Abstract
Wild edible plants (WEPs) such as Solanum nigrum L., Vigna membranacea A. Rich., Dioscorea praehensilis Benth., Trilepisium madagascariense DC., and Cleome gynandra L. are widely used for various forms of culinary and folk medicine in Southwest Ethiopia. However, the phytochemical content of these plants is not explored. Thus, this study aimed to determine the total phenols, flavonoids, antioxidants, vitamin C, and β-carotene in edible parts of the plants. Edible parts were oven-dried and extracted with methanol. Total phenolic content (TPC) and total flavonoid content (TFC) were determined using Folin Ciocalteu and Aluminium chloride colorimetric methods, respectively. In vitro, antioxidant activities were evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) tests. β-carotene and vitamin C content were assessed using spectrophotometric and titration, respectively. TPC ranged from 0.25 ± 0.06 mg GAE/g in D. praehensilis tuber to 35.73 ± 2.52 mg GAE/g in S. nigrum leaf, while TFC varied from 0.85 ± 0.03 to 11.25 ± 0.01 mg CE/g in D. praehensilis tuber and C. gynandra leaf. In the DPPH assay, the antioxidant value ranged from 50.09% in D. praehensilis tuber to 87.63% in S. nigrum leaf; while in the FRAP assay, the value ranged from 49.16 ± 2.13 in D. praehensilis tuber to 188.12 ± 1.13 mM Fe2+/100 g in S. nigrum leaf. Similarly, β-carotene content was recorded between 11.81 ± 0.00 mg/100g in D. praehensilis tuber to 34.49 ± 0.95 mg/100g in V. membranacea leaf. The concentration of vitamin C ranged from 10.00 ± 0.61 in D. praehensilis tuber to 45 ± 1.80 mg/100g in V. membranacea leaf. The results showed strong positive correlations between FRAP and TPC (r = 0.94), and FRAP and vitamin C (r = 0.93). S. nigrum and C. gynandra contain abundant levels of TPC and TFC. V.membranacea leaf contains a good source of vitamin C and β-carotene. These WEPs contribute a natural supply of dietary antioxidants that prevent oxidative stress.
Keywords: Antioxidant activity, β-carotene, Flavonoids, Phenolic content, Vitamin C
1. Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced from natural cellular processes or external sources such as pollution, cigarette smoke, radiation, and medication [1]. These two groups of reactive species degrade proteins, lipids, nucleic acids, and carbohydrates [2,3]. Although these reactive species have beneficial physiological roles at low to moderate concentrations, they are detrimental at greater concentrations and cause oxidative stress [3]. When there is high production of free radicals and insufficient endogenous antioxidant protection in the human body, the oxidative stress is more aggravated, which results in aging, cellular damage, diabetes, cancer, neurodegenerative, and cardiovascular disease [[4], [5], [6]]. Such oxidative stress-induced degenerative disease is inhibited or prevented by exogenous dietary antioxidants. Antioxidants are grouped into two: synthetic and natural antioxidants. The former group includes butylated hydroxyl toluene (BHT), butylated hydroxyl anisole (BHA), propyl gallate, and tributyl hydroquinone (TBHQ), that cause cancer and health disorder such as liver damage in humans [7]. Recently, food and pharmaceutical industries have focused on substituting these synthetic antioxidants with natural antioxidants that attribute safe, nutritional, and medicinal value [[7], [8], [9]]. Natural antioxidants have been extracted from plant sources, which are rich in phenolic, flavonoids, vitamin C, carotenoids, tannins, and proanthocyanins, and these phytochemicals protect the body against free radicals [10,11]. Among these plant sources, wild edible plants (WEPs) have untapped nutritional and bioactive constituents having the potential to prevent oxidative stress [5,12]. Phenols, flavonoids, vitamin C, and carotenoids are among the phytochemicals with antioxidant activity found in WEPs [[13], [14], [15], [16]]. In this regard, a wide diversity of WEPs available and have been utilized by rural communities of Ethiopia for multipurpose such as food, traditional medicine, and fodder [17,18]. Among WEPs, Solanum nigrum L. (Solanaceae), Vigna membranacea A. Rich. (Fabaceae), Dioscorea praehensilis Benth. (Dioscoraceae), Trilepisium madagascariense DC. (Moraceae), and Cleome gynandra L. (Capparidaceae) are consumed in southwest Ethiopia and are good candidates for their study of phytochemical and antioxidant activities. To the best of our knowledge, phytochemical and antioxidant data were not available on these WEPs in the county, but few or fragmented data are available on the nutraceutical value of S. nigrum, C.gynandra, and D. praehensilis in some parts of Africa. Few recent studies found on S. nigrum is rich in phytochemicals such as phenolics, flavonoids, pro-anthocyanidins, saponins, and, alkaloids, and these compounds imparted its medicinal properties [19,20]. Cleome gynandara had also a substantial amount of total phenolics, flavonoids, flavanols and proanthocyanins that inhibits the oxidative stress related diseases [21]. On the other side, the tubers of D. praehensilis contained considerable amount of total phenolic contents related to good antioxidant activity [22]. Although WEPs are extensively available and play a vital role in Ethiopia's rural communities as nutraceuticals, it is not yet known about their phytochemical and antioxidant activities. Therefore, this study aimed at evaluating total phenolic content (TPC), total flavonoid content (TFC), β-carotene, vitamin C content, and in vitro antioxidant activity of five WEPs, namely, S. nigrum, V. membranacea, D. praehensilis, T. madagascariense, and C. gynandra. In methanolic extracted samples, the TPC and TFC were analyzed using Folin Ciocalteu and Aluminium chloride colorimetric methods, respectively. In vitro antioxidant activities were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) tests. β-carotene and vitamin C content were evaluated using spectrophotometric and titration, respectively. Pearson correlation was used to determine the interrelationships between all the analyzed parameters. The study may provide a baseline data on application of these wild edible the plants in food and pharmaceuticals industry.
