diagnostic and prognostic features of TA-TMA using the Delphi method, and identified future directions of investigation. Consensus was reached on 4 key concepts: (1) TA-TMA can be diagnosed using clinical and laboratory criteria or tissue biopsy of kidney or gastrointestinal tissue; however, biopsy is not required; (2) consensus diagnostic criteria are proposed using the modified Jodele criteria with additional definitions of anemia and thrombocytopenia. TA-TMA is diagnosed when ≥4 of the following 7 features occur twice within 14 days: anemia, defined as failure to achieve transfusion independence despite neutrophil engraftment; hemoglobin decline by ≥1 g/dL or new-onset transfusion dependence; thrombocytopenia, defined as failure to achieve platelet engraftment, higher-than-expected transfusion needs, refractory to platelet transfusions, or ≥50% reduction in baseline platelet count after full platelet engraftment; lactate dehydrogenase (LDH) exceeding the upper limit of normal (ULN); schistocytes; hypertension; soluble C5b-9 (sC5b-9) exceeding the ULN; and proteinuria (≥1 mg/mg random urine protein-to-creatinine ratio [rUPCR]); (3) patients with any of the following features are at increased risk of nonrelapse mortality and should be stratified as high-risk TA-TMA: elevated sC5b-9, LDH ≥2 times the ULN, rUPCR ≥1 mg/mg, multiorgan dysfunction, concurrent grade II-IV acute graft-versus-host disease (GVHD), or infection (bacterial or viral); and (4) all allogeneic and pediatric autologous HCT recipients with neuroblastoma should be screened weekly for TA-TMA during the first 100 days post-HCT. Patients diagnosed with TA-TMA should be risk-stratified, and those with high-risk disease should be offered participation in a clinical trial for TA-TMA-directed therapy if available. We propose that these criteria and risk stratification features be used in data registries, prospective studies, and clinical practice across international settings. This harmonization will facilitate the investigation of TA-TMA across populations diverse in race, ethnicity, age, disease indications, and transplantation characteristics. As these criteria are widely used, we expect continued refinement as necessary. Efforts to identify more specific diagnostic and prognostic biomarkers are a top priority of the field. Finally, an investigation of the impact of TA-TMA-directed treatment, particularly in the setting of concurrent highly morbid complications, such as steroid-refractory GVHD and infection, is critically needed.
Keywords: Transplantation-associated, thrombotic microangiopathy, Risk stratification, Diagnostic criteria, Complement, Nonrelapse mortality
INTRODUCTION
Transplantation-associated thrombocytic microangiography (TA-TMA) is thought to be a disease of microvascular endothelial dysfunction and complement activation that can develop after a variety of insults, including inherent predisposition (endothelial dysfunction and complement genetic alterations), toxicity from the hematopoietic cell transplantation (HCT) preparative regimen or graft-versus-host disease (GVHD) prophylaxis, and complications such as GVHD itself and infection.1–5 These insults result in a proinflammatory, prothrombotic, and proapoptotic milieu in the microvasculature and formation of thrombosis, possibly leading to organ dysfunction. Renal insufficiency and/or failure is the most common organ manifestation of TA-TMA; however, the systemic nature of the pathophysiology can lead to the involvement of other organs, including the central nervous system, lungs, and gastrointestinal (GI) system. Clinical manifestations include refractory hypertension, posterior reversible encephalopathy syndrome, seizures, altered mental status, pulmonary hypertension, diffuse alveolar hemorrhage, abdominal pain, GI ischemia and bleeding, and serositis, including pericardial and/or pleural effusions.1,4,5
Although a renal biopsy allows for the definitive diagnosis of TA-TMA, this is rarely obtained in the post-HCT setting.6–8 Furthermore, the kidneys are not always impacted by TA-TMA. Histology of other organs impacted by TA-TMA are described and include the intestines9–12, brain13, lungs14,15, skin13, liver13, and testes.16 Regardless of organ involvement, all share similar vascular and endothelial changes seen on renal biopsies. In addition to vascular changes, multiple studies including children and adults and in centers worldwide describe gut histologic changes of intestinal TMA9–12.However, tissue diagnosis of TA-TMA in an organ other than the kidneys is not universal.
To date, there are no specific diagnostic biomarkers or biomarker panels for TA-TMA. Thus, TA-TMA is primarily a clinical diagnosis comprising nonspecific laboratory and clinical manifestations.17–21 Multiple criteria have been proposed, all of which include cardinal features of microangiopathy: anemia, thrombocytopenia, and evidence of RBC destruction. However, markers of RBC destruction vary by criteria; some require elevated lactate dehydrogenase (LDH) versus the presence of schistocytes, whereas others require organ dysfunction, inherently capturing more severe illness. Moreover, the various criteria include different numbers of features and differ in the required time frame when these features must appear. All diagnostic criteria rely on nonspecific features that (1) can be expected in the post-HCT setting (eg, anemia, thrombocytopenia), (2) can be attributed to medication side effects (eg, hypertension in patients on calcineurin inhibitors), or (3) can be attributed to concurrent comorbidities (eg, proteinuria in patients with hemorrhagic cystitis). These challenges have contributed to the lack of harmonization of the diagnostic criteria used for this disease.
In the absence of consensus for diagnostic criteria, the true incidence of TA-TMA is poorly defined, with the reported incidence ranging from .8% to 36%.7,22–24 Registry studies, including those from the Center for International Blood and Marrow Transplant Research and the European Society for Blood and Marrow Transplantation, typically rich resources for outcome studies in the HCT field, are inconclusive for investigating TA-TMA because of differences in the type of data collected from sites and likely underreporting from institutions who have differences in screening practices. Ongoing randomized controlled trials for novel interventions for GVHD in the HCT setting are not uniformly collecting information about TA-TMA. Even when data are collected, they are difficult to cross-reference or interpret given the different criteria used among institutions, and thus these sources cannot be used for secondary analysis.
Regardless of the diagnostic criteria used, multiple studies have demonstrated that nonrelapse mortality (NRM) is significantly higher in patients with TA-TMA compared to those without TA-TMA. Although some patients will have a self-remitting course, more than one-half of patients will progress to multiorgan dysfunction, with mortality rates typically exceeding 50% at 1 year post-HCT.1,25–27 TA-TMA is also associated with significant morbidity and healthcare utilization, including prolonged hospital stays, intensive care unit admissions, chronic kidney disease, and, among children with neuroblastoma, delays in the next phases of therapy.23,28,29
Typically an early complication of HCT, TA-TMA is diagnosed at a median of 22 to 100 days post-HCT.18,27 Risk factors for TA-TMA include second allogeneic HCT, tandem autologous HCT for neuroblastoma, medications, HCT indication, grade II-IV acute GVHD, bacterial and viral infections, myeloablative preparative regimens, and older transplant recipient age.30–34 Although many of the risk factors for developing TA-TMA confer increased NRM independently (eg grade II-IV acute GVHD), multiple studies have demonstrated that even after adjusting for these comorbidities, TA-TMA remains associated with increased NRM.19,35
Identifying patients with TA-TMA who are at the greatest risk for organ dysfunction and/or death and who will most benefit from TA-TMA-directed therapy is critical. Risk stratification facilitates treatment in those with high-risk diseases while sparing the potential toxicity and cost of therapy in those who may have a self-remitting course. However, these approaches also vary. There are 6 ongoing or recently completed interventional trials for TA-TMA (ClinicalTrials.gov identifiers NCT05148299, NCT04557735, NCT04247906, NCT04543591, NCT04784455, and NCT02222545), using 6 different eligibility criteria. Thus, there is a critical need for harmonization of definitions for diagnosis and risk stratification of TA-TMA.
The purpose of this panel was to propose consensus diagnostic and prognostic criteria for TA-TMA. Additionally, we identified knowledge gaps in the field and proposed a road-map for future directions. Outside of enrolling patients in TA-TMA-directed clinical trials when indicated and available, treatment was not discussed in this panel.
SUMMARY OF CONSENSUS KEY POINTS
TA-TMA is associated with significant mortality, particularly once multiorgan dysfunction has occurred, which is difficult to reverse. Thus, identifying patients early in the clinical course is critical.
