Highlights
-
•
The properties of gelatin film could be regulated by ultrasonic modification of the corresponding gelatin emulsion; the properties of gelatin film were related to those of gelatin emulsion.
-
•
Ultrasonic treatment could improve the properties of gelatin emulsion and the gelatin film, but excessive ultrasonic treatment would degrade their properties.
-
•
Ultrasonic treatment under the condition of 400w, 12 min, gelatin emulsion and gelatin film obtained the optimal performance.
-
•
The effect of ultrasonic treatment on gelatin film was manifested in the decrease of the film thickness, WVP, and WS, as well as the increase of TS and EAB, Tm and ΔH.
Keywords: Ultrasonic treatment, Gelatin emulsion, Regulation, Gelatin film, Correlation analysis
Abstract
Gelatin emulsion was an important process for preparing gelatin films. A gelatin film with water resistance and ductility could be prepared using gelatin emulsion, whereas the prepared gelatin film has several defects (e.g., low tensile strength and poor thermal stability). This study aimed to modify gelatin emulsion through ultrasonic treatment, then gelatin film was prepared by the modified gelatin emulsion. The results showed that: under the condition of ultrasonic treatment for 12 min at 400 w, zeta potential and viscosity of gelatin emulsion were the largest; thickness, water vapor permeability (WVP) and water solubility (WS) of corresponding gelatin film were the lowest, and the tensile strength (TS), elongation at break (EAB), denaturation temperature (Tm) and enthalpy value (ΔH) of corresponding gelatin film were the highest. The above result suggested that ultrasonic treatment can be used to prepare a gelatin film with better quality by regulating the properties of gelatin emulsion, and a certain correlation was found between the properties of gelatin emulsion and the properties of gelatin film.
1. Introduction
The mass production and use of conventional food packaging materials have caused several serious problems about energy, environmental protection and food safety over the past decades, and edible food packaging materials have aroused rising attention for their superior performance. Compared with conventional food packaging materials, edible films show the advantages as follows. 1) In general, edible film materials originate from abundant renewable resources in nature, and they can reduce human dependence on petroleum. 2) The processing and production of edible films is the deep processing and utilization of biological by-products, which is effective in saving resources and protecting the environment. 3) Edible films are easy to degrade in the environment, which is conducive to reducing environmental pollution caused by conventional packaging materials. 4) Edible films, a type of biomaterial, will not jeopardize human health. Edible film is a novel type of environmental protection packaging material. The research and development of edible films has great economic value, and it is conducive to environmental protection, such that edible film may replace petroleum-based packaging materials. Edible film materials originate from renewable and natural resources (e.g., proteins, polysaccharides, lipids, and all possible combinations) (Li et al., 2022, Li et al., 2022, Li et al., 2022).
The preparation of gelatin emulsion is critical process to the preparation of gelatin film. Gelatin is a type of protein-derived material. Gelatin film prepared from pure gelatin solution has several advantages (including high transparency, high rigidity, favorable biocompatibility, and no distinctive smell), whereas it has the disadvantages of poor water resistance, poor ductility, and being easy to dissolve in water. Gelatin film prepared with gelatin emulsion can overcome its shortcomings of poor water resistance, ductility, and water solubility. Moula Ali found that the palm oil modified-gelatin film had better ductility and water resistance (Moula Ali et al., 2019). Kim reported that the gelatin film added with cinnamon essential oil had antibacterial and antioxidant activities while exhibiting high ductility and water resistance (Kim et al., 2018). Moreover, the addition of lavender essential oil improved the antibacterial activity, ductility, and water resistance of the film (Jamróz et al., 2018). However, gelatin film modified with hydrophobic components resulted in common defects (e.g., the reduction of the tensile strength (TS) and the deterioration of the thermal stability) (Moula Ali et al., 2019). The properties of gelatin emulsion significantly affect the properties of the corresponding gelatin film. Therefore, the current research focus of gelatin based edible films is how to obtain high-quality gelatin films by adjusting the properties of gelatin emulsion.
Ultrasonic treatment of gelatin emulsion will affect gelatin protein and emulsion. On the one hand, ultrasonic treatment of gelatin protein can expand the tertiary structure of gelatin protein and promote the hydrophobic interaction of gelatin (Wang et al., 2021). On the other hand, ultrasonic treatment is a fast and effective method to form a stable emulsion, the mechanical vibration of ultrasound form an acoustic cavitation phenomenon, which makes rough droplets break into smaller droplets, reducing the size of the oil droplets in the gelatin emulsion, and enhancing the interaction between gelatin proteins (Ghosh et al., 2013). The innovative application of ultrasonic treatment to the preparation of film emulsion aims to obtain a gelatin emulsion and a gelatin film with excellent performance. Existing research has confirmed that ultrasonic treatment improves the properties of gelatin emulsion (Wang et al., 2022). However, the influence mechanism of ultrasonic treatment of gelatin emulsion on the properties of the corresponding gelatin film is still unclear, and the relationship between the properties of gelatin emulsion and the gelatin film is also unclear.
In this study, high-quality gelatin film was obtained by ultrasonic treatment of gelatin emulsion; The relationship between gelatin emulsion and gelatin film was studied by characterizing their properties, and the optimal conditions of ultrasonic treatment were determined. This study provided a solution for improving the properties of gelatin film and provided technical support to produce high-quality gelatin films.
2. Materials and methods
2.1. Materials
Tween-80 and glycerol were purchased from Sigma-Aldrich (Shanghai, China). Gelatin with 270 Bloom (Fish gelatin, Jiliding biotechnology company, Suzhou, China) was stored at 4℃. Soybean oil originated from Yihaijiali company (Shanghai, China).
2.2. Preparation of gelatin emulsion with ultrasonic treatment
8% fish gelatin (w/v, based on distilled water) dissolved in distilled water, and it was heated at 60℃ for 90 min till it completely dissolved. Moreover, 10% glycerin (w/w, based on gelatin), 50% soybean oil (w/w, based on gelatin), and 10% Tween-80 (w/w, based on gelatin) were introduced into the gelatin solution. The mixed solution was stirred with a magnetic stirrer for 3 min. Furthermore, the mixed solution was homogenized for 2 min using the homogenizer at 13 000 rpm, and gelatin emulsion before ultrasonic treatment was prepared (Li et al., 2022).