2. Materials and methods
2.1. Chemical reagents
Folin–Ciocalteu reagent, l-ascorbic acid, catechin, gallic acid, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), Sodium nitrite (NaNO2), Aluminum chloride (AlCl3), Sodium hydroxide (NaOH), metaphosphoric acid, butylated hydroxytoluene (BHT), acetic acid, 2,6-dichloroindophenol sodium salt, sodium bicarbonate (NaHCO3), metaphosphoric acid (HPO3), calcium chloride dihydrate (CaCl2.2H2O), β-carotene, hexane, acetone, ethanol, acetate buffer, 2,4,6-tri-2-pyridyl-s-triazine (TPTZ), and ferric chloride (FeCl3) were used for chemical analysis. All the chemicals and solvents used in this study were of analytical grade.
2.2. Plant materials
The WEPs were collected from May 2019 to March 2021 from Guraferda, Meinit Goldiye, and Meinit Shasha, located in the Bench-Maji Zone of Southwest Ethiopia. The plant specimens were identified by Mr. Melaku Wendafrash and placed in Ethiopiaʹs national herbarium. Some information about the five investigated plants is presented in Table 1.
Table 1.
List of wild edible plants commonly consumed by the Meinit community in Southwest Ethiopia (Authors’ own unpublished data).
| Scientific name | Botanical Family | Local name | Edible parts | Plant use | Voucher Number |
|---|---|---|---|---|---|
| Solanum nigrum L. | Solanaceae | Chaw | Leaf | Food and folk medicine | AY-24 |
| Vigna membranacea A. Rich. | Fabaceae | Shutamodoroy | Leaf/seed | Food | AY-16 |
| Dioscorea praehensilis Benth. | Dioscoraceae | Entut | Tuber | Food | AY-01 |
| Trilepisium madagascariense DC. | Moraceae | Gagut | Fruit | Food and folk medicine | AY-30 |
| Cleome gynandra L. | Capparidaceae | Tikawoch | Leaf | Food and folk medicine | AY-29 |
These data were collected from the interviews conducted with the Meinit communities.
2.3. Sample collection and preparation
The five commonly used edible wild plants, namely, S. nigrum, V. membranacea, D. praehensilis, T.madagascariense, and C. gynandra. were used for phytochemical and antioxidant analyses. The edible parts were collected from more than ten randomly selected plants that attained the optimum maturity stage. The composite sample was made from different locations and plants for each edible plants. Two-kilograms of freshly harvested edible plant part were collected for each plant, samples were put inside a plastic bag and packed in an ice box, and transported immediately to Jimma University, College of Agriculture and Veterinary Medicine (JUCAVM) for laboratory analyses. Seeds of C. gynandra were collected from Guraferda district and planted at the JUCAVM horticulture farm, and leaf samples, which attained optimum maturity or just before the flowering stage, were harvested.
All of the samples that have been collected were cleaned from dirt and extraneous material, washed, rinsed with distilled water, excess moisture removed using blotting paper, and trimmed. Then, the samples were dried in an oven (DHG-9203A, Shanghai, China) that was maintained at 45 °C for 18 h. The dried samples were milled with a laboratory miller (RRH-200, Zhejiang, China) and sieved with a sieve size of 0.5 mm. Finally, the powdered samples were packed in zip-lock plastic bags and kept in a refrigerator until needed for chemical analyses. For each analysis, a triplicate analysis result was produced.
2.4. Sample extraction
The dried powder samples were extracted following [23], with some modifications. A 0.5 g power was soaked for 24 h with 50 mL methanol (99.8% absolute methanol) in a capped bottle and shaken in an orbital shaker at 180 rpm at room temperature. The shaken sample was centrifuged at 10,000 rpm for 10 min and the supernatant was recovered.
2.5. Phytochemical contents
2.5.1. Determination of total phenolic content
The total phenolic contents (TPC) was determined using Folin–Ciocalteu method [24]. Briefly, extracted sample (0.50 mL) was added to a 1:10 diluted Folin–Ciocalteu reagent (2.5 mL). After 4 min, saturated sodium carbonate solution (about 75 g/L, 2 mL) was added. After 2 h of incubation at room temperature, the absorbance of the reaction mixture was measured at 760 nm against a blank (methanol) using UV–Vis Spectrophotometer (Shimadzu UV-2401PC). Gallic acid was used as a reference standard, and the results were expressed as milligram gallic acid equivalent (mg GAE)/g dry weight of plant material.
2.5.2. Determination of total flavonoids content
The total flavonoids contents (TFC)s of all the extracts were quantified following the protocol outlined by Ref. [24]. Initially, 700 μl of all the plant extracts were taken in different test tubes. To each extract, 2 mL of distilled water was added. Then, 150 μl of NaNO2 was added to each test tubes followed by incubation at room temperature for 6 min. After incubation, 150 μl of AlCl3 (10%) was added to each test tube. The test tubes were incubated for 6 min at room temperature. Then, 2 mL of 4% NaOH was added to all the test tubes which were made up to 5 mL using distilled water. The contents in all the test tubes were vortexed well and they were allowed to stand for 15 min at room temperature. The pink color developed due to the presence of flavonoids was read with a spectrophotometer at 510 nm. The level of flavonoids was calculated as milligram catechin equivalent per gram (mg CE/g).
