Abstract
There is a need to identify potentially useful biomarker(s) for the prediction of prognostic outcomes in patients diagnosed with gastric cancer. This meta-analysis provided updated evidence on the association of controlling nutritional status (CONUT) score with survival and other clinicopathological outcomes in patients with gastric cancer. PubMed and Scopus databases were systematically searched. The review included studies, observational in design, that were conducted among patients with gastric cancer and had documented the association of CONUT score with outcomes of interest. The primary outcomes of interest were overall survival (OS), cancer-specific survival (CSS) and recurrence-free survival (RFS) along with tumour size and extent (T status), nodal status (N status) and tumour staging (TNM staging). STATA was used for statistical analysis. The meta-analysis was conducted with 17 studies. The 5-year OS [hazard ratio (HR), 1.75; 95% confidence interval (CI): 1.55, 1.96], RFS (HR, 1.58; 95% CI: 1.30, 1.91) and CSS (HR, 1.89; 95% CI: 1.01, 3.52) were comparatively poorer in the high CONUT group, than in low CONUT group. High CONUT score was associated with increased risk of having T3/T4 tumour [odds ratio (OR), 1.64; 95% CI: 1.16, 2.34], N2/N3 nodal status (OR, 1.44; 95% CI: 1.17, 1.77) and stage III/IV tumour (OR, 1.64; 95% CI: 1.43, 1.88). The risk of microvascular invasion (OR, 1.46; 95% CI: 1.20, 1.77) and post-operative complications (OR, 1.64; 95% CI: 1.31, 2.06) was higher in those with high CONUT. There were no differences in the risk of poorly differentiated tumour and need for adjuvant chemotherapy between the two groups. Findings suggested that preoperative assessment of CONUT score may be included in the routine assessment of patients with gastric cancer due to its association with survival and other clinical as well as pathological outcomes.
Keywords: controlling nutritional status, immuno-nutritional biomarker, serum albumin, total cholesterol, lymphocyte count, gastric cancer
Introduction
In patients with cancer, malnutrition is a common occurrence, and can adversely affect the prognosis and other clinical outcomes (1,2). The nutritional status has also been shown to influence the post-treatment disease progression and survival (1). Therefore, numerous studies focused on identifying biomarkers that reflect the nutritional status and determining their prognostic role in various cancers. A recent addition to these biomarkers is the controlling nutritional status (CONUT) score that comprises of serum albumin, total cholesterol and total lymphocyte count (3). With the growing body of evidence on the possible role of nutrition in cancer progression and overall survival (OS), there has been an emerging interest in elucidating the predictive ability of CONUT score for clinical and survival outcomes.
There has been some indication from previous studies favoring the role of CONUT score in prediction of survival in patients with gastrointestinal (GI) and urinary tract cancers (4,5). A comprehensive systematic review involving 32 studies noted that the CONUT score provided enhanced indication of prognosis i.e., survival, cancer-specific survival and tumor progression in various types of cancers compared with other potential biomarkers (6). In subjects with high CONUT score, lower OS, cancer-specific survival (CSS), and recurrence-free survival (RFS) rates were reported by >85% of the included studies in cancer patients (6). A prognostic role of the CONUT score for prediction of OS, CSS and RFS was shown by ~92, 91 and 53% of the studies, respectively (6). However, as different cancers have their specific prognoses, it is important to carefully document the prognostic role of CONUT in organ-specific cancers (7).
A review by Takagi et al (7) that specifically focused on gastric cancers, pooled findings from 5 retrospective studies and found that in patients undergoing gastrectomy, the OS, rate of postoperative complications, clinical and other pathological parameters could be reliably predicted using preoperatively-assessed CONUT score (7). Several new studies on this issue have been published and the main goal of the current meta-analysis was to update the previous review by summarizing the existing data from studies dealing with gastric cancer, and to assess and document whether the CONUT score could predict tumor stage, survival and recurrence.
Materials and methods
Strategy for identification and selection of relevant studies
The protocol was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/; CRD42021287305) before starting the meta-analytic work. A systematic search was conducted to identify English language studies published before 1st March 2022. The databases searched were PubMed and Scopus. The search strategy used is presented in the Appendix. The search strategy incorporated the following: i) Controlling nutritional status score or CONUT or immuno-nutritional biomarker or serum albumin or total cholesterol or lymphocyte count and ii) stomach tumor or gastric tumor or gastric neoplasm or gastric malignancy or gastric carcinoma or gastric adenocarcinoma and iii) outcomes or mortality or survival or recurrence or prognosis. The primary outcomes of interest were: OS and RFS, CSS, tumor size and extent (T status), nodal status (N status), and tumor staging (TNM staging). Secondary outcomes of interest were tumor differentiation, need for adjuvant chemotherapy, microvascular invasion, hospital stay duration and postoperative complications. The study processes complied with the guidelines laid down in PRISMA (8).
Studies identified by the literature search were independently reviewed by two investigators after duplicate removal. After title and abstract review, full texts of the relevant studies were reviewed to identify studies that meet eligibility criteria. Any disagreements were resolved by discussion between the study authors. To identify additional studies for inclusion, bibliography sections of the selected studies were also reviewed.
Only studies that were performed on patients with gastric cancer and documented the association of CONUT scores (assessed prior to start of management) with outcomes of interest were considered. Observational studies i.e., case-control and cohort (either prospective or retrospective) and those that were performed using analysis of database or clinical records were considered for inclusion. Case-reports, conference abstracts or review articles were excluded. Studies that did not provide findings based on CONUT scores or did not provide data on the outcomes of interest were excluded. Studies that reported alteration in nutritional status secondary to poor eating as a result of any oral surgery or chronic illness other than gastric cancer were also excluded. There were no exclusions based on the modality of treatment received i.e., surgery only, chemotherapy or radiotherapy or immunotherapy only or a combination thereof.
Data extraction and quality assessment
A pre-tested data extraction sheet was used by two independent authors to extract data from relevant studies. In case of any discrepancy, the two authors discussed the issue at hand and achieved consensus. The adapted Newcastle-Ottawa Quality Assessment Scale (NOS) was used for assessing the quality of the included studies (9).
Statistical analysis
All the analysis was carried out using STATA software (StataCorp. 2017; Stata Statistical Software: Release 15. College Station, TX; StataCorp LP). The pooled findings were presented as hazards ratios (HR) or odds ratios (OR) in case the outcome was categorical and as weighted mean difference (WMD) for non-categorical/continuous outcomes. A 95% confidence interval (CI) was presented along with pooled estimates. Subgroup analyses were conducted for survival outcomes, based on a CONUT cut-off score of >3, study design (retrospective or prospective), and study sample size (>300 subjects). The degree of heterogeneity was assessed using I2 parameter and random-effects model was used in instances when the I2 value exceeded 40% (10). Egger's test was used to detect publication bias and a P-value of less than 0.05 for statistical significance (11). Additionally, funnel plots were also created to visually inspect presence or absence of publication bias.
