Abstract
Background: Various studies have demonstrated the incidence of hyponatremia in patients with Coronavirus Disease 2019 (COVID-19); however, to our knowledge, no study has assessed the difference in the incidence of hyponatremia in patients with and without COVID-19. Purpose: To compare the incidence of hyponatremia in patients requiring intensive care unit (ICU) admission with and without COVID-19 infection. Methods: This was a single-center, retrospective cohort study of patients with a diagnosis of pneumonia from February 2019 to January 2020, or a diagnosis of COVID-19 from June 2020 to May 2021. Included patients were matched on age and sex. The primary outcome was the incidence of hyponatremia within 72 h of admission. Secondary endpoints collected included severity of hyponatremia, symptomatic hyponatremia, and lowest serum sodium. Results: There were 99 and 104 patients included in pneumonia and COVID-19 arms, respectively. Twenty-nine patients in the pneumonia group and 56 patients in the COVID-19 group had a sodium level <134 mEq/L (29% vs 56%, RR 1.84, P < .01). The mean lowest serum sodium within 72 h of admission was 136.9 mEq/L in the pneumonia group and 134.5 mEq/L in the COVID-19 group (P < .01). Other notable findings included days of mechanical ventilation (3 days vs 8 days, respectively; P < .01), downgrade from the ICU (74.8% vs 59.6%, P = .02), ICU length of stay (4 days vs 10 days, P < .01), hospital length of stay (6 days vs 14 days, P < .01), and mortality (16.2% vs 39.4%, P < .01). Conclusion: Among critically ill patients with COVID-19, the risk of hyponatremia was significantly greater than the risk in critically ill patients with pneumonia.
Keywords: hyponatremia, COVID-19, pneumonia, critically ill
Introduction
Coronavirus Disease 2019 (COVID-19) has affected millions of people worldwide and increased the strain on healthcare systems. Patients with severe disease often develop complications ranging from severe respiratory distress to electrolyte disturbances. Hyponatremia is a well-documented complication among critically ill patients. In the absence of COVID-19, the literature reports an incidence of hyponatremia that ranges from almost 20% to 35% for patients in the medical intensive care unit (MICU).1,2 Hyponatremia is associated with adverse events such as seizures and other neurologic concerns as well as mortality.
Hyponatremia due to COVID-19 is an interesting phenomenon that has not been fully described in current literature. There is a proposed mechanism of pro-inflammatory cytokines such as interleukin (IL) 1b and 6 stimulating hypothalamic arginine vasopressin secretion. In healthy individuals, a rise in serum osmolarity, decreased blood volume or blood pressure, and certain medications can cause vasopressin to increase the amount of water that is retained by the kidneys. Antidiuresis and excess water retention cause the dilution of many electrolytes, especially sodium, and cause hyponatremia. The link between IL-6 and serum sodium levels has been demonstrated as an inverse relationship in multiple studies.3,4 It has been shown in nonhuman trials that the expression of vasopressin is suppressed or decreased when the IL-6 levels are deficient. 5
IL-6 levels are markedly increased during inflammation, but it is unclear if IL-6 alone induces a response or is one of the various cytokines involved. Serum ferritin and C-reactive protein (CRP) are also affected by inflammation. Ferritin is an acute-phase reactant often used to gauge whether interventions used to treat or mitigate inflammation are successful. Serum CRP, an acute-phase protein produced by the liver, increases in the setting of inflammation. The release of IL-6 causes increased production of both ferritin and CRP. 6
The incidence of hyponatremia in patients with COVID-19 has been described in various studies including a study of patients with COVID-19 community-acquired pneumonia in which the authors found that the overall rate of hyponatremia was 20.5%. 7 For most patients, the serum sodium fell between 130 and 134 mmol/L (16.7%) but values <120 mmol/L have been observed. In one study, the incidence of hyponatremia was 30% and a high incidence of mechanical ventilation was also observed. 4 The authors found that the severity of hyponatremia was associated with encephalopathy and reduced the probability of hospital discharge.
Although several trials have reported the incidence of hyponatremia in patients with COVID-19, there was no literature available assessing the difference in incidence for patients with and without COVID-19 at the time this study was commenced. Our study sought to examine whether a difference in the incidence of hyponatremia for patients with and without COVID-19 existed. In our study, patients with COVID-19 were compared to patients with a similar presentation and status (i.e., critically ill patients with pneumonia, but without a diagnosis of COVID-19). Other factors that might influence the incidence of hyponatremia such as concomitant medications and comorbid conditions were evaluated as secondary endpoints.