2.6. Determination of vitamin C content
The vitamin C content was determined following [25] 2,6-dichloroindophenol titration method 967.21. Briefly, a 0.1 g powder sample was extracted with 40 mL of 15 g of metaphosphoric acid (HPO3), mixed with 40 mL of acetic acid (Ac) in 500 mL deionized H2O. The extracted sample was filtered using Whatman number one filter paper. The filtrated sample was titrated using an indophenol solution made by dissolving 50 mg of 2,6-dichloroindophenol sodium salt and 42 mg of NaHCO3 to 200 mL of deionized water. The mixture was filtered through Whatman number one filter paper into umber bottle, and stored in refrigerator until use.
The standard solution of vitamin C was prepared by transferring 50 mg of vitamin C into 50 mL flask and diluted to volume using freshly prepared HPO3(Ac). The vitamin C content was calculated according to the following equation.
Where:
A = volume in mL of the 2,6-dichloroindophenol sodium salt solution used for the sample.B = volume in mL of the 2,6-dichloroindophenol sodium salt solution used for the blank.C = mass in mg of l-ascorbic acid equivalent to 1.0 mL of standard indophenol solution.S = weight of a sample taken (g).
40/10: 40 = volume of extract and 10 = volume of extract used for the determination.
2.7. Determination of β-carotene content
Extraction of β-carotene was done as described in Ref. [26] with minor modifications. Briefly,1 g of sample flour was mixed with 1g CaCl2.2H2O, 50 mL of 50% hexane, 25% acetone, and 25% ethanol containing 0.1% butyl hydroxy toluene (BHT) by shaking for 30 min at room temperature. After adding 15 mL of distilled water, the solution was mixed by a shaking for further 15 min. The organic phase, containing the β-carotene, was separated from the water phase using a separation funnel and filtered using Whatman number one filter paper. The extraction procedure was conducted under subdued light to avoid degradation of carotenoids. The stock β-carotene (Sigma Aldrich from the USA) standard solution was made by accurately weighing 0.01 g β-carotene in the solvent (50% hexane, 25% acetone, and 25% ethanol) used to extract samples and made the volume to 100 mL.
2.8. Antioxidant activity
2.8.1. Determination of antioxidant activity by DPPH assay
The determination of DPPH stable radical scavenging activities of the extracts and standards were evaluated based on the method described by Ref. [27]. Extracts (1 mL) of the different concentrations (0.2–0.56 mg/mL) made by reconstituting in respective solvents were added to DPPH solution (5 mL, 0.1 mM) in methanol and vortexed. After 20 min of reaction at 25 °C, the absorbance was measured at 517 nm against a blank (methanol) in a UV–Vis spectrophotometer (Shimadzu UV-2401PC). Methanolic DPPH solution (5 mL) without antioxidants was used as a control. The DPPH scavenging activity of the extract was expressed as IC50 (inhibitory concentration), that is, the concentration of the extract at which DPPH radicals were quenched by 50%. Ascorbic acid was used as the standard antioxidant. The percentage quenching of DPPH was calculated as follows:
2.8.2. Determination of antioxidant activity by ferric reducing antioxidant power (FRAP)
The FRAP analysis was conducted based on a modified method of [28]. Briefly, the FRAP reagent was made by mixing acetate buffer (pH 3.6, 300 mL), 10 mMol/L 2,4,6-tri-2-pyridyl-s-triazine (TPTZ) and 20 mMol/L FeCl3 at a ratio of 10:1:1 (v/v/v). Then, 150 μL of the extract solution was mixed with 4.5 mL of the FRAP reagent, and the mixture was incubated in a water bath at 37 °C for 10 min. The absorbance of the reacted mixture was measured by the UV–Vis spectrophotometer (Shimadzu UV-2401PC) at 593 nm, and the FRAP of each sample was calculated using a calibration curve with Fe2+ (Sigma-Aldrich Co, USA) as the standard.
2.9. Statistical analysis
The statistical analysis was done using MINITAB 17 and SAS 9.3 statistical software. The results were expressed as mean ± standard deviation. Each response variable was analyzed with analysis of variance (ANOVA). P-values (<0.05) indicated statistical significance; Tukey's post hoc test was used for mean separation. Pearson correlation analysis was performed to evaluate the relationship between some phytochemical (total phenols, flavonoids, vitamin C, and β-carotene), and antioxidant activity.
3. Results and discussions
3.1. Total phenolic content
Phenolic acids and flavonoids are two main phenolic compounds with antioxidant activity. The determination of total phenolic content (TPC) by the Folin-Ciocalteu method has limitation of overvaluation the phenolic content due to some non-phenolic reducing compounds, such as organic acids and sugars, could interfere the determination of total phenolic contents. Moreover, in Folin-Ciocalteu reagent might present different phenolics with varied responses such as gallic acid and rutin have similar behaviors, but several flavonoids present low absorption, which leads to an underestimation of various compounds [29]. The TPC of extracts varied significantly (P < 0.05) from 0.25 to 35.73 mg of gallic acid equivalent (GAE)/g on dry weight basis (dw) in D. praehensilis tuber and S. nigrum leaves, respectively (Table 2). The sample extract of S. nigrum showed the highest TPC, while the lowest quantity was recorded in D. praehensilis tuber. Comparable results were reported in Greece [30], who reported from 4.00 to 31.6 mg GAE/g dw, for Geranium purpureum and Nepeta melissifolia leaves, respectively. However, our study results were slightly lower than [5] who reported that TPC of WEPs in methanol extracts ranges from 72.06 mg to 292.65 mg GAE/g of Ipomoea aquatic and Alternanthera sessilis. These variations might be due to intrinsic (genetic), extrinsic (growing environment, agronomic practice) [31] factors and their interactions. Interestingly, S. nigrum, V. membranacea, and C. gynandra leaves contained a significant TPC among the studied WEPs, and this value supports potential antioxidant activities. The oxidative degenerative diseases may be prevented by plant extracts that are high in TPC.