Ultrasonic equipment (JY98-IIIDN, Ningbo Xinzhi, China) was used with variable amplitude rob (Φ6). Ultrasonic frequency and ultrasonic power were set to 20 kHz and 400 w respectively; ultrasonic working time was set as working 5 s, resting 5 s, then recycling in order; the ultrasonic time of the film emulsion were 0, 3, 6, 9, 12 and 15 min, this was equipment working time. The prepared film emulsion was used to determine the properties of the film emulsion.
2.2.1. Particle size of ultrasonically treated gelatin emulsion
The particle size of the film emulsion was examined by laser granulometery (LA-950 V2, Horiba, Japan), and deionized water was used as the dispersant. The respective group of the film emulsion was examined three times to calculate the average value (Wu et al., 2022, Wu et al., 2022).
2.2.2. Zeta potential of ultrasonically treated gelatin emulsion
Zeta potential was examined by Zetasizer Nano (ZS-90, Malvern, UK). The film emulsion was diluted 500 times with the corresponding pH water solution, and the temperature was set to 25℃. The respective group of gelatin emulsion was examined three times to calculate the average value (Huang et al., 2020).
2.2.3. Viscosity of ultrasonically treated gelatin emulsion
According to the method of SOW, the viscosity of the film solution was examined by rheometer (MCR102, Anton Paar, Austria). A stainless-steel cone plate with a diameter of 60 mm (with an angel of 1° and a gap of 0.1667 mm) was selected to measure film emulsion, the range of shear rate was set to 0.1–100 s−1, the strain was 1%, and the examined temperature was 25℃ (Lin et al., 2020).
2.2.4. Protein solubility of ultrasonically treated gelatin emulsion
Gelatin emulsion dissolved with three chemical solvents (e.g., 0.6 M NaCl (A), 0.6 M NaCl + 1.5 M urea (B), 0.6 M NaCl + 8 M urea (C)) that were selected for their capacity to cleave specific bonds. Proteins were partially solubilized in the above solutions to determine the existence of hydrogen bonds (difference between B and A), and hydrophobic interactions (difference between C and B). Gelatin emulsion (1 mL) was homogenized in 10 mL of each solution with a dispersing homogenizer for 2 min. The resulting homogenates were stirred at 4 °C for 1 h and centrifuged at 10 000 rpm for 15 min. The supernatant after centrifugation was adopted to detect the protein content. The respective group of samples was examined three times to determine the average value.
2.2.5. Microstructure of ultrasonically treated gelatin emulsion
A small amount of gelatin emulsion was placed on a slide, covered with a cover glass, and its microscope morphology was found by magnifying it 40 times under an optical microscope (BX43, Olympus, Japan) (Ren et al., 2019).
2.3. Preparation of ultrasonically treated gelatin film
Film solution (8 mL) treated with different ultrasonic times was poured on the plastic culture dish (90 × 90 mm2). Gelatin film was prepared using gelatin emulsion formed in a natural state. Subsequently, the prepared gelatin film was stored at 25 ± 2 °C and 50 ± 5% RH for 48 h before detection (Liu et al., 2017).
2.3.1. Thickness of ultrasonically treated gelatin film
The thickness of gelatin film was examined by a digital electronic micrometer. Ten points were randomly selected to measure the thickness of the respective group and the average thickness was calculated. Film thickness was used to calculate the tensile strength (TS) and water vapor permeability (WVP).
Water vapor permeability (WVP) of ultrasonically treated gelatin film
WVP of gelatin film was examined according to Papadaki et al. (2022). Anhydrous CaCl2 was dried at 105℃ for 2 h, then placed in a small glass cup 27 mm wide and 25 mm deep. The gelatin film was used to seal the glass, such that the relative humidity inside the glass cup was 0%. The sealed glasses cup was put into a closed container with saturated water vapor. The initial weight of the glass cup was recorded, and the changed weight of the glass cup was examined every 1 h at the test temperature of 25℃. The water vapor transmittance of gelatin film is expressed as:
| WVP (g∙m−1∙s−1∙Pa−1) = w∙l∙A-1∙t−1∙(P2-P1)-1 |
Where w denotes the increased weight of glass (g); l represents the thickness of the sealed film (m); A is the area of the sealed film (m2); t expresses the internal time between two weight measurements (s); (P2-P1) is the difference in saturated vapor pressure between the two sides of the film at 25℃. The respective group of samples was examined 4 times to determine the average value.
Mechanical properties of gelatin film
The mechanical properties of gelatin film were examined according to ASTM D882 (ASTM, 2012). Texture Analyzer (TA. TX-plus, Stable Micro System, UK) was used to test the mechanical properties of gelatin film. The parameters of tensile strength (TS) and elongation at break (EAB) were used to evaluate the mechanical properties of the film. Gelatin film was cut into strips 20 mm wide and 50 mm long and fixed on the two tongs of the texture analyzer. The promoted force was set to 5 g, and the test speed was set to 1 mm/s. TS and EAB are calculated as follows:
| TS (MPa) = Fmax/A |
| EAB (%) = (ΔL/L0) × 100% |
where Fmax represented the max force that need to apart the film (N), A was the area of cross-section (m2), ΔL was the length of the film after stretching (mm), L0 was the length of the film before stretching (L0 = 30 mm). The respective group of samples was examined 10 times to calculate the average value.
Water solubility (WS) of gelatin film
WS of gelatin film was examined according to existing research (Sucheta et al., 2019). Films were dried at 105℃ to obtain constant weight M1, and then the films were immersed in deionized water for 24 h with constant stirring. Lastly, the immersed films were put into an incubator at 105℃ for re-drying to determine constant weight M2. Water solubility of gelatin film is written as:
| WS (%) = (M1-M2)/M1 × 100% |
where M1 denotes the initial weight of the film before immersion; M2 represents the weight of the film after immersion.
Differential scanning Calorimetry (DSC) analysis of gelatin film
According to Tongnuanchan (Tongnuanchan et al., 2015), the thermal property of gelatin film was analysed by Differential Scanning Calorimetry analyzer (Nicolet 6700, Thermo Fisher, USA), and denaturation temperature (Tm) and enthalpy value (ΔH) were used to evaluate thermal stability. The film (2–5 mg) samples were accurately weighted, put into an aluminium crucible and sealed with an aluminium crucible lid, and then put into the differential scanning calorimeter analyser carefully. An empty aluminium crucible was placed together as a reference. The range of heating temperature was set from 20℃ to 120℃, and the heating rate was 10℃/min.
