Abstract
Objective
To observe the clinical efficacy and safety of the short-term administration of different doses of calcium polystyrene sulfonate in the treatment of hyperkalemia in patients with stage 3–5 non-dialysis chronic kidney disease.
Methods
A prospective, open, randomized, controlled, single-center clinical observation was conducted. In total, 107 patients were randomly assigned to receive calcium polystyrene sulfonate at 15 (group A) or 30 mg/day (group B) for 1 week. Patients were assessed on days 0, 3, and 7.
Results
After 3 days of treatment, the serum potassium levels in groups A and B had decreased by 0.68 ± 0.46 and 0.75 ± 0.43 mmol/L, respectively. After 7 days, the serum potassium levels in groups A and B had decreased by 0.64 ± 0.37 and 0.94 ± 0.49 mmol/L, respectively. Conversely, serum sodium, phosphorus, and calcium levels did not significantly change during the treatment period. Constipation was the most common adverse drug reaction, and no treatment-related serious adverse events were observed.
Conclusion
Calcium polystyrene sulfonate administered at a dose of 15 or 30 g/day can rapidly reduce potassium levels in patients with stage 3–5 non-dialysis chronic kidney disease without adverse effects on sodium, phosphorus, or calcium levels.
Keywords: Hyperkalemia, chronic kidney disease, calcium polystyrene sulfonate, potassium, calcium, phosphorus, sodium
Introduction
Hyperkalemia is defined as the elevation of serum potassium levels above the normal range. The mechanisms leading to hyperkalemia typically involve a combination of factors, such as increased intake of potassium-rich foods, disordered distribution between the intracellular and extracellular compartments, and abnormalities in potassium excretion. Hyperkalemia can cause electrophysiological disorders with severe clinical repercussions that can lead to death.1,2 The elimination of potassium ions is known to occur primarily through the kidneys. Chronic kidney disease (CKD) is the most common predisposing condition for increased potassium levels.2,3 The incidence of hyperkalemia varies between 2% and 35% among patients with CKD. Hyperkalemia has been associated with increased mortality in patients with CKD. This highlights the importance of maintaining serum potassium levels in the normal range at different stages of CKD.
Treatments for hyperkalemia include calcium gluconate, insulin, sodium bicarbonate, β-adrenergic antagonists, diuretics, and/or the initiation or intensification of dialysis. However, these measures are poorly tolerated in patients with advanced CKD because of the risks of increased blood pressure and fluid retention. 4 Sodium polystyrene sulfonate is effective in the treatment of mild hyperkalemia in patients with early-stage CKD. However, this treatment takes a long time to exert its efficacy. In addition to its delayed therapeutic effect, the drug has frequent side effects, such as gastrointestinal intolerance, hypocalcemia, and magnesium deficiency. Calcium polystyrene sulfonate is another resin that exchanges calcium for potassium. It also acts in the intestine, and it is administered orally. Studies revealed that calcium polystyrene sulfonate can reduce serum potassium levels without increasing risk of gastrointestinal events.
To better understand the clinical effects of calcium polystyrene sulfonate in reducing potassium levels in the short term, this study aimed to observe the clinical efficacy and safety of calcium polystyrene sulfonate in the treatment of hyperkalemia in patients with stage 3–5 non-dialysis CKD.
Materials and methods
Experimental design
This was a prospective, open, randomized, controlled, single-center clinical trial. The study protocol was approved by the Ethics Committee of Jinling Hospital (approval number: 2014ZFYJ-002) and registered with the Chinese Clinical Trials Registry (registration number: ChiCTR-TRC-14004901). The reporting of this study conforms to the CONSORT 5 statement.
