Abstract
Nutrigenomics attempts to characterize and integrate the relation between dietary molecules and gene expression on a genome-wide level. One of the biologically active nutritional compounds is vitamin D3, which activates via its metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) the nuclear receptor VDR (vitamin D receptor). Vitamin D3 can be synthesized endogenously in our skin, but since we spend long times indoors and often live at higher latitudes where for many winter months UV-B radiation is too low, it became a true vitamin. The ligand-inducible transcription factor VDR is expressed in the majority of human tissues and cell types, where it modulates the epigenome at thousands of genomic sites. In a tissue-specific fashion this results in the up- and downregulation of primary vitamin D target genes, some of which are involved in attenuating oxidative stress. Vitamin D affects a wide range of physiological functions including the control of metabolism, bone formation and immunity. In this review, we will discuss how the epigenome- and transcriptome-wide effects of 1,25(OH)2D3 and its receptor VDR serve as a master example in nutrigenomics. In this context, we will outline the basis of a mechanistic understanding for personalized nutrition with vitamin D3.
Keywords: Vitamin D, VDR, Vitamin D target genes, Epigenome, Transcriptome, Immune system, Personalized nutrition
Abbreviations
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
- ACVRL1
activin A receptor like type 1
- ATAC-seq
assay for transposase-accessible chromatin using sequencing
- BRD7
bromodomain containing 7
- CALB1
calbindin 1
- CAMP
cathelicidin antimicrobial peptide
- CAR
constitutive androstane receptor
- CCN
cyclin
- CD
cluster of differentiation
- CDKN
cyclin dependent kinase inhibitor
- CEBP
CCAAT enhancer binding protein
- ChIP-seq
chromatin immunoprecipitation sequencing
- CTCF
CCCTC binding factor
- CXCL
C-X-C motif chemokine ligand
- CYP
cytochrome P450
- DHCR7
7-dehydrocholesterol reductase
- DNMT
DNA methyltransferase
- EREG
epiregulin
- ESR
estrogen receptor
- FAIRE-seq
formaldehyde-assisted identification of regulatory elements followed by sequencing
- FBP1
fructose-bisphosphatase 1
- FGF23
fibroblast growth factor 23
- FN1
fibronectin 1
- FOS
Fos proto-oncogene, AP-1 transcription factor subunit
- FXR
farnesoid X receptor
- G0S2
G0/G1 switch 2
- GABPα
GA binding protein transcription factor α
- GC
GC vitamin D binding protein
- GR
glucocorticoid receptor
- GTEx
Genotype-Tissue Expression
- HAT
histone acetyltransferase
- HBEGF
heparin binding EGF like growth factor
- HDAC
histone deacetylase
- HLA
human leukocyte antigen
- IGF1
insulin-like growth factor 1
- IL
interleukin
- INSR
insulin receptor
- JUN
Jun proto-oncogene, AP-1 transcription factor subunit
- KDM
lysine demethylase
- KMT
lysine methyltransferase
- LILRB4
leukocyte immunoglobulin like receptor B4
- LMNA
lamin A/C
- LRRC25
leucine rich repeat containing 25
- LXR
liver X receptor
- MAPK13
mitogen-activated protein kinase 13
- MHC
major histocompatibility complex
- MYC
MYC proto-oncogene, BHLH transcription factor
- NAD
nicotinamide adenine dinucleotide
- NFE2L2
NFE2 like BZIP transcription factor 2, also called NRF2
- NGS
next-generation sequencing
- NINJ1
ninjurin 1
- NK
natural killer
- PARM1
prostate androgen-regulated mucin-like protein 1
- PBMC
peripheral blood mononuclear cell
- PFKFB4
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4
- Pol II
RNA polymerase II
- PTH
parathyroid hormone
- PXR
pregnane X receptor
- RNA-seq
RNA-sequencing
- ROS
reactive oxidative species
- RXR
retinoid X receptor
- SEMA6B
semaphorin 6B
- SPI1
spleen focus forming virus proviral integration oncogene, also called PU.1
- SRGN
serglycin
- STAB1
stabilin 1
- TAD
topologically associated domain
- TET
ten-eleven translocation
- TH
T helper
- THBD
thrombomodulin
- THEMIS2
thymocyte selection associated family member 2
- Treg
T regulatory
- TREM1
triggering receptor expressed on myeloid cells 1
- TRPV6
transient receptor potential cation channel subfamily V member 6
- TSS
transcription start site
- UV
ultraviolet
- VDR
vitamin D receptor
- VKORC1
vitamin K epoxide reductase complex subunit 1
1. Introduction
Nutritional genomics, also referred to as nutrigenomics, describes the relation between what we eat and how our genome reacts to this environmental trigger [1]. Nutrigenomics developed as a discipline for the epigenome- and transcriptome-wide description of the effects of diet in health and disease. Various next-generation sequencing (NGS) methods, such as ATAC-seq (assay for transposase-accessible chromatin using sequencing), FAIRE-seq (formaldehyde-assisted identification of regulatory elements followed by sequencing), ChIP-seq (chromatin immunoprecipitation sequencing) and RNA-seq (RNA-sequencing), are based on the knowledge of the complete sequence of genome, i.e., during the past 20 years these unbiased approaches for the assessment of the effects of dietary molecules on the epigenome and transcriptome could be developed [2]. In addition, nutrigenomics uses proteomic and metabolomic methods, such as mass spectroscopy, that are independent from NGS technologies, but at present they do not allow to detect the completeness of proteins and metabolites present in an investigated cell type or tissue [3]. Importantly, most nutrigenomic approaches integrate data from different omics levels being obtained by in vitro cell culture, model organisms and human intervention studies [4].
Molecules derived from our daily diet represent the major environmental influence, to which we are voluntarily exposed to. Many of these macro- and micronutrients, such as lipids and lipophilic vitamins, do not act only as a storage of energy, but have intra- and intercellular signaling properties that control a number of physiological processes, such as cellular metabolism and growth. A key aspect of nutrigenomics is to describe and mechanistically understand the signaling pathways of nutritional molecules. Since diet is a complex mixture of hundreds to thousands of biologically active compounds, the primary focus is taken often on individual molecules. Some of these compounds have a direct effect on gene expression, while others need to be first metabolized, in order to modulate the activity of transcription factors or chromatin modifying enzymes [5]. Examples are secondary metabolites like genistein from green tea, resveratrol from red grapes and curcumin from curcuma [6]. Another interesting example is the micronutrient vitamin D3 that we can take up from certain diets, such as fatty fish, but also produce endogenously, when we expose our skin to sufficient doses of ultraviolet (UV)–B radiation [7]. Importantly, when vitamin D3 is metabolized into 1,25(OH)2D3, it acts as a high affinity ligand for the transcription factor VDR, i.e., it has direct epigenome- and transcriptome-wide effects [8].
The key physiological functions of 1,25(OH)2D3 are the regulation of calcium homeostasis, which is essential for bone mineralization, and the modulation of the immune system by stimulating innate immunity and preventing overreactions of adaptive immunity [9,10]. In addition to these major, mechanistically well understood physiological function, vitamin D was reported to be involved in numerous other processes in health and disease. For example, vitamin D is suggested to delay cellular senescence via the reduction of oxidative stress [11].
For micronutrients like vitamin D3 often the question is raised, whether their serum levels are sufficient for obtaining maximal health benefits for the individual. A related question is, if there are interindividual variations in the need for the micronutrient, i.e., whether there is a need for personalized supplementation. Accordingly, this review will not only discuss nutrigenomics of vitamin D3 on the level of the compound's mechanistic function as a regulator of gene expression but will also address the personalized responses to the micronutrient in physiological settings like responses of the immune system.