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) analysis
ATR-FTIR (Nicolet 6700, Thermo Fisher, USA) was adopted to analyse the gelatin film, and the gelatin film was scanned under a crystal probe. The scanning wavelength was set to 4000–650 cm−1. The test temperature was ambient temperature (Romani et al., 2020).
Scanning electron microscope (SEM) analysis of gelatin film
The surface microstructure and cross-section microstructure of gelatin film were analyzed by SEM (S-3400N, Hitachi, Japan). The film sample was sprayed with gold to obtain better conductivity and fixed on the copper plate for scanning, and the accelerating voltage was 5 kv.
2.6. Data analysis
The data were analysed using Statistical Package for Social Science (SPSS) software. Moreover, the data were processed using variance analysis, multiple comparison analysis, and Pearson’s correlation coefficient analysis. p < 0.05 indicated a difference that achieved statistical significance. Graphs were generated using Origin software.
3. Results and discussions
3.1. Particle size of gelatin emulsion
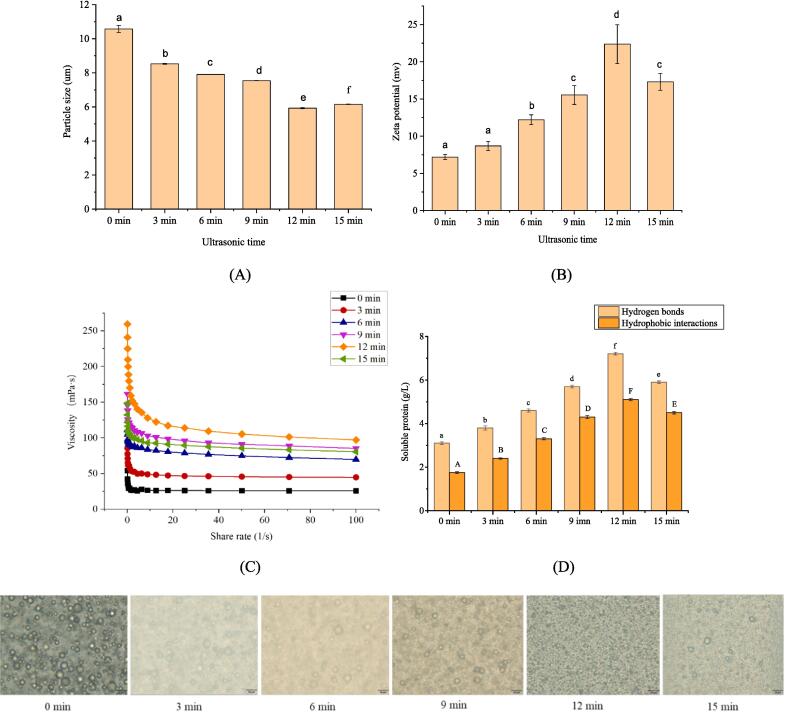
Fig. 1(A) presents the particle size of the ultrasonically treated gelatin emulsion. Ultrasonic treatment significantly affected the particle size of the oil droplets in gelatin emulsion. The particle size of oil droplets without ultrasonic treatment was the largest (10.57 μm); with the prolongation of ultrasonic time, the particle size of the oil droplets decreased first and then increased. The particle size of gelatin emulsion after ultrasonic treatment for 12 min represented the minimum particle size (5.92 μm); If the ultrasonic time reached 15 min, the particle size increased to 6.15 μm. This phenomenon that the particle size became stable or increased with the increase of the energy input could be termed the “over processing effect” (Li et al., 2022). The particle size of the oil droplet was a vital characteristic parameter since the particle size had an important impact on the performance of gelatin emulsion and corresponding gelatin film. Ultrasonic treatment had two effects on the particle size of emulsion. First, the shear force generated by cavitation caused the droplets to rupture and formed smaller droplets. Second, turbulence caused by mechanical vibration exploded dispersed phase droplets into a continuous phase, thus leading to the aggregation of droplets into larger droplets (Vankova et al., 2007). In general, the size of the oil droplets was controlled by the competition between two opposite processes. The oil droplet size was gradually reduced by ultrasonic treatment. If the ultrasonic treatment exceeded a certain limitation, the probability of collision between the oil droplets was increased, and the oil droplets gathered to increase their particle size. Under the treatment of ultrasound, the internal hydrophobic groups of gelatin protein were exposed, and the surface hydrophobicity was improved, which promoted the unfolding of gelatin protein at the oil/water interface, formed a stable network structure, enhanced the emulsification capacity of gelatin protein, thus promoting the formation of smaller particles of oil droplets, which were dispersed inside the emulsion; when the gelatin protein undergoes a longer ultrasonic treatment time, the gelatin protein aggregates and the emulsification capacity decreases. Thus, ultrasonic treatment could affect the particle size of the oil droplet in the gelatin emulsion, and the gelatin emulsion treated for 12 min achieved the smallest oil droplet size.
Fig. 1.
(A) Particle size of the gelatin emulsion treated with different ultrasonic times. (B) Zeta potential of the gelatin emulsion treated different ultrasonic times. (C) Viscosity of the gelatin emulsion treated with different ultrasonic times. (D) Soluble protein of the gelatin emulsion treated with different ultrasonic times. (E) Optical microscopy image of the gelatin emulsion treated with different ultrasonic times, and the scale line in the figure represent 20 μm.
3.2. Zeta potential of gelatin emulsion
Zeta potential of ultrasonically treated gelatin emulsion is showed in Fig. 1(B). Zeta potential without ultrasonic treatment was 7.19 mV; after ultrasonic treatment, zeta potential of gelatin emulsion increased. Zeta potential of gelatin emulsion reached the highest value (22.37 mV) under ultrasonic treatment for 12 min. If the ultrasonic time continued to increase, zeta potential of gelatin emulsion would decline. Zeta potential was related to the surface charge density of gelatin emulsion. The greater the absolute value of zeta potential, the better the stability of the solution (Yang et al., 2020). The ultrasonically treated gelatin emulsion achieved a higher zeta potential because ultrasound enhanced the structural unfolding of gelatin protein, such that more cationic groups were exposed to the surface of gelatin protein. As a result, zeta potential of gelatin emulsion was increased (Ma et al., 2019). However, if the ultrasound time was too long, gelatin protein would aggregate, thus resulting in the decreased exposure of cationic groups to the gelatin surface and the decreased zeta potential of gelatin emulsion.