Universal application of diagnostic criteria and risk stratification will allow for a better understanding of the incidence, risk factors, and outcomes and allow interpretation of the impact of TA-TMA-directed therapy. This is particularly critical with the advent of different therapeutic approaches under investigation in clinical trials.
TA-TMA can be diagnosed by renal biopsy, by intestinal biopsy, or clinically using the modified Jodele criteria. These criteria do not rely on the presence of organ dysfunction or on a single diagnostic criterion; this flexibility facilitates an earlier diagnosis. Limitations to the Jodele criteria include that they have been applied primarily to pediatric and young adult cohorts and include measurement of soluble C5b-9 (sC5b-9), a test that is not widely available. Nonetheless, the Jodele criteria have been validated in a prospective multi-institutional study. We have added definitions for anemia and thrombocytopenia to reduce ambiguity and facilitate standardized reporting. Harmonized reporting to registries should facilitate the potential future refinement of criteria as necessary.
Features associated with increased risk of NRM among those with TA-TMA should be classified as high-risk. The harmonization panel proposes that patients with any poor prognostic feature be stratified as high-risk TA-TMA. Poor prognostic features include elevated sC5b-9 (≥ upper limit of normal [ULN]), random urine protein-to-creatinine ratio (rUPCR) ≥1 mg/mg, organ dysfunction (as defined in Table 4), LDH ≥2 times the ULN, concurrent grade II-IV acute GVHD, or concurrent infections (bacterial or viral). In the absence of these features, TA-TMA is considered standard risk. Patients with high-risk TA-TMA should be enrolled in available clinical trials to determine the safety and efficacy of TA-TMA-directed therapy. Standard-risk patients can be observed closely.
Continued efforts to identify more specific diagnostic and prognostic biomarkers of TA-TMA are a top priority in the field.
All allogeneic HCT recipients and children undergoing autologous HCT for neuroblastoma should be regularly screened for TA-TMA until day 100 post-transplantation and thereafter in the presence of comorbidities, particularly GVHD and infections.
Further investigation is needed to understand the impact of TA-TMA-directed treatment on organ function and survival, particularly in the setting of other highly comorbid complications, such as GVHD and infection.
Table 4.
Risk Stratification of TMA into Standard-Risk and High-Risk
| Standard-Risk TMA | High-Risk TMA |
|---|---|
| Peak LDH <2 times ULN | Peak LDH >2 times ULN* |
| Spot rUPCR <1 mg/mg | Spot rUPCR ≥1 mg/mg |
| KDIGO stage I AKI | Any organ dysfunction developing in the setting of TMA except KDIGO stage I AKI (see Table 5) |
| Normal sC5b-9 | Elevated sC5b-9 (>ULN) |
| Concurrent acute GVHD grade II-IV* | |
| Concurrent systemic infection (bacterial or viral)* |
KDIGO indicates Kidney Disease Improving Global Outcomes.
International Consensus Risk stratification is a modification of Jodele et al.1, with additional risk factors indicated by an asterisk.
METHODS
The American Society for Transplantation and Cellular Therapy, Center for International Blood and Marrow Transplant Research, Asia-Pacific Blood and Marrow Transplantation, and European Society for Blood and Marrow Transplantation convened a Steering Committee to address challenging issues in HCT for which definitions vary or are unclear. Each society nominated 2 experts to form a TA-TMA Harmonization of Definitions Panel. Panel members included co-authors M.L.S., E.C., B.C., C.D., V.H., S.J., I.M., A.S., and V. S. The Steering Committee members included Y.A., B.E.S., P.A.C., J.K., N.K., P.L., U.P., R.S., A.S., and A.W. I.S.O. served as the project manager.
Panel members performed a literature search with a particular focus on articles from 2000 to 2022 to capture all currently reported TA-TMA diagnostic criteria and features associated with increased NRM. Peer-reviewed articles including ≥10 patients with TA-TMA and prognostic features reported in ≥2 articles were discussed within the panel. Diagnostic and prognostic criteria were evaluated with an emphasis on prospective validation and multicenter investigations.
After reviewing, summarizing, and presenting the literature, panel members used a modified Delphi method to facilitate the establishment of a consensus. This method is a group consensus strategy that systematically uses a literature review, the opinion of stakeholders, and the judgment of experts within a field to reach an agreement. Panel members voted on a statement with the following options for each diagnostic and prognostic criterion: (a) agree with the statement as written; (b) disagree with the statement, insufficient evidence; or (c) the statement should be refined. The statements were modified based on these discussions and then sent to the panel for members to respond anonymously. Each statement was scored using the Likert scale to specify the level of agreement in 5 points: 1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; and 5, strongly agree. Consensus was defined as 70% of members responding with 4 or 5 for a given statement. For questions that did not achieve consensus, further discussion and refinement of statements were done until consensus was achieved. Consensus was ultimately achieved on all statements. The panel periodically presented their findings to the Steering Committee, with revisions made throughout the process based on Steering Committee feedback.
The strength of the evidence supporting the consensus statement was assigned a category of evidence as defined as follows: category 1, high level of evidence such as randomized controlled trials with uniform consensus;, category 2A, lower level of evidence with uniform consensus; category 2B, lower level of evidence without a uniform consensus but with no major disagreement; and category 3, any level of evidence but with major disagreement.36 Meetings began in September 2021. An initial draft was proposed to the Harmonization Committee on April 24, 2022, and underwent 6 revisions.
DIAGNOSTIC TA-TMA CRITERIA
Current Knowledge
Currently, TA-TMA is primarily a clinical diagnosis. The Harmonization Panel considered the following proposed TA-TMA diagnostic criteria: City of Hope, Cho, Bone Marrow Transplant Clinical Trial Network (BMT CTN), Li, International Working Group (IWG), and Jodele (Table 1). To determine which diagnostic criteria should be used in the future, the panel prioritized diagnostic criteria that facilitate early identification of TA-TMA. Discussions centered on specific required criterion, including schistocytes, organ dysfunction, Coombs test, and timing of laboratory and clinical criteria.
Table 1.
Summary of Previously Proposed Diagnostic Criteria
| Parameter | Cho et al., 201017 | BMT-CTN, 200576 | IWG, 200721 | City of Hope, 201319 | Li et al., 201920 | Jodele et al., 201418 |
|---|---|---|---|---|---|---|
| Criteria | All features present at ≥2 time points | All features present | All features present | 4 criteria: definite TMA; 3 criteria: probable TMA | All features occurring within 24 h, overall TA-TMA microangiopathy, definite MAHA plus organ dysfunction | Renal biopsy consistent with TA-TMA or clinical diagnosis, ≥4 of 7 features at ≥2 time points in 14 d |
| Anemia or increased RBC transfusion needs | X | X | X | X | X | X |
| Thrombocytopenia with increased platelet transfusion needs, or a 50% decrease in platelet count postengraftment | X | X | X | X | X | X |
| Elevated LDH | X | X | X | X (≥2 times ULN) | X (≥2 times ULN) | X |
| Schistocytes | X (≥2 per HPF) | X (≥2 per HPF) | X (>4%) | X (present or nucleated RBCs) | X (>2 per HPF) | X (present) |
| Negative Coombs test | X | X | ||||
| Renal dysfunction | X | X (definite) | ||||
| Decreased haptoglobin | X | X | ||||
| Normal coagulation studies | X | X | ||||
| sC5b-9 >ULN | X | |||||
| Hypertension, ≥95th percentile for patients age <18 yr, ≥140/90 mmHg for patients age ≥18 yr | X | |||||
| rUPCR | X | |||||
| Renal biopsy | X (not required, but if obtained and consistent with TMA, sufficient for diagnosis) | |||||
| Neurologic dysfunction (encephalopathy, seizures) | X | X (definite) |
MAHA indicates microangiopathic hemolytic anemia; Abbreviations: high-power field (HPF), day (d), hour (h), upper limit of normal (ULN), lactate dehydrogenase (LDH), random urine protein to creatinine ratio (rUPCR)
Schistocytes as a diagnostic criterion
Of the 5 established criteria reviewed, the Cho, IWG, and BMT-CTN criteria require the presence of schistocytes to make a diagnosis of TA-TMA. However, there is significant interinstitutional and intrainstitutional variability in the reporting of schistocytes.37 While there are recommendations by the International Committee for Standardization in Hematology for evaluating schistocytes, these are derived from a healthy cohort and are not implemented widely in clinical laboratory testing.38 The range of expected schistocytes in the HCT setting are unknown. In some cases, schistocytes may be reduced in the circulation due to extravasation, and tissue diagnoses of TA-TMA have been reported in the absence of schistocytes.39 Although the presence of schistocytes is greatly supportive of the diagnosis, based on these data, the panel decided that it should not be mandated. Thus, these criteria were excluded. The only proposed criteria that do not mandate schistocytes are the Li and Jodele criteria.