Results
Literature search
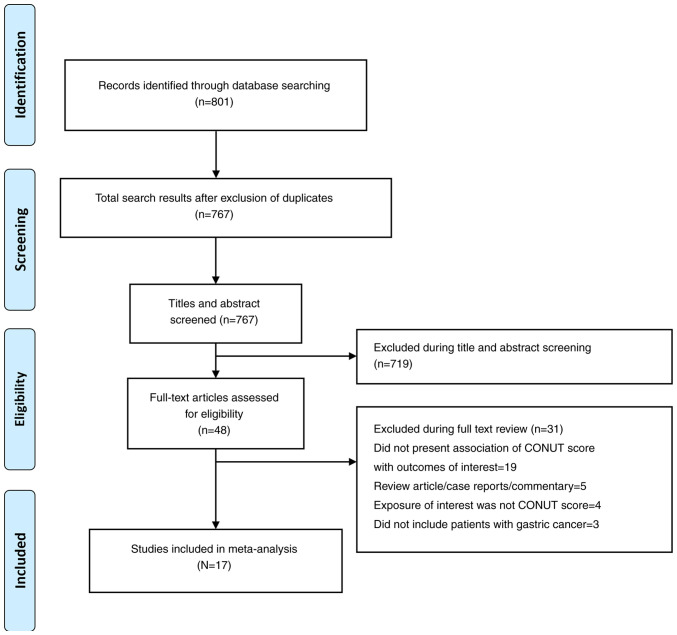
A total of 767 unique citations (i.e., after removal of duplicates) were identified by the search (Fig. 1). Through the title and abstract screening, 719 studies were removed and further 31 studies were excluded after review of full text. Finally, 17 studies were found relevant for inclusion in the meta-analysis. (12-28) (Tables I and II). Most included studies (n=14) were retrospective in design and utilized clinical databases or records, while the remaining three studies were prospective. A total of nine studies were performed in China, five in Japan, and individual studies in Turkey, Italy and South Korea. The studies used different cut-off values to categorize high and low CONUT scores. Most of the studies (n=12) had a median follow-up period of >24 months. Table SI presents the findings of the quality assessment and the quality of most studies was judged to be favorable.
Figure 1.
Selection process of the studies included in the review. CONUT, controlling nutritional status.
Table I.
Characteristics of the studies included in the meta-analysis.
| First author | Study design | Country | Participant characteristics | CONUT category | Sample size | Quality assessment score (NOS) | (Refs.) |
|---|---|---|---|---|---|---|---|
| Zhu et al | Retrospective data analysis | China | Patients with gastric cancer undergoing total gastrectomy (R0 resection- complete resection with negative margin); male (73%) and <65 years (66%); follow up period ranged from 5-15 years. | High (>3) vs. low (≤3) | 245 (high, 141; low, 104) | 9 | (12) |
| Galizia et al | Retrospective analysis of prospectively maintained database | Italy | Patients with gastric cancer (adenocarcinoma) undergoing total gastrectomy; male (59%) and ≤65years (53%); median follow up period of. 24.7 months | High (>1) vs. low (≤1) | 415 (high, 164; low, 251) | 9 | (13) |
| Sun et al | Prospective follow up | China | Patients with gastric cancer undergoing total gastrectomy (R0 resection); mean age of 60.4 years; male (73%); Mean BMI of 23 kg/m2; short term follow up of 30 days post-operatively | High (≥2) vs. low (<2) | 1479 (high, 852; low, 627) | 8 | (14) |
| Qian et al | Retrospective analysis of clinical records | China | Patients with gastric cancer undergoing laparoscopic gastrectomy; mean age of 63.4 years; male (73.8%); Mean BMI of 22.8 kg/m2; 38% with hypertension; short term follow up of 30 days post-operatively | High (>2.5) vs. low (<2.5) | 309 (high, 95; low, 214) | 8 | (15) |
| Jin et al | Retrospective analysis of data | China | Patients with gastric adenocarcinoma undergoing gastrectomy (38.6% with total and 48.5% with subtotal gastrectomy) along with neoadjuvant chemotherapy; median age of 61 years; male (74%); BMI of ≥18.5 kg/m2 (86%); median follow up of around 60 months | High (≥4) vs. low (≤3) | 267 (high, 85; low, 182) | 8 | (16) |
| Akagunduz et al | Retrospective review of medical records | Turkey | Patients with gastric carcinoma treated with FLOT (fluorouracil, leucovorin, oxaliplatin or docetaxel) chemotherapy; median age of 58.7 years; male (68.3%); median follow up of 11.2 months | High (>3) vs. low (≤3) | 161 (high, 105; low, 56) | 7 | (17) |
| Lin et al | Prospective follow up | China | Patients with gastric cancer treated with radical gastrectomy; median age of 60.8 years; male (75.3%); median BMI of 22.5 kg/m2; median follow up of 52 months | High (>2) vs. low (≤2) | 2182 (high, 478; low, 1704) | 8 | (18) |
| Huang et al | Prospective follow up | China | Patients with gastric cancer treated with curative gastrectomy (subtotal gastrectomy in 57%); median age of 73.3 years; male (77%); median BMI of 21.6 kg/m2; follow up of 12 months | High (≥2) vs. low (<2) | 357 (high, 204; low, 153) | 9 | (19) |
| Jeon et al | Retrospective review of records | South Korea | Patients who underwent curative gastrectomy; age ≥60 years (51.3%); male (66%); 33% with BMI ≥25 kg/m2; mean follow-up duration was 59.0 months | High (≥2) vs. low (<2) | 1307 (high, 414; low, 893) | 8 | (20) |
| Hirahara et al | Retrospective review of medical records | Japan | Patients who underwent curative gastrectomy; mean age of 70 years; male (69%); Mean BMI of 22 kg/m2; median follow-up duration was 35.3 months | High (≥3) vs. low (≤2) | 368 (high, 105; low, 263) | 8 | (21) |
| Kuroda et al | Retrospective review of medical records | Japan | Patients who underwent curative gastrectomy; age≥75 years (32%); male (64%); BMI of ≥18.5 kg/m2 (90%); median follow-up duration was 61.2 months | High (≥4) vs. low (≤3) | 416 (high, 62; low, 354) | 9 | (22) |
| Zheng et al | Retrospective design | China | Patients who underwent curative gastrectomy; Mean age of 61.1 years; male (75.8%); Mean BMI of 21.9 kg/m2; median follow-up duration was 60 months | High (≥2) vs. low (<2) | 532 (high, 241; low, 291) | 9 | (23) |
| Liu et al | Retrospective review of medical records | China | Patients who underwent curative gastrectomy followed by adjuvant chemotherapy with 5-fluorouracil based regimen; Age <60 years (59.4%); male (65.6%); median follow-up duration was 36 months | High (≥3) vs. low (≤2) | 697 (high, 217; low, 480) | 8 | (24) |
| Ryo et al | Retrospective review of clinical data | Japan | Patients who underwent curative gastrectomy; Mean age of around 67 years; male (69.4%); mean BMI of around 22 kg/m2; median follow-up duration was 49.2 months | High (≥2) vs. low (<2) | 626 (high, 289; low, 337) | 7 | (25) |
| Suzuki et al | Retrospective design | Japan | Patients who underwent curative gastrectomy; Mean age of around 80 years; male (67%); mean BMI of around 22.5 kg/m2; median follow-up duration was 47.0 months | High (≥5) vs. low (<5) | 211 (high, 36; low, 175) | 9 | (26) |
| Xiao et al | Retrospective design | China | Patients undergoing gastric surgery; Median age of 67 years; around 80% males; median follow up of 30 months | High (≥5) vs. low (<5) | 106 (high, 63; low, 43) | 9 | (27) |
| Aoyama et al | Retrospective design | Japan | Patients with curative surgery and adjuvant treatment; majority aged under 65 years (72%); males (66%) | High (≥2) vs. low (<2) | 331 (high, 110; low, 221) | 8 | (28) |
NOS, Newcastle Ottawa Scale score; CONUT, controlling nutritional status; BMI, body mass index.