3.3. Viscosity of gelatin emulsion
Viscosity of the ultrasonically treated gelatin emulsion is exhibited in Fig. 1(C). The experimental results indicated that ultrasonic treatment did not change the fluid characteristics of gelatin emulsion. η denoted the shear rate of gelatin emulsion. Ultrasonic treatment led to the increased shear rate of gelatin emulsion. At a lower shear rate, the gelatin solution had the characteristics of shear dilution, and it was non-Newtonian fluid (pseudoplastic fluid). On this basis, the gelatin emulsion had a higher apparent viscosity. The possible reason for this result is that the stable state of gelatin emulsion was not broken by lower shear pressure, such that the apparent viscosity was increased. With the increase of the shear rate, the apparent viscosity of the emulsion tended to be stable, exhibiting the characteristics of Newtonian fluid. The possible reason for the above phenomenon is that the flocculated particles in the gelatin emulsion had equal formation rate and disintegration rate, or the flocculated particles collapsed into a single liquid, so that the particles were kept in a relatively stable state (Floury et al., 2000). The value corresponding to the shear rate of 50 1/s (η50) was considered the viscosity value of gelatin emulsion (Nguyen et al., 2016). As depicted in Fig. 1(C), the viscosity of the ultrasonically treated gelatin emulsion was higher than that of non-ultrasonically treated gelatin emulsion. On the one hand, ultrasound facilitated the dispersion of large oil droplets into small oil droplets, accelerated the hydrogen bond interaction between gelatin proteins, and increased the viscosity of gelatin emulsion. On the other hand, ultrasound facilitated the unfolding of gelatin protein structure, enhanced the hydrophobic interaction, and increased the viscosity of gelatin emulsion. Under a specific ultrasonic power, the viscosity of gelatin emulsion tended to be increased with the extension of the ultrasonic time. The viscosity of gelatin emulsion was decreased when the ultrasonic time exceeded 12 min. The possible reason is that excessive ultrasound resulted in the aggregation of the oil droplets while increasing the size of the oil droplets and the blocking effect of large oil droplets on gelatin protein molecules. Furthermore, excessive ultrasound could lead to the aggregation of gelatin protein and the decrease of hydrophobic interaction (Wang et al., 2022).
3.4. Protein solubility of gelatin emulsion
Protein solubility of ultrasonically treated gelatin emulsion is displayed in Fig. 1(D). The protein solubility of gelatin emulsion in different solutions could be selected according to its ability to destroy specific types of bonds. Hydrophobic interactions and the hydrogen bond tended to be strengthened first and then weakened. Hydrophobic interactions and the hydrogen bond took on a critical significance to the stability of protein conformation. Ultrasonic treatment reduced the particle size of the oil droplets and enhanced the hydrogen bond interaction between gelatin proteins. Moreover, ultrasonic treatment unfolded the gelatin protein, exposed the buried polar groups, and facilitated the hydrophobic interaction of gelatin protein. When the ultrasonic time was extended to 12 min, the increase of the oil droplet size led to the decreased hydrogen bonding between proteins, and the folding of gelatin proteins led to weaker hydrophobic interactions between the proteins.
3.4. Microscopic morphology of gelatin emulsion under optical microscope
Fig. 1(E) shows the optical microstructure of the ultrasonically treated gelatin emulsion. The aggregation of the oil droplets was found in gelatin emulsion without ultrasonic treatment, particle size of oil droplets was the largest and the distribution was not uniform. In the gelatin emulsion treated with ultrasonic, the aggregation of the oil droplets gradually weakened, and the size of the oil droplets became smaller as the processing time extended; however, oil droplets further aggregation, and the size of the droplets increased with excessive ultrasonic treatment. The result achieved under the optical microscope indicated that the oil droplets in the 12 min-film emulsion were the smallest and most uniformly distributed.
3.5. Thickness of gelatin film
Table 1 lists the film thickness with ultrasonic treatment. The film thickness without ultrasonic treatment was the lowest, which was 66.00 × 10-3 mm. With the extension of the ultrasonic treatment time, the film thickness was decreased first and then increased. The minimum thickness of the film reached 44.00 × 10-3 mm after the ultrasonic treatment for 12 min. The film thickness after ultrasonic treatment was lower. The possible reasons for the above result are presented as follows. First, ultrasonic treatment facilitated the formation of cross-linking between gelatin protein molecules, resulting the film structure more compact. Second, smaller particle size of oil droplet were formed through ultrasonic treatment, and the smaller oil droplets had less hindrance on proteins, so that the gelatin film was thinner. Once the ultrasonic treatment time exceeded 12 min, the particle size of the oil droplets increased, the blocking effect of the oil droplets on proteins was increased, the cross-linking of proteins was decreased, and then the film thickness was increased.
Table 1.
Effects of different ultrasonic time on film thickness, water vapor permeability, tensile strength, elongation at break and water solubility.