Methods
This single-center, retrospective cohort study was performed at a 763-bed level I trauma center in southwest Virginia. It was undertaken as a Health Care Delivery Improvement Project, and as such was not reviewed as Human Subjects Research by the Institutional Review Board.
Population
Date ranges and specific intensive care unit (ICU)-level care units were used to gather patient information specific to both study arms. All patients with a diagnosis of pneumonia between February 1, 2019, and January 31, 2020, or a diagnosis of COVID-19 between June 1, 2020, and May 31, 2021 were screened for inclusion. Patients were then matched based on age and sex.
Exclusion criteria included admission for postoperative care or neurological disorders, any form of renal replacement therapy (RRT), pregnancy, malignancy, diabetic ketoacidosis (DKA), hyperosmolar hyperglycemia syndrome (HHS), readmission to the MICU, and patients dependent on extracorporeal membrane oxygenation (ECMO).
Endpoints
The primary outcome was the incidence of hyponatremia within 72 h of ICU admission. Hyponatremia was defined as serum sodium ≤134 mEq/L. Secondary outcomes included the severity of hyponatremia, symptomatic hyponatremia, lowest serum sodium, intervention for hyponatremia, concurrent use of medications known to cause hyponatremia, intubation due to hyponatremia, duration of mechanical ventilation, serum IL-6, serum ferritin, serum CRP, ICU length of stay (LOS), and hospital LOS.
Intervention for hyponatremia was defined as administration or restriction of fluids, administration of sodium tablets, urea, loop diuretics, 3% sodium chloride, or vasopressin receptor antagonists.
Statistical Analysis
Power was calculated based on previous studies noting a 16.2% incidence of hyponatremia in the MICU versus 20.5% for all patients diagnosed with COVID-19 (only 18.4% of this population was in the ICU).1,7 Other studies reported rates of hyponatremia upon admission to the ICU of 34.3%. 2 Given the increased likelihood of hyponatremia in patients with COVID-19 and critical illness (cytokine storm influencing IL-6 and thus arginine vasopressin secretion), it was estimated that a population size of 349 patients would be needed to provide 80% power to detect a 15% statistically significant difference. A P value of .05 was considered statistically significant.
All analyses were performed with de-identified data and were performed using Microsoft Excel. For continuous data, a Student t test was used. For nominal data, a χ2 test was used. For ordinal data and continuous data that were not normally distributed, a Mann-Whitney U test was used.
Results
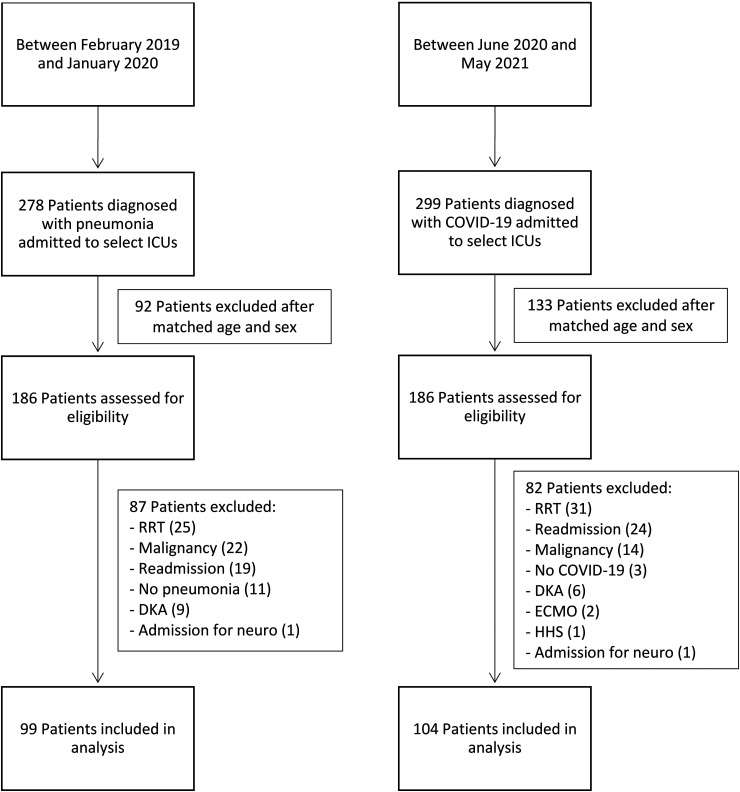
Five-hundred and seventy-seven patients with a diagnosis of COVID-19 or pneumonia were admitted to 1 of 3 select MICU floors during the specified time frames (Figure 1). Three hundred and seventy-two patients were eligible for analysis of exclusion after both groups were matched based on age and sex. Of those 372 patients, 56 required RRT, 43 had a prior ICU admission or were transferred from an outside facility, 36 had a past medical history significant for malignancy, 15 were admitted for DKA, 14 were identified to not have pneumonia or COVID-19, 2 were admitted for a neurological disorder, 2 required ECMO, and 1 was admitted for HHS and was, therefore, excluded.