Table II.
Key findings from the studies included in the meta-analysis.
| First author | Key outcomes (high vs. low) | (Refs.) |
|---|---|---|
| Zhu et al | Overall survival (5-year): HR, 2.03; (95% CI: 1.12, 2.95) | (12) |
| Disease free survival (5-year): HR, 3.12; (95% CI: 1.24, 4.99) | ||
| Cancer-specific survival (5-year): HR, 0.89; (95% CI: 0.52, 1.50) | ||
| Poor differentiation: OR, 1.53; (95% CI: 0.89, 2.64) | ||
| T3/4: OR, 0.80; (95% CI: 0.48, 1.34) | ||
| N2/3: OR, 0.83; (95% CI: 0.50, 1.38) | ||
| Stage III/IV: OR, 2.00; (95% CI: 1.19, 3.36) | ||
| Need for adjuvant chemotherapy: OR, 0.41; (95% CI: 0.24, 0.68) | ||
| Microvascular invasion: OR, 1.55; (95% CI: 0.93, 2.58) | ||
| Galizia et al | Overall survival (5-year): HR, 1.28; (95% CI: 0.79, 2.05) | (13) |
| Disease free survival (5-year): HR, 1.46; (95% CI: 0.68, 3.16) | ||
| Sun et al | Post-operative complication: OR, 1.16; (95% CI: 1.08, 1.24) | (14) |
| Stage III/IV: OR, 1.51; (95% CI: 1.22, 1.87) | ||
| Length of hospital stay [mean (sd); days]: 12.3 (6.0); 11.1 (4.6) | ||
| Qian et al | Post-operative complication: OR, 2.43; (95% CI: 1.22, 4.86) | (15) |
| T3/4: OR, 2.33; (95% CI: 1.38, 3.94) | ||
| N2/3: OR, 2.07; (95% CI: 1.26, 3.38) | ||
| Stage III/IV: OR, 2.56; (95% CI: 1.55, 4.24) | ||
| Length of hospital stay [mean (sd); days]: 14.1 (0.7); 11.6 (0.5) | ||
| Jin et al | Overall survival (5-year): HR, 1.62; (95% CI: 1.11, 2.36) | (16) |
| Disease free survival (5-year): HR, 1.61; (95% CI: 1.11, 2.34) | ||
| Poor differentiation: OR, 1.39; (95% CI: 0.74, 2.61) | ||
| T3/4: OR, 2.02; (95% CI: 1.11, 3.69) | ||
| N2/3: OR, 1.05; (95% CI: 0.61, 1.80) | ||
| Stage III: OR, 1.22; (95% CI: 0.73, 2.05) | ||
| Need for adjuvant chemotherapy: OR, 1.16; (95% CI: 0.51, 2.64) | ||
| Post-operative complication: OR, 1.08; (95% CI: 0.54, 2.18) | ||
| Akagunduz et al | Poor differentiation: OR, 1.91; (95% CI: 0.98, 3.71) | (17) |
| T3/4: OR, 0.26; (95% CI: 0.06, 1.21) | ||
| N2/3: OR, 1.43; (95% CI: 0.72, 2.84) | ||
| Overall survival (5-year): HR, 2.40; (95% CI: 1.03, 5.54) | ||
| Lin et al | Post-operative complications: OR, 1.81; (95% CI: 1.04, 3.18) | (18) |
| Overall survival (5-years): HR, 1.69; (95% CI: 1.45, 1.98) | ||
| Huang et al | Post-operative complications: OR, 2.69; (95% CI: 1.63, 4.45) | (19) |
| Overall survival (1-year): HR, 2.91; (95% CI: 0.91, 9.31) | ||
| Stage III/IV: OR, 1.51; (95% CI: 0.98, 2.32) | ||
| Length of hospital stay [mean (sd); days]: 18.7 (10.8); 15.7 (9.1) | ||
| Microvascular invasion: OR, 1.30; (95% CI: 0.85, 1.98) | ||
| Jeon et al (20) | Overall survival (5-year): HR, 2.23; (95% CI: 1.07, 4.66) | |
| Stage III: OR, 1.47; (95% CI: 1.05, 2.04) | ||
| Hirahara et al | Overall survival (5-year): HR, 2.44; (95% CI: 1.46, 4.07) | (21) |
| Poor differentiation: OR, 1.21; (95% CI: 0.77, 1.90) | ||
| T3/4: OR, 2.70; (95% CI: 1.69, 4.32) | ||
| N2/3: OR, 1.67; (95% CI: 1.01, 2.78) | ||
| Stage III: OR, 1.94; (95% CI: 1.17, 3.23) | ||
| Need for adjuvant chemotherapy: OR, 1.26; (95% CI: 0.76, 2.07) | ||
| Post-operative complication: OR, 1.73; (95% CI: 1.07, 2.80) | ||
| Kuroda et al | Overall survival (5-year): HR, 2.72; (95% CI: 1.74, 4.25) | (22) |
| Disease/recurrence-free survival (5-year): HR, 2.63; (95% CI: 1.16, 5.97) | ||
| Cancer-specific survival (5-year): HR, 4.13; (95% CI: 1.62, 10.54) | ||
| Poor differentiation: OR, 1.76; (95% CI: 1.01, 3.06) | ||