| Ultrasonic time (min) | Thickness (×10-3 mm) | WVP (×10-11 g⋅m-1⋅s-1⋅Pa-1) | TS (MPa) | EAB (%) | WS (%) |
|---|---|---|---|---|---|
| 0 | 66.00 ± 4.00 a | 5.09 ± 0.16 a | 20.37 ± 5.29 a | 115.52 ± 32.40 a | 0.163 ± 0.0030 a |
| 3 | 63.00 ± 5.50 a | 4.81 ± 0.09 b | 22.06 ± 4.10 ab | 131.30 ± 26.64 ab | 0.151 ± 0.0067 ab |
| 6 | 61.00 ± 4.50 a | 4.66 ± 0.04 b | 27.87 ± 1.15 ab | 152.76 ± 13.96 ab | 0.147 ± 0.0033 ab |
| 9 | 51.00 ± 10.00 b | 3.85 ± 0.16 c | 35.90 ± 5.15 b | 195.96 ± 42.01 bc | 0.138 ± 0.0131 bc |
| 12 | 44.00 ± 3.80 bc | 3.21 ± 0.14 d | 55.39 ± 10.18 c | 333.26 ± 28.69 d | 0.122 ± 0.0111 c |
| 15 | 55.00 ± 9.70 bc | 3.40 ± 0.75 d | 38.21 ± 3.56 b | 225.06 ± 63.29 c | 0.143 ± 0.0025 ab |
3.6. Water vapor permeability (WVP) of gelatin film
Table 1 lists the water vapor permeability of gelatin film after ultrasonic treatment. Gelatin film without ultrasonic treatment achieved the highest WVP (5.09 × 10-11 g·m−1·s−1·Pa−1). After ultrasonic treatment, the WVP of gelatin film was decreased first and then increased, and the WVP of 12 min film was the lowest (3.21 × 10-11g·m−1·s−1·Pa−1). In general, the poor hydrophobicity of gelatin film limited its application in industrial field. Existing research has suggested that strengthening the hydrophobicity of gelatin film is beneficial to improve its application value (Zheng et al., 2022). For gelatin protein, ultrasonic treatment enhanced the crosslinking degree between gelatin proteins and reduced the permeability of water vapor (Schmid & Müller, 2019); Besides, moderate ultrasound resulted in the changes in the tertiary structure of protein, which made the protein unfolded, thus enhancing the surface hydrophobicity of gelatin film (Meng et al., 2021). For oil droplet, ultrasonic treatment converted larger oil droplets into smaller oil droplets, thus increasing the specific surface area of hydrophobic components and reducing the absorption and diffusion ability of water vapor on the gelatin film; as a result, the WVP value of gelatin film was decreased (Arfat et al., 2014). Fabra found a similar process in the experiment. Adding lipid to the film significantly reduced the WVP of the film, and the WVP value was decreased with the decrease of the particle size (Fabra et al., 2011). However, the experimental result indicated that the WVP value was decreased after the ultrasonic treatment time exceeded 12 min. The possible reason for the above result is the protein reaggregation caused by excessive ultrasonic treatment, thus embedding the hydrophobic region into the protein molecules again and reducing the surface hydrophobicity of gelatin emulsion. Furthermore, excessive ultrasound resulted in the formation of the large oil droplet particles, the specific surface area of the oil droplet was decreased, and the WVP of gelatin film was decreased.
3.7. Water solubility (WS) of gelatin film
The WS of the ultrasonically treated film is listed in Table 1. The WS of gelatin film without ultrasonic treatment was the highest (0.163%). With the extension of the ultrasonic treatment time, the WS of gelatin film was decreased first and then increased, and the WS of gelatin film was the lowest (0.122%) at 12 min. The WS reflected the water resistance of gelatin film. The lower the WS, the stronger the water resistance of gelatin film. The size of the oil droplets was related to the tightness of the internal structure of gelatin film. The addition of the oil components reduced the degree of cross-linking between proteins, forming a loose structure of gelatin film (Song et al., 2018). Compared with the gelatin film without ultrasonic treatment, gelatin film after ultrasonic treatment had smaller oil droplets and a more uniform dispersion state; at this point, the proteins in the gelatin film formed stronger cross-linking, increased water resistance of gelatin film, and finally decreased WS of gelatin film. Gelatin film treated with ultrasonic for 12 min had the smallest particle size, which showed the lowest WS.
3.8. Tensile strength (TS) and elongation at break (EAB) of gelatin film
Table 1 exhibits the TS and EAB of gelatin film treated with different ultrasonic time. The gelatin films without ultrasonic treatment achieved the lowest TS (20.37 MPa) and EAB (11.5%); ultrasonic treatment enhanced the TS and EAB of gelatin film. Both of the above properties tended to be increased first and then decreased. The TS of gelatin film was the highest (55.39 MPa) and the EAB was the highest (333.26%) after ultrasonic treatment for 12 min. Compared with the non-ultrasonic treatment, TS and EAB in 12 min-film were increased by 2.5 times and 30 times, respectively.
TS and EAB indicated the mechanical properties of gelatin film. The higher the TS, the stronger the rigidity; the higher the EAB, the better the ductility. For gelatin components, ultrasonic treatment enhanced the non-covalent bond interaction between gelatin molecules, thus increasing the TS of gelatin film (Yang et al., 2021). The researcher suggested that ultrasonic treatment can facilitate the formation of ordered protein molecules, such that a rigid structure was formed (Wu et al., 2022). This macroscopic performance was that the TS of gelatin film became stronger.
The ductility of gelatin film was correlated with the content and distribution of hydrophobic components in the gelatin film. The interaction between non-polar molecules (e.g., oil) was weaker than that between polar molecules (e.g., protein molecules). The addition of non-polar components to polar molecules reduced the interaction between polar chains and formed flexible regions, thus reducing the TS of gelatin film and increasing the EAB of the film. The larger oil droplets in the gelatin film dispersed into smaller oil droplets after ultrasonic treatment, and the smaller oil droplets had less resistance to the interaction of gelatin molecules. Moreover, the oil droplets in the ultrasonically treated film were smaller in size and more uniform in dispersion. In addition, the gelatin film with smaller oil droplets had poor fluidity, and the film was not easy to break suddenly in the tensile process. This macroscopic performance was that the EAB of gelatin film was enhanced. A similar conclusion was summarized that the mechanical properties of gelatin film could be affected by oil droplets of different sizes (Fabra et al., 2011). After ultrasonic treatment for over 12 min, the size of the oil droplets was increased, the uniformity of the oil droplets became worse, and the gelatin film was prone to sudden fracture, thus leading to the deterioration of TS and EAB. Accordingly, ultrasonic treatment affected the mechanical properties of gelatin film (e.g., TS and EAB) by affecting the cross-linking of gelatin molecules, the ordered structure inside gelatin molecules, and the particle size of the oil droplets. The smaller the particle size of gelatin film, the stronger the TS of gelatin film, and the higher the EAB.