2. Vitamin D and its metabolites
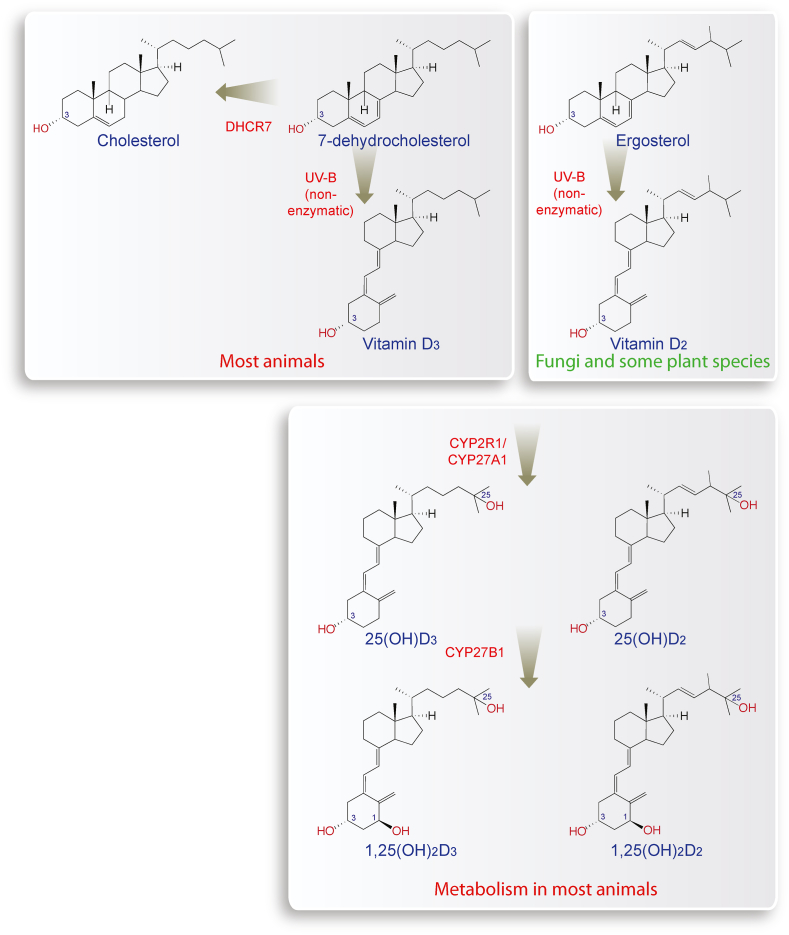
In keratinocytes of human skin, a reaction takes place that converts 7-dehydrocholesterol, which is a direct precursor of cholesterol, into pre-vitamin D3 (Fig. 1, left). The latter is a thermodynamically unstable molecule that rapidly isomerizes into vitamin D3 [12]. This reaction is non-enzymatic but requires energy provided by UV-B (290–315 nm) radiation. Interestingly, at excessive UV-B exposure pre-vitamin D3 can transform into the compounds tachysterol and lumisterol, in order not to produce too high amounts of vitamin D3 [13].
Fig. 1.
Synthesis of vitamin D3and vitamin D2. 7-dehydrocholesterol is the second last metabolite of the cholesterol biosynthesis pathway, which also reacts into vitamin D3, when it is exposed to UV-B (top left). In plants, vitamin D2 is synthesized based on ergosterol (top right). The liver enzymes CYP2R1 and CYP27A1 convert both vitamin D3 and vitamin D2 into 25(OH)D3 and 25(OH)D2, respectively (bottom). In the kidneys, CYP27B1 adds a hydroxy group to C1 of both molecules resulting in the nuclear hormones 1,25(OH)2D3 and 1,25(OH)2D2, which both activate the transcription factor VDR.
All cholesterol-producing species are able to synthesize vitamin D3, when they are exposed to sunlight of sufficient intensity. However, species living in a cholesterol-rich environment, such as blow flies and tapeworms, gave up energy and oxygen consuming cholesterol synthesis [14]. Interestingly, also UV-B-radiated plants and mushrooms produce a vitamin D isomer, but since they use the sterol ergosterol as a precursor, the outcome is vitamin D2 [15] (Fig. 1, right). In contrast to vitamins C and E, vitamin D has no scavenging function for reactive oxidative species and other free radicals. However, the absorption of UV-B by 7-dehydrocholesterol functions as a shield against radiation damage in animals and plants. Therefore, even simple eukaryotes, such as phytoplankton, synthesize vitamin D3 as a side product of a sun-shielding effect but they do not use vitamin D3 for any endocrine function [16]. Interestingly, vitamin D3 production in phytoplankton is the main reason why the molecules accumulate in the marine food chain [17].
Both vitamin D3 and vitamin D2 are biologically inert secosteroids with an open B-ring in their sterol backbone that differ only in their side chain. In human intestine, vitamin D3 is taken up more effectively [18] but both vitamin D isomers are used for supplementation and food fortification [19]. In the bloodstream both compounds (as well as their metabolites) are bound to the serum glycoprotein GC (GC vitamin D binding protein) and transported from keratinocytes (when endogenously produced) or enterocytes (when taken up by diet) to the liver [20]. The enzymes CYP2R1 (cytochrome P450 family 2 subfamily R member 1) in microsomes and CYP27A1 in mitochondria hydroxylate both vitamin D3 and vitamin D2 at C-25 leading to the pre-hormones 25-hydroxyvitamin D3 (25(OH)D3) and 25(OH)D2 [21] (Fig. 1, bottom). In proximal tubule cells of the kidneys, the enzyme CYP27B1 hydroxylates both metabolites at C-1, which creates the nuclear hormones 1,25(OH)2D3 and 1,25(OH)2D2, respectively [22,23], that bind already at a concentration of 0.1 nM to the nuclear receptor VDR [24]. In addition to 1,25(OH)2D3 production in the kidneys, cells of the innate immune system like dendritic cells, macrophages and monocytes, keratinocytes and osteoblasts express the CYP27B1 gene and can synthesize 1,25(OH)2D3 for autocrine and paracrine purposes [25].
Since the metabolite 25(OH)D3 (Fig. 1) is with a serum half-life of more than 14 days the metabolically most stable and abundant vitamin D compound [26], it is used as a biomarker indicating the individual's vitamin D status [27]. Serum concentrations of less than 50 nM 25(OH)D3 (20 ng/ml) are considered as insufficient [28], because they significantly increase the risk for musculoskeletal disorders in children (rickets) and adults (osteomalacia and fractures) [29]. Furthermore, vitamin D insufficiency contributes to a number of immunological disorders, such as multiple sclerosis [30,31], rheumatoid arthritis [32], inflammatory bowel disease [33], type I diabetes [34], and is associated with severe consequences from infections with the intracellular bacterium Mycobacterium tuberculosis [35,36], influenza virus or severe acute respiratory syndrome coronavirus type 2 [37,38]. In order to obtain a clinical benefit from these non-skeletal effects of vitamin D, the vitamin D status should be in the range of 75–100 nM (30–40 ng/ml) 25(OH)D3 [39].
During winter times in the Northern hemisphere, there is above a latitude of 38°N a period of 1–5 months, in which the UV-B component of sunlight reaching the surface is too low for vitamin D3 synthesis (“vitamin D winter”). Therefore, the migration of our species out of Africa as well as modern lifestyle characterized by predominant indoor activities [40] made vitamin D3 a micronutrient that needs to be obtained by diet or supplemented by pills. Since average human diet does not contain much fatty fish (the main source of vitamin D3 in diet [41]) or UV-B-irradiated mushrooms [42], it is low in vitamin D3 or vitamin D2. In order to prevent vitamin D deficiency, it is recommended to take at least 25 μg (1000 IU) vitamin D3 per winter day [43], but daily doses of up to 100 μg are considered to have a positive effect on health. However, caution needs to be taken, since long-term overdosing with vitamin D3 or its metabolites can cause hypercalcemia and tissue calcification [44].
In summary, vitamin D3 can be synthesized endogenously in UV-B exposed skin, but due to human migration and lifestyle changes it became a physiologically important micronutrient that needs to be taken up via diet or directly supplemented. A sufficient vitamin D status is essential for the health of our bones and immune system [28].