Table 2.
Total phenol, total flavonoid and some vitamin content of five wild edible plants (dw).
| Wild edible plants | TPC (mg GAE/g) | TFC (mg CE/g) | β-carotene (mg/100g) | Vitamin C (mg/100g) |
|---|---|---|---|---|
| S. nigrum | 35.73 ± 2.52a | 8.65 ± 0.03b | 19.15 ± 0.43d | 35.90 ± 3.60b |
| V. membranacea | 27.23 ± 0.07b | 5.35 ± 0.03c | 34.49 ± 0.95a | 45.00 ± 1.80a |
| D. praehensilis | 0.25 ± 0.06d | 0.85 ± 0.03e | 11.81 ± 0.00e | 10.00 ± 0.61d |
| T. madagascariense | 22.97 ± 0.16c | 2.07 ± 0.02d | 24.92 ± 0.63c | 26.07 ± 1.50c |
| C. gynandra | 29.03 ± 0.00b | 11.25 ± 0.01a | 28.67 ± 0.63b | 23.40 ± 1.91c |
| CV (%) | 4.91 | 0.44 | 2.57 | 7.79 |
| LSD (p < 0.05) | 3.04 | 0.07 | 1.64 | 5.89 |
Note: Values are means ± standard deviations of three replicate measurements, TPC = total phenolic content expressed as gallic acid equivalent (mg GAE/g), TFC = total flavonoid content expressed as mg catechin equivalent (CE/g), CV% = coefficient of variation in percentage, and LSD = least significant difference. Values in a column followed by a different superscript letter are significantly different (p < 0.05).
3.2. Total flavonoid content
Flavonoids are phenolic compounds that are known for their free radical scavenging activities that prevent oxidative stress. The total flavonoid content (TFC) was presented as mg catechin equivalent (CE) per gram dry weight extract of the five WEPs (Table 2). The TFC in methanolic extracts ranged from 0.85 to 11.25 mg CE/g in D. praehensilis tuber and C. gynandra leaf. Among studied WEPs, C. gyanandra and S. nigrum leaves contained higher amount of TFC while T. madagascariense fruit and D. praehensilis tuber showed the least amount of TFC.
These values were consistent with the result of [16] who reported from 0.30 in Helminthostachys zeylanica to 6.13 mg CE/g in Gonostegia hirta leaves methanolic extracts. Moreover, results from the present study were slightly higher than previous report by Ref. [20] who reported 0.36 in Sonchus asper and 0.98 mg QE/g in S. nigrum leaf. However, the TFC in the present study was much lower than those reported by Ref. [32] who reported 25.88 to 89.78 mg CE/g for methanolic extracts of green leafy vegetables of wild origin of Montia Fontana and Rumex induratus, respectively. The common error in TFC value might be attributed to genetic and/or environmental factors and their interactions, type of plant part used, stage of maturity, and method of analysis followed. Thus, comparing the TFC of WEPs from varied data sources is challenging.
3.3. Beta-carotene content
The results of the β-carotene content of the five WEPs are presented in Table 2. The β-carotene content of the WEPs varied significantly (p < 0.05) and ranged between 11.81 and 34.49 mg/100g (dw). The highest amount of β-carotene was found in V. membranacea leaf, while the lowest was recorded in D. praehensilis tuber. The results were in agreement with the earlier work of [33], who reported 14.67 in Cucurbita maxima leaves to 39.86 mg/100g dw in Moringa oleifera leaves. Similar results also reported by Ref. [34] ranging from 15 in Hibiscus sabdariffa seed to 42 mg/100g in Raphanus sativus leaves (dw). The findings in the present study were however lower than those reported for other WEPs like Berberis lyceum leaves (91.54) to Nasturtium officinale aerial part (209.60) mg/100g but higher than those reported for Grewia trichocarpa fruit (0.06) to Talinum portulacifolium leaves (0.275) mg/100g [26,35]. However, V. membranacea leaf, C. gynandra leaf, and T. madagascariense fruit contained a reasonable amount of β-carotene implying that the studied WEPs might be used for nutrient diversification and as a natural antioxidant sources for consumers.