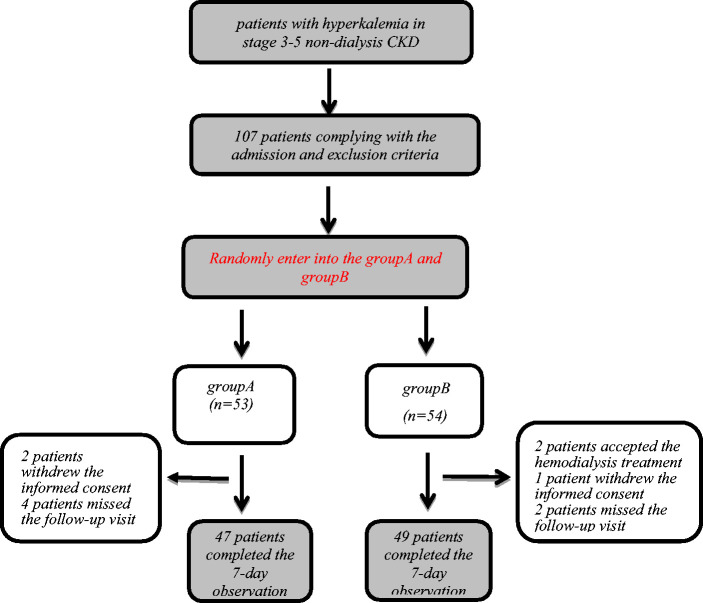
Random numbers were generated by statistical professionals using a computer, and patients were randomly assigned to group A (calcium polystyrene sulfonate 15 g/day) or B (calcium polystyrene sulfonate 30 g/day) at a 1:1 ratio. Patients were treated for 1 week. Patient visits were performed on days 0, 3, and 7 (Figure 1). Each patient was given a health education on a low-potassium diet.
Figure 1.
Study flow chart.
Subjects
Patients aged 18 to 65 years with stage 3–5 non-dialysis CKD were assessed for eligibility according to their serum potassium levels (5.0–6.0 mmol/L) as measured in their usual follow-up visits in the Outpatient Department of the National Clinical Research Center for Kidney Diseases from May 2014 to April 2020 and on the day of randomization. All patients signed a written informed consent form prior to enrollment, and the study adhered to the Declaration of Helsinki.
The exclusion criteria included severe arrhythmia or electrocardiogram findings suggestive of high sharp T waves, hypercalcemia (calcium ion concentration >2.5 mmol/L), severe metabolic acidosis (bicarbonate ion concentration/CO2 binding force ratio of less than 18 mmol/L or less than 40% volume), intestinal stenosis or alimentary ulcer, active tumors, and allergy or intolerance to calcium polystyrene sulfonate or other ion-exchange resin drugs.
Drug combination
Drugs that can cause elevated serum levels, including angiotensin-converting enzyme inhibitors(ACEIs)/angiotensin receptor blockers (ARBs)/β-receptor blockers, potassium-sparing diuretics (spironolactone, amiloride), non-steroidal anti-inflammatory drugs, potassium-containing laxatives (sodium potassium, carboxin, dietary fiber), and digoxin were not permitted during the study. However, if such drugs were prescribed prior to enrollment, their dosages and administration schedules were not changed during the trial.
Demographic data, clinical baseline characteristics, combined medication use, and adverse events were recorded.
Statistical methods
According to the literature, 6 the expected mean decrease in serum potassium levels was 1 ± 0.55 mmol/L after 3 days of treatment with calcium polystyrene sulfonate (15 g/day). Previous studies found that the ability of calcium polystyrene sulfonate to reduce potassium levels was dose-dependent. 7 A superiority test was proposed. Assuming an expected decrease of 1.3 mmol/L with a daily calcium polystyrene sulfonate dosage of 30 g, a one-sided α was 0.05, and test power (1 − β) of 0.8, the required sample size was 43 cases per group, and thus, 86 cases (43 × 2 = 86) were needed. Assuming a withdrawal rate of 20%, the total sample size was approximately 107 patients.
SPSS 20.0 software (IBM Corp., Armonk, NY, USA ) was used to evaluate the changes of serum potassium levels in each treatment group after treatment by Wilcoxon’s rank sum test. All statistical tests were two-tailed, and P < 0.05 denoted statistical significance. The safety analysis was mainly descriptive analysis. The incidence of adverse events and serious adverse events was described and calculated.
Results
Grouping and baseline characteristics of the subjects
From 1 May 2014 to 30 April 2020, patients who underwent follow-up in the Outpatient Department of the National Clinical Research Center for Kidney Disease in Jinling Hospital were recruited. Of these, 107 patients who met the enrollment criteria were recruited, including 53 patients in group A and 54 patients in group B. Meanwhile, 101 completed the day 3 observation, and 96 patients completed 7 days of treatment, including 47 patients in group A and 49 patients in group B. Eleven patients withdrew from the study for the following reasons: change in the treatment regimen, three patients; receipt of hemodialysis, two patients; and missed follow-up visits, six patients (Figure 1).