| T3/4: OR, 2.33; (95% CI: 1.34, 4.04) | ||
| N2/3: OR, 2.29; (95% CI: 1.16, 4.52) | ||
| Stage III: OR, 2.73; (95% CI: 1.44, 5.20) | ||
| Post-operative complication: OR, 1.54; (95% CI: 0.88, 2.71) | ||
| Microvascular invasion: OR, 1.90; (95% CI: 1.10, 3.27) | ||
| Zheng et al | Overall survival (5-year): HR, 1.36; (95% CI: 0.98, 1.88) | (23) |
| Disease/recurrence-free survival (5-year): HR, 1.36; (95% CI: 1.00, 1.88) | ||
| Poor differentiation: OR, 1.10; (95% CI: 0.76, 1.61) | ||
| Stage III: OR, 1.66; (95% CI: 1.17, 2.34) | ||
| Microvascular invasion: OR, 1.51; (95% CI: 1.02, 2.24) | ||
| Need for adjuvant chemotherapy: OR, 1.12; (95% CI: 0.80, 1.58) | ||
| Liu et al | Cancer-specific survival (5-year): HR, 1.55; (95% CI: 1.08, 2.23) | (24) |
| Poor differentiation: OR, 0.64; (95% CI: 0.41, 1.01) | ||
| Stage III: OR, 1.20; (95% CI: 0.83, 1.73) | ||
| T3/4: OR, 1.62; (95% CI: 0.86, 3.08) | ||
| N2/3: OR, 1.57 ; (95% CI: 1.11, 2.21) | ||
| Post-operative complication: OR, 1.30; (95% CI: 0.90, 1.89) | ||
| Ryo et al | Overall survival (5-year): HR, 1.74; (95% CI: 1.26, 2.41) | (25) |
| Disease/recurrence-free survival (5-year): HR, 1.33; (95% CI: 0.98, 1.81) | ||
| Poor differentiation: OR, 0.83; (95% CI: 0.60, 1.14) | ||
| Stage III: OR, 1.55; (95% CI: 1.13, 2.13) | ||
| T3/4: OR, 1.53 ; (95% CI: 1.01, 2.32) | ||
| N2/3: OR, 1.33 ; (95% CI: 0.97, 1.82) | ||
| Microvascular invasion: OR, 1.30; (95% CI: 0.88, 1.94) | ||
| Post-operative complication: OR, 1.28; (95% CI: 0.91, 1.81) | ||
| Need for adjuvant chemotherapy: OR, 0.80; (95% CI: 0.58, 1.10) | ||
| Length of hospital stay [mean (sd); days]: 14.0 (3.9); 13.0 (4.1) | ||
| Suzuki et al | Overall survival (5-year): HR, 2.12; (95% CI: 1.18, 3.69) | (26) |
| Cancer-specific survival (5-year): HR, 3.75; (95% CI: 1.30, 10.43) | ||
| Poor differentiation: OR, 1.16; (95% CI: 0.55, 2.43) | ||
| Stage III: OR, 3.82; (95% CI: 1.57, 9.33) | ||
| Post-operative complication: OR, 2.22; (95% CI: 1.07, 4.60) | ||
| Length of hospital stay [mean (sd); days]: 22.0 (2.7); 19.0 (1.5) | ||
| Xiao et al | Overall survival (5-year): HR, 1.19; (95% CI: 0.74, 1.92) | (27) |
| Post-operative complication: OR, 3.75; (95% CI: 1.72, 8.17) | ||
| Length of hospital stay [mean (sd); days]: 25.6 (7.5); 24.3 (7.4) | ||
| Aoyama et al | Overall survival (5-year): HR, 1.95; (95% CI: 1.10, 3.45) | (28) |
| Disease/recurrence-free survival (5-year): HR, 1.71; (95% CI: 1.00, 2.93) |
OR, odds ratio; CI, confidence interval; HR, hazard ratio.
CONUT score and survival outcomes
5-year OS in the group of patients with the high CONUT scores was comparatively lower than in the group with low CONUT scores (HR, 1.75; 95% CI: 1.55, 1.96; n=14; I2=11.9%) (Fig. 2). Similarly, 5-year RFS (HR, 1.58; 95% CI: 1.30, 1.91; n=7; I2=17.6%) and 5-year cancer-specific survival (HR, 1.89; 95% CI: 1.01, 3.52; n=4; I2=73.2%) was lower in the high CONUT group compared with the low CONUT group (Fig. 2). There was no evidence of publication bias on Egger's test and also on visual inspection of the funnel plots (Table SII; Fig. S1, Fig. S2 and Fig. S3).
Figure 2.
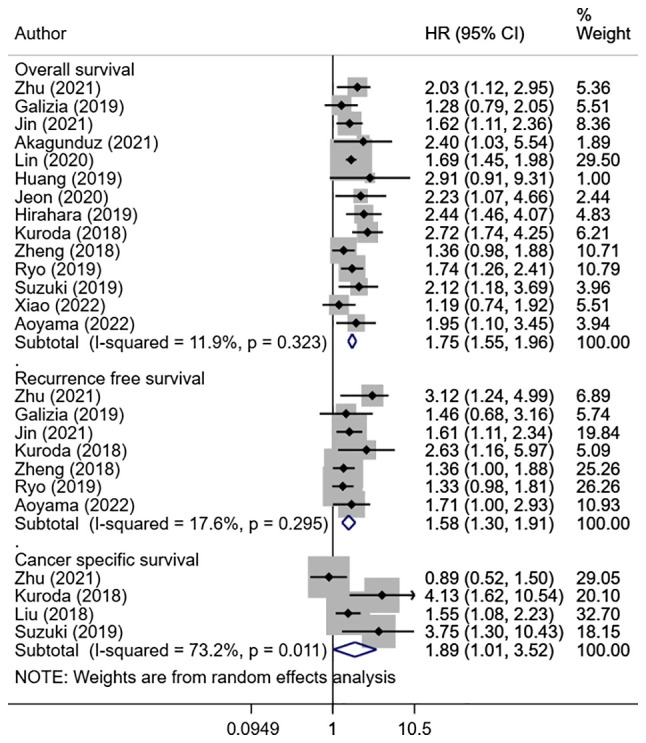
Overall survival, recurrence-free survival and cancer-specific survival in high CONUT score group compared with low CONUT score group. CONUT, controlling nutritional status; HR, hazard ratio; CI, confidence interval.
