Key Points
Question
Does radiosurgery improve pain response compared with conventional external beam radiation therapy?
Findings
In this phase 3 randomized clinical trial of 339 patients, the primary end point of pain response at 3 months was not improved in the spine radiosurgery group. There were no spinal cord complications at 2-year follow-up after spine radiosurgery.
Meaning
Radiosurgery was not found to be superior to in terms of pain response at 3 months and, in fact, worse pain response was observed compared with conventional external beam radiation therapy.
Abstract
Importance
Spine metastasis can be treated with high-dose radiation therapy with advanced delivery technology for long-term tumor and pain control.
Objective
To assess whether patient-reported pain relief was improved with stereotactic radiosurgery (SRS) as compared with conventional external beam radiotherapy (cEBRT) for patients with 1 to 3 sites of vertebral metastases.
Design, Setting, and Participants
In this randomized clinical trial, patients with 1 to 3 vertebral metastases were randomized 2:1 to the SRS or cEBRT groups. This NRG 0631 phase 3 study was performed as multi-institutional enrollment within NRG Oncology. Eligibility criteria included the following: (1) solitary vertebral metastasis, (2) 2 contiguous vertebral levels involved, or (3) maximum of 3 separate sites. Each site may involve up to 2 contiguous vertebral bodies. A total of 353 patients enrolled in the trial, and 339 patients were analyzed. This analysis includes data extracted on March 9, 2020.
Interventions
Patients randomized to the SRS group were treated with a single dose of 16 or 18 Gy (to convert to rad, multiply by 100) given to the involved vertebral level(s) only, not including any additional spine levels. Patients assigned to cEBRT were treated with 8 Gy given to the involved vertebra plus 1 additional vertebra above and below.
Main Outcomes and Measures
The primary end point was patient-reported pain response defined as at least a 3-point improvement on the Numerical Rating Pain Scale (NRPS) without worsening in pain at the secondary site(s) or the use of pain medication. Secondary end points included treatment-related toxic effects, quality of life, and long-term effects on vertebral bone and spinal cord.
Results
A total of 339 patients (mean [SD] age of SRS group vs cEBRT group, respectively, 61.9 [13.1] years vs 63.7 [11.9] years; 114 [54.5%] male in SRS group vs 70 [53.8%] male in cEBRT group) were analyzed. The baseline mean (SD) pain score at the index vertebra was 6.06 (2.61) in the SRS group and 5.88 (2.41) in the cEBRT group. The primary end point of pain response at 3 months favored cEBRT (41.3% for SRS vs 60.5% for cEBRT; difference, −19 percentage points; 95% CI, −32.9 to −5.5; 1-sided P = .99; 2-sided P = .01). Zubrod score (a measure of performance status ranging from 0 to 4, with 0 being fully functional and asymptomatic, and 4 being bedridden) was the significant factor influencing pain response. There were no differences in the proportion of acute or late adverse effects. Vertebral compression fracture at 24 months was 19.5% with SRS and 21.6% with cEBRT (P = .59). There were no spinal cord complications reported at 24 months.
Conclusions and Relevance
In this randomized clinical trial, superiority of SRS for the primary end point of patient-reported pain response at 3 months was not found, and there were no spinal cord complications at 2 years after SRS. This finding may inform further investigation of using spine radiosurgery in the setting of oligometastases, where durability of cancer control is essential.
Trial Registration
ClinicalTrials.gov Identifier: NCT00922974
This randomized clinical trial assesses whether patient-reported pain relief was improved with stereotactic radiosurgery compared with conventional external beam radiotherapy for patients with 1 to 3 sites of vertebral metastases.
Introduction
Vertebral metastases are a common complication of cancer, causing back pain and leading to serious clinical consequences from compression fracture or spinal cord compression. Treatment for spine metastases not requiring or amenable to surgery has been conventional external beam radiotherapy (cEBRT) with palliative radiation doses.1 Previous studies of pain palliation of bone metastases comparing various cEBRT dose regimens demonstrated equivalent efficacy.2,3,4 Pain relief was modest at 50% to 60%,5 with a median duration of 4 months.6,7
With improved image guidance, immobilization and targeting techniques, and use of intensity or volumetric modulation, stereotactic radiosurgery (SRS) has been used to deliver a highly conformal dose covering the vertebral metastasis with a steep dose gradient to the spinal cord. Early institutional experiences assessing SRS for vertebral metastases have demonstrated safety, rapid and durable pain relief of 80% to 90%, and long-term tumor control using various dose regimens including 16 Gy to 24 Gy in 1 fraction, 24 Gy in 2 fractions, 24 Gy to 27 Gy in 3 fractions, and 20 Gy to 35 Gy in 5 fractions (to convert Gy to rad, multiply by 100).8,9,10,11,12,13,14,15 These studies suggest that SRS treatment may improve pain relief and local tumor control over cEBRT.
This phase 2 and 3 randomized clinical trial, NRG Oncology’s RTOG 0631, assessed whether patient-reported pain relief was improved with SRS as compared with cEBRT for patients with 1 to 3 sites of vertebral metastases (see Supplement 1 for the trial protocol). The phase 2 study results confirmed the feasibility and safety of SRS to treat vertebral metastases in the multi-institutional cooperative group setting.16 Herein, the phase 3 results assessing patient reported pain relief and treatment safety on long-term follow-up are reported.