3. Physiological role of vitamin D and VDR
VDR is one of some 1600 transcription factors encoded by our genome, but stands out from this large family of regulatory proteins by being directly modulated in its activity by a small lipophilic molecule like 1,25(OH)2D3. This property makes VDR very comparable to the receptors ESR (estrogen receptor) and GR (glucocorticoid receptor) that have large medical impact, because they are activated by the female sex steroid estrogen and the stress hormone cortisol, respectively. All together there are only 13 classical endocrine members within the superfamily of nuclear receptors. Interestingly, VDR's closest relatives within the superfamily are the adopted orphan receptors PXR (pregnane X receptor), CAR (constitutive androstane receptor), FXR (farnesoid X receptor) and LXR (liver X receptor) α and β [45]. All six nuclear receptors bind and get activated by moderate levels of the cholesterol derivatives bile acids and/or oxysterols [[46], [47], [48], [49]]. However, only VDR learned some 550 million years ago to accommodate with high affinity 1,25(OH)2D3 [50].
The receptors FXR and LXRs are well known for the regulation of lipid metabolism pathways, while PXR and CAR control more specifically xenobiotic detoxification pathways. This suggests that very likely the evolutionary first role of VDR was the regulation of metabolic pathways, such as those controlled by CYP enzymes [51]. It is likely that in this context VDR and its ligand got an impact on attenuating oxidative stress, e.g., by modulating the expression of the NFE2L2 (NFE2 like BZIP transcription factor 2) gene, the encoded protein of which is often referred to as NRF2 [11].
One of the most prominently responding vitamin D target genes is CYP24A1, which encodes for an enzyme that initiates the degradation of 1,25(OH)2D3 and 25(OH)D3. Moreover, investigating CYP24A1 gene regulation provides molecular insight into the coordinated mechanistic actions of 1,25(OH)2D3 in the kidney that regulate mineral homeostasis [52]. In contrast, CYP27B1 encodes for an enzyme that is essential for 1,25(OH)2D3 production, while CYP19A1 is the gene of the aromatase enzyme catalyzing the last step in estrogen biosynthesis. Both genes are downregulated vitamin D targets. Other important vitamin D target genes with metabolic function are FBP1 (fructose-bisphosphatase 1) [53] and PFKFB4 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4) [54], which encode for enzymes with key functions in gluconeogenesis.
VDR became an important regulator of immunity, since both innate and adaptive cells need substantial amounts of energy for their differentiation and proliferation [55]. For example, inducing tolerogenic properties to dendritic cells requires the reprogramming of their glucose metabolism via the upregulation of PFKFB4. Vitamin D supports monocytes and macrophages in their fight against tuberculosis [56] through increasing the levels of the anti-microbial peptide CAMP (cathelicidin antimicrobial peptide) [57] or the plasma membrane-anchored glycoprotein CD14 (cluster of differentiation 14) that functions as co-receptor for Toll-like receptors [58]. In parallel, vitamin D prevents that cells of the adaptive immune system overreact. This involves the reduction of TH (T helper) 1 cell counts and the increase of Treg (T regulatory) and TH2 cells [59,60]. Most cell types of the immune system show a fast turnover, in order to quickly respond to environmental changes [61]. For example, macrophages coordinate pathways of inflammation, metabolism and general stress response via vitamin D-triggered changes of their epigenome and transcriptome. Vitamin D stimulation causes first an increase and later the resolution of inflammation [62]. Mechanistically, this is mediated by a shift of M1 into M2 macrophages [63,64].
Epigenomic programming through VDR also happens during hematopoiesis, where the receptor acts together with the pioneer transcription factors SPI1 (spleen focus forming virus proviral integration oncogene) and CEBP (CCAAT enhancer binding protein) α as key regulators of myeloid differentiation [65]. Vitamin D affects the growth of hematopoietic stem cells [66] by regulating a family of CXCL (C-X-C motif chemokine ligand) genes, which are all located in gene cluster on chromosome 4. This cluster contains the up-regulated genes CXCL1, CXCL5, CXCL7, CXCL8 and EREG (epiregulin) and the down-regulated genes CXCL9, CXCL10 and PARM1 (prostate androgen-regulated mucin-like protein 1) [9]. Further examples of immune related vitamin D target genes are ACVRL1 (activin A receptor like type 1), CD93, CEBPB, MAPK13 (mitogen-activated protein kinase 13), FN1 (fibronectin 1), NINJ1 (ninjurin 1), LRRC25 (leucine rich repeat containing 25), LILRB4 (leukocyte immunoglobulin like receptor B4), SEMA6B (semaphorin 6B), THBD (thrombomodulin), SRGN (serglycin), TREM1 (triggering receptor expressed on myeloid cells 1) and THEMIS2 (thymocyte selection associated family member 2) and, most of which encode for membrane proteins or secreted proteins [67]. In T cells VDR antagonizes the action of the transcription factors NFAT, AP1 and NFκB, so that the major growth factor for adaptive immune cells, the cytokine IL (interleukin) 2, is produced in lower amounts [68]. In dendritic cells, vitamin D inhibits their differentiation, maturation and the immuno-stimulatory capacity via the downregulation of the genes for the co-stimulatory molecules CD40, CD80 and CD86 [69]. Finally, a real “hotspot” of vitamin D targets is the cluster of HLA (human leukocyte antigen) genes encoding for major histocompatibility complex (MHC) proteins of classes I and II [9]. In total, 10 of the 12 genes that encode for both chains of MHC class II receptors are downregulated by vitamin D9. This is a central mechanism how vitamin D reduces the risk for autoimmune diseases.
Since immune and transformed cells use the same pathways for controlling their growth [66,70], 1,25(OH)2D3 and the VDR are able to inhibit cancer cell proliferation. Key vitamin D targets in cell cycle regulation are the upregulated tumor suppressor genes CDKN1A (cyclin dependent kinase inhibitor 1A) and CDKN1B, the cyclins CCNC (cyclin C), CCND1, and G0S2 (G0/G1 switch 2) and the downregulated oncogenes MYC (MYC proto-oncogene, BHLH transcription factor), JUN (Jun proto-oncogene, AP-1 transcription factor subunit), FOS (Fos proto-oncogene, AP-1 transcription factor subunit), JUND and JUNB [[71], [72], [73], [74], [75], [76], [77], [78]]. Thus, the anti-proliferative effect of 1,25(OH)2D3 and its synthetic analogues on cell lines of solid cancers and the differentiation-inducing effect on leukemia cell lines, which have been studied for 40 years [79,80], as well as the ability to induce apoptosis in many cell types, are related to vitamin D's function controlling the fate of immune cells [81]. Thus, the main effect of vitamin D against cancer is not inhibiting the growth of existing tumors but the stimulation of cytolytic T cells to detect and eliminate transformed cells already in an early stage [82].
In general, vitamin D is best known for regulating calcium homeostasis, which is essential for bone metabolism [83]. Accordingly, PTH (parathyroid hormone), FGF23 (fibroblast growth factor 23), CALB1 (calbindin 1) and TRPV6 (transient receptor potential cation channel subfamily V member 6) are key vitamin D targets genes encoding for proteins with impact on calcium metabolism and bone turnover [84]. In the kidneys, there is an interesting regulatory network between 1,25(OH)2D3, PTH and FGF23, in which vitamin D downregulates PTH but upregulates FGF23, while both PTH and FGF23 inhibit 1,25(OH)2D3 synthesis by downregulating CYP27B1 gene expression [85,86]. Since 1,25(OH)2D3 is primarily synthesized in the kidney, PTH is produced in the parathyroid gland and FGF23 in bone, in this metabolically important regulatory network vitamin D cannot be replaced by other regulatory molecules. This explains why vitamin D deficiency has primarily a bone dysfunction phenotype [87]. Moreover, the regulatory network is even further extended by the finding that insulin and IGF1 (insulin-like growth factor 1) downregulate FGF23 production [88]. This is further complicated by the observation that the INSR (insulin receptor) gene is upregulated by 1,25(OH)2D3 [89]. Thus, vitamin D signaling has several connections with insulin signaling and may provide a hint how vitamin D deficiency may increase the risk for type 2 diabetes and the metabolic syndrome [90,91].