The baseline clinical characteristics of the two groups of subjects are presented in Table 1. The 107 patients included 64 men (59.8%) with an average age of 51 ± 13.4 years. Meanwhile, 89 patients also presented with hypertension, whereas 16 patients had coincident diabetes. The mean serum creatinine level was 3.31 ± 1.57 mg/dL, and the estimated glomerular filtration rate (eGFR) was 24.2 ± 13 mL/minute/1.73 m2. The mean hemoglobin level was 11.30 ± 1.92 g/dL. Fifty-four patients were treated with RAAS inhibitors, and 28 patients received β-receptor blockers for more than 2 weeks. Sixty-three patients underwent renal biopsy for the definitive diagnosis, and the cohort included 31 patients with IgA nephropathy, 16 patients with diabetic nephropathy, 7 patients with lupus nephritis, 3 patients with focal segmental glomerulosclerosis, 2 patients with purpura nephritis, 1 patient with autosomal dominant polycystic kidney, 1 patient with anti-GBM nephritis, 1 patient with POEMS syndrome, 1 patient with heavy chain deposition disease, and 44 patients with CKD of unknown cause. All patients were routinely followed up in the outpatient clinic, and all patients had stable blood glucose levels, stable blood pressure, and stable disease before enrollment.
Table 1.
Baseline clinical characteristics of the subjects.
| Total (n = 107) | Group A (n = 53) | Group B (n = 54) | P | |
|---|---|---|---|---|
| Male, n (%) | 64 (59.8) | 33 (62.3) | 31 (57.4) | 0.694 |
| Age, years | 51 ± 13.4 | 53 ± 12.9 | 49 ± 13 | 0.134 |
| Serum potassium, mmol/L | 5.57 ± 0.27 | 5.57 ± 0.26 | 5.58 ± 0.27 | 0.081 |
| CKD staging | ||||
| Stage 3 CKD, n (%) | 31 (29) | 13 (24.5) | 18 (33.3) | 0.603 |
| Stage 4 CKD, n (%) | 42 (39.3) | 22 (41.5) | 20 (37) | 0.602 |
| Stage 5 CKD, n (%) | 34 (31.8) | 18 (34) | 16 (29.6) | 0.385 |
| Serum creatinine, mg/dL | 3.31 ± 1.57 | 3.40 ± 1.55 | 3.21 ± 1.59 | 0.438 |
| eGFR, mL/minute/1.73 m2 | 24.2 ± 13 | 23.2 ± 13 | 25.2 ± 13 | 0.4 |
| Hypertension, n (%) | 89 (83.2) | 43 (81.1) | 46 (85.2) | 0.614 |
| Diabetes, n (%) | 16 (15) | 11 (20.8) | 5 (9.3) | 0.097 |
| Hemoglobin, g/dL | 11.30 ± 1.92 | 11.21 ± 2.18 | 11.39 ± 1.63 | 0.851 |
| ACEIs/ARBs, n (%) | 54 (50.5) | 22 (41.5) | 32 (59.3) | 0.068 |
| β-receptor blockers, n (%) | 28 (26.2) | 18 (34) | 10 (18.5) | 0.081 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
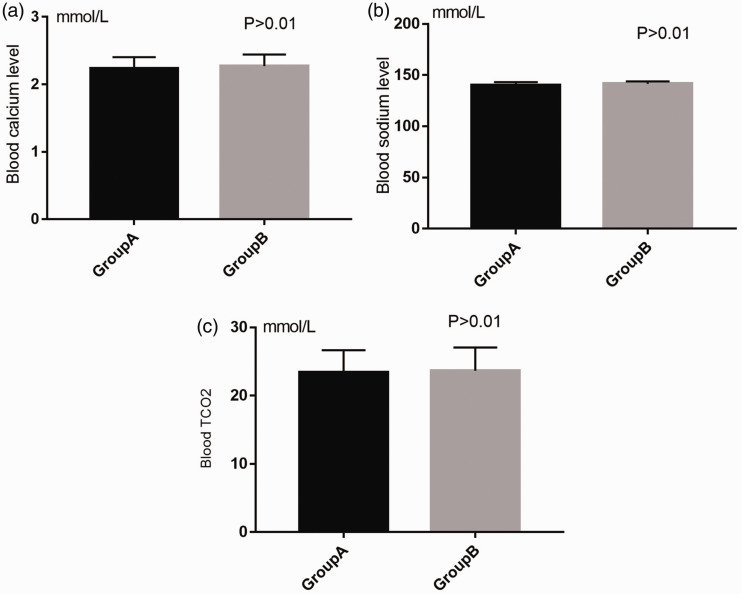
The mean serum potassium levels of the patients in groups A and B at baseline were 5.57 ± 0.26 and 5.58 ± 0.27 mmol/L, respectively (P = 0.081), and eGFR in these groups were 23.2 and 25.2 mL/minute/1.73 m2, respectively. Serum potassium, sodium, TCO2, and calcium levels did not differ between the groups at baseline (Figure 2).