Methods
Study Eligibility
In this randomized clinical trial, patients who were aged at least 18 years, had a Zubrod score (a measure of performance status ranging from 0 to 4, with 0 being fully functional and asymptomatic, and 4 being bedridden) of 0 to 2, and had 1 to 3 treatment-naive vertebral metastases were eligible. Other criteria included the following: (1) solitary vertebral metastasis, (2) 2 contiguous vertebral levels involved, or (3) maximum of 3 separate sites. Each site may involve up to 2 contiguous vertebral bodies. Clinically asymptomatic spine metastases detected on screening magnetic resonance imaging (MRI) were included, provided that the lesion was no more than 20% of each vertebral marrow volume. Every patient was required to have a baseline pain score of at least 5, using the 0 to 10 Numerical Rating Pain Scale (NRPS). Pain medication, including narcotics, was allowed. If there were more than 1 spine lesion with the same maximal pain score, the most cephalad level was considered the index lesion for pain evaluation. Epidural lesions with at least a 3-mm gap between the tumor and spinal cord, and paraspinal masses (<5 cm) directly attached to the vertebral body, were eligible. Radioresistant cancers (melanoma, renal cell carcinoma, soft tissue sarcoma) were included. Tumors with vertebral compression fractures with more than 50% height loss and/or bony retropulsion were excluded. Patients with systemic or visceral metastases or uncontrolled primary tumors were included when the estimated survival time was longer than 6 months. All participating centers, located in the US and internationally, had institutional review board approval, and all enrolled patients signed informed consent.
Study Design
Patients were assigned 2:1 between the SRS experimental group and the cEBRT control group using permuted-block randomization.17 There was no blinding of patients or physicians. Patients were stratified by number of metastases (1 vs 2-3), intended SRS dose (16 Gy vs 18 Gy), and tumor histologic examination results (radioresistant vs other).
In the SRS group, a single dose of 16 Gy or 18 Gy was prescribed to the margin of target volume encompassing the involved vertebral bone. Prescription isodose line selection was according to institutional preference, meeting the spinal cord dose constraints defined as either 10 Gy to V10% of the partial spinal cord volume from 5 mm to 6 mm superior to 5 mm to 6 mm inferior to the target spine or 10 Gy to V0.35cc.14 In the cEBRT group, a single dose of 8 Gy, prescribed to the isocenter, was delivered to the involved vertebral level(s) including 1 additional vertebral level above and below the target spine using 2-dimensional or 3-dimensional treatment planning. Central dosimetric review of the radiation plan was performed before treatment in the first 3 SRS patients from each participating institution. The study followed Consolidated Standards of Reporting Trials Extension (CONSORT Extension) reporting guideline. This analysis includes data extracted on March 9, 2020.
Study End Points
The primary end point was patient-reported pain response at 3 months at the index vertebral level using the NRPS.18 Complete response was a pain score of 0 with no increase in narcotic pain medication and no progressive pain at the other treated spine. Partial response was an improvement of at least 3 points from the baseline pain score, with no increase in narcotic medication. Stable response was defined as the same pain score or within 2 points of the baseline pain score. Progressive response was an increase of 3 points from the baseline pain score at any treated spine level. Patients with complete or partial pain relief were considered responders. Patients with complete or partial pain relief at the index site but a progressive response at the secondary site(s) were considered nonresponders. In addition to evaluating pain for eligibility, NRPS was collected the day prior to radiation therapy, weekly for the first month, and then at 3, 6, 12, and 24 months with clinical and neurological examinations and spine MRIs.
Secondary end points included rapidity and duration of pain response, adverse events (AEs) as measured by the Common Toxicity Criteria Adverse Events, version 3.0, long-term effects on the spinal cord, and vertebral compression fractures on follow-up spine MRIs. Quality of life (QoL) was measured by the Functional Assessment of Cancer Therapy-General (FACT-G), the Brief Pain Inventory (BPI), and EuroQOL–5 Dimension (EQ-5D), collected at baseline and at 1, 3, 6, and 12 months from randomization.
Statistical Analyses
The pain response proportion for cEBRT was estimated from a subgroup of spine vertebral metastases from NRG/RTOG 9714, where the rate partial or complete pain relief was 51% at 3 months. It was hypothesized that radiosurgery would increase this percentage. Based on the 1-sided Fisher exact test with α = .025 and 2:1 randomization, 228 patients were required to detect a 40% relative improvement in the response proportion from 51% to 70% by SRS with 80% statistical power. Assuming inflation of 35% for ineligibility, death, and patient noncompliance, the target accrual was 352 patients. Differences in pain response rates between groups were tested using the Fisher exact test. All other categorical variables were compared between groups using χ2 tests, while continuous variables were compared using t tests. Sensitivity analyses were conducted for NRPS response assuming alive patients missing 3-month NRPS scores were either all responders or nonresponders, excluding pain at secondary sites and pain medication in the definition across all time points, and using a 2-point threshold in the definition of complete or partial response instead of 3 points. A proportional odds mixed effects model was run for the NRPS score using maximum likelihood estimation with adaptive quadrature, cumulative logit link function, random intercepts, and unstructured correlation structure to account for the repeated nature of the data. Similarly, mixed effects models were run for the FACT-G total score using maximum likelihood estimation and a compound symmetry correlation structure. In addition to treatment group and stratification factors, other clinically relevant baseline variables were considered for model inclusion to adjust for biases due to missing data. Multiple imputation models with 30 imputations were run using the Markov Chain Monte Carlo method to impute missing data for alive patients. Rapidity of pain relief was defined as the date from randomization to the date of pain response. Duration of pain response was defined as the date of first complete or partial response to the date of first progressive response. Overall survival and rapidity and duration of pain response were estimated using Kaplan-Meier method, with differences between arms tested using the stratified log-rank test. Cumulative incidence was used to estimate time to develop spinal cord signs on MRI with death without an event as a competing risk and between group differences tested using Gray’s test. Apart from the primary end point, all analyses used a 2-sided significance level of P < .05. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute).