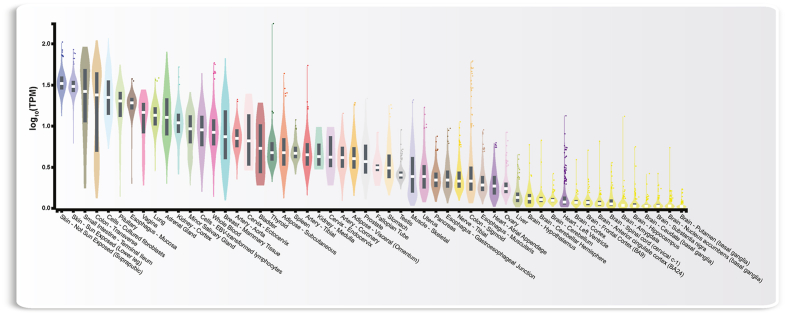
An alternative approach to judge the physiological impact of VDR and its ligand 1,25(OH)2D3 is to compare the expression of the VDR gene in various human tissues and cell types. At present, the best source of such data is the big biology project GTEx (Genotype-Tissue Expression, https://gtexportal.org), through which gene expression data from 54 tissues obtained from 948 post-mortem donors are available [92] (Fig. 2). Interestingly, highest VDR expression is found in tissues of vitamin D3 production (skin) and resorption (small intestine), while lowest levels of VDR mRNA is found in different regions of the brain. Between these extremes basically all investigated tissues show intermediate VDR expression. These data suggest that 1,25(OH)2D3 should have an impact on the physiology of most human tissues and cell types.
Fig. 2.
Expression of the VDR gene in 54 different human tissues. Normalized RNA-seq data are shown in TPM (transcripts per million) and sorted by descending tissue expression. Box plots display the median as well as 25th and 75th percentiles. Points indicate outliers that are 1.5 times above or below interquartile range. Data are based on GTEx analysis release V8 (dbGaP Accession phs000424.v8.p2) accessed on January 29, 2023 [92].
Taken together, the transcription factor VDR is the only high affinity target of 1,25(OH)2D3. This suggests that the functional profile of VDR and vitamin D are nearly identical showing pleiotropic actions related to metabolism, in particular calcium homeostasis, as well as immunity, cellular growth and differentiation. Interestingly, anti-cancer actions of vitamin D are based on the same mechanisms and genes that control immune cells.
4. Impact of the epigenome
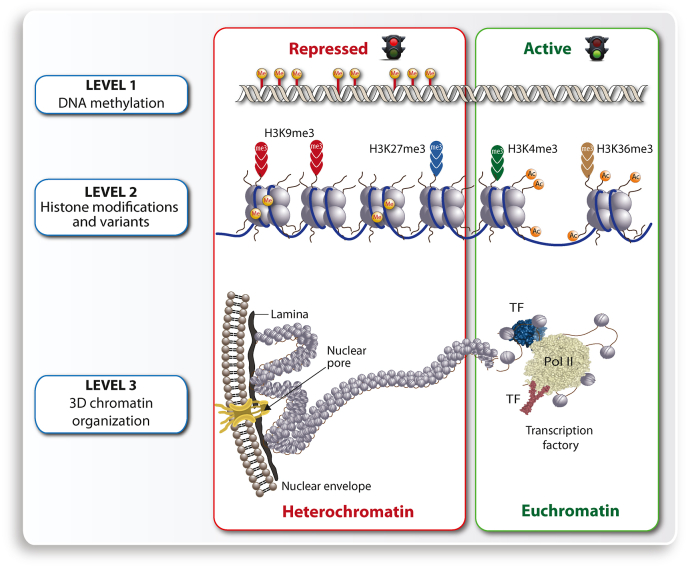
Chromatin, the three-dimensional complex of genomic DNA and nucleosome-forming histones, is the physical expression of epigenetics [93,94]. The location of regulatory regions of a gene within loosely packed chromatin (euchromatin) or densely packed chromatin (heterochromatin) determines, if a given gene will be transcribed [95]. Thus, chromatin accessibility is the major determinant for gene expression, since it allows transcription factors to bind to enhancer regions and Pol II (RNA polymerase II) to transcription start site (TSS) regions, also referred to as core promoters. Chromatin accessibility, which can be determined by FAIRE-seq and ATAC-seq, is regulated on all three major levels of the epigenome, which are DNA methylation, post-translational histone modifications like methylation and acetylation and the 3-dimensional structure of chromatin [96] (Fig. 3). Euchromatin is found preferentially in the center of the nucleus and is composed of histone proteins that are mostly acetylated as well as of genomic DNA that has a low methylation level. In contrast, heterochromatin shows the opposite profile, i.e., it is often located close to the nuclear membrane and formed by methylated histones and highly methylated DNA [97].
Fig. 3.
Epigenetic layers. Three different layers of chromatin organization are DNA methylation (top), histone modification (center) and 3-dimensional chromatin structure (bottom). The layers represent either heterochromatin including inactive genes (left) as well as euchromatin containing active genes (right).
On the genome-wide level the collection of all epigenetic changes causes epigenomic programming of the respective tissue or cell type. Epigenomic programming events are most prominent during embryogenesis where major decision on the formation of the different tissues and organs of the embryo are taken [98]. However, epigenomic programming also occurs during differentiation of adult cells, e.g., in the lifelong replacement of cells of bone marrow, colon and skin. Importantly, epigenetic changes do not cause any alterations to the genome and are mostly reversible [99].
Many epigenetic changes are the result of signal transduction cascades that are often triggered by extracellular signals, such as growth factors, cytokines and peptide hormones. In most cases, a transient signal results only in a transient epigenetic change, but the more often a signal is repeated, the more likely it causes a persistent epigenetic change. In this way, patterns of histone modifications or DNA methylation can last for days, months or even years [100]. Thus, the epigenome is able to preserve effects of cellular perturbations as epigenetic drifts [101,102]. For example, a continuous lifestyle of healthy diet and sufficient physical activity results in different epigenomes of metabolic organs than unhealthy diet combined with low physical activity. In this context, epigenomic programming is based on positive and negative learning events and represents the long-term memory of these lifestyle decisions that may even be transferred via epigenetic memory of germ cells to the next generation [103].
The molecular mediators of epigenetic changes are chromatin modifying enzymes that add (“write”), remove (“erase”) or interpret (“read”) post-translational histone modifications or DNA methylation [104]. These are histone acetyltransferases (HATs) and lysine methyltransferases (KMTs) that add acetyl and methyl groups, respectively, to lysines of histone proteins. In contrast, histone deacetylases (HDACs) and lysines demethylases (KDMs) remove them. The methylation of genomic DNA at cytosines is performed by DNA methyltransferases (DNMTs), while TET (ten-eleven translocation) enzymes start the process of erasing the methyl groups by a cascade of oxidation reactions and the involvement of DNA repair enzymes. Interestingly, these chromatin modifying enzymes depend in their activity on intermediate metabolites, such as acetyl-CoA, NAD+ (nicotinamide adenine dinucleotide) and α-ketoglutarate [105], i.e. the redox and metabolic state of a cell has a direct effect on its chromatin and epigenome [106,107]. Accordingly, chromatin modifying enzymes function as sensors of metabolic information, i.e., if cells are in a fasting or feeding state.
In summary, chromatin accessibility is the key epigenetic determinant for the controlling gene expression. Extra- and intracellular signals, many of which derive from the exposure with nutritional molecules, are able to initiate an epigenome-wide programming process that can lead to long-term memory based on persistent chromatin changes.
5. Nutritional epigenomics at the example of vitamin D
The discipline nutritional epigenomics studies how dietary molecules affect gene expression via modulation of the epigenome [108]. Importantly, diet-induced epigenomic changes are often transient and reversible, i.e., in contrast to largely irreversible cell fate decisions during cellular differentiation they are dynamic. This insight should allow to develop strategies how appropriate lifestyle decisions can lead to healthy, disease-free aging [109]. Vitamin D affects via its nuclear receptor VDR the epigenome of many tissues and cell types and represents a master example of nutritional epigenomics [8].