3.4. Vitamin C content
Vitamin C content of S. nigrum leaf, V. membranacea leaf, C. gynandra leaf, and T. madagascariense fruit varied slightly (p < 0.05), as shown in Table 2. The study results for vitamin C content of the five WEPs fluctuated between 10 in D. praehensilis tuber to 45 mg/100g (dw) in S. nigrum leaf. The V. membranacea contained the highest vitamin C content, followed by S. nigrum leaf, while the lowest amount of vitamin C was found in D. praehensilis tuber. The results of this findings were slightly higher than previous report by Ref. [36] who reported from 1 to 10 mg/100g (dw) of vitamin C in Corchorus olitorius and Citrullus lanatus, respectively. However, the highest level of vitamin C (260 mg/100 g) in Rumex vesicarius leaves [37], and from 73.08 to 172.49 mg/100g in Gardenia erubescens and Sclerocarya birrea fruits, respectively [38], were also reported previously. The variation in vitamin C content may be differences in species, growing environment, stage of harvesting, edible parts used and method of the analytical technique. Vitamin C play a significant role in human body, these as antioxidant, healthy skin, and iron absorption. The V. membranacea fruit has a relatively higher vitamin C content as compared to the other WEPs included in the present investigation, and is a better source of vitamin C and antioxidant activity.
3.5. Antioxidant activity
Two different in vitro assays such DPPH, and FRAP were used to evaluate the antioxidant activity of the five selected WEPs.
3.5.1. DPPH assay
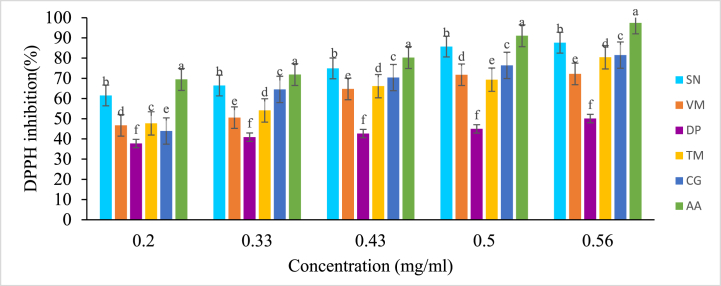
Antioxidants in the extracts react with DPPH and convert 1, 1-diphenyl-2- picrylhydrazyl (deep violet color) to 1, 1-diphenyl-2-picrylhydrazine, a stable molecule (yellow color or bleached product) by accepting an electron or hydrogen radical at a very rapid rate resulting in a decrease in absorbance at 517 nm. The results in Fig. 1 reveal that the DPPH scavenging activities of WEPs extracts and ascorbic acid (A.A.) standard were concentration dependent. The radical scavenging activity of the extracts increased with increasing concentration. At a concentration of 0.56 mg/mL, the DPPH scavenging activity of S.nigrum extract was 87.63%, while that of the ascorbic acid was 97.44% (Fig. 1).
Fig. 1.
Inhibition (%) of DPPH at different concentrations of WEPs extracts and standard ascorbic acid. Results are expressed as the mean ± standard deviation (n = 3). The bars with different letters are significantly different (P < 0.05) by one-way ANOVA followed by Tukey's test. SN = S.nigrum, VM = V. membranacea, DP = D. praehensilis, TM = T.madagascariense, CG= C.gynandra and AA = Ascorbic acid.
The scavenging activity increased with the concentration of extract in the DPPH assay. These results closely agreed with earlier reports [39,40].The DPPH scavenging activity of methanolic extracts of five WEPs is presented in Table 3. The highest inhibition of DPPH was recorded at the concentration of 0.56 mg/mL. There was significant (p < 0.05) variation in DPPH inhibition by the five WEPs, and the results varied between 50.09 and 87.63% in different concentrations. The highest DPPH scavenging activity was recorded by S. nigrum leaf while the lowest by D. praehensilis tuber. Among the studied WEPs, the highest DPPH inhibition was recorded for S. nigrum (87.63%) followed by C. gynandra (81.48%), T.madagascariense (80.41%), and V. membranacea (72.18%). However, DPPH inhibition in all sample extracts was significantly lower than the standard ascorbic acid (97.44%). Similar results were reported by Refs. [4,34] who reported that DPPH inhibition ranged from 28.56 to 72.6% in ethanol extract of Grewia carpinifolia leaves; from 50.01 in Hibiscus sabdariffa to 73.66% in Raphanus sativus methanolic extracts of leaves. However, the antioxidant activities of the WEPs included in the present investigation were slightly lower than Becium dhofarense shoot (93.1%) of the DPPH assay reported by Ref. [41]. It is known that plant extract with high antioxidant potential has a higher reducing activity of oxidative stress. Antioxidant potential of these WEPs could be used to reduce oxidative stress.
Table 3.
Antioxidant activity of five wild edible plants.
| Plants | DPPH | IC50 | FRAP (mM of Fe2+/g) |
|---|---|---|---|
| S. nigrum | 87.63 ± 0.10b | 0.08 ± 0.01e | 188.12 ± 1.13a |
| V. membranacea | 72.18 ± 0.09e | 0.26 ± 0.08b | 163.55 ± 3.55b |
| D. praehensilis | 50.09 ± 0.05f | 0.62 ± 0.04a | 49.16 ± 2.13e |
| T. madagascariense | 80.41 ± 0.09d | 0.25 ± 0.00c | 97.08 ± 2.51d |
| C. gynandra | 81.48 ± 0.09c | 0.23 ± 0.07d | 131.85 ± 0.74c |
| Ascorbic acid | 97.44 ± 0.10a | 0.011 ± 0.00f | – |
| CV | 0.11 | 0.36 | 1.96 |
| LSD | 0.16 | 0.01 | 3.65 |
Note: Results are expressed as mean ± SD (n = 3), DPPH = 2,2-diphenyl-1-picrylhydrazyl expressed in percent, IC50 = inhibitory concentration, FRAP = Ferric Reducing Antioxidant Power, CV% = coefficient of variation percentage, and LSD = least significant difference. Values in the same column followed by a different superscript letter are significantly different (p < 0.05).