3.9. DSC analysis of gelatin film
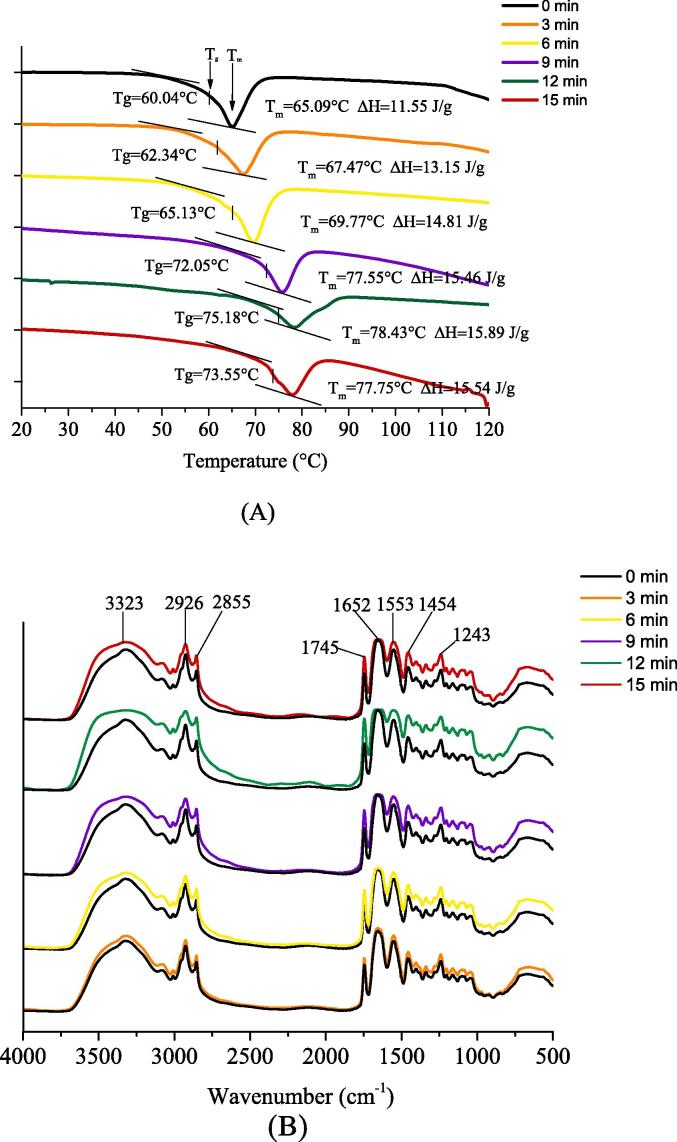
Fig. 2(A) presents DSC analysis of the ultrasonically treated gelatin film. The values of Tg Tm and ΔH without ultrasonic treatment were 60.04℃ 65.09℃ and 11.55 J/g, respectively; with the extension of the ultrasonic time, Tg Tm and ΔH were increased first and then decreased. Gelatin films after ultrasonic treatment for 12 min achieved the highest Tg(75.18℃) Tm (78.43℃) and the highest ΔH (15.89 J/g). Tg Tm represented the glass transition temperature, the melting peak temperature respectively, and ΔH represented enthalpy change of the thermal transition process in the heating process. The higher the values of Tg Tm and ΔH, the better the thermal stability of gelatin film and the lower the fluidity of the internal structure. Sothornvit suggested that the addition of the oily components would release the rigid structure of gelatin film, if the oil droplet size was larger, the effect on the rigid structure of gelatin film was increased (Sothornvit & Krochta, 2005). In DSC experiments, the chemical bonds required to maintain the natural (folded) conformation of proteins were broken since the exposure of proteins gradually increased the temperatures, thus leading to thermal denaturation (Andlinger et al., 2021). The ultrasonically treated gelatin film had higher Tg Tm and ΔH value since ultrasonic treatment formed smaller oil droplet particles, the stability of gelatin film structure was better, and the fluidity was poor. Moreover, since ultrasonic treatment enhanced the cross-linking between gelatin proteins, and the hydrogen bond and hydrophobic interaction used to maintain the conformation of gelatin proteins were enhanced, the gelatin proteins at this time required higher temperatures to undergo thermal denaturation. If the ultrasonic time exceeds 12 min, ultrasonic treatment was excessive, the oil droplet size inside the gelatin film was increased larger, and the cross-linking degree of gelatin protein was decreased, thus leading to the deterioration of the thermal stability of gelatin film. Furthermore, Tg Tm and ΔH value tended to be decreased.
Fig. 2.
(A) DSC analysis of the gelatin film treated with different ultrasonic times. (B) FTIR analysis of the gelatin film treated with different ultrasonic times.
3.10. ATR-FTIR analysis of gelatin film
Fig. 2(B) presents ATR-FTIR analysis of the ultrasonically treated gelatin film. All gelatin films had similar vibrations since the respective film had equal composition and content. The vibration at 3323 cm−1 was amide A, indicating the stretching of N—H and hydrogen bonds (Kong & Yu, 2007). In general, the vibration of amide-A was nearly 3300 cm−1, amide-A in the gelatin film moved to a higher wave number (3323 cm−1). The possible reason for this result is that the addition of soybean oil reduced the protein–protein interaction, thus weakening the hydrogen bonding between junction proteins. The vibration at 3083 cm−1 was amide-B, representing the stretching vibration of C—H. The amplitudes at 2879 cm−1 and 2931 cm−1 represented the asymmetric vibration and symmetric vibration of C—H bond in CH2 and CH3 of aliphatic compounds, respectively; these amplitudes also appeared in most lipids, and the intensity of amplitude represented the hydrophobic intensity of the film. The amplitude of the ultrasonically treated gelatin film increased at the above two peaks, and the amplitude in 12 min-film was the strongest, indicating the strongest hydrophobicity of gelatin film. The vibration at 1745 cm−1 represented the C O stretching vibration of aldehyde or ester carbonyl (Tongnuanchan et al., 2016). Amide-I was located at 1655–1660 cm−1, representing C O stretching vibration. Amide II was located at 1552 cm−1, and it was generated by the bending vibration of N—H. Amide III at 1240 cm−1 represented the interaction between the bending vibration of N—H and the stretching vibration of C—N (Li et al., 2022). As depicted in Fig. 2(B), the amplitude difference was the most significant difference among all gelatin films, and the above differences arose from the time difference of ultrasonic treatment.
The amplitude of gelatin film was correlated with hydrophobicity interaction of gelatin molecules. Macroscopically, the hydrophobicity interaction of gelatin molecules are expressed as TS and WVP of gelatin film. The gelatin film without ultrasonic treatment achieved the highest WVP and the lowest TS. As the ultrasonic treatment time was extended, the WVP value was first decreased and then increased, and the TS value was first increased and then decreased. WVP and TS reached the minimum and maximum values respectively after ultrasonic treatment for 12 min, i.e., the gelatin film achieved the optimal water vapor resistance and tensile properties.