Results
Enrollment
A total of 353 patients were enrolled from September 2011 to August 2017. Fourteen patients did not meet the eligibility criteria and were excluded from analysis (Figure 1). Twenty-six patients withdrew consent, but all available data were included in the analysis. A total of 339 patients (mean [SD] age of SRS group vs cEBRT group, respectively, 61.9 [13.1] years vs 63.7 [11.9] years; 114 [54.5%] male in SRS group vs 70 [53.8%] male in cEBRT group) were analyzed.
Figure 1. CONSORT Diagram.
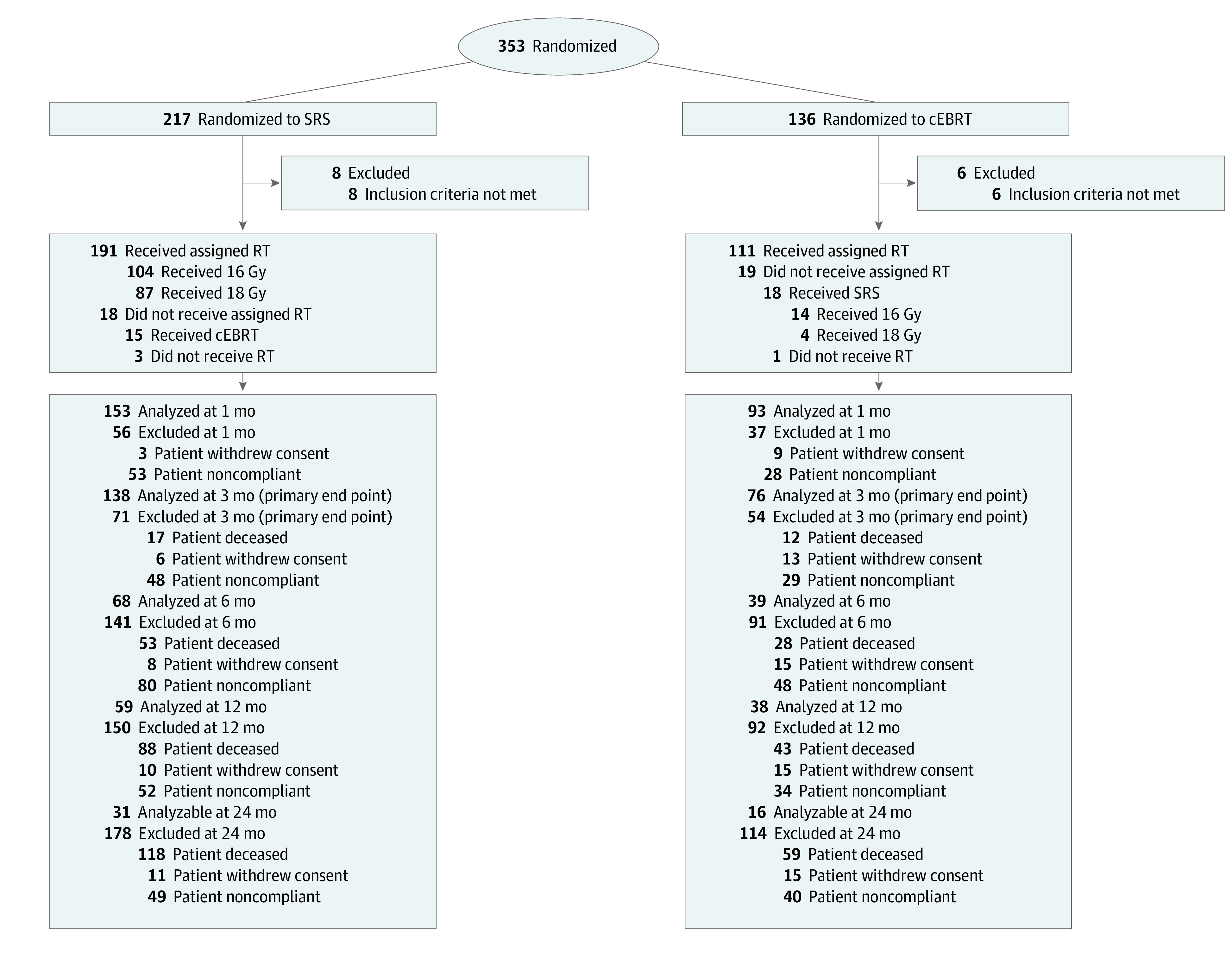
Abbreviations: cEBRT, conventional external beam radiotherapy; RT, radiation therapy; SRS, stereotactic radiosurgery.