Figure 1.
Patient selection.
Baseline Characteristics
Baseline characteristics were similar between groups (Table 1), with the exception of weight, BMI, APACHE II score, liver disease, and alcohol abuse. The COVID-19 group had a significantly higher weight (91.8 kg vs 77.1 kg, P < .01) and BMI (33 kg/m2 vs 26.8 kg/m2, P < .01) compared to the pneumonia group. The pneumonia group had a significantly higher APACHE II score (16 vs 12, P < .01) upon admission. The pneumonia group also had significantly more individuals with a past medical history of liver disease (20 vs 1, P < .01) and alcohol abuse (18 vs 7, P = .01).
Table 1.
Baseline Characteristics.
| Characteristic | Pneumonia (n = 99) | COVID-19 (n = 104) | P value |
|---|---|---|---|
| Age (years), median (IQR) | 62.9 (57.3-71.7) | 66.8 (59.3-75.4) | .1524 |
| Male sex, n (%) | 64 (64.6) | 57 (54.8) | .1533 |
| White, n (%) | 76 (76.8) | 79 (76) | .8925 |
| Weight (kg), median (IQR) | 77.1 (64.5-98.4) | 91.8 (80.2-109) | .0001 |
| BMI (kg/m2), median (IQR) | 26.8 (23.3-32.2) | 33 (28-37.8) | .0001 |
| APACHE II Score, median (IQR) | 16 (11-20) | 12 (8-17) | .0045 |
| Diabetes, n (%) | 33 (33.3) | 44 (42.3) | .1878 |
| Heart failure, n (%) | 27 (27.3) | 18 (17.3) | .0875 |
| Liver disease, n (%) | 20 (20.2) | 1 (1) | .0001 |
| Pancreatitis, n (%) | 3 (3) | 3 (3) | - |
| Hypothyroidism, n (%) | 16 (16.2) | 15 (14.4) | .7307 |
| Alcohol abuse, n (%) | 18 (18.2) | 7 (6.7) | .0131 |
Abbreviations: IQR, interquartile range; BMI, body mass index; APACHE, acute physiology and chronic health evaluation.
Endpoints
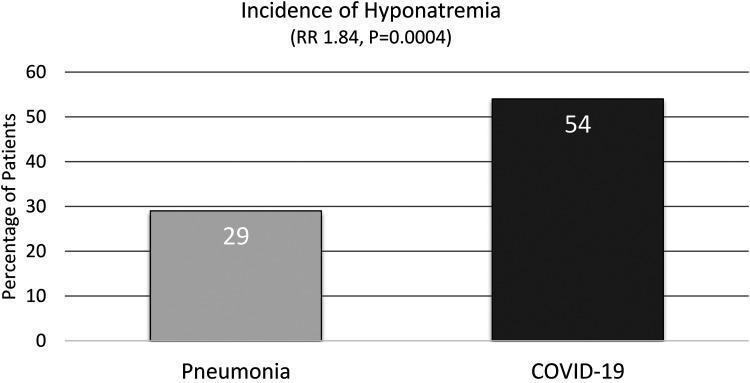
The primary and secondary outcomes are listed in Table 2. The primary outcome of the incidence of hyponatremia was significantly higher in the COVID-19 group compared to the pneumonia group (Figure 2). Twenty-nine patients in the pneumonia group and 56 patients in the COVID-19 group had a sodium level ≤134 mEq/L (29% vs 56%, P < .01, RR 1.84, 95% CI 1.29-2.62).
Table 2.
Endpoints.
| Variables | Pneumonia (n = 99) | COVID-19 (n = 104) | P value |
|---|---|---|---|
| Incidence of hyponatremia, n (%) | 29 (29.3) | 56 (53.8) | .0004 |
| Lowest serum sodium (mEq/L), median (IQR) | 137 (134-141) | 134 (132.7-137) | .0004 |
| Hyponatremia treatment, n (%) | 6 (6.1) | 7 (6.7) | .8454 |
| Need for mechanical ventilation, n (%) | 32 (42.7) | 43 (57.3) | .1849 |
| Mechanical ventilation (days), median (IQR) | 3 (2-3.5) | 8 (2-13) | .0002 |
| Mortality, n (%) | 16 (16.2) | 41 (39.4) | .0002 |
| Downgrade from ICU, n (%) | 74 (74.8) | 62 (59.6) | .0219 |
| ICU LOS (days), median (IQR) | 4 (2-5) | 10 (3-14.7) | <.0001 |
| Hospital LOS (days), median (IQR) | 6 (4-12) | 14 (8-21) | <.0001 |
Abbreviations: IQR, interquartile range; LOS, length of stay; ICU, intensive care unit.