Organ dysfunction as a diagnostic criterion
The BMT-CTN and Li criteria also require either renal and/or neurologic dysfunction. Although these features have prognostic implications, organ dysfunction may be later manifestations of the disease, and their inclusion may impede early diagnosis.
Negative Coombs test as a diagnostic criterion
The Cho and BMT-CTN criteria require a negative Coombs test as a diagnostic feature. Although TA-TMA is not an antibody-mediated disease, it is possible that patients can have concurrent positive Coombs tests for other reasons, such as i.v. immunoglobulin administration or ABO incompatibility. Although TA-TMA needs to be considered very carefully in the setting of a positive Coombs test, this alone should not definitively rule out TA-TMA.
Timing of diagnostic criterion
The Cho, BMT-CTN, IWG, City of Hope, and Li criteria require that all features be present simultaneously to diagnose TA-TMA. However, there is evidence that TA-TMA features develop over time, with some features lagging by 4 weeks. The Jodele criteria allow for a 14-day period to meet the criteria18; thus, we considered these criteria in more detail.
Jodele criteria
The Jodele criteria require the presence of ≥ 4 of 7 features at 2 different time points within 14 days: anemia, thrombocytopenia, elevated LDH, schistocytes, hypertension (blood pressure ≥140/90 mmHg in patients age ≥18 years or ≥99th percentile in those age <18 years), sC5b-9 ≥ ULN, and rUPCR≥1 mg/mg.6 No single criterion is deemed essential or critical for a diagnosis, and there is inherent flexibility in allowing patients to have 4 different features. Furthermore, these criteria have been used prospectively and in a multicenter setting.23 Because patients can be anemic and thrombocytopenic for up to 100 days post-HCT, we needed to clarify the definitions of anemia and thrombocytopenia in the TA-TMA diagnostic criteria. The proposed consensus diagnostic criteria from the Harmonization Panel are listed in Table 3.
Table 3.
Clinical Manifestations, Diagnostic Workup, and Considerations in TA-TMA
| Organ System | Clinical Manifestations | Diagnostic Workup | Supportive of TA-TMA Diagnosis | Clinical Considerations |
|---|---|---|---|---|
| Hematologic | Bleeding, fatigue | Complete blood count | Anemia, defined as (1) failure to achieve RBC transfusion independence despite evidence of neutrophil engraftment (in absence of AIHA or PRCA from donor recipient major ABO mismatch); (2) hemoglobin decline from baseline by ≥1 g/dL; or (3) new onset of transfusion dependence Thrombocytopenia, defined as (1) failure to achieve platelet engraftment with greater than expected platelet transfusion needs; (2) refractory to platelet transfusions; or (3) a 50% reduction in baseline platelet count after initial platelet engraftment | In the early post-HCT period, there are multiple etiologies for protracted cytopenias including conditioning regimen, engraftment failure, sinusoidal obstructive syndrome, GVHD, severe infections, GI bleeding, etc. Because TA-TMA often develops in the early post-HCT period, it may overlap with these complications. TA-TMA should be considered in the context of these multiple etiologies, and additional testing should be performed to support or rule out its presence. |
| LDH | Above ULN for age on ≥2 occasions | Elevated LDH is not specific and needs to be interpreted within the context of other laboratory test results. However, serum LDH is almost always persistently elevated in TA-TMA. | ||
| Haptoglobin | Below lower limit of normal for age | Low haptoglobin is consistent with hemolysis. However, haptoglobin is an acute-phase reactant, and in HCT recipients with ongoing inflammation, haptoglobin may be falsely elevated. Thus, low haptoglobin is helpful to confirm hemolysis, but normal or elevated haptoglobin does not rule out hemolysis. | ||
| Direct Coombs test | Negative | A positive direct Coombs test can occur in patients after receipt of i.v. immunoglobulin and certain medications or can be present in ABO-mismatched donors and recipients. A positive test does not rule out TA-TMA, but the diagnosis should be questioned and additional testing performed. | ||
| Manual review of peripheral blood smear | Schistocytes (any presence) | Although there are published guidelines defining pathologic schistocyte numbers, these are derived from the healthy population. Whether these data can be extrapolated to the HCT population is unknown. The presence of more than 2 schistocytes per HPF is highly supportive of a microangiopathic process; however, the absence of schistocytes does not rule out TA-TMA. | ||
| ADAMTS-13 Activity | ≥10% | <10% ADAMTS-13 activity is diagnostic of TTP. Although ADAMTS-13 should be checked as part of the TMA evaluation, we do not recommend delaying TA-TMA treatment for this laboratory result, as TTP (<10% activity) is extremely rare in the HCT setting. | ||
| Coagulation studies | Normal coagulation studies | Coagulation studies are recommended to rule out disseminated intravascular coagulation when considering a diagnosis of TA-TMA. However, it is important to recognize that PT/PTT could be abnormal in the setting of poor nutrition and/or liver dysfunction as well, and that abnormal coagulation tests from other causes can coexist in TA-TMA. | ||
| Renal | Hypertension | Serum creatinine | ≥2 times pretransplantation baseline or a ≥50% decrease in eGFR using serum creatinine or cystatin C | AKI is not necessary for the diagnosis of TA-TMA and can be absent in earlier stages. However, TA-TMA should always be included in the differential diagnosis in patients who develop AKI after HCT. |
| rUPCR | ≥1 mg/mg | De novo proteinuria can be an early sign of developing renal TA-TMA, often occurring before AKI becomes apparent. In patients with concurrent hemorrhagic cystitis/hematuria, urine protein may be difficult to interpret; however, viral infections (including BK virus and adenovirus) are themselves known risk factors for TA-TMA. | ||
| Hypertension | Age <18 yr, BP ≥99th percentile; Age ≥18 yr, BP ≥140/90 mmHg | Hypertension is relatively common among adults as a baseline medical condition and is often exacerbated by calcineurin inhibitors or steroids after allogeneic HCT. TA-TMA should be considered if the hypertension is out of proportion to expected, and there is need for additional antihypertensive agents (especially 2 or more agents) to maintain control of BP. | ||
| Cardiopulmonary | Shortness of breath, hypoxia, hemoptysis, or diffuse alveolar hemorrhage | Echocardiogram | Elevated right ventricular pressure concerning for pulmonary hypertension, right-sided heart failure | Patients with respiratory symptoms should undergo echocardiography to evaluate for pulmonary hypertension. |
| Chest imaging | Pleural effusion or pericardial effusion on chest X-ray | Serositis leading to pericardial and/or pleural effusions is a known manifestation of TA-TMA. Effusions also can occur in the context of volume overload from VOD/SOS or heart failure. | ||
| Bronchoscopy/BAL | Alveolar hemorrhage | If clinically indicated, bronchoscopy and BAL analysis can identify alveolar hemorrhage and evaluate for infectious organisms. Patients with pulmonary TMA can manifest with diffuse alveolar hemorrhage, with associated poor outcomes. | ||
| GI | Abdominal pain, bloody stools | EGD/colonoscopy | Biopsy results consistent with TA-TMA | Acute GVHD and intestinal TA-TMA symptoms overlap, Intestinal TMA should be considered in patients with existing intestinal acute GVHD who develop severe pain, GI bleeding, or evidence of bowel ischemia. GI pathologists should be alerted to specifically assess for evidence of TA-TMA in the GI biopsies of patients with acute GVHD. |
| Central nervous system | Seizures, altered mental status, visual changes/cortical blindness | MRI/A | Imaging findings consistent with PRES, hemorrhage, or thrombosis | MRI/A findings with or without evidence of PRES or other focal findings may be seen but are not required for the diagnosis of neurologic TA-TMA. Neurologic involvement of TA-TMA should be strongly considered in patients with altered mental status and known TA-TMA regardless of imaging results. Consider CSF analysis to rule out infectious organisms. |
| Lumbar puncture | CSF negative for leukocytosis or infection; may have elevated CSF protein | |||
| EEG | Seizures | |||
| Miscellaneous | — | Complement studies | Elevated sC5b-9 | Elevated sC5b-9 alone is not diagnostic of TA-TMA but is very helpful in supporting the diagnosis if other clinical parameters consistent with TMA are present. Normal C5b-9 levels do not rule out TA-TMA. |
| Biopsy | Kidney or GI biopsy with characteristic TA-TMA features | Regardless of clinical criteria, tissue evidence of TA-TMA is sufficient for diagnosis. There is insufficient evidence of histology of other organs, including skin, liver, etc, or of stains (ie, complement deposition) to diagnose TA-TMA in tissue. |
eGFR indicates estimated glomerular filtration rate; TTP, thrombotic thrombocytopenia purpura; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome; BAL, bronchoalveolar lavage; EGD, esophagogastroduodenoscopy; MRI/A, magnetic resonance imaging/angiography; PRES, posterior reversible encephalopathy; CSF, cerebrospinal fluid; EEG, electroencephalography., GI; gastrointestinal
Of note, the Jodele diagnostic criteria allow for proteinuria detected on random urinalysis or rUPCR. Because urinalysis can be falsely positive in the presence of hematuria, mucus, or dilute or concentrated urine—all of which possible in the post-HCT setting—the consensus was to use rUPCR only for diagnostic criteria.40 In addition, there are some reports using ≥ 5 of 7 criteria; however, requiring 5 or more features was intended to identify a severe phenotype, not for use in diagnosis.41 Without objective, specific diagnostic markers, patients may be overidentified or underidentified. The Jodele criteria allow for earlier identification of patients and likely capture more patients than the other criteria24,42; thus, risk stratification is key to determining whether treatment is indicated.