3.11. SEM analysis of gelatin film
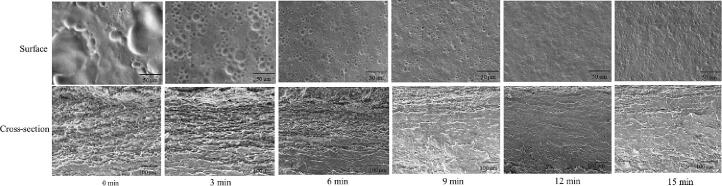
Fig. 3 presents the SEM micromorphology (surface photograph and cross-section photograph) of the ultrasonically treated gelatin film. In the surface micromorphology, the gelatin film without ultrasonic treatment had larger oil droplets with an uneven surface. After ultrasonic treatment, the oil droplets in the gelatin film became smaller, and the surface structure of the film became uniform and smooth. Gelatin film with ultrasonic treatment for 12 min had the smallest oil droplets particles and the most uniform surface. The cross-section micromorphology indicated that the gelatin film without ultrasonic treatment was relatively loose and rough, the gelatin film after ultrasonic treatment became more compact, and the ultrasonically treated gelatin film for 12 min had the most compact structure. The tighter the film structure, the worse its fluidity, and the better its thermal stability; the tighter the film structure, the stronger the interaction between the proteins, and the stronger the tensile strength. The microscopic performance of gelain film confirmed its macroscopic properties (e.g., tensile property and thermal stability). The optical microscopic image of gelatin emulsion was related to the scanning electron microscope image of gelatin film. The smaller the oil droplet size in the gelatin emulsion, the smoother the surface structure of the corresponding gelatin film, without obvious oil droplets, and the more compact the cross-sectional structure of the corresponding gelatin film.
Fig. 3.
Scanning electron microscopy (SEM) analysis of the gelatin film treated with different ultrasonic time, surface microstructure and cross-section microstructure.
3.12. Pearson’s correlation coefficients between properties of gelatin emulsion and related properties of gelatin film
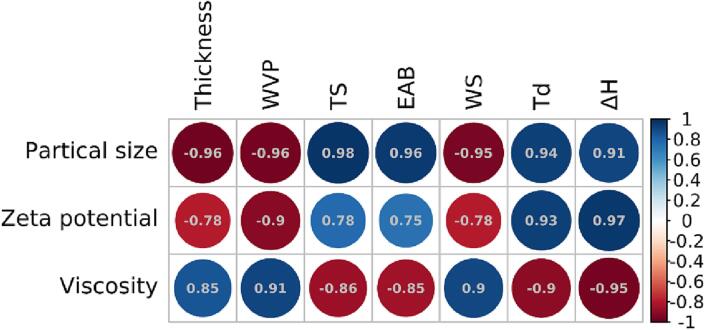
As depicted in Fig. 4, a certain correlation was found between the properties of the film emulsion (particle size, zeta potential, and viscosity) and the properties of the related gelatin film (film thickness, WVP, TS, EAB, WS, Tm, and ΔH). The result indicated that the properties of gelatin film were controlled by the properties of gelatin emulsion. The higher the absolute value of Pearson’s correlation coefficient, the stronger the correlation. A correlation coefficient with an absolute value of 1.0 was considered to be a complete linear correlation, whereas a correlation coefficient with an absolute value of 0.8 and 1.0 was considered to be a strong correlation. The particle size and viscosity of gelatin emulsion were significantly correlated with major film performance indexes (thickness, WVP, TS, EAB, WS, Tm, and ΔH). The particle size of gelatin emulsion affected the interaction of proteins and fluidity inside the gelatin film, which was manifested in the variations of mechanical properties, thermal properties, and other properties. Zeta potential was significantly correlated with minor films’ performance indexes (WVP, Tm, and ΔH).
Fig. 4.
Pearson’s correlation analysis between properties of the gelatin emulsion and properties of the gelatin film.
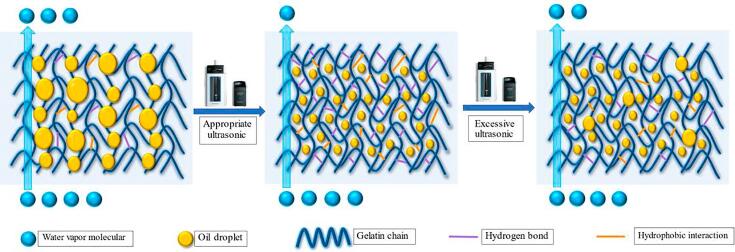
3.13. Schematic model
The schematic model in Fig. 5 was adopted to illustrate the effect of ultrasonic treatment on the properties of gelatin emulsion and related gelatin film based on the examined results above. The effect of ultrasonic treatment on gelatin emulsion primarily indicated that particle size was decreased, zeta potential and viscosity were increased, and the hydrogen bond and hydrophobic interaction between proteins were increased, too. The effect of ultrasonic treatment on the related gelatin film was manifested in the decrease of film thickness, WVP and WS, as well as the increase of TS, EAB, Tm, and ΔH. If the time of ultrasonic treatment went beyond the critical limitation, particle size was increased, zeta potential and viscosity were decreased; the film thickness, WVP and WS were increased, and TS, EAB, Tm and ΔH were decreased.
Fig. 5.
Schematic model illustrating the effect of ultrasonic treatment on properties of the gelatin film.
4. Conclusion
The properties of gelatin emulsion and related gelatin film were significantly affected by ultrasonic treatment, ultrasonic control of gelatin emulsion performance could realize the control of gelatin film performance. Ultrasonic treatment could reduce the particle size of oil droplets and increase zeta potential and viscosity of gelatin emulsion, enhance the hydrogen bond interaction and hydrophobic interaction between proteins. Moreover, the effect of ultrasonic treatment on gelatin film was manifested in the decrease of the film thickness, WVP, and WS, as well as the increase of TS and EAB, Tm and ΔH. However, the excess ultrasonic treatment led to the aggregation of the oil droplets in gelatin emulsion, thus resulting in the deterioration of the film properties. The experimental results indicated that gelatin film had optimal performance under ultrasonic treatment of 400 w for 12 min.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the China Agriculture Research System (CARS-45), Guizhou Provincial Natural Science Foundation (ZK [2022] 360), the National Natural Science Foundation of China (No. 32160576), and the Research Funding Project of Guizhou Medical University (J [2021] 013).
Contributor Information
Hong-Shun Yang, Email: chmynghs@nus.edu.sg.
Zong-Cai Tu, Email: tuzc_mail@aliyun.com.
Data availability
No data was used for the research described in the article.