Figure 2.
Incidence of hyponatremia.
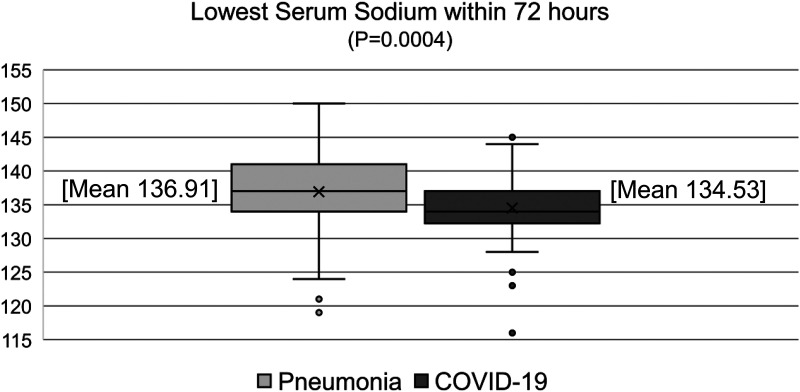
Multiple secondary outcomes were significantly different between pneumonia and COVID-19 groups. The mean lowest serum sodium within 72 h of admission was 136.9 mEq/L in the pneumonia group and 134.5 mEq/L in the COVID-19 group (P < .01) (Figure 3). Other notable significant differences between pneumonia and COVID-19 groups were days of mechanical ventilation (median, 3 days vs 8 days, P < .01), downgrade from the ICU (74.8% vs 59.6%, P = .02), ICU LOS (median, 4 days vs 10 days, P < .01), hospital LOS (median, 6 days vs 14 days, P < .01), and mortality (16.2% vs 39.4%, P < .01). The overall rate of treatment for hyponatremia was not different between pneumonia and COVID-19 groups (6.1% vs 6.7%, P = .845).
Figure 3.
Lowest serum sodium within 72 h.
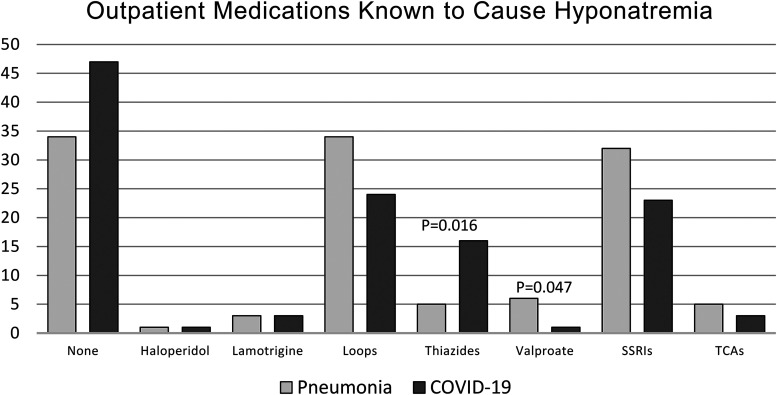
As represented in Figure 4, the only significant differences in prior to admission medications known to cause hyponatremia were thiazide diuretics and valproate sodium. Five patients (5.1%) in the pneumonia group and 16 patients (15.4%) in the COVID-19 group reported taking thiazide diuretics (P = .016). Six patients (6.1%) in the pneumonia group and 1 patient (1%) in the COVID-19 group reported taking valproate sodium (P = .046).
Figure 4.
Incidence of the use of outpatient medications known to cause hyponatremia.
Discussion
Hyponatremia is a commonly observed electrolyte disturbance that is associated with high morbidity and mortality. This study, which only included critically ill patients, revealed a combined incidence of hyponatremia of 41.8%.
Frontera and colleagues evaluated the prevalence, predictors, and impacts that varying degrees of hyponatremia had on outcomes compared to normonatremia in patients with COVID-19. Moderate and severe hyponatremia, defined as Na 121-129 mEq/L and Na ≤ 120 mEq/L, respectively, occurred in 7% and 1% of the study population. 4 The combined prevalence of moderate and severe hyponatremia in our study was similar at 10.6% of patients with COVID-19.