Patient and Tumor Characteristics
Across both arms, most patients had only 1 vertebral metastasis (76.7%) and received pain medication (87.3%) (Table 1). In the SRS group, the intended dose was 16 Gy (55%) or 18 Gy (45%). There were significantly more patients with a Zubrod performance status score of 2 in the SRS group compared with the cEBRT group (22.0% vs 10.0%, P = .02). There were significantly more patients with Zubrod scores of 2 in the SRS group, whose NRPS reporting compliance was significantly lower across time compared with patients with Zubrod scores of 0 to 1 (eTable 1 in Supplement 2). Patient refusal was the most common reason for noncompliance (eTable 2 in Supplement 2). The baseline mean (SD) pain score at the index vertebra was 6.06 (2.61) in the SRS group and 5.88 (2.41) in the cEBRT group.
Table 1. Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| SRS (n = 209) | cEBRT (n = 130) | |
| Age, y | ||
| Mean (SD) [range] | 61.9 (13.1) [23-93] | 63.7 (11.9) [32-91] |
| Baseline numerical rating pain scale (NRPS) | ||
| Median (range) | 7 (5-10) | 7 (5-10) |
| Sex | ||
| Female | 95 (45.5) | 60 (46.2) |
| Male | 114 (54.5) | 70 (53.8) |
| Racea | ||
| Asian | 12 (5.7) | 3 (2.3) |
| Black or African American | 20 (9.6) | 20 (15.4) |
| White | 169 (80.9) | 101 (77.7) |
| Otherb | 2 (1.0) | 2 (1.6) |
| Not reported/unknown | 6 (2.9) | 4 (3.0) |
| Ethnicity | ||
| Hispanic or Latino | 10 (4.8) | 7 (5.4) |
| Not Hispanic or Latino | 194 (92.8) | 118 (90.8) |
| Unknown | 5 (2.4) | 5 (3.8) |
| Zubrod performance statusc | ||
| 0 | 51 (24.4) | 34 (26.2) |
| 1 | 112 (53.6) | 83 (63.8) |
| 2 | 46 (22.0) | 13 (10.0) |
| No. of spine metastases | ||
| 1 | 160 (76.6) | 100 (76.9) |
| 2-3 | 49 (23.4) | 30 (23.1) |
| Location of index spine metastasis | ||
| C1 through C7 | 9 (4.3) | 11 (8.5) |
| T1 through T12 | 101 (48.3) | 69 (53.1) |
| L1 through L5 | 99 (47.4) | 50 (38.5) |
| Pain medication, yes | 181 (86.6) | 115 (88.5) |
| Type of tumor | ||
| Radioresistant tumor | 29 (13.9) | 15 (11.5) |
| Other | 180 (86.1) | 115 (88.5) |
| Intended SRS single-fraction dose | ||
| 16 Gyd | 115 (55.0) | NA |
| 18 Gyd | 94 (45.0) | NA |
Abbreviations: cEBRT, conventional external beam radiotherapy; NA, not applicable; NRPS, Numerical Rating Pain Scale; SRS, stereotactic radiosurgery.
Race was reported by the investigator and is a requirement of the National Cancer Institute using the categories provided.
Other includes American Indian and Alaska Native (1 patient in SRS), Native Hawaiian or Other Pacific Islander (1 patient in cEBRT), and more than 1 race (1 patient in each group).
Zubrod score is a measure of performance status ranging from 0 to 4, with 0 being fully functional and asymptomatic, and 4 being bedridden.
To convert Gy to rad, multiply by 100.
Pain Response
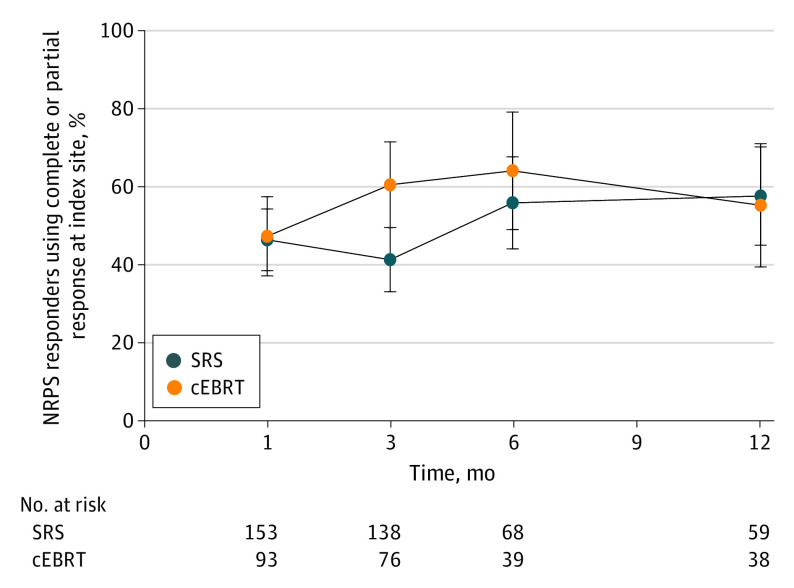
No evidence of SRS improvement over cEBRT was observed in the proportion of pain responses at 3 months (41.3% [57 of 138] vs 60.5% [46 of 76], respectively; 1-sided P = .99; Figure 2). The estimated between-group difference in the proportions of pain response is −19 percentage points (95% CI, −32.9 to −5.5), providing evidence of materially worse SRS pain response due to the exclusion of zero in the CI and the direction being in favor of cEBRT. Sensitivity analyses for the primary end point at 3 months showed similar results between SRS and cEBRT (eTable 3 in Supplement 2). No difference was found for the mean (SD) change from baseline in pain score at the index site at 3 months (−2.98 [3.34] for SRS vs −3.83 [2.97] for cEBRT; P = .07). In patients with 2 to 3 spine sites, the mean (SD) change in pain score of the secondary index site was similar at 3 months (−1.50 [3.13] for SRS [n = 24], and −2.64 [4.72] for cEBRT [n = 11]; P = .40).
