Summary
Background
Anti-programmed cell death protein 1 antibodies plus multikinase inhibitors have shown encouraging activity in several tumour types, including colorectal cancer. This study assessed regorafenib plus nivolumab in patients with microsatellite stable/mismatch repair-proficient metastatic colorectal cancer.
Methods
This single-arm, open-label, multicentre phase 2 study enrolled adults from 13 sites in the USA with previously treated advanced microsatellite stable/mismatch repair-proficient metastatic colorectal cancer. Eligible patients had known extended RAS and BRAF status, progression or intolerance to no more than two (for extended RAS mutant) or three (for extended RAS wild type) lines of systemic chemotherapy and an Eastern Cooperative Oncology Group performance status of 0 or 1. Regorafenib 80 mg/day was administered orally for 3 weeks on/1 week off (increased to 120 mg/day if 80 mg/day was well tolerated) with intravenous nivolumab 480 mg every 4 weeks. Primary endpoint was objective response rate. Secondary endpoints included safety, overall survival, and progression-free survival. Exploratory endpoints included biomarkers associated with antitumour activity. Patients who received at least one dose of study intervention were included in the efficacy and safety analyses. Tumour assessments were carried out every 8 weeks for the first year, and every 12 weeks thereafter until progressive disease/end of the study, and objective response rate was analysed after all patients had met the criteria for primary completion of five post-baseline scans and either 10-months’ follow-up or drop out. This trial is registered with ClinicalTrials.gov, number NCT04126733.
Findings
Between 14 October 2019 and 14 January 2020, 94 patients were enrolled, 70 received treatment. Five patients had a partial response, yielding an objective response rate of 7% (95% CI 2.4–15.9; p = 0.27). All responders had no liver metastases at baseline. Median overall survival (data immature) and progression-free survival were 11.9 months (95% CI 7.0–not evaluable) and 1.8 months (95% CI 1.8–2.4), respectively. Most patients (97%, 68/70) experienced a treatment-related adverse event; 51% were grade 1 or 2, 40% were grade 3, 3% were grade 4, and 3% were grade 5. The most common (≥20%) events were fatigue (26/70), palmar-plantar erythrodysesthesia syndrome (19/70), maculopapular rash (17/70), increased blood bilirubin (14/70), and decreased appetite (14/70). Higher baseline expression of tumour biomarkers of immune sensitivity correlated with antitumour activity.
Interpretation
Further studies are warranted to identify subgroups of patients with clinical characteristics or biomarkers that would benefit most from treatment with regorafenib plus nivolumab.
Funding
Bayer/Bristol Myers Squibb.
Keywords: Regorafenib, Nivolumab, Microsatellite stable, Mismatch repair-proficient, Metastatic colorectal cancer
Research in context.
Evidence before this study
Combinations of anti-programmed cell death protein 1 (PD-1) antibodies, such as nivolumab, and multikinase inhibitors, such as regorafenib, have shown encouraging activity in renal cell carcinoma, endometrial cancer, and other solid tumours. In a phase 1b study of Japanese patients with microsatellite stable/mismatch repair-proficient colorectal cancer, the combination of regorafenib plus nivolumab demonstrated a manageable safety profile and significant antitumour activity with a confirmed objective response rate of 33%, which is higher than the objective response rates demonstrated by regorafenib or anti-PD-1 monotherapy.
Added value of this study
Our phase 2 study was the largest prospective study of an anti-PD-1 antibody plus a multikinase inhibitor to date. In patients from the USA with microsatellite stable/mismatch repair-proficient metastatic colorectal cancer, regorafenib plus nivolumab demonstrated an objective response rate of 7%, with all tumour responses observed in patients without liver metastases. Regorafenib plus nivolumab had a manageable safety profile that was consistent with the known safety profiles of both treatments, with the exception of a higher incidence of grade 3 rash in our study. Lastly, high expression of biomarkers for pre-existing immune sensitivity in tumour samples and lower expression of biomarkers related to angiogenesis in peripheral blood samples trended with better clinical outcomes, although these results should be considered hypothesis generating only.
Implications of all the available evidence
Although our study did not meet its primary endpoint in an unselected cohort of patients with microsatellite stable/mismatch repair-proficient metastatic colorectal cancer, the demonstrated level of activity in a subset of patients without liver metastases warrants further investigation to identify subgroups of patients with clinical characteristics and/or biomarkers who would benefit most from treatment with regorafenib plus nivolumab.