CONUT score and clinicopathological outcomes
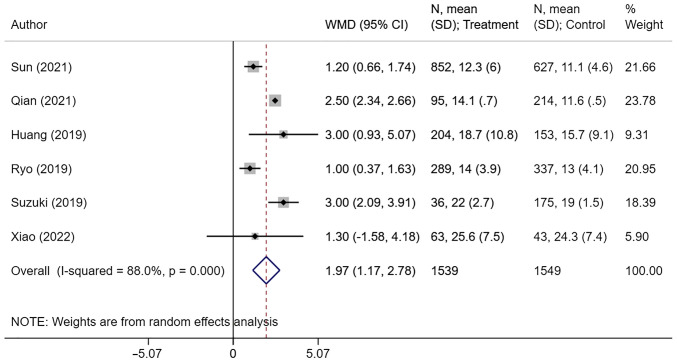
High CONUT score was associated with an increased risk of T3 or T4 tumor status (OR, 1.64; 95% CI: 1.16, 2.34; n=8; I2=67.0%), N2 or N3 nodal status (OR, 1.44; 95% CI: 1.17, 1.77; n=8; I2=33.4%), and stage III or IV tumor (OR, 1.64; 95% CI: 1.43, 1.88; n=12; I2=26.8%) (Fig. 3). The pooled risk of microvascular invasion (OR, 1.46; 95% CI: 1.20, 1.77; n=5; I2=0.0%) and post-operative complications (OR, 1.64; 95% CI: 1.31, 2.06; n=11; I2=67.6%) was higher in patients with high CONUT scores compared with the low CONUT group (Fig. 4). There were no differences in terms of poorly differentiated tumor risk (OR, 1.15; 95% CI: 0.91, 1.46; n=9; I2=50.1%) and need for adjuvant chemotherapy (OR, 0.87; 95% CI: 0.60, 1.26; n=5; I2=69.1%) between the two groups (Fig. 4). Those with high CONUT scores had longer hospital stays (in days) compared with those with low CONUT scores (WMD, 1.97; 95% CI: 1.17, 2.78; n=6; I2=88.0%) (Fig. 5). Post-operative complications reported by the studies mainly consisted of surgical site infections, post-operative active haemorrhages, intra-abdominal abscesses, septic shock and organ dysfunction. Egger's test did not indicate presence of publication bias for the outcomes considered, except for risk of post-operative complications (Table SII). The funnel plots for each of the aforementioned outcomes have been presented as Fig. S4, Fig. S5, Fig S6, Fig. S7 and Fig. S8.
Figure 3.
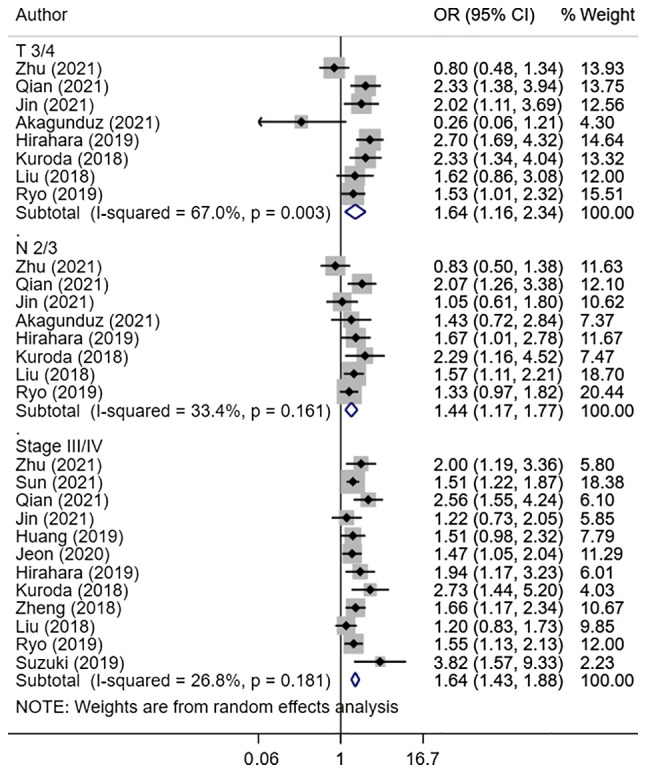
Risk of advanced tumor size and extent (T3/4), nodal metastasis (N2/3) and tumor stage (III/IV) between high and low controlling nutritional status score group. OR, odds ratio; CI, confidence interval.
Figure 4.
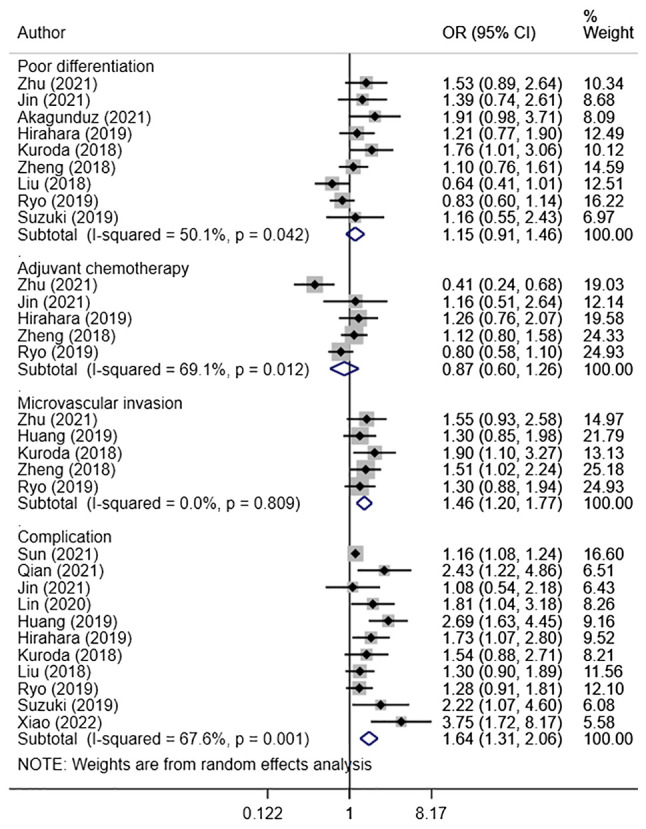
Risk of poor tumor differentiation, need for adjuvant chemotherapy, microvascular invasion and complication between high and low controlling nutritional status score group. OR, odds ratio; CI, confidence interval.
Figure 5.
Length of hospital stay (in days) between high and low controlling nutritional status score group. CI, confidence interval; WMD, weighted mean difference.