Figure 2. Numerical Rating Pain Scale Response Proportion at the Index Spine With No Increase in Pain Medication and No Progressive Pain at the Other Treated Spine.
The error bars indicate 95% CIs. Abbreviations: cEBRT, conventional external beam radiotherapy; NRPS, Numerical Rating Pain Scale; SRS, stereotactic radiosurgery.
At 12 and 24 months, 46.6% and 29.0% of alive eligible patients, respectively, completed the NRPS (eTable 2 in Supplement 2). No analyses were conducted using the 24-month time point due to low compliance. At 12 months, pain response from SRS improved and cEBRT response plateaued, without significant differences (57.6% [34 of 59] vs 55.3% [21 of 38]; P = .49) (Figure 2). The sensitivity analysis of the index spine showed no differences between groups at all time points (eTable 4 in Supplement 2). The results indicate no difference in the appearance of new metastases between SRS and cEBRT (43.5% vs 43.9%, respectively; P = .96) or progression of known metastases (34.0% vs 42.3%; P = .12). Survival rates at 12 and 24 months were 44.3% and 31.5% for the SRS group and 53.1% and 31.5% for the cEBRT group (hazard ratio = 0.91; 95% CI, 0.69-1.20; P = .51).
Modeling Pain Response
Modeling of complete case data, supported by imputed data, did not show a significant treatment effect at 3 months in favor of cEBRT (least squares mean odds ratio [OR] = 2.02; 95% CI, 0.97-4.19; P = .06; eTable 4 in Supplement 2). Modeling showed that the most predictable factor for pain control was a Zubrod score of 0, but Zubrod performance status was no longer significant on multiple imputation (OR = 0.62; 95% CI, 0.37-1.06; P = .08). Radioresistant tumors responded similarly compared with tumors with other histologic examination results in both models. There were no significant differences between treatment groups in the rapidity of or duration of pain response (eTable 6A and eTable 6B in Supplement 2).
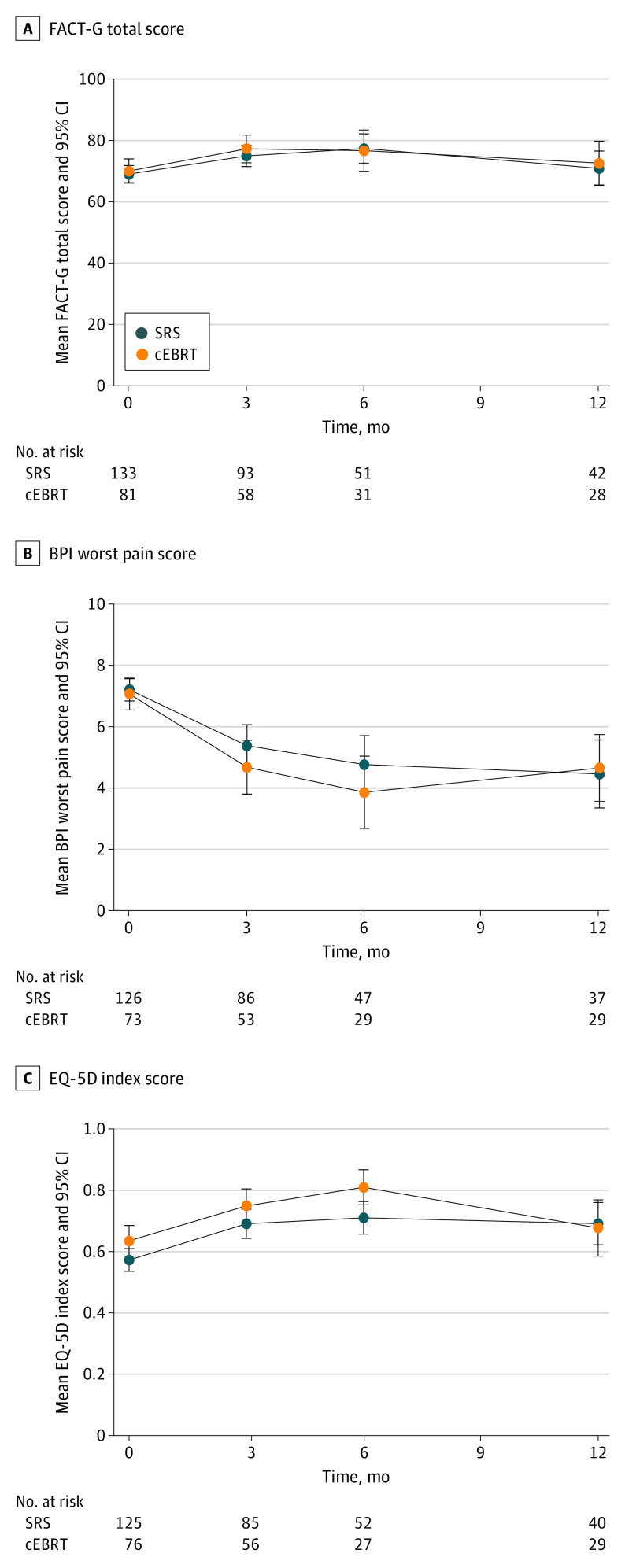
Quality of Life
No significant differences were found in change scores between groups for the FACT-G total score, EQ-5D index score, or the BPI worst pain score at any time point (Figure 3). The FACT-G total score longitudinal model did not demonstrate a difference between groups, which was confirmed on multiple imputation (eTable 7A and 7B in Supplement 2).
