Abstract
Erdheim-Chester disease (ECD) is a rare form of L group histiocytosis, accounting for up to 1500 cases to date worldwide, which mainly affects men between their 5th and 7th decade of life. The most frequent manifestations are bone involvement, perirenal infiltration with an evocating appearance of “hairy kidneys”, and a “coated aorta” aspect. Lung involvement in ECD is less common and includes pleural infiltration and interstitial lung disease. Herein, we report the case of a 76-year-old woman with recurrent pleuropneumonia revealing ECD.
Keywords: Erdheim-Chester, Histiocytosis, Pleuropneumonia
1. Introduction
Erdheim-Chester disease (ECD) is a rare form of L group histiocytosis, accounting for up to 1500 cases to date worldwide, which mainly affects men between their 5th and 7th decade of life [1]. The most frequent manifestations are bone involvement, perirenal infiltration with an evocating appearance of “hairy kidneys” [2], and a “coated aorta” aspect. Lung involvement in ECD is less common and includes pleural infiltration and interstitial lung disease. Herein, we report the case of a 76-year-old woman with recurrent pleuropneumonia revealing ECD.
2. Case presentation
A 76-year-old nonsmoking woman was admitted for recurrent pleuropneumonia. She had a history of Crohn's disease treated by adalimumab, of relapsing Clostridium difficile colitis successfully treated with fecal microbiota transplantation, and of chronic myelomonocytic leukemia diagnosed five years earlier, which did not require specific treatment. She had been hospitalized nine months before admission for right lower lobe pneumonia associated with a right pleural effusion. Broncho-alveolar lavage fluid was sterile and showed no alveolitis. She had been successfully treated with levofloxacin for ten days. One week before admission, she consulted for a cough and fever. In addition, the patient complained of ongoing fatigue and night sweats for the last 2 years, and of right chest pain.
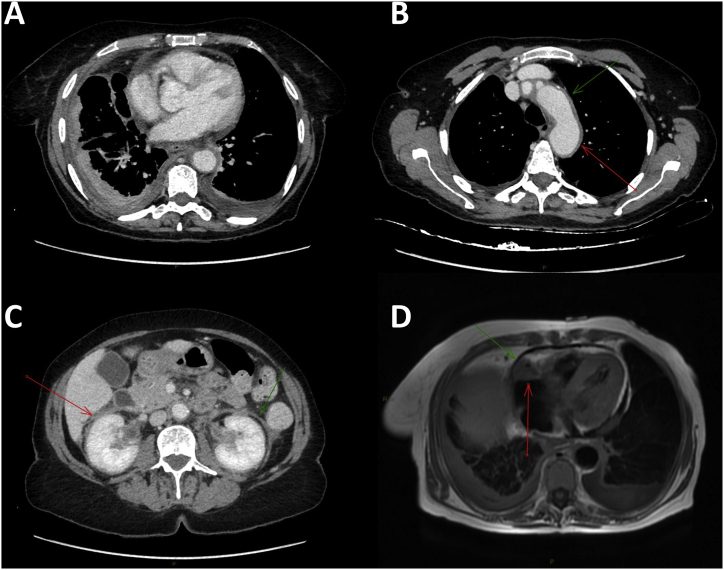
On physical examination, pulmonary auscultation revealed right basal crackles. Clinical exam was otherwise normal. Laboratory findings showed an increase monocyte counts (1.1 103 cells/mL) and an increased C-reactive protein level (43 mg/dL). Full body computed tomodensitometry (CT) scan showed a thickening of the right basal parietal pleura, a perivascular aortic infiltration and peri-renal fat infiltration with mesenteric adenomegaly (Fig. 1). A cardiac MRI showed pseudo-tumoral infiltration of the atrioventricular groove sheathing the right coronary artery (Fig. 1).
Fig. 1.
A. Computed tomodensitometry showing a thickening of the right basal parietal pleura. B. Computed tomodensitometry showing a perivascular aortic infiltration. C. Abdominal computed tomodensitometry showing a perivascular aortic infiltration (“coated aorta” aspect) and a peri-renal infiltration (“hairy kidneys” aspect). D. Cardiac magnetic resonance imaging showing a pseudo-tumoral infiltration of atrioventricular groove and a right pleural effusion.
Diagnosis of Erdheim-Chester disease (ECD) was suspected because of the history of chronic myelomonocytic leukemia, a coated aorta, a hairy kidney aspect, and a pseudo-tumoral infiltration of the atrioventricular groove. Biopsies of the peri-renal fat found CD1a- CD163+ histiocytes in a perirenal fibrosis area and the BRAFV600E mutation was evidenced, confirming the diagnosis. After evaluation in a referral center for ECD, a treatment with cobimetinib was initiated. BRAF inhibitors could not be used in this patient because a NRAS mutation was detected by a Next-Generation Sequencing technique. After six months, all symptoms had resolved, including cough, chest pain and fatigue. CT-scan showed complete regression of the right pleural effusion, however revealed splenomegaly and thoracic lymphadenopathies that were not present on previous CT-scans. Cobimetinib treatment was stopped. Biopsy sample of a lymphadenopathy confirmed the diagnosis of angioimmunoblastic T-cell lymphoma, of which the patient died one year later.
3. Discussion
Erdheim-Chester disease (ECD) is a rare form of L group histiocytosis characterized by the xanthomatous infiltration of involved tissues by foamy CD1a-, CD68+, CD163+, S100- histiocytes, in a background of inflammatory stroma [1]. It is a rare disease of unknown origin accounting for up to 1500 cases to date worldwide, which mainly affects men between their 5th and 7th decade of life [2].