Subgroup analyses
OS and RFS were consistently lower in patients with high CONUT scores compared with the low CONUT group when analyses were restricted to studies with retrospective design, larger sample size (>300), and studies that used a CONUT score cut-off of >3. Due to a limited number of prospective studies, pooled outcomes were only possible for OS. The present results revealed that OS (HR, 1.71; 95% CI: 1.46, 1.99; n=2; I2=0.0%) was lower in patients with high CONUT scores (Table III).
Table III.
Subgroup analysis for overall survival, cancer-specific survival and recurrence-free survival.
| Parameter | Overall survival | Cancer-specific survival | Recurrence-free survival |
|---|---|---|---|
| High controlling nutritional status score >3 | HR, 2.15; (1.74, 2.52); (n=7; I2=0.0%)a | HR, 1.89; (1.01, 3.52); (n=4; I2=73.2%)a | HR, 2.14; (1.37, 3.33); (n=3; I2=39.0%)a |
| Prospective design | HR 1.71 (1.46, 1.99); (n=2; I2=0.0%)a | - | - |
| Retrospective design | HR 1.85 (1.57, 2.18); (n=12; I2=23.0%)a | HR 1.89 (1.01, 3.52); (n=4; I2=73.2%)a | HR 1.58 (1.30, 1.91); (n=7; I2=17.6%)a |
| Sample size >300 | HR 1.80 (1.51, 2.13); (n=9; I2=37.3%)a | HR 2.29 (0.89, 5.87); (n=2; I2=72.7%) | HR 1.40 (1.17, 1.70); (n=5; I2=0.0%)a |
aP<0.05. HR, hazard ratio. Data is presented as pooled effect size (95% confidence interval); (n=total number of studies; I2).
Discussion
The main goal of this meta-analysis was to update the previous evidence on the predictive value of CONUT scores in estimating tumor stage, survival and recurrence of gastric cancer. Through the inclusion of 17 studies, the review found that the high CONUT score in gastric cancer patients was associated with worse 5-year OS, RFS and cancer-specific survival compared with patients with a low CONUT score. Moreover, a high CONUT score was associated with an increased risk of having T3/T4 tumor status, N2/N3 nodal status and stage III/IV tumor. The pooled risk of microvascular invasion and post-operative complications was higher in patients with the high CONUT group. These findings support the previous review by Takagi et al (7) where the authors, using 5 studies, showed that the CONUT score, assessed pre-operatively, was an independent and reliable indicator of OS and postoperative complications as well as stage of tumor and extent of microvascular invasion (7).
The inclusion criteria were similar to that of Takagi et al (7); however, it was more explicit. For instance, it has been specifically mentioned that the present review would include only observational studies and would not consider case-reports or conference abstracts. It was also mentioned that subjects with evident cause of poor nutrition that is unrelated to gastric cancer would be excluded. Therefore, studies that reported alteration in nutritional status secondary to poor eating as a result of any oral surgery or chronic illness other than gastric cancer were excluded. While the review by Takagi et al (7) included studies with gastric cancer subjects undergoing curative resection, the present review did not have exclusions based on the modality of treatment received i.e., surgery only, chemotherapy or radiotherapy or immunotherapy only or a combination thereof. The current meta-analysis was not conducted to challenge the aforementioned review by Takagi et al but instead, to update the evidence base through inclusion of more recently published studies on this issue. The fact that the findings of the present study are largely similar to those reported by the previous review provides assurance that these insights could potentially be used to inform the current clinical practice.
Studies have indicated that cancer prognosis and survival rates may be influenced, to a certain extent, by the host's nutritional status as well as the underlying inflammatory status (29). Furthermore, the nutritional status may impact the immune status and metabolic health in patients with cancer (30,31). Particularly, the immune status is known to affect tumor recurrence (32). Therefore, there is a continuous effort to explore biomarkers of nutritional and immune status that could be used as prognostic indicators of various types of malignancies. A commonly utilized marker to reflect nutritional status is the prognostic nutritional index (PI) that is derived from serum albumin values and total lymphocyte count (33). Studies on PNI have shown that it could be a modest predictor of post-operative complications and overall prognosis in various cancers including GI cancers (34,35). In terms of the immune biomarker, other potential candidates have also been explored and tested (36-38). The findings of the current meta-analysis suggest that the CONUT score could be used as a potentially useful biomarker for the prediction of prognostic outcomes in patients diagnosed with gastric cancer. Available evidence suggests that the CONUT score is probably more accurate, compared with other prognostic factors and indicators (39-41). For instance, in a study among subjects with hepatocellular carcinoma undergoing curative hepatectomy, CONUT score exhibited a higher area under the curve value (61.8%), when compared with other immune-nutritional parameters including PNI (59.9%), neutrophil to lymphocyte ratio (57.5%) and platelet to lymphocyte ratio (49.4%) for prediction of OS (39). Similarly, for the prediction of post-operative pulmonary complications in patients with resectable non-small cell lung cancer, the CONUT showed a higher area under curve (64.0%) than other prognostic models such as PNI (61%) and Glasgow prognostic score (57%) (40).
The higher accuracy of the CONUT score, when compared with the other commonly used nutritional marker i.e., PNI could be related to the higher emphasis on peripheral lymphocyte count and additional measurement of total serum cholesterol level. Reduced cholesterol levels (hypocholesterolaemia) may be an indicator of advancing tumour progression (42). There is an increased expression of LDL receptors in tumour cells which leads to increased uptake of circulating LDL cholesterol, thereby leading to hypocholesterolaemia (43). This uptake of LDL cholesterol is required for increased tumour growth (44,45). There is further indication of altered cell membrane fluidity due to hypocholesterolaemia, thereby affecting the mobility of cell surface receptors and their ability to transmit transmembrane signals (46). This leads to a situation wherein even in the presence of adequate number of immunocompetent cells present, the immunological function is compromised (47). Based on these considerations, CONUT is considered to be more sensitive than PNI as a prognostic indicator.
The underlying mechanisms linking CONUT score with prognostic outcomes in gastric cancer patients have not been fully understood. However, previous evidence indicates that there may be a link between individual components of CONUT score and gastric cancer outcomes. For instance, serum albumin is a prominent indicator of nutritional status and systemic inflammation that is associated with survival in gastric cancer patients (48,49). Numerous studies show that in patients with GI cancers serum cholesterol is strongly correlated with tumor progression and survival (50-52). Similarly, total lymphocyte count is related to prognosis in gastric cancer (53). An interesting observation identified by previous studies is that the sensitivity and specificity of the total CONUT score is usually higher than that of each of its three individual components (22,54,55). The present review revealed that the CONUT score was associated with TNM staging as well as tumor size (T) and nodal status (N). However, it remains unclear whether these findings relating to the CONUT score were a cause or a result of the tumor progression.