Additional research is needed to support a diagnosis of TA-TMA in the absence of clinical criteria in organs other than the kidney and intestines. A limitation of the Jodele criteria is that they have been applied primarily in pediatric and young adult cohorts. Harmonization and widespread application of these criteria in diverse cohorts will allow any needed modification over time.
Gaps in Knowledge and Future Directions
Based on the proposed pathophysiology of the disease, some candidate diagnostic biomarkers have been tested in TA-TMA (Figure 1). Suppression of tumorgenicity 2 (ST2) is a marker of endothelial damage, and elevations are associated with the severity of GVHD and NRM.43,44 Some studies have suggested that ST2 is also elevated in TA-TMA.45 Because TA-TMA often co-occurs with GVHD, further investigation is needed to determine the diagnostic utility of ST2.
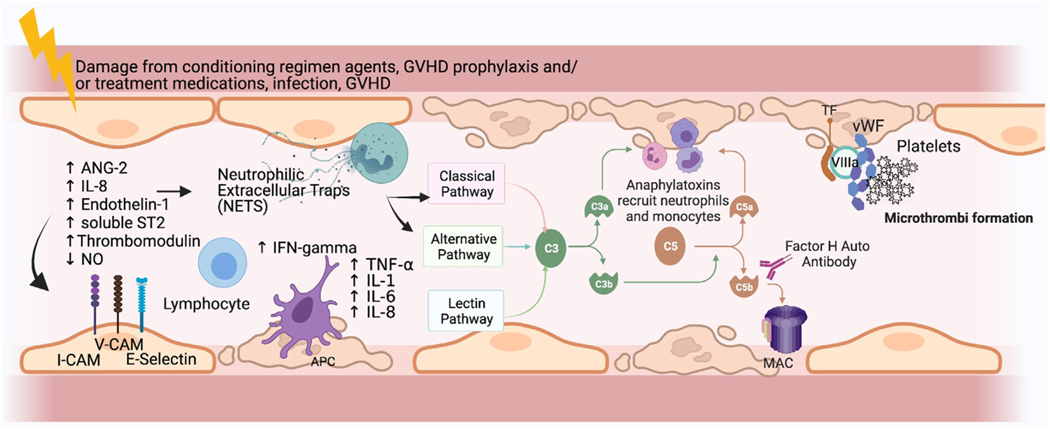
Figure 1.
Proposed pathophysiology of TA-TMA and potential biomarkers. Endothelial cells (ECs) are thought to be damaged by chemotherapy administered as part of the preparative regimen, medications, and complications of BMT. Damaged ECs release ANG-2, leading to vascular destabilization and release of IL-8. Local recruitment of antigen-presenting cells (APCs) and lymphocytes results from the expression of increased adhesion molecules by activated APCs. APCs also express TNF-α and INF-γ, which further activate neutrophils and T cells, which then release neutrophil extracellular traps (NETs). This leads to the activation of a component of the alternative complement pathway, C3b, which binds to the endothelial surface, leading to formation of the membrane activating complex (MAC) on ECs, further contributing to direct ongoing damage of the ECs. The formation of microthrombi ensues next, whereby tissue factor released from the damaged ECs binds factor VIIA and von Willebrand factor (vWF), promoting platelet activation and subsequent thrombus formation. Microthrombi then lead to organ ischemia and end-organ damage (eg, renal failure). Markers of any of these pathways may have diagnostic or prognostic implications in TA-TMA. sC5b-9 and dsDNA (marker of NETs) have been tested for diagnostic utility, but the other markers have not. Potential biomarkers include soluble selectins, markers of endothelial injury, inflammatory cytokines, components of complement cascade other than sC5b-9, and markers of thrombosis. V-CAM indicates vascular cell adhesion molecule; I-CAM, intercellular adhesion molecule (Figure created using Biorender). Figure modified with permission from Schoettler, et al. Current Opinion Hematology, 2021. Schoettler M, Chonat S, Williams K, Lehmann L. Emerging therapeutic and preventive approaches to transplant-associated thrombotic microangiopathy. Curr Opin Hematol. 2021 Nov 1;28 (6):408–416. doi: 10.1097/MOH.0000000000000687. PMID: 34534983.
Neutrophil extracellular traps (NETs), measured indirectly as double-stranded DNA (dsDNA), are known to activate complement and to be a driver of TA-TMA.46,47 Although elevated dsDNA levels early in the course of HCT (day 0 and day 7) predict the later development of TA-TMA, there is no compelling evidence supporting the diagnostic utility of this biomarker at this time.
Improved understanding of the drivers of TA-TMA may reveal additional candidate diagnostic markers. Outside of patient-derived serum on cultured endothelial cells on a plate, there are no in vitro models of TA-TMA or any animal models for interrogating pathophysiology or biomarkers.48 However, there are models of other complement-mediated TMAs that could be leveraged to further understand TA-TMA.49,50 The combination of the development of such in vitro models and large in vivo studies would help identify specific biomarkers of disease. This has been identified as an urgent need and high priority to move this field forward. In addition to blood markers, biomarkers in additional fluids (eg, urine) and noninvasive diagnostic imaging markers of disease can be explored.
Until the development of sensitive and specific laboratory biomarkers, TA-TMA will remain a diagnosis made primarily using laboratory and clinical criteria. Reliance on clinical criteria has inherent limitations as noted in other disease (eg, hemophagocytic lymphohistiocytosis, sinusoidal obstructive syndrome, engraftment syndrome), however the limitations of nonspecific criteria are amplified in HCT because there may be multiple concurrent potential non-TMA etiologies for laboratory abnormalities and clinical features. Furthermore, many of these HCT complications, such as steroid-refractory GVHD and sinusoidal obstruction syndrome, have shared features of endothelial injury. Given the spectrum of endothelial injury post-HCT51–53, it can be difficult to appropriately identify patients with TA-TMA, particularly early in its course.24,54 This is highlighted by discrepancies in autopsy data demonstrating histologic findings of TA-TMA in patients who were not diagnosed clinically.13 The panel summarized clinical, laboratory, and imaging findings that are often present in patients in whom TA-TMA is being considered, along with the diagnostic considerations associated with these findings (Table 2).
Table 2.