Figure 3. Quality of Life Over Time.
The error bars indicate 95% CIs. A, Functional Assessment of Cancer Therapy – General (FACT-G) total score. B, Brief Pain Index (BPI) worst pain score. C, EuroQol–5 Dimension (EQ-5D) index score. Abbreviations: cEBRT, conventional external beam radiotherapy; QoL, quality of life; SRS, stereotactic radiosurgery.
Adverse Events
Any grade acute AEs related to treatment within 3 months showed no differences between arms (7.7% vs 7.7%; P = .99). Similarly, no differences were seen in late effects (5.6% vs 3.4%; P = .38). There were 3 patients who experienced grade 4 late AEs related to treatment (2 on the SBRT group and 1 on the cEBRT group attributed to sepsis/lymphopenia; eTable 8 in Supplement 2). The proportion of vertebral compression fractures at 24 months was 19.5% after SRS and 21.6% after cEBRT (P = .59; eTable 9 in Supplement 2). The spinal cord signal change on MRI at 24 months was 3.6% after SRS and 1.7% after cEBRT (P = .38; Table 2). There were no clinical signs of acute or late spinal cord complications reported.
Table 2. Time to Develop Spinal Cord Signs on Magnetic Resonance Imaging.
| Mo | SRS | cEBRT | ||||
|---|---|---|---|---|---|---|
| Cumulative estimate, % (95% CI) | Cumulative events | At riska | Cumulative estimate, % (95% CI) | Cumulative events | At riska | |
| 0 | NA | 0 | 209 | NA | 0 | 130 |
| 3 | 0.5 (0.0-2.5) | 1 | 163 | 1.7 (0.3-5.4) | 2 | 96 |
| 6 | 1.5 (0.4-3.9) | 3 | 125 | 1.7 (0.3-5.4) | 2 | 76 |
| 9 | 2 (0.7-4.7) | 4 | 107 | 1.7 (0.3-5.4) | 2 | 64 |
| 12 | 2 (0.7-4.7) | 4 | 87 | 1.7 (0.3-5.4) | 2 | 58 |
| 15 | 2.5 (0.9-5.4) | 5 | 73 | 1.7 (0.3-5.4) | 2 | 44 |
| 18 | 3 (1.2-6.2) | 6 | 64 | 1.7 (0.3-5.4) | 2 | 40 |
| 21 | 3.6 (1.6-6.9) | 7 | 60 | 1.7 (0.3-5.4) | 2 | 37 |
| 24 | 3.6 (1.6-6.9) | 7 | 48 | 1.7 (0.3-5.4) | 2 | 23 |
| Total | NA | 7 of 209 | NA | NA | 2 of 130 | NA |
| P value, Gray (2-sided) | NA | NA | .38 | NA | NA | NA |
Abbreviations: cEBRT, conventional external beam radiotherapy; NA, not applicable; SRS, stereotactic radiosurgery.
Patients at risk are those without an event or competing event.
Discussion
The NRG/RTOG 0631 trial represents the first phase 2 and 3 multicenter randomized clinical trial to assess the safety and efficacy of technologically advanced SRS for the treatment of vertebral metastases. The primary phase 3 end point of improved pain relief with SRS at 3 months was not met, and the study showed improvement of cEBRT over SRS with no statistical difference. The SRS pain relief proportion was only half of the hypothesized rate used to power this trial which had been based on single institution spine SRS experiences.8,9,10,11,12 It is not clear whether this lower response can be attributed to patient selection, inclusion of occult spine metastases, or study conduct differences as compared with the other reports. The SRS treatment in this study did appear to be impactful for radioresistant tumors as there was no difference in pain control using SRS between radioresistant tumors and tumors with other histologic examination results.