The present findings support the use of CONUT to assess nutritional status and consider this at the time of planning management for patients with gastric cancer. It will provide an opportunity to improve the nutritional status of those with malnutrition, before starting and/or during treatment, which in turn could considerably maximize the efficacy of the treatment provided, i.e., either surgery and/or a combination of surgery and chemotherapy. The use of CONUT-based nutritional assessment is not meant to alter the standard current management practices for gastric cancer. However, it does help to identify patients that are nutritionally compromised and would therefore need nutritional management in order to improve their OS or reduce the risk of recurrence. Assessment of CONUT score will also help to identify patients at risk of complications and these could be followed up and monitored closely.
There are certain limitations that should be considered while interpreting the findings. The majority of the included studies were retrospective and it is possible that certain important variables (possibly certain confounders) may not have been adjusted for in the analysis. Specifically, factors including body mass index and metabolic diseases such as diabetes mellitus have been shown to influence the outcomes in various cancers, including GI cancers (56,57). Additionally, most of the included studies were from Asian countries and consequently, the external validity of the findings remains restricted. This calls for more evidence from developed western settings. Another important limitation is that the studies used different cut-offs to label high and low CONUT scores. This could have led to the heterogeneity noted in certain of the outcomes. Further, large epidemiological studies are needed to carefully define the optimal cut-offs for CONUT scores that effectively predict the prognosis and risk of complication in patients with gastric cancer. Certain studies in the present meta-analysis included patients with gastric adenocarcinoma only, while others had a more heterogeneous population with different histologic types of cancer. Furthermore, the described treatment was also heterogeneous, with patients receiving either total gastrectomy, sub-total/partial gastrectomy, or only chemotherapy. The studies did not provide stratified findings based on these differences i.e., histologic types and treatment offered. This introduced a potential variability in the association of CONUT with prognostic and clinic-pathological outcomes.
In conclusion, the current meta-analysis included a total of 17 studies and reported an increased risk of poor survival outcomes tumor progression, advanced tumor stage, microvascular invasion and post-operative complications in gastric cancer patients with a high CONUT score. The results of the present meta-analysis suggested that in patients diagnosed with gastric cancer, the CONUT score could be an easy-to-use indicator to reflect the nutritional status and may be considered for routine assessment. Additionally, the CONUT score could be helpful as a prognostic biomarker in gastric carcinoma and thereby, aid decision making for appropriate management.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021287305), reference no. CRD42021287305.
Authors' contributions
All authors took part in substantial contributions to the study. JY and MW designed the study. JY was the main contributor to the work. JQ and XL participated in the collection and analysis of clinical data. JY was involved in writing the original draft. MW was involved in review and substantial editing of the paper. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69:1151–1176. doi: 10.1080/01635581.2017.1367947. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH. Nutritional issues in patients with cancer. Intest Res. 2019;17:455–462. doi: 10.5217/ir.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Ulíbarri JI, González-Madroño A, de Villar NGP, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 4.Liang RF, Li JH, Li M, Yang Y, Liu YH. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin Chim Acta. 2017;474:155–158. doi: 10.1016/j.cca.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang X. Controlling nutritional status score, a promising prognostic marker in patients with gastrointestinal cancers after surgery: A systematic review and meta-analysis. Int J Surg. 2018;55:39–45. doi: 10.1016/j.ijsu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: A systematic review. Adv Nutr. 2021;12:234–250. doi: 10.1093/advances/nmaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi K, Domagala P, Polak WG, Buettner S, Wijnhoven BPL, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. BMC Surgery. 2019;19(129) doi: 10.1186/s12893-019-0593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372(n71) doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Hospital Research Institute, 2014. [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, London, 2022. www.training.cochrane.org/handbook. [Google Scholar]
- 11.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X, Zhao Y, Ma F, Wu S. Controlling nutritional status score predict the individualized survival of patients with gastric cancer. Asia Pac J Clin Nutr. 2021;30:51–59. doi: 10.6133/apjcn.202103_30(1).0007. [DOI] [PubMed] [Google Scholar]
- 13.Galizia G, Auricchio A, de Vita F, Cardella F, Mabilia A, Basile N, Orditura M, Lieto E. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Ann Ital Chir. 2019;90:404–416. [PubMed] [Google Scholar]
- 14.Sun F, Zhang C, Liu Z, Ai S, Guan W, Liu S. Controlling nutritional status (CONUT) score as a predictive marker for short-term complications following gastrectomy of gastric cancer: A retrospective study. BMC Gastroenterol. 2021;21(107) doi: 10.1186/s12876-021-01682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian Y, Liu H, Pan J, Yu W, Lv J, Yan J, Gao J, Wang X, Ge X, Zhou W. Preoperative controlling nutritional status (CONUT) score predicts short-term outcomes of patients with gastric cancer after laparoscopy-assisted radical gastrectomy. World J Surg Oncol. 2021;19(25) doi: 10.1186/s12957-021-02132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H, Zhu K, Wang W. The predictive values of pretreatment controlling nutritional status (CONUT) score in estimating short- and long-term outcomes for patients with gastric cancer treated with neoadjuvant chemotherapy and curative gastrectomy. J Gastric Cancer. 2021;21:155–168. doi: 10.5230/jgc.2021.21.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akagunduz B, Demir M, Atcı MM. Controlling nutritional status (CONUT) score is a prognostic factor for patients with gastric cancer treated by perioperative FLOT. J Gastrointest Cancer. 2022;53:571–580. doi: 10.1007/s12029-021-00664-4. [DOI] [PubMed] [Google Scholar]
- 18.Lin JX, Lin LZ, Tang YH, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, et al. Which nutritional scoring system is more suitable for evaluating the short- or long-term prognosis of patients with gastric cancer who underwent radical gastrectomy? J Gastrointest Surg. 2020;24:1969–1977. doi: 10.1007/s11605-019-04360-4. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Huang Y, Lu M, Sun W, Sun X, Chen X, Li L, Chandoo A, Li L. Controlling nutritional status (CONUT) score is a predictor of post-operative outcomes in elderly gastric cancer patients undergoing curative gastrectomy: A prospective study. Cancer Manag Res. 2019;11:9793–9800. doi: 10.2147/CMAR.S233872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon CH, Park KB, Jung YJ, Seo HS, Park CH, Song KY, Lee HH. Modified controlling nutritional status score: A refined prognostic indicator depending on the stage of gastric cancer. Surg Oncol. 2020;34:261–269. doi: 10.1016/j.suronc.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, Yamamoto T, Taniura T. Controlling nutritional status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: A propensity score-matched analysis. Surg Endosc. 2019;33:4143–4152. doi: 10.1007/s00464-019-06723-z. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204–212. doi: 10.1007/s10120-017-0744-3. [DOI] [PubMed] [Google Scholar]