Figure 2.
Comparison of potassium, sodium, total TCO2, and calcium levels at baseline between the two groups.
Curative effect analysis
Changes in serum potassium levels in the two groups after treatment
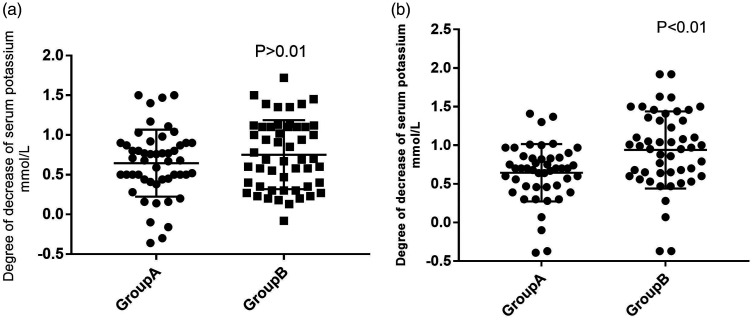
After 3 days of treatment with calcium polystyrene sulfonate, serum potassium levels were 0.68 ± 0.46 and 0.75 ± 0.43 mmol/L in groups A and B, respectively (P = 0.320). The proportions of patients whose serum potassium levels decreased to normal after 3 days of treatment were 60% and 70.6% in groups A and B, respectively, with no significance difference between the groups. The serum potassium levels of four patients (8%) were higher than the baseline level after 3 days of treatment (Table 2).
Table 2.
Comparison of curative effects in the two groups.
| Group | Group A | Group B | P |
|---|---|---|---|
| Number of included patients | 53 | 54 | |
| Serum potassium level at baseline, mmol/L | 5.57 ± 0.26 | 5.58 ± 0.27 | 0.801 |
| Number of patients who completed 3 days of treatment | 50 | 51 | |
| Decrease of serum potassium levels, mmol/L | 0.68 ± 0.46 | 0.75 ± 0.43 | 0.320 |
| Number of patients with standard serum potassium (%) | 30 (60%) | 36 (70.6%) | 0.300 |
| Number of patients who do not achieve standard serum potassium levels (%) | 20 (40%) | 15 (29.4%) | 0.300 |
| Number of patients with increased serum potassium levels versus baseline (%) | 4 (8%) | 1 (2%) | |
| Number of patients who completed 7 days of treatment | 47 | 49 | |
| Decrease of serum potassium levels, mmol/L | 0.64 ± 0.37 | 0.94 ± 0.49 | 0.001 |
| Number of patients with standard serum potassium levels (%) | 27 (57.4%) | 38 (77.6%) | 0.049 |
After 7 days of calcium polystyrene sulfonate treatment, serum potassium levels had decreased by 0.64 ± 0.37 and 0.94 ± 0.49 mmol/L in groups A and B, respectively (P = 0.001, Figure 3). The proportions of patients whose serum potassium decreased to normal after 7 days of treatment were 57.4% and 77.6% in groups A and B, respectively (P = 0.049, Table 2).
Figure 3.
The changes of serum potassium levels in the two groups after 3 and 7 days of treatment.
After 3 days of treatment, serum sodium, total TCO2, and calcium levels did not differ between the groups (Figure 4).
Figure 4.
Comparison of sodium, TCO2, and calcium levels after 3 days of treatment between the two groups.
Safety analysis
Six patients were lost to follow-up. Of the remaining 101 patients, only two patients (1.98%) developed constipation, both of whom were in group A. No serious adverse reactions or deaths related to the treatment were found.