DPPH assay is further expressed by half-maximal inhibitory concentration (IC50). The IC50 value is defined as the inhibitory concentration of the crude extract that scavenges 50% of reactive oxygen species or inhibits the process of oxidation process by 50%. It was calculated using the concentration-inhibition activity curve. It is inversely related to antioxidant capacity, and a lower IC50 value signals better antioxidant activity. The lowest IC50 values (μg/mL) for the WEPs were recorded for S. nigrum (0.08), C. gynandra (0.23), V. membranacea (0.26), T. madagascariense (0.25) whereas D. praehensilis tuber (0.62) showed the highest IC50 value among the studied WEPs (Table 3). Thus, S. nigrum leaves had the lowest IC50 value among the WEPs tested, indicating its stronger antioxidant activity. However, all the plants under investigation had IC50 values higher than those of vitamin C (0.011).
3.5.2. Ferric reducing antioxidant power (FRAP)
FRAP assay measures the changes in absorbance at 593 nm from colorless blue-colored Fe2+-tripyridyltriazine solution formed by the action of electron-donating antioxidants (reductants) in the plant extract. Methanolic extracts of S. nigrum, V. membranacea, and C. gynandra leaves showed higher FRAP than D. praehensilis tuber and T. madagascariense fruit plant extracts. The FRAP of all samples varied significantly (P < 0.05) and ranged from 49.16 ± 2.13 to 188.12 ± 1.13 mM Fe2+/g (dw) (Table 3). Among the WEPs, the highest FRAP value was recorded from S. nigrum followed by V. membranacea, C.gynandra, T. madagascariense fruit, and D. praehensilis tuber. Compared to the sample extracts, S. nigrum, and V. membranacea demonstrated a greater ability to reduce ferric ion (Fe3+) to ferrous ion (Fe2+). In a separate investigation, the FRAP value ranged from 12.84 to 119.97 mM Fe2+/g of methanolic extract of Schimatoglottis ahmadii stem and Heckeria umbellatum leaf, respectively [16].
3.6. Correlation between phytochemical and antioxidant activity
Table 4 displays the correlation results between phytochemical contents and antioxidants of five wild edible plants. The five correlation scales were categorized by Ref. [42] namely: very weak (r = 0.0–0.19), weak (r = 0.20–0.39), moderate (r = 0.40–0.59), strong (r = 0.60–0.79), and very strong (r = 0.80–1.0). The findings demonstrated very strong relationships between TPC and TFC of WEPs (r = 0.83), as well as between FRAP and TPC (r = 0.94), and FRAP and vitamin C (r = 0.93) and these relationships had also a high significant (p < 0.01). The DPPH value and TPC had a weak correlation (r = 0.25) and revealed non-significant (p < 0.05). According to some previous findings, TPC and antioxidant activity were correlated in the DPPH assay [5], but there was little correlation between TPC and antioxidant activity in the phosphomolybdenum assay model [41], and there was no direct correlation between TPC and antioxidant activity in Rancimat assay [9]. However, TPC, TFC, β-carotene, and vitamin C correlate better with the FRAP assay results recorded in the present investigation. These differences could be attributed to the different plant species, growing conditions, and analytical assay used.
Table 4.
Correlation between phytochemical contents (total phenolic content, total flavonoids, β-carotene and vitamin C) and antioxidant activity.
| Parameters | Phytochemical contents |
Antioxidant activity |
|||||
|---|---|---|---|---|---|---|---|
| TPC | TFC | β-carotene | Vitamin C | DPPH | IC50 | FRAP | |
| TPC | 1 | ||||||
| TFC | 0.83 (0.00) | 1 | |||||
| β-carotene | 0.78 (0.00) | 0.62 (0.01) | 1 | ||||
| Vitamin C | 0.86 (0.00) | 0.58 (0.01) | 0.86 (0.00) | 1 | |||
| DPPH | 0.25 (0.32) | 0.18 (0.47) | −0.21 (0.40) | −0.07 (0.79) | 1 | ||
| IC50 | −0.32 (0.20) | −0.22 (0.38) | −0.12 (0.65) | −0.06 (0.83) | −0.98 (0.00) | 1 | |
| FRAP | 0.94 (0.00) | 0.79 (0.00) | 0.80 (0.00) | 0.93 (0.00) | 0.01 (0.98) | −0.12 (0.65) | 1 |
Note: Pearson's correlation coefficient (r) and p-values (p < 0.05) are noted in parentheses. TPC = total phenolic content, TFC = total flavonoid content, DPPH = 2,2-diphenyl-1-picrylhydrazyl, FRAP = Ferric Reducing Antioxidant Power, IC50 = half-maximal inhibitory concentration.
The study provides insight on nutraceutical sources of WEPs for users. However, the stage of maturity, seasonal variation, methods employed, and unavailable previous literature were potential limitation of this study that made discrepancies of the study findings.
4. Conclusions
In this study, the total phenolic content, total flavonoids, antioxidant activity, vitamin C, and β-carotene of methanolic extracts of five WEPs (S. nigrum, V. membranacea, D. praehensilis, T. madagascariense, and C. gynandra) were determined. While C. gynandra leaves displayed a significant amount of total flavonoids, S. nigrum leaf extract had the highest total phenolic and antioxidant activity. Similar to this, V. membranacea leaf extract has shown a moderate level of antioxidant activity, phenolic and flavonoid content, as well as the highest levels of vitamin C and β-carotene. The findings demonstrated a strong linear correlation between FRAP and total phenolic contents, as well as between FRAP and vitamin C. S. nigrum and C. gynandra plant extracts showed the good source of total phenolics, flavonoids, and antioxidant activities, and are potential natural antioxidants that can help to treat oxidative stress-related disease. Agronomic use of these wild edible plants for agriculture warrants further research. Additionally, it is necessary to conduct research on these plants for their in vivo antioxidant activity and toxicological makeup.