TMA Harmonization Panel Consensus Recommended Diagnostic Criteria, Modified Jodele Criteria
| Biopsy-proven disease (kidney or GI) or | |
| Clinical criteria: must meet ≥4 of the following 7 criteria within 14 days at 2 consecutive time points | |
| Anemia* | Defined as one of the following: 1. Failure to achieve transfusion independence for pRBCs despite evidence of neutrophil engraftment 2. Hemoglobin decline from patient’s baseline by 1 g/dL 3. New onset of transfusion dependence Rule out other causes of anemia, such as AIHA and PRCA |
| Thrombocytopenia* | Defined as one of the following: 1. Failure to achieve platelet engraftment 2. Higher than expected platelet transfusion needs 3. Refractoriness to platelet transfusions 4. 50% reduction or greater in baseline platelet count after full platelet engraftment |
| Elevated LDH | >ULN for age |
| Schistocytes | Present |
| Hypertension | >99th percentile for age (<18 yr), or systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg (≥18 yr) |
| Elevated sC5b-9 | ≥ULN |
| Proteinuria | ≥1 mg/mg rUPCR |
pRBCs indicates packed red blood cells; AIHA, autoimmune hemolytic anemia; PRCA, pure red cell aplasia; BP, blood pressure. upper limit of normal; ULN, random urine protein to creatinine ratio (rUPCR)
Indicates clarification from published Jodele et al. criteria1.
Although a previous consensus group recommended using the Jodele criteria for the diagnosis of TA-TMA55, this has not been widely adopted, and institutions continue to use different diagnostic criteria in clinical practice. Herein we identify some barriers that have prevented the widespread adoption of these criteria and propose approaches to address them. First, application of the Jodele criteria has been well described in children and young adults but not yet in older adult cohorts. 56,57 Large studies in patients of diverse age, race, ethnicity, and underlying HCT indication are needed to determine whether these differences impact the application of criteria in older adults. sC5b-9, a marker of terminal complement activation, is a nonspecific finding, and elevations are noted in other diseases.58,59 sC5b-9 testing is not widely available, which can lead to delayed results; moreover, if the sample is not processed appropriately, complement activation can continue in the tube, resulting in inaccurate results. As such, for the Jodele criteria to become widely applicable, it is crucial that the sC5b-9 test become more widely available, especially because sC5b-9 is also an important prognostic marker in this disease.
PROGNOSTIC FEATURES OF TA-TMA
Current Knowledge
Significant advances in identifying poor prognostic factors have been made, particularly in pediatric cohorts. After reviewing the literature in both pediatric and adult cohorts, the panel identified the following features associated with increased NRM in TA-TMA patients: LDH ≥2 times the ULN for age, elevated sC5b-9 (>ULN), proteinuria (rUPCR ≥1 mg/mg), multiorgan dysfunction, concurrent grade II-IV acute GVHD, and concurrent infections. The Harmonization Panel proposes that patients with any of these poor prognostic features be stratified as high-risk TA-TMA. In the absence of these features, TA-TMA is considered standard risk (Table 4). TA-TMA-directed interventions should be considered in patients with high-risk TA-TMA, ideally in the setting of clinical trials.
Elevated sC5b-9
In a prospective study of children by Jodele et al.18, sC5b-9 exceeding the upper limit of laboratory normal range at the time of clinical diagnosis of TA-TMA was associated with an increased risk of NRM, particularly when combined with proteinuria. Elevated sC5b-9 level and its association with NRM were subsequently demonstrated in another cohort, although another group failed to demonstrate this association.60 In adults, the sC5b-9 cutoff for prognosis is unknown, although one study suggested ≥300 mg/dL.26
Proteinuria
Proteinuria, defined as rUPCR ≥1 mg/mg measured on 2 occasions, is an early sign of renal endothelial injury and dysfunction. Proteinuria was associated with increased NRM in children and young adults with TA-TMA in 2 studies, particularly when sC5b-9 was also elevated.18,24,29 In 2 studies of adults, proteinuria on day 100 post-HCT has been associated with an increased risk of NRM, but TA-TMA was not screened for or reported in these studies61,62, and thus whether proteinuria is a poor prognostic factor in older adults with TA-TMA remains unclear.
Evidence of microangiopathy
LDH is an enzyme found in nearly all living cells, and elevated levels are nonspecific but typically indicate cell or tissue damage. Multiple studies have demonstrated that an elevated LDH, from ≥2 times the ULN in both children and adults with TA-TMA, is associated with increased NRM24,63,64, although one study could not confirm this finding.18 The ratio of LDH divided by the platelet count, or the so-called “TTP index,” has also been reported as a prognostic marker for TA-TMA.65 Schistocytes ≥4.9% are part of a scoring system that identifies TA-TMA patients with increased NRM.65 Schistocytes ≥8 HPF in a univariate analysis also was associated with NRM in a univariate analysis, although it was not associated with mortality in a multivariate analysis.17 Given the previously described limitations of schistocytes, the panel proposed using LDH >2 times the ULN for risk stratification.
Organ dysfunction
Acute kidney injury (AKI) is not necessary for the diagnosis of TA-TMA, but its presence is associated with increased NRM in children and adults.17,24 Although different definitions of AKI were used in these studies, the panel proposed defining AKI as one of the following: an increase in serum creatinine ≥2 times over baseline prior to HCT conditioning or a 50% decline in the estimated or measured glomerular filtration rate using either serum creatinine or cystatin C. TA-TMA with GI bleeding also has been associated with increased NRM in multiple pediatric and adult studies.41,66,67 Poor outcomes of TA-TMA in the setting of neurologic6, pulmonary26, and hepatic67 dysfunction also have been reported, although the data are less robust, perhaps because these clinical syndromes are rare or underappreciated. Jodele et al.41 proposed a risk stratification incorporating high-risk definitions of organ dysfunction involving the renal, pulmonary, cardiovascular, central nervous, and GI systems as well as serositis (Table 5) and have demonstrated decreased survival in these patients.
Table 5.
Definitions of Multiorgan Dysfunction in TA-TMA
| Organ | Manifestations |
|---|---|
| Renal | ≥50% reduction in GFR from pre-HCT conditioning value calculated by serum creatinine or cystatin-C or increase in serum creatinine ≥2 times baseline |
| Pulmonary | Any need for positive-pressure ventilation (noninvasive or invasive) for ≥24 hours in the absence of definite etiology (i.e., adenovirus pneumonia, fluid overload, or severe sepsis), diffuse alveolar hemorrhage |
| Cardiovascular | Pulmonary hypertension diagnosed by a cardiologist using cardiac catheterization, or pulmonary hypertension diagnostic criteria on echocardiography |
| Serositis | Clinically significant serositis (pleural or pericardial effusions or ascites) requiring medical therapy (ie, diuretics) or drainage in the absence of other causes (eg, VOD/SOS, congestive heart failure) |
| Central nervous system | Confusion, altered mental status, seizures with or without imaging evidence of posterior reversible encephalopathy syndrome (PRES) |
| GI | GI bleeding and/or intestinal strictures requiring medical or surgical interventions |
Concurrent comorbidities
TA-TMA, acute GVHD, and infections are all risk factors for mortality post-HCT when adjusted for one another and for other comorbidities.19,24,54 However, multiple studies have demonstrated that among patients with TA-TMA, the co-occurrence of acute GVHD is associated with an increased risk of NRM.6,26,68,69 Concurrent viral and bacterial infections also are associated with increased NRM in patients with TA-TMA.22,24,70
Endothelial activation
The endothelial activation and stress index (EASIX) score, calculated as [LDH × creatinine]/platelet count on day 0, is associated with poor overall survival in HCT.71 Although a high EASIX score on day 0 is associated with the later development of TA-TMA in adults, it was not associated with increased NRM among patients with TA-TMA.71,72 The EASIX score likely is not specific to TA-TMA but is rather a reflection of cellular damage and comorbidities. It may be less predictive in pediatric patients, given the nonlinear relationship between EASIX score and age.71 Other markers of endothelial dysfunction, including thrombomodulin73, ANG-226, circulating endothelial cells74, and von Willebrand factor73, have been associated with prognosis in single-center studies but need to be confirmed in other cohorts.
Older age
Older HCT recipient age is associated with increased NRM among patients with TA-TMA compared to those without TA-TMA, although no explicit age cutoff has been identified. Some studies have demonstrated differences in NRM between patients age ≥18 years and those age <18 years27, between patients age ≥50 years and those age <50 years31, and other studies have demonstrated increasing hazard ratio by year or decade of age.54,70
Drug-induced TA-TMA
The combination of sirolimus and a calcineurin inhibitor (cyclosporine or tacrolimus) is a known risk factor for developing TA-TMA.75 In the absence of other risk factors, drug-induced TA-TMA has a good prognosis.20,54,76
Patients with high-risk TA-TMA should be offered TA-TMA-directed therapy, preferably enrolled in a clinical trial. Patients with standard risk TA-TMA can be observed closely.