Discussion
The total prevalence of CKD in China is as high as 10.8%, and the total number of patients is estimated to be as high as 120 million. The incidence of hyperkalemia in patients with CKD is much higher than that in patients without CKD. 8 The main causes of hyperkalemia in patients with CKD include decreased eGFR, decreased renal potassium excretion, a high potassium diet in patients with relative residual renal function, metabolic acidosis, and combined use of ACEIs/ARBs or other potassium-sparing drugs. 9 Studies have revealed that with the decrease of eGFR, the mean serum potassium levels in patients with stages 3, 4, and 5 CKD were 4.36 ± 0.49, 4.50 ± 0.55, and 4.69 ± 0.73 mmol/L, respectively. 10 Patients with hyperkalemia have no specific clinical symptoms, and the illness can manifest as muscle weakness and limb numbness, as well as fatal arrhythmias in severe cases.11,12 Appropriate prevention and timely treatment are extremely important.
At present, emergency treatments for severe hyperkalemia include calcium gluconate, insulin, sodium bicarbonate, β-adrenergic antagonists, diuretics, and dialysis. It is worth noting that these measures might be poorly tolerated in patients with advanced CKD because of the risk of increased blood pressure and fluid retention. 4
At the beginning of the study protocol design, exchange resins can be used (calcium polystyrene sulfonate, sodium polystyrene sulfonate). Sodium polystyrene sulfonate is a resin that exchanges sodium for potassium, calcium, and ammonia and acts on the distal portion of the colon.
Potassium-lowering sodium ion exchange resin was first used in the clinic in 1953, and it has been widely used in clinics in recent years. Clinical trials illustrated that this resin is effective in the treatment of mild hyperkalemia in patients with early-stage CKD. However, it has side effects, such as gastrointestinal intolerance, hypocalcemia, magnesium deficiency, and intestinal necrosis. One study found that patients with CKD and eGFR <30 mL/minute who used sodium polystyrene sulfonate had a higher risk of gastrointestinal events, including gastrointestinal hemorrhage. 13
Calcium polystyrene sulfonate (trade name, Kalimate) is another resin that exchanges calcium for potassium. It acts in the intestine, and it is administered orally. Similar to sodium polystyrene sulfonate, calcium polystyrene sulfonate is a resin that acts in the colon, exchanging potassium for calcium, and its efficacy in patients with hyperkalemia has been proven.14,15 The main side effect is constipation. Sodium polystyrene sulfonate did not affect serum calcium and phosphorus levels, nor did it cause serious adverse events such as sodium retention and elevated blood pressure and side effects such as functional liver and kidney lesions,16–18 making it useful for correcting calcium and phosphorus metabolism disorders in patients with kidney disease.
Our study evaluated 107 patients who were randomly assigned to receive 15 or 30 mg/day calcium polystyrene sulfonate for 1 week. Serum potassium levels were reduced by 0.68 to 0.75 mmol/L. After 7 days of treatment, the reduction of serum potassium levels and the proportion of patients whose serum potassium levels reached the standard level were higher in group B than in group A. The proportions of patients whose serum potassium reached the standard were 60% and 70.6% in groups A and B, respectively. In this study, constipation was observed in only two patients, and the incidence of adverse reactions in the gastrointestinal tract was only 1.98%, with all cases occurring in group A. This suggested that calcium polystyrene sulfonate was safe for short-term treatment, and it can be used as an effective treatment for hyperkalemia.
The limitations of our study included a lack of difference in efficacy in the two drug doses after 3 days of treatment. The reason could be that the effects of health education, low-potassium diet consumption, and potassium intake were also weaker in the short term, and thus, there was no difference in the potassium reduction effect between the two groups. Meanwhile, a dose-dependent effect was observed after 1 week of treatment, which was consistent with that reported in the literature.
In conclusion, although hyperkalemia is a metabolic complication often observed in patients with CKD that is associated with severe outcomes, calcium polystyrene sulfonate has been demonstrated to be effective and well tolerated in the reduction of potassium levels.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231167516 for Efficacy and safety of calcium polystyrene sulfonate in patients with hyperkalemia and stage 3–5 non-dialysis chronic kidney disease: a single-center randomized controlled trial by Xia Wang, Dacheng Chen, Xia Song, Jinquan Wang and Haitao Zhang in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231167516 for Efficacy and safety of calcium polystyrene sulfonate in patients with hyperkalemia and stage 3–5 non-dialysis chronic kidney disease: a single-center randomized controlled trial by Xia Wang, Dacheng Chen, Xia Song, Jinquan Wang and Haitao Zhang in Journal of International Medical Research
Acknowledgment
We are grateful to Kowa Pharmaceutical (China) Co., Ltd. for providing calcium polystyrene sulfonate. We thank Professor Hao Bao of Jinling Hospital for critically reviewing our manuscript and verifying the results.