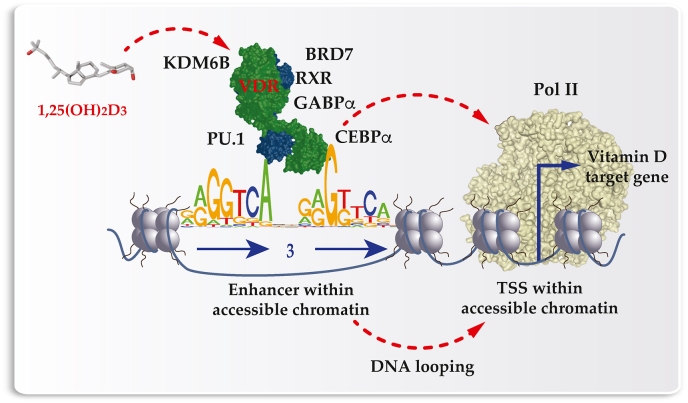
The established model of vitamin D signaling [110] suggests that VDR, like PXR, CAR, FXR, and LXR [111], is supported by RXR (retinoid X receptor) in the binding to genomic DNA. For VDR these are preferentially DR3-type response elements formed by a direct repeat of two hexameric motifs in a distance of three nucleotides (Fig. 4). The complete set of all genomic VDR binding sites, referred as VDR cistrome [112], had been determined by ChIP-seq in cell lines representing B lymphocytes [113], monocytes [53,114], colorectal cancer [71], hepatic stellate cells [115] and macrophages [116]. In the absence of ligand VDR binds in these cellular models to some 200–2000 sites, while after ligand stimulation the number increases in average 2.5-fold [116]. Since the binding of transcription factors is an epigenetic effect, the ligand-dependent VDR binding to thousands of genomic sites demonstrates epigenome-wide effect of vitamin D8.
Fig. 4.
Principles of vitamin D signaling. VDR is activated by 1,25(OH)2D3 and interacts with a number of nuclear proteins, such as RXR, PU.1, CEBPα, GABPα, KDM6B and BRD7, and with genomic regions formed by DR3-type binding sites within enhancer regions. Activated VDR bridges via Mediator complex with Pol II binding to TSS regions of vitamin D target genes. In net effect, the expression of the target genes is up- or downregulated.
The binding of 1,25(OH)2D3 to the ligand-binding domain within VDR causes a conformational change to the receptor [117]. This has the effect that VDR loses the contact with co-repressor proteins [118], but enables the binding to co-activators proteins [119]. Some of the co-factors have chromatin modifying activity themselves, whereas others act as a bridge to chromatin modifying enzymes. For example, in a ligand-dependent fashion VDR interacts with the chromatin modifying enzymes KDM6B and the chromatin remodeling protein BRD7 (bromodomain containing 7). Accordingly, vitamin D affects the histone markers H3K4me3 (active TSSs) and H3K27ac (active chromatin) [120,121]. In human monocytes, a stimulation with 1,25(OH)2D3 affects the accessibility of some 4500 chromatin loci within human monocytes, more than 500 of which are promoters and 2500 are enhancer regions, as determined by FAIRE-seq and ChIP-seq [122].
VDR can bind its preferred binding motifs when they are located within accessible chromatin, i.e., VDR is a “settler”-type of transcription factor. In contrast, “pioneer factors” [123] have response elements that are short enough to be accessed even in the presence of nucleosomes. The pioneer transcription factors SPI1 (also called PU.1), CEBPα and GABPα (GA binding protein transcription factor α) help VDR to access its genomic binding sites [121,124,125]. In turn, vitamin D stimulation has been shown to affect the binding of the pioneer factors to their genomic target regions [126]. Interestingly, also the VDR gene has been shown to be a target of epigenetic regulation [127]. This includes hypermethylation of the VDR gene promoter, in particular in the context of cancer, which leads to reduced VDR expression and responsiveness to vitamin D stimulation [128]. A downregulation of VDR expression is also observed in the context of infectious diseases, such as HIV-1 (human immunodeficiency virus 1) infection [129] and tuberculosis [130], as well as in autoimmune disorders rheumatoid arthritis [131], systemic lupus erythematosus [132] and Crohn's disease [133].
Another interesting epigenome-wide effect of vitamin D is the modulation of CTCF (CCCTC binding factor) binding at some 1300 genomic sites [134]. The chromatin organizing protein CTCF is essential in the formation of chromatin loops, which defines the borders of the more than 2000 topologically associated domains (TADs) [135], into which the human genome is subdivided. Importantly, a gene can be regulated by a transcription factor binding to an enhancer region, when the enhancer and the TSS of the gene are located within the same TAD. In this way, changes in chromatin looping have any effect on gene expression.
Taken together, vitamin D had been shown to affect the epigenome via the modulation of transcription factor binding as well as on the level of histone markers, chromatin accessibility and 3-dimensional chromatin organization.
6. Personalized response to vitamin D
The “big biology” project 1000 Genomes (www.internationalgenome.org) demonstrated that humans differ from each other by some 4–5 million single nucleotide variants, 0.7 million insertions/deletions and about 1000 larger copy number variations [136]. A minor proportion of these variants contribute to the risk for common diseases or explain the responsiveness to natural and synthetic signaling molecules. For example, effectiveness of the anti-coagulant drug warfarin is determined by variants in the genes VKORC1 (vitamin K epoxide reductase complex subunit 1) and CYP2C9 [137]. Thus, there are low, mid and high responders to warfarin and suggesting different doses for the prescription of the drug.
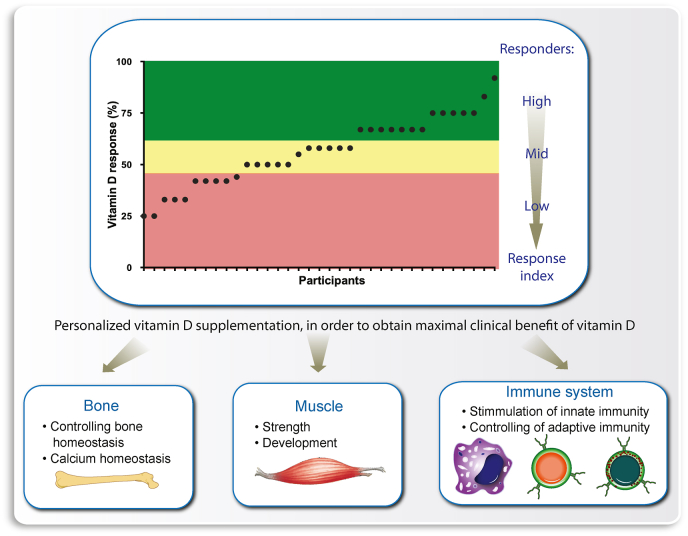
In principle, this concept seems to apply also for vitamin D [138] as suggested by the vitamin D3 intervention studies VitDmet (NCT01479933, ClinicalTrials.gov) [[139], [140], [141], [142]] and VitDbol (NCT02063334) [143,144]. Individuals were found to show a personalized reaction to vitamin D3 supplementation, which allows them to differentiate themselves into high, mid and low responders (Fig. 5). On the level of vitamin D target gene regulation and other vitamin D-triggered molecular parameters, some 25% of the investigated cohorts showed to be low responders [40]. This finding implies that low responders need a higher dose of daily vitamin D3 supplementation than suggested by population-based recommendations and guidelines. In contrast, high vitamin D responders should benefit even from a low vitamin D status and better tolerate European winters with low or no endogenous vitamin D3 production. Therefore, high vitamin D responders should suffer less frequently from infections [145], autoimmune diseases [146] and cancer [147], because vitamin D protects against these diseases (Fig. 5).
Fig. 5.
The vitamin D response index concept. Determining the vitamin D response index of an individual will allow personalized supplementation with vitamin D3, in order to obtain optimal clinical benefits, such as prevention of osteoporosis, sarcopenia and autoimmune diseases.