The results of this study show that the risk of hyponatremia is significantly greater for patients with COVID-19 compared to those with pneumonia. The mean serum sodium level within 72 h was also shown to be significantly lower in the COVID-19 group. For the pneumonia group, our findings are similar to those of previous studies of critically ill patients with an incidence of hyponatremia of 29%.1,2 Of note, the overall rate of treatment for hyponatremia was not different between the 2 groups, potentially indicating a lack of clinical significance.
There were several other significantly different endpoints such as ventilation days, ICU downgrade, ICU LOS, and mortality, but these were expected given the higher BMI and severity of illness seen in patients with COVID-19 requiring ICU-level care. Further research is necessary to investigate whether any of these endpoints are affected by the rate or severity of hyponatremia in patients with COVID-19.
The use of concomitant medications known to cause hyponatremia was documented based on available home medication lists. Specific medications included carbamazepine, oxcarbazepine, haloperidol, lamotrigine, loop diuretics, thiazide diuretics, valproate sodium, and selective serotonin reuptake inhibitors. Prior to admission medications were evaluated rather than hospital-administered medications due to hyponatremia generally occurring weeks to months after initiation of these medications. A further review of hospital-administered medications compared to prior to admission medications would need to be conducted to delineate a difference in this confounding variable.
There were other notable differences in baseline characteristics that have been associated with an increased risk of hyponatremia. Patients in the pneumonia arm were more likely to have a history of liver disease and alcohol abuse, both of which can cause hyponatremia. Patients with pneumonia were also more likely to take valproate sodium compared to those with COVID-19. Despite these differences, patients with pneumonia were found to have a lower incidence of hyponatremia compared to those with COVID-19. Thiazide use, however, was significantly more common in the COVID-19 arm which could have contributed to the differences in hyponatremia between the groups and is a limitation of this observational study.
Our study has several additional limitations. First, the study was developed to compare the incidence of hyponatremia between critically ill patients with pneumonia and critically ill patients with COVID-19 within 72 h of ICU admission. Because of this short window, effects such as mortality or LOS cannot be attributed to these early sodium values. Further research documenting mean sodium levels throughout hospital stay between groups would need to be conducted to suggest causation. Second, the lack of IL-6 values for both groups did not allow for an adequate comparison. Furthermore, only a few pneumonia patients had ferritin and CRP values reported compared to most of the COVID-19 patients. Third, it should be noted that a majority of patients were Caucasian (76.8% in pneumonia and 76% in COVID-19), making these data more difficult to extrapolate. Fourth, this study was not intended to assess volume status and treatment effects, which could have impacted sodium levels. It should also be noted that serum sodium levels were not corrected for glucose levels, which could have resulted in dilutional hyponatremia in patients with hyperglycemia. To account for the impacts of severe hyperglycemia, patients were excluded if they were admitted for DKA or HHS. Lastly, the methods for this study included a 4-month gap between groups to help eliminate confounding variables but could have introduced historical bias.
Strengths of this study include the diversity of endpoints explored and overall similar baseline characteristics between groups, except for variations in weight, as well as inclusion and exclusion criteria, which limited potential confounders for hyponatremia. Serum sodium levels were measured using direct ion selective electrodes and, therefore, were not affected by lipemia or hyperproteinemia. These data, to our knowledge, have not been researched in combination and warrant further investigation.
Conclusions
This study found that critically ill patients with COVID-19 had a significantly higher incidence of hyponatremia compared to critically ill patients with pneumonia, but no difference in the rate of treatment for hyponatremia. Over half of those in the COVID-19 group demonstrated hyponatremia, which is a much higher incidence compared to previous studies. Given the potential for morbidity and mortality associated with hyponatremia, further exploration into this correlation is warranted.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Benjamin D Gustafson https://orcid.org/0000-0003-4625-8763
References
- 1.Sim JK, Ko RE, Na SJ, Suh GY, Jeon K. Intensive care unit-acquired hyponatremia in critically ill medical patients. J Transl Med. 2020;18(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padhi R, Panda BN, Jagati S, Patra SC. Hyponatremia in critically ill patients. Indian J Crit Care Med. 2014;18(2):83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Hyponatremia PA. IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Investig. 2020;43:1137-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera JA, Valdes E, Huang Jet al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med. 2020;48(12):1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benrick A, Schéle E, Pinnock SBet al. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol. 2009;21(7):620-628. [DOI] [PubMed] [Google Scholar]
- 6.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61-75. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta Met al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Front Endocrinol (Lausanne). 2020:11. [DOI] [PMC free article] [PubMed] [Google Scholar]