Author contribution statement
Abebe Yimer: Performed the experiments; Wrote the paper.
Sirawdink Fikereyesus Forsido: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Getachew Addis: Analyzed and interpreted the data; Wrote the paper.
Abebe Ayelign: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors acknowledge Jimma University, College of Agriculture and Veterinary Medicine (JUCAVM) supports a finance for this research study.
References
- 1.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health lien. Int. J. Biomed. Sci. 2008;4(2):89–96. doi: 10.17094/ataunivbd.483253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parcheta M., Świsłocka R., Orzechowska S., Akimowicz M., Choińska R., Lewandowski W. Recent developments in effective antioxidants: the structure and antioxidant properties. Materials. 2021;14(8):1–24. doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh, Mahadi F., Roy A., Sharma P. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J. Clin. Biochem. 2009;24(4):324–342. doi: 10.1007/s12291-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adebiyi O.E., Olayemi F.O., Ning-Hua T., Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci. 2017;6(1):10–14. doi: 10.1016/j.bjbas.2016.12.003. [DOI] [Google Scholar]
- 5.Aryal S., Baniya M.K., Danekhu K., Kunwar P., Gurung R., Koirala N. Total Phenolic content, Flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. 2019;8(4) doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A., Li S., Li H., Xu D., Xu X., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Fuct. Foods. 2013;6(3 1 9) – 3 3 0. [Google Scholar]
- 7.Alam M.K., Rana Z.H., Islam S.N., Akhtaruzzaman M. Total phenolic content and antioxidant activity of methanolic extract of selected wild leafy vegetables grown in Bangladesh : a cheapest source of antioxidants. Slovak J. Food Sci. 2019;13(1):287–293. [Google Scholar]
- 8.Mehmood A., Shah M.H., Li T., Fu X., Guo X. Ethnomedicinal values , phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015;162:333–345. doi: 10.1016/j.jep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 9.Tiveron A.P., Melo P.S., Bergamaschi K.B., Vieira T.M.F.S., Arce M.A.B.R., Alencar S.M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content , antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015;9(4):449–454. [Google Scholar]
- 11.Karthishwaran K., Obaid S., Obaid S., Shamisi A., Kurup S., Sakkir S., Cheruth A.J. Free-radical-scavenging and antioxidant capacities with special emphasis on enzyme activities and in vitro studies in Caralluma flava N . E . Br. Biotechnol. Biotechnol. Equip. 2018;32(1) doi: 10.1080/13102818.2017.1379362. [DOI] [Google Scholar]
- 12.Alam M.A., Nadirah T.A., Mohsin G.M., Saleh M., Moneruzzaman K.M., Aslani F., Juraimi A.S., Alam M.Z. To. Antioxidant compounds , antioxidant activities , and mineral contents among underutilized vegetables. Int. J. Veg. Sci. 2020:1931–5279. doi: 10.1080/19315260.2020.1748785. [DOI] [Google Scholar]
- 13.Alirezalu A., Salehi P., Ahmadi N., Sonboli A., Aceto S., Maleki H.H., Ayyari M., Alirezalu A., Salehi P., Ahmadi N., et al. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp .) from different regions of Iran. Int. J. Food Prop. 2018;21(1):452–470. doi: 10.1080/10942912.2018.1446146. [DOI] [Google Scholar]
- 14.Ebrahimzadeh M.A., Nabavi S.M.N., Fazel S.N., Bahramian F., Bekhradnia A.R. Antioxidant and free radical scavenging activity of Hyssopus officinalis, Viola odorata, Buxus hyrcana and Colchicum speciosum. Pak. J. Pharm. Sci. 2008;23(1):29–34. [PubMed] [Google Scholar]
- 15.Ereifej K.I., Feng H., Rababah T., Almajwal A., Alu M., Gammoh S.I., Oweis L.I. Chemical composition , phenolics , anthocyanins concentration and antioxidant activity of ten wild edible plants. Food Nutr. Sci. 2015;6:581–590. [Google Scholar]
- 16.Wong J.Y., Matanjun P., Ooi Y.B.H., Chia K.F. Evaluation of antioxidant activities in relation to total phenolics and flavonoids content of selected malaysian wild edible plants by multivariate analysis. Int. J. Food Prop. 2014;17(8):1763–1778. doi: 10.1080/10942912.2012.724756. [DOI] [Google Scholar]
- 17.Adamu E., Asfaw Z., Demissew S., Baye K. Antioxidant activity and anti-nutritional factors of selected wild edible plants collected from northeastern Ethiopia. Foods. 2022;11(15):1–13. doi: 10.3390/foods11152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addis G., Asfaw Z., Singh V., Woldu Z., Baidu-Forson J., Bhattacharya S. Dietary values of wild and semi-wild edible plants in Southern Ethiopia. Afr. J. Food Nutr. Sci. 2013;13(2) [Google Scholar]
- 19.Ogundola Adijat F., Bvenura Callistus, Ehigie Adeola F., AJA Effects of soil types on phytochemical constituents and antioxidant properties of Solanum nigrum. South Afr. J. Bot. 2022;151:325–333. doi: 10.1016/j.sajb.2022.09.048. [DOI] [Google Scholar]