The molecular basis of the vitamin D response index is not yet fully understood. In analogy to the findings about warfarin, it may be primarily explained by genetic variants. In fact, variations in the genes involved in vitamin D transport and metabolism, such as GC, DHCR7 (7-dehydrocholesterol reductase), CYP2R1 and CYP24A1, can explain some of the interindividual differences in the vitamin D status [148]. For example, the UV-B-driven conversion of 7-dehydrocholesterol to vitamin D3 depends critically on DHCR7 gene expression [149] (Fig. 1). Individuals with low DHCR7 activity have more 7-dehydrocholesterol in their skin and therefore a higher level of endogenously produced vitamin D3 even at lower intensity of UV-B exposure. However, the vitamin D response index appears not depend on 25(OH)D3 serum levels of the investigated individuals, i.e., it does not depend on respective genetic variants.
Peripheral blood mononuclear cells (PBMCs) are a mixture of B and T cells, NK (natural killer) cells and monocytes, of which the latter are the most vitamin D-responsive component. A study on the dose-dependent changes of the transcriptome of PBMCs, as determined by RNA-seq, in response to the stimulation with 1,25(OH)2D3 indicated an average EC50-value of 0.48 nM for 206 vitamin D target genes [150]. However, not all vitamin D target genes respond equally, but there are high responding genes like HBEGF (heparin binding EGF like growth factor) [151] and G0S2 [72] that get activated already at 0.1 nM 1,25(OH)2D3, while genes like LMNA (lamin A/C) [152] and STAB1 (stabilin 1) [10] need concentrations of 1 nM and higher. Since ligand-dependent gene expression is an epigenetic event, the different ligand sensitivity of vitamin D target genes suggests that interindividual differences in the vitamin D response index are also based, at least to some extent on epigenetics.
In summary, nutrigenomics of vitamin D responsiveness suggests a personalized vitamin D3 supplementation advice. Moreover, a stratification of vitamin D intervention studies based on an individual's vitamin D response index may allow a better evaluation of the protective role of vitamin D on common diseases, such as cancer and cardiovascular disease [153].
7. Conclusions
Nutrition provides our body not only with molecules that serve as sources of energy [154], but some of these compounds directly communicate with our epigenome via the regulation of transcription factor and chromatin modifier activity [155]. The vitamin D and its metabolites are a special group of dietary molecules that have direct effects on gene regulation and therefore represent a master example of nutrigenomics. Vitamin D3 intervention studies represent nutrigenomic experiments, in which the action of vitamin D can be investigated under human in vivo conditions. For example, longitudinal epigenome- and transcriptome-wide analysis, such as vitamin D-triggered changes in chromatin accessibility [156] or target gene regulation [157], can be performed with PBMCs without the need of any further in vitro culture.
Vitamin D connects cellular metabolism with immunity [158,159] and has in this way pleiotropic physiological impact. The daily communication between diet and the epigenomes of metabolic organs, such as in skeletal muscle, adipose tissue, pancreas and liver, modulates gene regulatory networks that keep our body in homeostasis and prevent the onset of non-communicable diseases. Therefore, personalized vitamin D3 supplementation should be implemented in precision nutrition, in order to prevent age- and lifestyle-related diseases. This may apply in particular to disorders related to chronic inflammation.
Financial support and sponsorship
This publication is part of the WELCOME2 project that has received funding from the European Union's Horizon2020 research and innovation program under grant agreement no. 952601.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Data availability
No data was used for the research described in the article.
References
- 1.Carlberg C., Ulven S.M., Molnár F. Springer textbook; 2020. Nutrigenomics: How Science Works. [DOI] [Google Scholar]
- 2.Meyer C.A., Liu X.S. Identifying and mitigating bias in next-generation sequencing methods for chromatin biology. Nat. Rev. Genet. 2014;15:709–721. doi: 10.1038/nrg3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson J.F., et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American Heart Association. Circ Cardiovasc Genet. 2016;9:291–313. doi: 10.1161/HCG.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenech M., et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J. Nutrigenetics Nutrigenomics. 2011;4:69–89. doi: 10.1159/000327772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller M., Kersten S. Nutrigenomics: goals and strategies. Nat. Rev. Genet. 2003;4:315–322. doi: 10.1038/nrg1047. [DOI] [PubMed] [Google Scholar]
- 6.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol. Res. : off. j. Italian Pharmacol. Soc. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Bendik I., Friedel A., Roos F.F., Weber P., Eggersdorfer M. Vitamin D: a critical and essential micronutrient for human health. Front. Physiol. 2014;5:248. doi: 10.3389/fphys.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlberg C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017;453:14–21. doi: 10.1016/j.mce.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Hanel A., Carlberg C. Time-resolved gene expression analysis monitors the regulation of inflammatory mediators and attenuation of adaptive immune response by vitamin D. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malmberg H.R., Hanel A., Taipale M., Heikkinen S., Carlberg C. Vitamin D treatment sequence is critical for transcriptome modulation of immune challenged primary human cells. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.754056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sosa-Diaz E., Hernandez-Cruz E.Y., Pedraza-Chaverri J. The role of vitamin D on redox regulation and cellular senescence. Free Radic. Biol. Med. 2022;193:253–273. doi: 10.1016/j.freeradbiomed.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Tremezaygues L., et al. Cutaneous photosynthesis of vitamin D: an evolutionary highly-conserved endocrine system that protects against environmental hazards including UV-radiation and microbial infections. Anticancer Res. 2006;26:2743–2748. [PubMed] [Google Scholar]
- 13.Holick M.F. third ed. 2011. Photobiology of Vitamin D. Vitamin D; pp. 13–22. [DOI] [Google Scholar]
- 14.Capell-Hattam I.M., Brown A.J. Sterol evolution: cholesterol synthesis in animals is less a required trait than an acquired taste. Curr. Biol. 2020;30:R886–R888. doi: 10.1016/j.cub.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Jasinghe V.J., Perera C.O., Barlow P.J. Bioavailability of vitamin D2 from irradiated mushrooms: an in vivo study. Br. J. Nutr. 2005;93:951–955. doi: 10.1079/bjn20051416. [DOI] [PubMed] [Google Scholar]
- 16.Holick M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 17.Bouillon R., Suda T. Vitamin D: calcium and bone homeostasis during evolution. BoneKEy Rep. 2014;3:480. doi: 10.1038/bonekey.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripkovic L., et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006;92:33–38. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Bikle D.D., Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman A.W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 23.Bikle D., Christakos S. New aspects of vitamin D metabolism and action - addressing the skin as source and target. Nat. Rev. Endocrinol. 2020 doi: 10.1038/s41574-019-0312-5. [DOI] [PubMed] [Google Scholar]
- 24.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metabol. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Hewison M., et al. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 26.Zerwekh J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 27.Hollis B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 28.Institute-of-Medicine . National Academies Press; Washington, DC: 2011. Dietary Reference Intakes for Calcium and Vitamin D. [PubMed] [Google Scholar]
- 29.Bouillon R., et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sintzel M.B., Rametta M., Reder A.T. Vitamin D and multiple sclerosis: a comprehensive review. Neurol. Ther. 2018;7:59–85. doi: 10.1007/s40120-017-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramagopalan S.V., et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery L.E., Raza K., Hewison M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat. Rev. Rheumatol. 2016;12:201–210. doi: 10.1038/nrrheum.2015.140. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher J., Cooper S.C., Ghosh S., Hewison M. The role of vitamin D in Inflammatory bowel disease: mechanism to management. Nutrients. 2019;11 doi: 10.3390/nu11051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Infante M., et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11 doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S.J., et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des. Dev. Ther. 2017;11:91–102. doi: 10.2147/DDDT.S79870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rook G.A. The role of vitamin D in tuberculosis. Am. Rev. Respir. Dis. 1988;138:768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- 37.Charoenngam N., Shirvani A., Holick M.F. Vitamin D and its potential benefit for the COVID-19 pandemic. Endocr. Pract. : off. j. Amer. College Endocrinol. Amer Assoc. Clin Endocrinol. 2021;27:484–493. doi: 10.1016/j.eprac.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maghbooli Z., et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Pludowski P., et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Carlberg C. Molecular approaches for optimizing vitamin D supplementation. Vitam. Horm. 2016;100:255–271. doi: 10.1016/bs.vh.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z., et al. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J. Steroid Biochem. Mol. Biol. 2007;103:642–644. doi: 10.1016/j.jsbmb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urbain P., Singler F., Ihorst G., Biesalski H.K., Bertz H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur. J. Clin. Nutr. 2011;65:965–971. doi: 10.1038/ejcn.2011.53. [DOI] [PubMed] [Google Scholar]