Gaps in Knowledge and Future Directions
As with the diagnostic criteria, high-risk features of TA-TMA are not specific. Early markers or features that predict NRM in patients with TA-TMA before the development of organ dysfunction are needed. Genetic predisposition for severe disease has been explored. In 2 studies, variants of complement genes were associated with severe disease; however, these were small cohorts and included variants of unknown significance.2,3 Additional large studies are needed to determine whether recipient or donor genetic variations increase the risk of developing TA-TMA or are associated with severe disease. Like the diagnostic criteria, if the mechanisms and drivers of TA-TMA are better understood, these may have prognostic implications.
The studies summarized above are single-center retrospective studies enriched with pediatric patients, and many used differing diagnostic criteria. In addition to the limitations of sC5b-9 summarized above, the expected ranges of sC5b-9 in the inflammatory milieu of HCT are unknown, and there are limited data regarding the appropriate cutoffs for prognosis in adults. Adult patients are expected to have more baseline comorbidities, such as chronic kidney disease, diabetes, and hypertension, prior to HCT that increase the risk of baseline proteinuria.56,57 Whether or how this impacts TA-TMA prognostic criteria is unknown. Using harmonized prognostic criteria, large prospective pragmatic multi-institutional efforts are needed to confirm the poor prognostic features currently described. Such studies should overcome many of these limitations.
NRM in TA-TMA is multifactorial, and more investigation is needed to understand the links between TA-TMA and concurrent highly morbid complications. Serum from patients with acute GVHD induces endothelial damage in in vitro models77, and there is emerging evidence of endothelial activation and damage in acute GVHD45,78 Some common post-HCT infections also are known to invade the endothelium and result in damage, including cytomegalovirus, BK virus, and Candida79. Additional studies are needed to understand the mechanistic overlap of these diseases. Achieving consensus on high-risk patients who merit TA-TMA-directed therapy is the first step to designing clinical trials and understanding the impact of TA-TMA-directed therapy on organ function and survival, particularly in patients with multiple comorbidities.
PROSPECTIVE SCREENING FOR TA-TMA
Current Knowledge
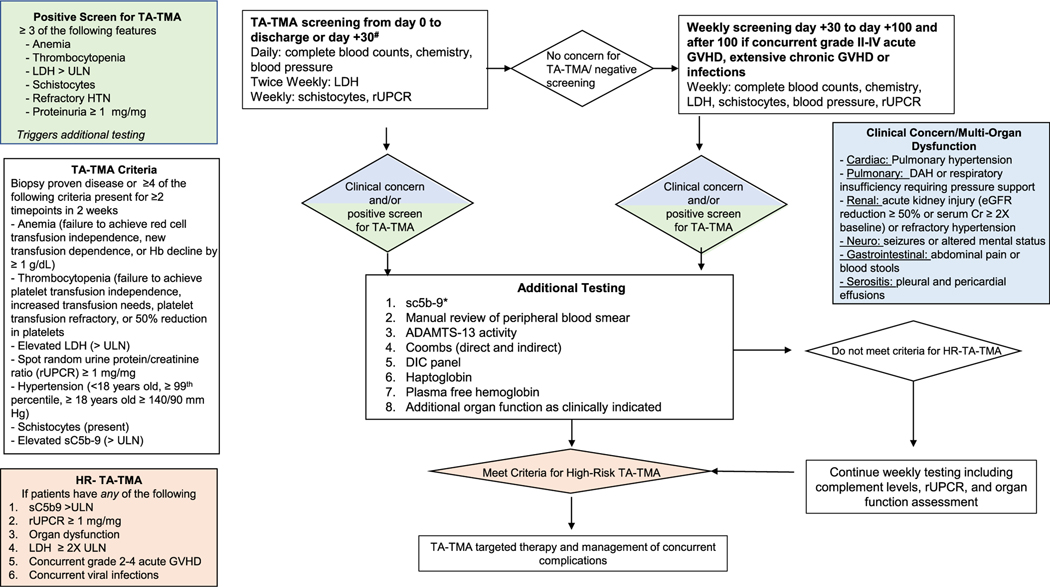
The diagnosis of TA-TMA requires vigilance and a high index of suspicion from clinicians. There is a general consensus that TA-TMA is often underdiagnosed.24,54 To improve early recognition and diagnosis, there is sufficient rationale to support prospective screening for TMA in allogeneic HCT recipients and pediatric autologous HCT recipients with an underlying diagnosis of neuroblastoma.23,32 TA-TMA typically occurs early in the post-HCT period, before day 100, and later diagnoses often are associated with GVHD and infections.31 Thus, we propose that patients be routinely screened in the first 100 days post-HCT, with a low threshold for screening beyond this time point when patients have laboratory features of TA-TMA and concurrent complications. Standard screening should include at least weekly complete blood count, LDH, rUPCR, and blood smears for schistocytes. These are nonspecific laboratory markers; however, when they persist without other explanations and occur simultaneously, TA-TMA should be strongly considered, and if ≥3 of 6 screening features are positive (anemia, thrombocytopenia, elevated LDH, schistocytes, refractory hypertension, or proteinuria), additional laboratory workup should be pursued (Figure 2). Patients who meet diagnostic criteria for TA-TMA should then be risk-stratified. Triggers of TA-TMA, such as GVHD and infections, should be aggressively managed. TA-TMA-directed therapy should be considered in patients with high-risk disease, preferably via enrollment in a clinical trial if available. Patients who do not have high-risk disease should continue to be monitored closely with weekly serial urine and complement testing. Figure 1
Figure 2.
Recommended screening and diagnostic workup for TA-TMA. There is sufficient evidence supporting routine screening of all allogeneic and pediatric autologous HCT recipients with an underlying diagnosis of neuroblastoma through day 100 post-HCT. Screening also should be considered after day 100 in patients who develop a known risk factor for TA-TMA, including acute GVHD, chronic GVHD, or infection. In patients with ≥3 abnormal screening laboratory test results or clinical manifestations or organ dysfunction concerning for TA-TMA, additional testing should be done. In patients who meet the criteria for high-risk TA-TMA, treatment with TA-TMA-directed therapy should be considered. Patients who do not meet high-risk criteria should be monitored closely, and treatment may be initiated at the discretion of the clinician if cytopenia or other manifestations persist. Triggers of TA-TMA (eg, infection, GVHD) should be aggressively managed. *sC5b-9 is not available at all centers. If sC5b-9 testing is not available, screening of urine and other organ function should continue, and treatment offered to patients meeting high-risk TA-TMA criteria.
Gaps in Knowledge and Future Directions
Routine screening is not yet incorporated universally in all institutions. LDH and rUPCR might not be done routinely at all centers, but they are readily available, low-cost tests necessary to assess for TA-TMA. In the absence of screening, TA-TMA is usually diagnosed at later stages of the disease, resulting in significant morbidity. Furthermore, implementing standard screening is vital to understand the true incidence of disease, define the phenotypic spectrum, and further identify prognostic criteria—all of which are key to moving the field forward. Although sC5b-9 is costly and not widely available, it is recommended for risk stratification only in patients who meet the criteria for TA-TMA. In pediatric cohorts who are prospectively screened, the incidence of TA-TMA is ~20% to 30% using the Jodele criteria.18,23 Assuming similar findings in other cohorts, the access and cost implications of implementing widespread screening for TA-TMA and sending sC5b-9 more frequently are unknown and need to be explored.
If a patient meets the criteria, additional testing should be sent for risk stratification. Patients with high-risk features should be offered TA-TMA-directed therapy, preferably via a clinical trial. Patients should be screened after day 100 in the presence of known risk factors for TA-TMA, including GVHD and infections.
Roadmap for the Future
The acknowledged limitation of sC5b-9 testing availability notwithstanding, the panel recommends that the modified Jodele criteria be universally adopted going forward for diagnosis of TA-TMA and that the harmonization prognostic criteria be used for risk stratification in retrospective, prospective, and interventional studies. Harmonizing TA-TMA diagnostic and prognostic criteria internationally is the first step, but we expect that these criteria will be further refined as more data from diverse centers are collected.