In terms of patient selection, the trial eligibility was broad allowing Zubrod performance status of 0 to 2 and up to 3 noncontiguous sites of vertebral metastases. There were significantly more patients with Zubrod status of 2 in the SRS group, whose NRPS reporting compliance was significantly lower across time compared with patients with a Zubrod status of 0 to 1. This imbalance between groups may have contributed to the unexpected results, as modeling showed that the most predictable factor for pain control was a Zubrod status of 0, which was no longer significant on multiple imputation. Indeed, performance status is an important predictor of pain response after radiotherapy of bone metastases.19
Since this study opened, use of spine MRI had evolved to be standard practice for the diagnosis of small spine metastases. The RTOG 0631 trial was therefore revised to include patients with clinically occult small metastatic lesions. This may have contributed to patients experiencing symptoms at untreated adjacent spine, and the inability to explicitly discriminate the precise location of pain. Extension of tumor at the immediately adjacent spine has been reported in approximately 5% of patients after radiosurgery.20 Additionally, this study was developed before the use of the spinal instability neoplastic score (SINS) for evaluation of spine metastases.21 Therefore, the initial high pain score may have had a mechanical pain component, ultimately not responding to radiation therapy.
The choice of the primary end point for this trial (patient-reported pain improvement of at least 3 points at the index vertebral body with no increase in pain medication and no progressive pain response of any other treated vertebral metastases) may have contributed to the lower pain response in the SRS group. The International Bone Metastases Consensus Working Party defined pain response as the reduction of at least 2 points at the treated site with maximum pain cited.22 The stringent pain response criterion was chosen for this trial based on the unique clinical manifestation of painful spine metastasis that may progress to spinal cord compression if not treated properly, as well as the striking SRS pain response reported from institutional studies. The target volume was also different between RTOG 0631 treatment groups. The SRS treatment was directed to only the involved index spine, whereas cEBRT included 1 additional vertebral body above and below the target. How the inclusion of multiple sites and variation of target volumes of the treated vertebral levels affected the patient-reported pain relief results is difficult to discern.
While the study was being conducted, a randomized phase 2 trial was performed in Heidelberg, Germany comparing pain response of 24 Gy SRS vs cEBRT of 30 Gy in 10 fractions in 55 patients with 2 noncontiguous vertebral metastases excluding radioresistant tumors.23 The primary end point was pain relief of at least 2 points using the BPI at 3 months without analgesic increase. Although a significant pain response at 3 months was not demonstrated, the complete pain response proportion was 43.5% with SRS, as compared with 17.4% reported with cEBRT (P = .06). A randomized multicenter trial of Canada and Australia involving 229 patients with painful spine metastases compared 24 Gy in 2 fraction SRS vs 20 Gy in 5 fraction cEBRT.24 This study allowed treatment of 3 consecutive vertebral bodies with SRS. Using BPI–based worst pain score with at least 2 points representing improvement, the results showed a significantly superior complete pain response rate of 35% for SRS vs 14% for cEBRT at 3 months (P < .001), and 32% vs 16% at 6 months (P = .004), respectively. It is difficult to compare our trial and findings with these other studies as the measurement and definition of pain response varied.
Limitations
The SRS treatment ultimately appeared to address long-term pain relief, with complete pain relief in 61.3% at 12 months. This long-term result suggests that SRS may provide durable pain control, albeit results are subject to missing data. Indeed, the major limitation of this study is that patient compliance of pain reporting was low. At 24 months, only 47 patients completed the NRPS. This fact may cause bias, and therefore 2-year pain response is not included in the final analysis. A recent meta-analysis of SRS for spine metastases including more than 59 unique publications demonstrated 1-year local control rates of approximately 80% to 90% in the de novo setting.25
Conclusions
Notably, the RTOG 0631 randomized clinical trial was used as a proving ground for centers, including community practices, to carry out more sophisticated and technology intensive radiotherapy treatments under the guidance of rigorous quality assurance. There were no clinical or radiographic spinal cord toxic effects noted at 2 years after SRS. This finding represents a valuable prospective report of long-term spinal cord tolerance after SRS. It confirms that SRS doses of 10 Gy to V10% of the partial spinal cord volume from 5 mm to 6 mm superior to 5 mm to 6 mm inferior to the target spine and 10 Gy to V0.35cc of the spinal cord are safe to use in clinical practice.14 This information may inform future clinical trials, such as those assessing SRS for epidural tumor decompression or for spinal cord compression,26 and for treating oligometastases where durable local tumor control improves survival.27,28 Importantly, these results demonstrate prospectively, that 1-year and 2-year survival rates of patients with spine metastases treated with SRS are encouraging and underscore the need to continue to find the optimal SRS radiation dose and fractionation for patients experiencing vertebral metastases.