- 20.Afolayan A.J., Jimoh F.O. Nutritional quality of some wild leafy vegetables in South Africa. Int. J. Food Sci. Nutr. 2009;60(5):424–431. doi: 10.1080/09637480701777928. [DOI] [PubMed] [Google Scholar]
- 21.Sowunmi L.I., Afolayan A.J. Phytochemical constituents and antioxidant properties of acetone extract of cleome gynandra (L.) growing in the eastern cape, South Africa. Afr. J. Tradit., Complementary Altern. Med. 2015;12(3):1–8. doi: 10.4314/ajtcam.v12i3.1. [DOI] [Google Scholar]
- 22.Bukatuka F., Ngombe K., Mutwale K., Moni B., Makengo K., Pambu L., Bongo N., Mbombo M., Musuyu M., Maloueki U., et al. Bioactivity and nutritional values of some Dioscorea species traditionally used as medicinal foods in Bandundu, DR Congo. Eur. J. Med. Plants. 2016;14(1):1–11. doi: 10.9734/ejmp/2016/25124. [DOI] [Google Scholar]
- 23.Korekar G., Stobdan T., Arora R. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L .) Kern. Func. Genotype. 2011:376–383. doi: 10.1007/s11130-011-0246-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 25.AOAC. Association of Official Analytical Chemists . Washington, DC, USA; 2005. Official Methods of Analysis of AOAC International. [Google Scholar]
- 26.Shad A.A., Shah H.U., Bakht J. Ethnobotanical assessment and nutritive potential of wild food plants. J. Anim. Plant Sci. 2013;23(1):92–99. [Google Scholar]
- 27.Singh R.P., Murthy K.N.C., Jayaprakasha G.K. Studies on the antioxidant activity of Pomegranate (Punica granatum) Peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 28.Benzie I.F.F. Strain JJ. The ferric reducing ability of Plasma (FRAP) as a measure of ‘“ antioxidant power ”’: the FRAP assay. Anal. Biochem. 1996;76:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Fu L., Xu B.T., Xu X.R., Qin X.S., Gan R.Y., Li H Bin. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules. 2010;15(12):8602–8617. doi: 10.3390/molecules15128602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proestos C., Lytoudi K., Mavromelanidou O.K., Zoumpoulakis P., Sinanoglou V.J. 2013. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils; p. 11. –22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrikanta A., Kumar A., Govindaswamy V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015;52(1):383–390. doi: 10.1007/s13197-013-0993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira C., Barros L., Carvalho A.M., Ferreira I. Nutritional composition and bioactive properties of commonly consumed wild greens: potential sources for new trends in modern diets. Food Res. Int. 2011;44(9):2634–2640. doi: 10.1016/j.foodres.2011.05.012. [DOI] [Google Scholar]
- 33.Djuikwo V.N.D., Ejoh R.A., Gouado I., Mbofung C.M., Tanumihardjo S.A. Determination of major carotenoids in processed tropical leafy vegetables indigenous to africa. Food Nutr. Sci. 2011;2(8):793–802. doi: 10.4236/fns.2011.28109. [DOI] [Google Scholar]
- 34.Keyata E.O., Tola Y.B., Bultosa G., Forsido S.F. Phytochemical contents, antioxidant activity and functional properties of Raphanus sativus L, Eruca sativa L. and Hibiscus sabdariffa L. growing in Ethiopia. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2021.e05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anywar G.U., Oryem-Origa H., Kamatenesi-Mugisha M. Proximate nutrient composition of some wild edible medicinal plants from Uganda. Afr. J. Food Nutr. Sci. 2017;17(3):12214–12225. doi: 10.18697/ajfand.79.15590. [DOI] [Google Scholar]
- 36.Jaarsveld P Van, Faber M., Heerden I Van, Wenhold F., Jansen W., Rensburg V., Averbeke W Van. Analysis Nutrient content of eight African leafy vegetables and their potential contribution to dietary reference intakes. J. Food Compos. Anal. 2014;33(1):77–84. doi: 10.1016/j.jfca.2013.11.003. [DOI] [Google Scholar]
- 37.Alfawaz M.A. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J. Food Compos. Anal. 2006;19(6–7):552–555. doi: 10.1016/j.jfca.2004.09.004. [DOI] [Google Scholar]
- 38.Achaglinkame M.A., Aderibigbe R.O., Hensel O., Sturm B., Korese J.K. Nutritional characteristics of four underutilized edible wild fruits of dietary interest in Ghana. Foods. 2019;8(104):1–12. doi: 10.3390/foods8030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuda T., Tsunekawa M., Goto H., Araki Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005;18(7):625–633. doi: 10.1016/j.jfca.2004.06.015. [DOI] [Google Scholar]
- 40.Ismail A., Hong T.S. Antioxidant activity of selected commercial seaweeds. Malays. J. Nutr. 2002;8(2):167–177. [PubMed] [Google Scholar]
- 41.Marwah R.G., Fatope M.O., Mahrooqi R Al, Varma G.B., Abadi H Al, Al-burtamani S.K.S. Vol. 101. 2007. pp. 465–470. (Food Chemistry Antioxidant Capacity of Some Edible and Wound Healing Plants in Oman). [DOI] [Google Scholar]
- 42.Evans J.D. Thomson Brooks/Cole Publishing Co; 1996. Straightforward Statistics for the Behavioral Sciences. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.