- 43.Holick M.F., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 44.Cheskis B.J., Freedman L.P., Nagpal S. Vitamin D receptor ligands for osteoporosis. Curr. Opin. Invest. Drugs. 2006;7:906–911. [PubMed] [Google Scholar]
- 45.Krasowski M.D., Ni A., Hagey L.R., Ekins S. Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol. Cell. Endocrinol. 2011;334:39–48. doi: 10.1016/j.mce.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makishima M., et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 47.Makishima M., et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 48.Staudinger J.L., et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo G.L., et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield G.K., et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144:2704–2716. doi: 10.1210/en.2002-221101. [DOI] [PubMed] [Google Scholar]
- 51.Hanel A., Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem. Pharmacol. 2020;173 doi: 10.1016/j.bcp.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Meyer M.B., Pike J.W. Genomic mechanisms controlling renal vitamin D metabolism. J. Steroid Biochem. Mol. Biol. 2023;228 doi: 10.1016/j.jsbmb.2023.106252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heikkinen S., et al. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39:9181–9193. doi: 10.1093/nar/gkr654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanherwegen A.S., et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019;187:134–145. doi: 10.1016/j.jsbmb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Vanherwegen A.S., Gysemans C., Mathieu C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017;453:52–67. doi: 10.1016/j.mce.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Chun R.F., Liu P.T., Modlin R.L., Adams J.S., Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front. Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanoni I., Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu M., McComish B.J., Burdon K.P., Taylor B.V., Körner H. The association between vitamin D and multiple sclerosis risk: 1,25(OH)2D3 induces super-enhancers bound by VDR. Front. Immunol. 2019;10:488. doi: 10.3389/fimmu.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dankers W., Colin E.M., van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front. Immunol. 2016;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlberg C., Velleuer E. Springer Textbook; 2022. Molecular Immunology: How Science Works. [Google Scholar]
- 62.Zanoni I., Tan Y., Di Gioia M., Springstead J.R., Kagan J.C. By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity. 2017;47:697–709 e693. doi: 10.1016/j.immuni.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoeksema M.A., de Winther M.P. Epigenetic regulation of monocyte and macrophage function. Antioxidants Redox Signal. 2016;25:758–774. doi: 10.1089/ars.2016.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang S., Cai J., Li Y., Yang R. 1,25Dihydroxyvitamin D3 induces macrophage polarization to M2 by upregulating T cell Igmucin 3 expression. Mol. Med. Rep. 2019;19:3707–3713. doi: 10.3892/mmr.2019.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novershtern N., et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortes M., et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17:458–468. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koivisto O., Hanel A., Carlberg C. Key vitamin D target genes with functions in the immune system. Nutrients. 2020;12 doi: 10.3390/nu12041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeitelhofer M., et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E1678–E1687. doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barragan M., Good M., Kolls J.K. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyer M.B., Goetsch P.D., Pike J.W. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012;26:37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmer H.G., et al. Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 73.Wood R.J., Tchack L., Angelo G., Pratt R.E., Sonna L.A. DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol. Genom. 2004;17:122–129. doi: 10.1152/physiolgenomics.00002.2003. [DOI] [PubMed] [Google Scholar]
- 74.Salehi-Tabar R., et al. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18827–18832. doi: 10.1073/pnas.1210037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinkkonen L., Malinen M., Saavalainen K., Väisänen S., Carlberg C. Regulation of the human cyclin C gene via multiple vitamin D3-responsive regions in its promoter. Nucleic Acids Res. 2005;33:2440–2451. doi: 10.1093/nar/gki502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saramäki A., Banwell C.M., Campbell M.J., Carlberg C. Regulation of the human p21waf1/cip1 gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saramäki A., et al. Cyclical chromatin looping and transcription factor association on the regulatory regions of the p21 (CDKN1A) gene in response to 1α,25-dihydroxyvitamin D3. J. Biol. Chem. 2009;284:8073–8082. doi: 10.1074/jbc.M808090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toropainen S., Väisänen S., Heikkinen S., Carlberg C. The down-regulation of the human MYC gene by the nuclear hormone 1α,25-dihydroxyvitamin D3 is associated with cycling of corepressors and histone deacetylases. J. Mol. Biol. 2010;400:284–294. doi: 10.1016/j.jmb.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 79.Abe E., et al. Differentiation of mouse myeloid leukemia cells induced by 1α,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colston K., Colston M.J., Fieldsteel A.H., Feldman D. 1,25-dihydroxyvitamin D3 receptors in human epithelial cancer cell lines. Cancer Res. 1982;42:856–859. [PubMed] [Google Scholar]
- 81.Balomenos D., et al. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat. Med. 2000;6:171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Lostao L., Anel A., Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015;21:5047–5056. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 83.Doherty A.H., Ghalambor C.K., Donahue S.W. Evolutionary physiology of bone: bone metabolism in changing environments. Physiology. 2015;30:17–29. doi: 10.1152/physiol.00022.2014. [DOI] [PubMed] [Google Scholar]
- 84.van de Peppel J., van Leeuwen J.P. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014;5:137. doi: 10.3389/fphys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Driel M., van Leeuwen J. Vitamin D and bone: a story of endocrine and auto/paracrine action in osteoblasts. Nutrients. 2023;15 doi: 10.3390/nu15030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Latic N., Erben R.G. Interaction of vitamin D with peptide hormones with emphasis on parathyroid hormone, FGF23, and the renin-angiotensin-aldosterone system. Nutrients. 2022;14 doi: 10.3390/nu14235186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veldurthy V., et al. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4 doi: 10.1038/boneres.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bar L., et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23) Proc. Natl. Acad. Sci. U. S. A. 2018;115:5804–5809. doi: 10.1073/pnas.1800160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanel A., et al. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-78288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarthy K., et al. Association between vitamin D deficiency and the risk of prevalent type 2 diabetes and incident prediabetes: a prospective cohort study using data from the Irish Longitudinal Study on Ageing (TILDA) EClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melguizo-Rodriguez L., et al. Role of vitamin D in the metabolic syndrome. Nutrients. 2021;13 doi: 10.3390/nu13030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim-Hellmuth S., et al. Cell type-specific genetic regulation of gene expression across human tissues. Science. 2020;369 doi: 10.1126/science.aaz8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlberg C., Molnár F. second ed. Springer; 2016. The Impact of Chromatin. Mechanisms Of Gene Regulation; pp. 17–34. [DOI] [Google Scholar]
- 94.Michael A.K., Thoma N.H. Reading the chromatinized genome. Cell. 2021;184:3599–3611. doi: 10.1016/j.cell.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 95.Beisel C., Paro R. Silencing chromatin: comparing modes and mechanisms. Nat. Rev. Genet. 2011;12:123–135. doi: 10.1038/nrg2932. [DOI] [PubMed] [Google Scholar]
- 96.Rivera Chloe M., Ren B. Mapping human epigenomes. Cell. 2013;155:39–55. doi: 10.1016/j.cell.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hathaway N.A., et al. Dynamics and memory of heterochromatin in living cells. Cell. 2012;149:1447–1460. doi: 10.1016/j.cell.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perino M., Veenstra G.J. Chromatin control of developmental dynamics and plasticity. Dev. Cell. 2016;38:610–620. doi: 10.1016/j.devcel.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Carlberg C., Molnár F. second ed. Springer; 2016. The Epigenome. Mechanisms Of Gene Regulation; pp. 159–172. [DOI] [Google Scholar]