- 23.Zheng ZF, Lu J, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Zheng CH, et al. Preoperative skeletal muscle index vs the controlling nutritional status score: Which is a better objective predictor of long-term survival for gastric cancer patients after radical gastrectomy? Cancer Med. 2018;7:3537–3547. doi: 10.1002/cam4.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Zhang D, Lin E, Chen Y, Li W, Chen Y, Sun X, Zhou Z. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric cancer. BMC Cancer. 2018;18(699) doi: 10.1186/s12885-018-4616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryo S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: Analysis of a multi-institutional data set. Ann Surg Oncol. 2019;26:456–464. doi: 10.1245/s10434-018-07121-w. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Kanaji S, Yamamoto M, Oshikiri T, Nakamura T, Kakeji Y. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg. 2019;43:1076–1084. doi: 10.1007/s00268-018-04889-6. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Q, Li X, Duan B, Li X, Liu S, Xu B, Shi S, Zhang J, Qin H, Duan X, et al. Clinical significance of controlling nutritional status score (CONUT) in evaluating outcome of postoperative patients with gastric cancer. Sci Rep. 2022;12(93) doi: 10.1038/s41598-021-04128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoyama T, Komori K, Nakazano M, Hara K, Tamagawa H, Kazama K, Hashimoto I, Yamada T, Maezawa Y, Segami K, et al. The clinical influence of the CONUT score on survival of patients with gastric cancer receiving curative treatment. In Vivo. 2022;36:942–948. doi: 10.21873/invivo.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 30.Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9(1055) doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alifano M, Mansuet-Lupo A, Lococo F, Roche N, Bobbio A, Canny E, Schussler O, Dermine H, Régnard JF, Burroni B, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9(e106914) doi: 10.1371/journal.pone.0106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Alzahrani NA, Chua TC, Huo YR, Liauw W, Morris DL. Impacts of preoperative serum albumin level on outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23:2411–2418. doi: 10.1245/s10434-016-5172-9. [DOI] [PubMed] [Google Scholar]
- 33.Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 34.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58:1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 35.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PubMed] [Google Scholar]
- 36.Mellor KL, Powell AGMT, Lewis WG. Systematic review and meta-analysis of the prognostic significance of neutrophil-lymphocyte ratio (NLR) after R0 gastrectomy for cancer. J Gastrointest Cancer. 2018;49:237–244. doi: 10.1007/s12029-018-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang LX, Wei ZJ, Xu AM, Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320–327. doi: 10.1016/j.ijsu.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 38.Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128–138. doi: 10.1016/j.ijsu.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Lin ZX, Ruan DY, Jia CC, Wang TT, Cheng JT, Huang HQ, Wu XY. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol. 2020;22:370–380. doi: 10.1007/s12094-019-02137-4. [DOI] [PubMed] [Google Scholar]
- 40.Lee SC, Lee JG, Lee SH, Kim EY, Chang J, Kim DJ, Paik HC, Chung KY, Jung JY. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (CONUT) score in patients with resectable non-small cell lung cancer. Sci Rep. 2020;10(12385) doi: 10.1038/s41598-020-68929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(e0132488) doi: 10.1371/journal.pone.0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cengiz O, Kocer B, Sürmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12:CR240–CR247. [PubMed] [Google Scholar]
- 43.Niendorf A, Nägele H, Gerding D, Meyer-Pannwitt U, Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int J Cancer. 1995;61:461–464. doi: 10.1002/ijc.2910610405. [DOI] [PubMed] [Google Scholar]
- 44.Vitols S, Gahrton G, Björkholm M, Peterson C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: evidence from studies in patients with leukaemia. Lancet. 1985;2:1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- 45.Notarnicola M, Altomare DF, Correale M, Ruggieri E, D'Attoma B, Mastrosimini A, Guerra V, Caruso MG. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology. 2005;68:371–374. doi: 10.1159/000086977. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Hurng J, Rateri DL, Daugherty A, Schmid-Schönbein GW, Shin HY. Membrane cholesterol modulates the fluid shear stress response of polymorphonuclear leukocytes via its effects on membrane fluidity. Am J Physiol Cell Physiol. 2011;301:C451–C460. doi: 10.1152/ajpcell.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver MF. Serum cholesterol-the knave of hearts and the joker. Lancet. 1981;2:1090–1095. doi: 10.1016/s0140-6736(81)91286-1. [DOI] [PubMed] [Google Scholar]
- 48.Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381–389. doi: 10.1245/s10434-006-9093-x. [DOI] [PubMed] [Google Scholar]
- 49.Crumley ABC, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34:2393–2398. doi: 10.1007/s00268-010-0641-y. [DOI] [PubMed] [Google Scholar]
- 50.Okuyama H, Ichikawa Y, Sun Y, Hamazaki T, Lands WEM. Cancer and all-cause mortalities are lower in the higher total cholesterol groups among general populations. World Rev Nutr Diet. 2007;96:37–54. doi: 10.1159/000097806. [DOI] [PubMed] [Google Scholar]
- 51.Strasak AM, Pfeiffer RM, Brant LJ, Rapp K, Hilbe W, Oberaigner W, Lang S, Borena W, Concin H, Diem G, et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: A prospective 19-year follow-up study. Ann Oncol. 2009;20:1113–1120. doi: 10.1093/annonc/mdn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou P, Li B, Liu B, Chen T, Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin Chim Acta. 2018;477:94–104. doi: 10.1016/j.cca.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 53.Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Yamamoto M, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Prognostic significance of pre- and postoperative lymphocyte counts in patients with gastric cancer. Dig Surg. 2019;36:137–143. doi: 10.1159/000486581. [DOI] [PubMed] [Google Scholar]
- 54.Tokunaga R, Sakamoto Y, Nakagawa S, Ohuchi M, Izumi D, Kosumi K, Taki K, Higashi T, Miyamoto Y, Yoshida N, et al. CONUT: A novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32:99–106. doi: 10.1007/s00384-016-2668-5. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Bao L, Wang W, Wang Q, Pan Y, Gao X. Prognostic impact of the Controlling Nutritional Status score following curative nephrectomy for patients with renal cell carcinoma. Medicine (Baltimore) 2018;97(e13409) doi: 10.1097/MD.0000000000013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallagher EJ, LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karczewski J, Begier-Krasińska B, Staszewski R, Popławska E, Gulczynska-Elhadi K, Dobrowolska A. Obesity and the risk of gastrointestinal cancers. Dig Dis Sci. 2019;64:2740–2749. doi: 10.1007/s10620-019-05603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021287305), reference no. CRD42021287305.