Author contributions: Professor Haitao Zhang contributed to study conception and design and held oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. Xia Wang designed the study, prepared materials, performed data collection and analysis, and wrote and revised the article. Dacheng Chen and Xia Song performed data collection and revised the manuscript. Jinquan Wang was responsible for the provision of patients.
The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Kowa Pharmaceutical(China) Co., Ltd. provided financial support for the research, writing, and publication of this article. The funding body played no role in the collection, analysis, and interpretation of the data or the writing of this manuscript.
ORCID iD: Wang Xia https://orcid.org/0000-0003-0683-2492
Data availability statement
All data related to this case report are documented within this manuscript.
References
- 1.Hayes J, Kalantar-Zadeh K, Lu JL, et al. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract 2012; 120: c8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmar Vega L, Galabia ER, Bada da Silva J, et al. Epidemiology of hyperkalemia in chronic kidney disease. Nefrologia (Engl Ed) 2019; 39: 277–286. [DOI] [PubMed] [Google Scholar]
- 3.Cowan AC, Gharib EG, Weir MA.Advances in the management of hyperkalemia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017; 26: 235–239. [DOI] [PubMed] [Google Scholar]
- 4.Cupisti A, Brunori G, Di Iorio BR, et al. Nutritional treatment of advanced CKD: twenty consensus statements. J Nephrol 2018; 31: 457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz KF, Altman DG, Moher D.CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu G, Lin H, et al. Calcium polystyrene sulfonate in treating hyperkalemia patients with chronic kidney disease:a m ulticenter clinical study. Chinese Journal of Nephrology 2013; 29: 419–422. [Google Scholar]
- 7.Tomino Y, Yamazaki T, Shou I, et al. Dose-response to a jelly preparation of calcium polystyrene sulfonate in patients with hyperkalemia–changes in serum potassium levels with or without a RAAS inhibitor. Clin Nephrol 2007; 68: 379–385. [DOI] [PubMed] [Google Scholar]
- 8.Shemer J, Modan M, Ezra D, et al. Incidence of hyperkalemia in hospitalized patients. Isr J Med Sci 1983; 19: 659–661. [PubMed] [Google Scholar]
- 9.Palmer BF.Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351: 585–592. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh MF, Wu IW, Lee CC, et al. Higher serum potassium level associated with late stage chronic kidney disease. Chang Gung Med J 2011; 34: 418–425. [PubMed] [Google Scholar]
- 11.An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care 2012; 16: R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepin J, Shields C.Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract 2012; 14: 1–17; quiz 17–18. [PubMed] [Google Scholar]
- 13.Kim GH.Pharmacologic Treatment of Chronic Hyperkalemia in Patients with Chronic Kidney Disease. Electrolyte Blood Press 2019; 17: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panarelli NC.Drug-induced injury in the gastrointestinal tract. Semin Diagn Pathol 2014; 31: 165–175. [DOI] [PubMed] [Google Scholar]
- 15.Joo M, Bae WK, Kim NH, et al. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (Kalimate) in a uremic patient. J Korean Med Sci 2009; 24: 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nepal M, Bucaloiu ID, Norfolk ER.Hypernatremia in a patient treated with sodium polystyrene sulfonate. Int J Nephrol Renovasc Dis 2010; 3: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujino Y, Inoue Y, Onodera M, et al. [Hypokalemic myopathy with severe constipation in a patient routinely administered sodium polystyrene sulfonate and the spherical carbon adsorbent]. Chudoku Kenkyu 2013; 26: 49–53. [PubMed] [Google Scholar]
- 18.Thomas A, James BR, Landsberg D.Colonic necrosis due to oral kayexalate in a critically-ill patient. Am J Med Sci 2009; 337: 305–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231167516 for Efficacy and safety of calcium polystyrene sulfonate in patients with hyperkalemia and stage 3–5 non-dialysis chronic kidney disease: a single-center randomized controlled trial by Xia Wang, Dacheng Chen, Xia Song, Jinquan Wang and Haitao Zhang in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231167516 for Efficacy and safety of calcium polystyrene sulfonate in patients with hyperkalemia and stage 3–5 non-dialysis chronic kidney disease: a single-center randomized controlled trial by Xia Wang, Dacheng Chen, Xia Song, Jinquan Wang and Haitao Zhang in Journal of International Medical Research
Data Availability Statement
All data related to this case report are documented within this manuscript.