- 100.Li J., et al. Metabolic control of histone acetylation for precise and timely regulation of minor ZGA in early mammalian embryos. Cell Discov. 2022;8:96. doi: 10.1038/s41421-022-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su C., et al. 3D chromatin maps of the human pancreas reveal lineage-specific regulatory architecture of T2D risk. Cell Metabol. 2022;34:1394–1409 e1394. doi: 10.1016/j.cmet.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruno S., Williams R.J., Del Vecchio D. Epigenetic cell memory: the gene's inner chromatin modification circuit. PLoS Comput. Biol. 2022;18 doi: 10.1371/journal.pcbi.1009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitz-James M.H., Cavalli G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022;23:325–341. doi: 10.1038/s41576-021-00438-5. [DOI] [PubMed] [Google Scholar]
- 104.Carlberg C., Molnár F. second ed. Springer; 2016. Chromatin Modifiers. Mechanisms Of Gene Regulation; pp. 129–145. [DOI] [Google Scholar]
- 105.Gut P., Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489–498. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 106.Chen C., Wang Z., Qin Y. Connections between metabolism and epigenetics: mechanisms and novel anti-cancer strategy. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.935536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai Z., Ramesh V., Locasale J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020;21:737–753. doi: 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlberg C., Ulven S.M., Molnár F. Springer Textbook; 2016. Nutrigenomics. [Google Scholar]
- 109.Carlberg C., Molnár F. Springer; 2018. Human Epigenomics. [Google Scholar]
- 110.Pike J.W., et al. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J. Steroid Biochem. Mol. Biol. 2007;103:389–395. doi: 10.1016/j.jsbmb.2006.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mangelsdorf D.J., Evans R.M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 112.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramagopalan S.V., et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neme A., Seuter S., Carlberg C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. Biochim. Biophys. Acta. 2017;1860:952–961. doi: 10.1016/j.bbagrm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Ding N., et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuoresmäki P., Väisänen S., Neme A., Heikkinen S., Carlberg C. Patterns of genome-wide VDR locations. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molnár F., Peräkylä M., Carlberg C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J. Biol. Chem. 2006;281:10516–10526. doi: 10.1074/jbc.M513609200. [DOI] [PubMed] [Google Scholar]
- 118.Polly P., et al. VDR-Alien: a novel, DNA-selective vitamin D3 receptor-corepressor partnership. Faseb. J. 2000;14:1455–1463. doi: 10.1096/fj.14.10.1455. [DOI] [PubMed] [Google Scholar]
- 119.Herdick M., Carlberg C. Agonist-triggered modulation of the activated and silent state of the vitamin D3 receptor by interaction with co-repressors and co-activators. J. Mol. Biol. 2000;304:793–801. doi: 10.1006/jmbi.2000.4267. [DOI] [PubMed] [Google Scholar]
- 120.Nurminen V., Neme A., Seuter S., Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim. Biophys. Acta. 2018;1861:697–705. doi: 10.1016/j.bbagrm.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Nurminen V., Neme A., Seuter S., Carlberg C. Modulation of vitamin D signaling by the pioneer factor CEBPA. Biochim. Biophys. Acta. 1862:96–106. doi: 10.1016/j.bbagrm.2018.12.004. 2019. [DOI] [PubMed] [Google Scholar]
- 122.Seuter S., Neme A., Carlberg C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016;44:4090–4104. doi: 10.1093/nar/gkv1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zaret K.S., Carroll J.S. Pioneer transcription factors: establishing competence for gene expression. Gene Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seuter S., Neme A., Carlberg C., Epigenomic P.U. 1-VDR crosstalk modulates vitamin D signaling. Biochim. Biophys. Acta. 2017;1860:405–415. doi: 10.1016/j.bbagrm.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 125.Seuter S., Neme A., Carlberg C. ETS transcription factor family member GABPA contributes to vitamin D receptor target gene regulation. J. Steroid Biochem. Mol. Biol. 2018;177:46–52. doi: 10.1016/j.jsbmb.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 126.Carlberg C. Vitamin D and is target genes. Nutrients. 2022;14 doi: 10.3390/nu14071354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saccone D., Asani F., Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561:171–180. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 128.Pilon C., et al. Methylation status of vitamin D receptor gene promoter in benign and malignant adrenal tumors. Internet J. Endocrinol. 2015 doi: 10.1155/2015/375349. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chandel N., Malhotra A., Singhal P.C. Vitamin D receptor and epigenetics in HIV infection and drug abuse. Front. Microbiol. 2015;6:788. doi: 10.3389/fmicb.2015.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang C., et al. The methylation state of VDR gene in pulmonary tuberculosis patients. J. Thorac. Dis. 2017;9:4353–4357. doi: 10.21037/jtd.2017.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hussain M.Z., et al. Genetic and expression deregulation of immunoregulatory genes in rheumatoid arthritis. Mol. Biol. Rep. 2021;48:5171–5180. doi: 10.1007/s11033-021-06518-3. [DOI] [PubMed] [Google Scholar]
- 132.Sun J., Zhang S., Liu J.S., Gui M., Zhang H. Expression of vitamin D receptor in renal tissue of lupus nephritis and its association with renal injury activity. Lupus. 2019;28:290–294. doi: 10.1177/0961203319826704. [DOI] [PubMed] [Google Scholar]
- 133.Matos C., et al. Downregulation of the vitamin D receptor expression during acute gastrointestinal graft versus host disease is associated with poor outcome after allogeneic stem cell transplantation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1028850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neme A., Seuter S., Carlberg C. Vitamin D-dependent chromatin association of CTCF in human monocytes. Biochim. Biophys. Acta. 2016;1859:1380–1388. doi: 10.1016/j.bbagrm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Dixon J.R., et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Genomes Project C., et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rieder M.J., et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 138.Carlberg C., Haq A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018;175:12–17. doi: 10.1016/j.jsbmb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 139.Carlberg C., et al. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilfinger J., et al. Primary vitamin D receptor target genes as biomarkers for the vitamin D3 status in the hematopoietic system. J. Nutr. Biochem. 2014;25:875–884. doi: 10.1016/j.jnutbio.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 141.Ryynänen J., et al. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Mol. Nutr. Food Res. 2014;58:2036–2045. doi: 10.1002/mnfr.201400291. [DOI] [PubMed] [Google Scholar]
- 142.Saksa N., et al. Dissecting high from low responders in a vitamin D3 intervention study. J. Steroid Biochem. Mol. Biol. 2015;148:275–282. doi: 10.1016/j.jsbmb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 143.Vukic M., et al. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seuter S., et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017;174:314–321. doi: 10.1016/j.jsbmb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Mangin M., Sinha R., Fincher K. Inflammation and vitamin D: the infection connection. Inflamm. Res. 2014;63:803–819. doi: 10.1007/s00011-014-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Salzer J., et al. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79:2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- 147.Fleet J.C., DeSmet M., Johnson R., Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang X., et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018;9:260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prabhu A.V., Luu W., Li D., Sharpe L.J., Brown A.J. DHCR7: a vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 2016;64:138–151. doi: 10.1016/j.plipres.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 150.Hanel A., Veldhuizen C., Carlberg C. Gene-regulatory potential of 25-hydroxyvitamin D3 and D2. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.910601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Seuter S., Pehkonen P., Heikkinen S., Carlberg C. Dynamics of 1α,25-dihydroxyvitamin D-dependent chromatin accessibility of early vitamin D receptor target genes. Biochim. Biophys. Acta. 2013;1829:1266–1275. doi: 10.1016/j.bbagrm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 152.Kreienkamp R., et al. Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes. Oncotarget. 2016:30018–30031. doi: 10.18632/oncotarget.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manson J.E., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y., Michalak M., Agellon L.B. Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med. 2018;91:95–103. [PMC free article] [PubMed] [Google Scholar]
- 155.Ordovas J.M., Ferguson L.R., Tai E.S., Mathers J.C. Personalised nutrition and health. Br. Med. J. 2018;361:k2173. doi: 10.1136/bmj.k2173. bmj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carlberg C., et al. In vivo response of the human epigenome to vitamin D: a proof-of-principle study. J. Steroid Biochem. Mol. Biol. 2018;180:142–148. doi: 10.1016/j.jsbmb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 157.Neme A., et al. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019;188:71–76. doi: 10.1016/j.jsbmb.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 158.Verstuyf A., Carmeliet G., Bouillon R., Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 159.Carlberg C. The physiology of vitamin D-far more than calcium and bone. Front. Physiol. 2014;5:335. doi: 10.3389/fphys.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.