ECD pathogenesis is not yet fully understood. Recently, the discovery of activating mutations of BRAF in half of ECD patients, has increased the comprehension of the disease. Mutations of BRAF in early multipotent myelomonocytic precursors or in tissue-resident histiocytes results in the activation of the RAS-RAF-MEK-ERK pathway and, therefore, an accelerated proliferation and survival of cells. ECD is thus now considered to be a disease of the monocyte/macrophage lineage. A recent study showed a prevalence of 10% of myeloid neoplasms and/or myelodysplastic syndromes in ECD, the most common being is chronic myelomonocytic leukemia [3].
Diagnosis is evocated on clinical and radiological features and confirmed by histologic findings. Some of the most striking symptoms of ECD are the long bone involvement (80–95%), with bilateral and symmetric cortical osteosclerosis, as well as the perirenal infiltration on CT scan, with an evocating appearance of “hairy kidneys” [2]. A “coated aorta” aspect, and a right atrium pseudo-tumoral infiltration, and xanthelasma are also suggestive of the diagnosis. Central nervous system involvement (including focal mass lesions, infiltration of the pituitary gland with diabetes insipidus, or retro-orbital mass) is associated with poor prognosis.
Lung involvement in ECD is less common and includes pleural infiltration and interstitial lung disease. Patients may complain of non-specific dyspnea, chest pain and/or chronic cough. Pleural involvement has been reported in 15–40% of ECD patients [4]. Unilateral or bilateral pleural effusion is the most commonly described radiological finding. Focal or diffuse thickening of the visceral pleura is less frequent. Lung parenchymal involvement occurs 30–50% of cases with an interstitial infiltration by foamy histiocytes through lymphatic spreading resulting in non-specific fissural, peribronchovascular and interlobular septal thickening or more rarely ground glass opacities, micronodules, or parenchymal condensation. Very few patients complain of persistent pulmonary symptoms, such as respiratory failure, and little change to pulmonary functional tests are generally observed. However, lung involvement has been identified as an independent poor prognostic factor [5].
Diagnosing ECD is challenging, particularly because some clinical manifestations lack specificity. Histological analysis is necessary to confirm the diagnosis and retrieve foamy histiocytic infiltration of polymorphic granuloma and fibrosis or xanthogranulomatosis, with CD68−and CD163-positive and CD1a-negative immunostaining. Tissue genetic testing should be systematic to determine the BRAF mutational status.
For many years, steroids and cytotoxic drugs (anthracyclines, vinca alkaloids and cyclophosphamide) were given as first line treatments before considering autologous hematopoietic stem cell transplantation. Interferon alpha has demonstrated clinical and radiological efficacy in ECD, but its use is limited by many side effects. Recently, targeted therapy such as the BRAF inhibitors vemurafenib and dabrafenib showed unprecedented clinical improvement [6]. Cobimetinib, a MEK inhibitor, is also a highly promising treatment, independent of the BRAF mutational status [7].
In our patient, recurrent pleuropneumonia was the only symptomatic manifestation, apart from the altered general condition. The diagnosis was suspected because of the context of chronic myelomonocytic leukemia, and the presence of other manifestations of ECD (peri-renal, aortic, and cardiac), which were asymptomatic and only revealed by imaging. This case is interesting because it shows that lung involvement can be the first manifestation of ECD.
Ethics approval and consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. All methods were carried out in accordance with CARE Guidelines.
Declaration of interests
None.
Contributors
All authors contributed to the manuscript. YK and YN wrote the first version of the manuscript. All authors critically revised and approved the final version of the manuscript.
Funding
None.
Handling Editor: DR AC Amit Chopra
References
- 1.Emile J.-F., Abla O., Fraitag S., Horne A., Haroche J., Donadieu J., et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroche J., Cohen-Aubart F., Amoura Z. Erdheim-Chester disease. Blood. 2020;135:1311–1318. doi: 10.1182/blood.2019002766. [DOI] [PubMed] [Google Scholar]
- 3.Papo M., Diamond E.L., Cohen-Aubart F., Emile J.-F., Roos-Weil D., Gupta N., et al. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130:1007–1013. doi: 10.1182/blood-2017-01-761718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun A.-L., Touitou-Gottenberg D., Haroche J., Toledano D., Cluzel P., Beigelman-Aubry C., et al. Erdheim-Chester disease: CT findings of thoracic involvement. Eur. Radiol. 2010;20:2579–2587. doi: 10.1007/s00330-010-1830-7. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Aubart F., Emile J.-F., Carrat F., Helias-Rodzewicz Z., Taly V., Charlotte F., et al. Phenotypes and survival in Erdheim-Chester disease: results from a 165-patient cohort. Am. J. Hematol. 2018;93:E114–E117. doi: 10.1002/ajh.25055. [DOI] [PubMed] [Google Scholar]
- 6.Diamond E.L., Subbiah V., Lockhart A.C., Blay J.-Y., Puzanov I., Chau I., et al. Vemurafenib for BRAF V600-mutant erdheim-chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4:384–388. doi: 10.1001/jamaoncol.2017.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen Aubart F., Emile J.-F., Maksud P., Galanaud D., Cluzel P., Benameur N., et al. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br. J. Haematol. 2018;180:150–153. doi: 10.1111/bjh.14284. [DOI] [PubMed] [Google Scholar]