Trial Protocol
eTable 1. Zubrod Performance Status for Patients who Completed NRPS vs. Those Who Did Not
eTable 2. Numerical Rating Pain Scale (NRPS) Patient Compliance
eTable 3. Sensitivity Analyses of Pain Response at 3 Months
eTable 4. Change Of NRPS Pain Score And Pain Response Of The Index Spine Only
eTable 5. Repeated Measures Proportional Odds Mixed Effects Model for Ordinal NRPS Score
eTable 6. A) Rapidity of Pain Response. B) Duration of Pain Response
eTable 7. Repeated Measures Mixed Effects Model for FACT-G Total Score
eTable 8. Number of Patient with an Adverse Event by Category, Term, and Grade Definitely, Probably, or Possibly Related to Protocol Treatment
eTable 9. Time to Compression Fracture
Data sharing statement
References
- 1.Mizumoto M, Harada H, Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys. 2011;79(1):208-213. doi: 10.1016/j.ijrobp.2009.10.056 [DOI] [PubMed] [Google Scholar]
- 2.Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the Radiation Therapy Oncology Group. Cancer. 1982;50(5):893-899. doi: [DOI] [PubMed] [Google Scholar]
- 3.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804. doi: 10.1093/jnci/dji139 [DOI] [PubMed] [Google Scholar]
- 4.Bone Pain Trial Working Party . 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52(2):111-121. doi: 10.1016/S0167-8140(99)00097-3 [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol). 2012;24(2):112-124. doi: 10.1016/j.clon.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79(2):524-530. doi: 10.1016/j.ijrobp.2009.10.073 [DOI] [PubMed] [Google Scholar]
- 7.Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375. doi: 10.1200/JCO.2005.04.754 [DOI] [PubMed] [Google Scholar]
- 8.Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35(3):292-298. doi: 10.1016/j.jpainsymman.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32(2):193-199. doi: 10.1097/01.brs.0000251863.76595.a2 [DOI] [PubMed] [Google Scholar]
- 10.Degen JW, Gagnon GJ, Voyadzis JM, et al. 882 Improved pain control and stable quality of life after CyberKnife stereotactic radiosurgery of spinal tumors. Neurosurgery. 2005;57:424. doi: 10.1093/neurosurgery/57.2.424 [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484-490. doi: 10.1016/j.ijrobp.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 12.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7(2):151-160. doi: 10.3171/SPI-07/08/151 [DOI] [PubMed] [Google Scholar]
- 13.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97(8):2013-2018. doi: 10.1002/cncr.11296 [DOI] [PubMed] [Google Scholar]
- 14.Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628-636. doi: 10.1002/cncr.22442 [DOI] [PubMed] [Google Scholar]
- 15.Gerszten PC, Burton SA, Quinn AE, Agarwala SS, Kirkwood JM. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg. 2005;83(5-6):213-221. doi: 10.1159/000091952 [DOI] [PubMed] [Google Scholar]
- 16.Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4(2):76-81. doi: 10.1016/j.prro.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365-375. doi: 10.1016/0021-9681(74)90015-0 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157-162. doi: 10.1016/S0304-3959(99)00101-3 [DOI] [PubMed] [Google Scholar]
- 19.van der Velden JM, Peters M, Verlaan JJ, et al. Development and internal validation of a clinical risk score to predict pain response after palliative radiation therapy in patients with bone metastases. Int J Radiat Oncol Biol Phys. 2017;99(4):859-866. doi: 10.1016/j.ijrobp.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 20.Ryu S, Rock J, Rosenblum M, Kim JH. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg. 2004;101(suppl 3):402-405. doi: 10.3171/sup.2004.101.supplement3.0402 [DOI] [PubMed] [Google Scholar]
- 21.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35(22):E1221-E1229. doi: 10.1097/BRS.0b013e3181e16ae2 [DOI] [PubMed] [Google Scholar]
- 22.Chow E, Hoskin P, Mitera G, et al. ; International Bone Metastases Consensus Working Party . Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730-1737. doi: 10.1016/j.ijrobp.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 23.Sprave T, Verma V, Förster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128(2):274-282. doi: 10.1016/j.radonc.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 24.Sahgal A, Myrehaug SD, Siva S, et al. ; trial investigators . Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22(7):1023-1033. doi: 10.1016/S1470-2045(21)00196-0 [DOI] [PubMed] [Google Scholar]
- 25.Glicksman RM, Tjong MC, Neves-Junior WFP, et al. Stereotactic ablative radiotherapy for the management of spinal metastases: a review. JAMA Oncol. 2020;6(4):567-577. doi: 10.1001/jamaoncol.2019.5351 [DOI] [PubMed] [Google Scholar]
- 26.Ryu S, Rock J, Jain R, et al. Radiosurgical decompression of metastatic epidural compression. Cancer. 2010;116(9):2250-2257. [DOI] [PubMed] [Google Scholar]
- 27.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830-2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell KG, Farooqi A, Ludmir EB, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non–small-cell lung cancer. Clin Lung Cancer. 2020;21(1):37-46.e7. doi: 10.1016/j.cllc.2019.07.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Zubrod Performance Status for Patients who Completed NRPS vs. Those Who Did Not
eTable 2. Numerical Rating Pain Scale (NRPS) Patient Compliance
eTable 3. Sensitivity Analyses of Pain Response at 3 Months
eTable 4. Change Of NRPS Pain Score And Pain Response Of The Index Spine Only
eTable 5. Repeated Measures Proportional Odds Mixed Effects Model for Ordinal NRPS Score
eTable 6. A) Rapidity of Pain Response. B) Duration of Pain Response
eTable 7. Repeated Measures Mixed Effects Model for FACT-G Total Score
eTable 8. Number of Patient with an Adverse Event by Category, Term, and Grade Definitely, Probably, or Possibly Related to Protocol Treatment
eTable 9. Time to Compression Fracture
Data sharing statement