Recommendations for studies in the next 3 to 5 years
Apply diagnostic and prognostic criteria proposed by the Harmonization of Definitions panel across international registries and interventional trials to assess the incidence and impact of TA-TMA.
Develop in vitro and in vivo models of TA-TMA to further understand the mechanism of disease and serve as a platform for interrogating novel therapies.
Conduct multi-institutional, collaborative blood biomarker studies to identify and validate diagnostic and prognostic biomarkers of TA-TMA and determine the specificity of biomarkers with concurrent complications, including VOD, GVHD, and infection.
Investigate the links between TA-TMA, acute GVHD, and infections, with a focus on understanding the impact of TA-TMA-directed therapy on organ dysfunction and survival in the presence of concomitant GVHD and infection.
Enroll children and adults on clinical trials to investigate the safety and efficacy of TA-TMA directed therapies.
Consensus Key Point 1:
TA-TMA is independently associated with significant morbidity and mortality (Category 2A)
Consensus Key Point 2:
Harmonization of diagnostic criteria and risk stratification in TA-TMA are critical to better understand the incidence, risk factors, and outcomes of the disease and the impact of TA-TMA-directed therapy (Category 2A)
Consensus Key Point 3:
TA-TMA can be diagnosed via histology (renal or intestinal) or clinically using modified Jodele criteria (Category 2B)
Consensus Key Point 4:
The following features are associated with increased NRM in patients with TA-TMA and are considered high-risk features: elevated sC5b-9 (>ULN), rUPCR (≥1 mg/mg), elevated LDH (≥2 times ULN), grade II-IV acute GVHD, infections (viral or bacterial), and organ dysfunction (Category 2A).
Consensus Key Point 5:
Specific diagnostic and prognostic biomarkers or biomarker panels are needed for TA-TMA (Category 2A)
Consensus Key Point 6:
Additional studies are needed to determine the impact of TA-TMA directed therapy in patients with high-risk disease, particularly in those with concurrent severe GVHD and infection (Category 2A).
Consensus Key Point 7:
All allogeneic and pediatric autologous HCT recipients with neuroblastoma should be routinely screened for TA-TMA through day 100 (Category 2B)
Financial disclosure:
No other authors have an financial disclosures.
Abbreviations:
- TA-TMA
Transplant Associated Thrombotic Microangiopathy
- MOD
Multiorgan Dysfunction
- LDH
Lacate Dehydrogenase
- ULN
Upper Limit of Normal
Footnotes
Authorship statement: All authors participated in the consensus statements. M.S. and S.V. wrote the manuscript, and all authors participated in edits.
Conflict of interest statement:
B.E.S. reports consulting for Mallinckrodt and Orca Bio; C.E.D. reports consulting for Omeros and Alexion; S.V. reports serving on an advisory board for Omeros; S. J. is named on US Patent 10,815,296 B2 as a principal investigator for a drug provided by Alexion and consulting for and honoraria from Omeros, SOBI, Alexion, and MedScape; and V.H. reports consulting for Alexion and Omeros and research funding from CareDx, Jazz Pharmaceuticals, and Omeros.
REFERENCES
- 1.Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019; 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavriilaki E, Touloumenidou T, Sakellari I, et al. Pretransplant genetic susceptibility: clinical relevance in transplant-associated thrombotic microangiopathy. Thromb Haemost. 2020;120:638–646. [DOI] [PubMed] [Google Scholar]
- 3.Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant associated thrombotic microangiopathy. Blood. 2016;127:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavriilaki E, Sakellari I, Batsis I, et al. Transplant-associated thrombotic microangiopathy: incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation. Clin Transplant. 2018;32: e13371. [DOI] [PubMed] [Google Scholar]
- 7.Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med. 2016;7:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren M, Jodele S, Dandoy C, et al. A complete histologic approach to gastrointestinal biopsy from hematopoietic stem cell transplant patients with evidence of transplant-associated gastrointestinal thrombotic microangiopathy. Arch Pathol Lab Med. 2017;141:1558–1566. [DOI] [PubMed] [Google Scholar]
- 10.Gavriilaki E, Sakellari I, Karafoulidou I, et al. Intestinal thrombotic microangiopathy: a distinct entity in the spectrum of graft-versus-host disease. Int J Hematol. 2019;110:529–532. [DOI] [PubMed] [Google Scholar]
- 11.El-Bietar J, Warren M, Dandoy C, et al. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall SA, Zhao Q, Yearsley M, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv. 2018;2:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada R, Nemoto T, Ohashi K, et al. Distribution of transplantation-associated thrombotic microangiopathy (TA-TMA) and comparison between renal TA-TMA and intestinal TA-TMA: autopsy study. Biol Blood Marrow Transplant. 2020;26:178–188. [DOI] [PubMed] [Google Scholar]
- 14.Jodele S, Hirsch R, Laskin B, Davies S, Witte D, Chima R. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19:202–207. [DOI] [PubMed] [Google Scholar]
- 15.Schoettler ML, Saldana BD, Berkenkamp L, et al. Pulmonary manifestations and vascular changes in pediatric transplantation-associated thrombotic microangiopathy. Transplant Cell Ther. 2023;29:45.e1–45.e8. 10.1016/j.jtct.2022.09.026. Epub 2022 Oct 4. PMID: 36202334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabulski A, Szabo S, Myers KC, Davies SM, Jodele S. Testicular thrombotic microangiopathy: an unrecognized complication. Pediatr Blood Cancer. 2021;68:e29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. [DOI] [PubMed] [Google Scholar]
- 18.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:653.. 645-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shayani S, Palmer J, Stiller T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Wu Q, Davis C, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. 2019;25:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruutu T, Barosi G, Benjamin RJ, et al. , et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. [DOI] [PubMed] [Google Scholar]
- 22.Ramgopal A, Sridar S, Dalal J, Kalpatthi R. Thrombotic microangiopathy: multi-institutional review of pediatric patients who underwent HSCT. J Pers Med. 2021;11:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dandoy CE, Rotz S, Alonso PB, et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv. 2021;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoettler M, Lehmann LE, Margossian S, et al. Risk factors for transplant-associated thrombotic microangiopathy and mortality in a pediatric cohort. Blood Adv. 2020;4:2536–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jodele S, Sabulski A. Transplant-associated thrombotic microangiopathy: elucidating prevention strategies and identifying high-risk patients. Expert Rev Hematol. 2021;14:751–763. [DOI] [PubMed] [Google Scholar]
- 26.Li A, Bhatraju PK, Chen J, et al. Prognostic biomarkers for thrombotic microangiopathy after acute graft-versus-host disease: a nested case-control study. Transplant Cell Ther. 2021;27:308.e1–308.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82:638–644. [DOI] [PubMed] [Google Scholar]
- 28.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation associated thrombotic microangiopathy. Blood. 2011;118:1462.. 1452. [DOI] [PubMed] [Google Scholar]
- 29.Laskin BL, Goebel J, Davies SM, et al. Early clinical indicators of transplant-associated thrombotic microangiopathy in pediatric neuroblastoma patients undergoing auto-SCT. Bone Marrow Transplant. 2011;46:682–689. [DOI] [PubMed] [Google Scholar]
- 30.Elfeky R, Lucchini G, Lum SH, et al. New insights into risk factors for transplant-associated thrombotic microangiopathy in pediatric HSCT. Blood Adv. 2020;4:2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasu S, Bostic M, Zhao Q, et al. Acute GVHD, BK virus hemorrhagic cystitis and age are risk factors for transplant-associated thrombotic microangiopathy in adults. Blood Adv. 2022;6:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jodele S, Dandoy CE, Myers K, et al. High-dose carboplatin/etoposide/melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. 2018;53:1311–1318. [DOI] [PubMed] [Google Scholar]
- 33.Daly AS, Hasegawa WS, Lipton JH, Messner HA, Kiss TL. Transplantation-associated thrombotic microangiopathy is associated with transplantation from unrelated donors, acute graft-versus-host disease and veno-occlusive disease of the liver. Transfus Apher Sci. 2002;27:3–12. [DOI] [PubMed] [Google Scholar]