References
- Andlinger D.J., Röscheisen P., Hengst C., Kulozik U. Foods. 2021;10(4):796. doi: 10.3390/foods10040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfat Y.A., Benjakul S., Prodpran T., Sumpavapol P., Songtipya P. Food Hydrocolloids. 2014;41:265–273. doi: 10.1016/j.foodhyd.2014.04.023. [DOI] [Google Scholar]
- ASTM (2012). Standard Test Method for Tensile Properties of Thin Plastic Sheeting, D882-12 (pp. 162-170). Philadelphia: American Society for Testing and Materials.
- Fabra M.J., Perez-Masia R., Talens P., Chiralt A. Food Hydrocolloids. 2011;25(5):1112–1121. doi: 10.1016/j.foodhyd.2010.10.008. [DOI] [Google Scholar]
- Floury J., Desrumaux A., Lardières J. Innovative Food Science & Emerging Technologies. 2000;1(2):127–134. doi: 10.1016/S1466-8564(00)00012-6. [DOI] [Google Scholar]
- Ghosh V., Mukherjee A., Chandrasekaran N. Ultrasonics Sonochemistry. 2013;20(1):338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Huang T., Tu Z., Zou Z., Shangguan X., Wang H., Bansal N. Food Hydrocolloids. 2020;102 doi: 10.1016/j.foodhyd.2019.105552. [DOI] [Google Scholar]
- Jamróz E., Juszczak L., Kucharek M. International Journal of Biological Macromolecules. 2018;14:1094–1101. doi: 10.1016/j.ijbiomac.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Kim H., Beak S., Song K.B. LWT. 2018;96:583–588. doi: 10.1016/j.lwt.2018.06.016. [DOI] [Google Scholar]
- Kong J., Yu S. Acta Biochimica Et Biophysica Sinica. 2007;39(8):549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Li N., Wang T., Yang X., Qu J., Wang N., Wang L.…Han C. Ultrasonics Sonochemistry. 2022;86 doi: 10.1016/j.ultsonch.2022.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tu Z., Sha X., Li Z., Li J., Huang M. Food Chemistry: X. 2022 doi: 10.1016/j.fochx.2022.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tu Z., Sha X., Ye Y., Li Z. Journal of Food Science and Technology. 2022;59(2):815–824. doi: 10.1007/s13197-021-05080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., An F., He H., Geng F., Huang Q. Food Hydrocolloids. 2020;114(5) doi: 10.1016/j.foodhyd.2020.106555. [DOI] [Google Scholar]
- Liu J., Liu S., Wu Q., Gu Y., Kan J., Jin C. Food Hydrocolloids. 2017;73:90–100. doi: 10.1016/j.foodhyd.2017.06.035. [DOI] [Google Scholar]
- Ma X., Yan T., Hou F., Chen W., Miao S., Liu D. Ultrasonics Sonochemistry. 2019;59 doi: 10.1016/j.ultsonch.2019.104748. [DOI] [PubMed] [Google Scholar]
- Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P.…Liu L. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112272. [DOI] [Google Scholar]
- Moula Ali A.M., Prodpran T., Benjakul S. Food Hydrocolloids. 2019;96:123–133. doi: 10.1016/j.foodhyd.2019.05.019. [DOI] [Google Scholar]
- Nguyen P.T., Bhandari B., Prakash S. Journal of Food Engineering. 2016;168:27–34. doi: 10.1016/j.jfoodeng.2015.07.011. [DOI] [Google Scholar]
- Papadaki A., Manikas A.C., Papazoglou E., Kachrimanidou V., Lappa I., Galiotis C.…Kopsahelis N. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132604. [DOI] [PubMed] [Google Scholar]
- Ren Z., Chen Z., Zhang Y., Lin X., Li B. Food Hydrocolloids. 2019;96:322–330. doi: 10.1016/j.foodhyd.2019.05.015. [DOI] [Google Scholar]
- Romani V.P., Martins V.G., Goddard J.M. Food Control. 2020;109 doi: 10.1016/j.foodcont.2019.106946. [DOI] [Google Scholar]
- Academic Press; 2019. pp. 407–437. [Google Scholar]
- Song X., Zuo G., Chen F. International Journal of Biological Macromolecules: Structure, Function and Interactions. 2018;107:1302–1309. doi: 10.1016/j.ijbiomac.2017.09.114. [DOI] [PubMed] [Google Scholar]
- Acadeimic Press; 2005. pp. 403–433. [Google Scholar]
- Sucheta, Rai S.K., Chaturvedi K., Yadav S.K. Food Hydrocolloids. 2019;91:127–135. doi: 10.1016/j.foodhyd.2019.01.022. [DOI] [Google Scholar]
- Tongnuanchan P., Benjakul S., Prodpran T., Pisuchpen S., Osako K. Food Hydrocolloids. 2016;56:93–107. doi: 10.1016/j.foodhyd.2015.12.005. [DOI] [Google Scholar]
- Tongnuanchan P., Benjakul S., Prodpran T., Nilsuwan K. Food Hydrocolloids. 2015;48:248–259. doi: 10.1016/j.foodhyd.2015.02.025. [DOI] [Google Scholar]
- Vankova N., Tcholakova S., Denkov N.D., Ivanov I.B., Vulchev V.D., Danner T. Journal of Colloid & Interface Science. 2007;312(2):363–380. doi: 10.1016/j.jcis.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang N., Li N., Ji X., Zhang H., Yu D.…Wang L. Ultrasonics Sonochemistry. 2022;82 doi: 10.1016/j.ultsonch.2021.105871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huang Y., Zhou B., Xu W., Xiang X., Huang Q.…Li S. Ultrasonics Sonochemistry. 2021;75 doi: 10.1016/j.ultsonch.2021.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xiang X., Liu L., An F., Geng F., Huang Q.…Wei S. LWT. 2022;171 doi: 10.1016/j.lwt.2022.114155. [DOI] [Google Scholar]
- Wu Y., Zhang Y., Duan W., Wang Q., An F., Luo P.…Huang Q. Journal of Food Engineering. 2022;319 doi: 10.1016/j.jfoodeng.2021.110908. [DOI] [Google Scholar]
- Yang D., Gao S., Yang H. Food Hydrocolloids. 2020;99 doi: 10.1016/j.foodhyd.2019.105317. [DOI] [Google Scholar]
- Yang Z., Zhou H., Bai Y. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130058. [DOI] [PubMed] [Google Scholar]
- Zheng H., Zhao M., Dong Q., Fan M., Wang L., Li L. Food Hydrocolloids. 2022;132 doi: 10.1016/j.foodhyd.2022.107849. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.