- 34.Schoettler M, Stenger EO, Spencer K, et al. Sickle cell disease is a risk factor for transplant-associated thrombotic microangiopathy in children [e-pub ahead of print]. Blood Adv. 10.1182/bloodadvances.2022008058, Accessed 11/22/2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoettler ML, Lehmann LE, Margossian S, et al. Transplantation-associated thrombotic microangiopathy (TA-TMA): lessons learned from a large pediatric center. Biol Blood Marrow Transplant. 2019;25:S18–S19. suppl. [Google Scholar]
- 36.Desai A, Go RS, Poonacha T. Level of scientific evidence supporting NCCN guidelines: is there evidence of progress? J Clin Oncol. 2019;37 (suppl):14. [Google Scholar]
- 37.Moiseev IS, Tsvetkova T, Aljurf M, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54:1022–1028. [DOI] [PubMed] [Google Scholar]
- 38.Zini G, d’Onofrio G, Erber WN, et al. 2021 update of the 2012 ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes: impact and revisions. Int J Lab Hematol. 2021;43:1264–1271. [DOI] [PubMed] [Google Scholar]
- 39.Wirtschafter E, VanBeek C, Linhares Y. Bone marrow transplant-associated thrombotic microangiopathy without peripheral blood schistocytes: a case report and review of the literature. Exp Hematol Oncol. 2018;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Physician. 2000;62:1333–1340. [PubMed] [Google Scholar]
- 41.Jodele S, Dandoy CE, Lane A, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moiseev IS, Tsvetkova TG, Ruutu T. Practical review of current approaches to diagnosis and treatment of transplant-associated thrombotic microangiopathy. Cell Ther Transplant. 2021;10:17–25. [Google Scholar]
- 43.Major-Monfried H, Renteria AS, Pawarode A, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131:2846–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotz SJ, Dandoy CE, Davies SM. ST2 and endothelial injury as a link between GVHD and microangiopathy. N Engl J Med. 2017;376:1189–1190. [DOI] [PubMed] [Google Scholar]
- 46.Gloude NJ, Khandelwal P, Luebbering N, et al. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood. 2017;130:1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arai Y, Yamashita K, Mizugishi K, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1683–1689. [DOI] [PubMed] [Google Scholar]
- 48.Elhadad S, Chapin J, Copertino D, Van Besien K, Ahamed J, Laurence J. MASP2 levels are elevated in thrombotic microangiopathies: association with microvascular endothelial cell injury and suppression by anti-MASP2 antibody narsoplimab. Clin Exp Immunol. 2021;203:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palomo M, Blasco M, Molina P, et al. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol. 2019;14:1732.. 1719-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda Y, Gullipalli D, Song WC. Modeling complement-driven diseases in transgenic mice: values and limitations. Immunobiology. 2016;221:1080–1090. [DOI] [PubMed] [Google Scholar]
- 51.Luft T, Dreger P, Radujkovic A. Endothelial cell dysfunction: a key determinant for the outcome of allogeneic stem cell transplantation. Bone Marrow Transplant. 2021;56:2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eftychidis I, Sakellari I, Anagnostopoulos A, Gavriilaki E. Endothelial dysfunction and vascular complications after allogeneic hematopoietic cell transplantation: an expert analysis. Expert Rev Hematol. 2021;14: 831–840. [DOI] [PubMed] [Google Scholar]
- 53.Hildebrandt GC, Chao N. Endothelial cell function and endothelial-related disorders following haematopoietic cell transplantation. Br J Haematol. 2020;190:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postalcioglu M, Kim HT, Obut F, et al. Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uderzo C, Jodele S, El Missiry M, et al. Transplant-associated thrombotic microangiopathy (TA-TMA) and consensus-based diagnostic and therapeutic recommendations: which TA-TMA patients to treat and when? J Bone Marrow Res. 2014;2:3. [Google Scholar]
- 56.Rosenstock JL, Pommier M, Stoffels G, Patel S, Michelis MF. Prevalence of proteinuria and albuminuria in an obese population and associated risk factors. Front Med (Lausanne). 2018;5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol. 2010;21:2027.. 2020-. [DOI] [PubMed] [Google Scholar]
- 58.Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117:25018–25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gavriilaki E, Mainou M, Christodoulou I, et al. In vitro evidence of complement activation in patients with sickle cell disease. Haematologica. 2017;102:e481–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavriilaki E, Sakellari I, Chatzikonstantinou T, et al. Endothelial and complement activation as predictors of survival in adult allogeneic hematopoietic cell transplantation. Hemasphere. 2021;5:e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hingorani S, Gooley T, Pao E, Sandmaier B, McDonald G. Urinary cytokines after HCT: evidence for renal inflammation in the pathogenesis of proteinuria and kidney disease. Bone Marrow Transplant. 2014;49:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hingorani SR, Seidel K, Lindner A, Aneja T, Schoch G, McDonald G. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biol Blood Marrow Transplant. 2008;14:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uderzo C, Fumagalli M, De Lorenzo P, et al. Impact of thrombotic thrombocytopenic purpura on leukemic children undergoing bone marrow transplantation. Bone Marrow Transplant. 2000;26:1005–1009. [DOI] [PubMed] [Google Scholar]
- 64.Holler E, Kolb HJ, Hiller E, et al. Microangiopathy in patients on cyclosporine prophylaxis who developed acute graft-versus-host disease after HLA-identical bone marrow transplantation. Blood. 1989;73:2018–2024. [PubMed] [Google Scholar]
- 65.Zeigler ZR, Shadduck RK, Nemunaitis J, Andrews DF, Rosenfeld CS. Bone marrow transplant-associated thrombotic microangiopathy: a case series. Bone Marrow Transplant. 1995;15:247–253. [PubMed] [Google Scholar]
- 66.Zhang XH, Liu X, Wang QM, et al. Thrombotic microangiopathy with concomitant GI aGVHD after allogeneic hematopoietic stem cell transplantation: risk factors and outcome. Eur J Haematol. 2018;100:171–181. [DOI] [PubMed] [Google Scholar]
- 67.Ye Y, Zheng W, Wang J, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35:821–827. [DOI] [PubMed] [Google Scholar]
- 68.Cho BS, Min CK, Eom KS, et al. Clinical impact of thrombotic microangiopathy on the outcome of patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:813–820. [DOI] [PubMed] [Google Scholar]
- 69.Kraft S, Bollinger N, Bodenmann B, et al. High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease. Bone Marrow Transplant. 2019;54:540–548. [DOI] [PubMed] [Google Scholar]
- 70.Heybeli C, Sridharan M, Alkhateeb HB, et al. Characteristics of late transplant-associated thrombotic microangiopathy in patients who underwent allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2020;95:1170–1179. [DOI] [PubMed] [Google Scholar]
- 71.Luft T, Benner A, Terzer T, et al. EASIX and mortality after allogeneic stem cell transplantation. Bone Marrow Transplant. 2020;55:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shouval R, Fein JA, Shouval A, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeigler ZR, Rosenfeld CS, Andrews DF 3rd, et al. Plasma von Willebrand factor antigen (vWF:AG) and thrombomodulin (TM) levels in adult thrombotic thrombocytopenic purpura/hemolytic uremic syndromes (TTP/HUS) and bone marrow transplant-associated thrombotic microangiopathy (BMT-TM). Am J Hematol. 1996;53:213–220. [DOI] [PubMed] [Google Scholar]
- 74.Erdbruegger U, Woywodt A, Kirsch T, Haller H, Haubitz M. Circulating endothelial cells as a prognostic marker in thrombotic microangiopathy. Am J Kidney Dis. 2006;48:564–570. [DOI] [PubMed] [Google Scholar]
- 75.Labrador J, López-Corral L, López-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014;49:684–690. [DOI] [PubMed] [Google Scholar]
- 76.Cutler C, Henry NL, Magee C, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:551–557. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Sanchez J, Hamelmann H, Palomo M, et al. Acute graft-vs-host disease-associated endothelial activation in vitro is prevented by defibrotide. Front Immunol. 2019;10:2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordes S,Mokhtari Z,Bartosova M,et al.Endothelialdamageanddysfunction inacutegraft-versus-hostdisease. Haematologica.2021;106:2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konradt C, Hunter CA. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur J Immunol. 2018; 48:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]