Introduction
Regorafenib is approved for use in patients with previously treated metastatic colorectal cancer (mCRC) based on overall survival (OS) benefit.1, 2, 3 The immune checkpoint inhibitors pembrolizumab and nivolumab (anti-programmed cell death protein 1 [PD-1] antibodies) are approved as monotherapies for the treatment of patients with microsatellite instability—high or mismatch repair-deficient mCRC.4,5 In the context of mismatch repair (MMR)-deficient/microsatellite instability—high mCRC, immune sensitivity relies on high mutational burden due to the deficiency of the MMR system, along with immune escape owing to the upregulation of several immune checkpoints.6 Together, these features afford an inflamed tumour microenvironment that is highly sensitive to immune checkpoint inhibitors.7 However, early studies showed no efficacy signals in patients with microsatellite stable (MSS)/MMR-proficient (pMMR) mCRC.8 The lack of T-cell tumour infiltration along with reduced expression of checkpoint proteins are perceived to be major drivers of resistance to immune checkpoint inhibitors.9 One strategy to improve outcomes is combining immune checkpoint inhibitors with a multikinase inhibitor targeting the vascular endothelial growth factor pathway, which together are predicted to have complementary antitumour effects and potential enhancement of tumour-directed immune response.
Combinations of anti-PD-1 antibodies and multikinase inhibitors targeting the vascular endothelial growth factor pathway have shown encouraging activity in renal cell carcinoma, endometrial cancer, and other solid tumours.10, 11, 12 In preclinical tumour models of colon cancer, the combination of regorafenib and an anti-PD-1 antibody was more active than either drug alone.13 In the phase 1b REGONIVO trial of Japanese patients with advanced gastric cancer or MSS/pMMR CRC, the combination of regorafenib and nivolumab demonstrated a manageable safety profile and significant antitumour activity with a confirmed objective response rate (ORR) of 33% in patients with MSS/pMMR CRC,14 which is higher than the ORRs demonstrated by regorafenib or anti-PD-1 monotherapy.8,15,16 In addition, several studies interrogated the combination of multikinase inhibitors with PD-1 or programmed cell death-ligand 1 (PD-L1) inhibitors, but with lower response rates than reported in the REGONIVO trial.14,17, 18, 19, 20 In view of the aforementioned biological complexities and limited studies, predictive biomarkers could help to accurately select immune checkpoint inhibitor responders with MSS/pMMR CRC. Furthermore, the identification of patients with distinct clinical features may also help tailor the use of immune checkpoint inhibitor-based combination therapy in patients with MSS/pMMR mCRC. This phase 2 study therefore aimed to further assess the safety and efficacy of the combination of regorafenib plus nivolumab in patients from the USA with advanced or metastatic MSS/pMMR CRC, and explored how the presence of liver metastases (LM) and skin toxicities affects response.
Methods
Study design
The primary endpoint of this open-label, single-arm, phase 2 study was to evaluate the ORR (investigator assessed) of regorafenib administered in combination with nivolumab. Secondary endpoints included assessing incidence and severity of adverse events (AEs), duration of response, duration of stable disease (SD), disease control rate, progression-free survival (PFS), and OS. Exploratory aims were to characterise the pharmacokinetics of the study drugs when used in combination, to assess efficacy using immune-related response criteria, and to identify biomarkers in baseline tumour materials and blood that might be associated with response. The study was conducted at 13 sites in the USA.
Key eligibility criteria
Eligible patients were adults aged ≥18 years with confirmed advanced, metastatic, or progressive MSS/pMMR CRC; known extended RAS and BRAF status; progression (radiologically or clinically) or intolerance to no more than two (for extended RAS mutant) or three (for extended RAS wild type) lines of systemic chemotherapy; and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Microsatellite status was performed per local standard of practice, for example, immunohistochemistry and/or polymerase chain reaction and next-generation sequencing.
Major exclusion criteria included a history of previous treatment with regorafenib or any form of immunotherapy to treat cancer. Full inclusion and exclusion criteria are listed in the protocol (Section 5.1).
Drug administration and dose escalation procedure
Regorafenib was administered orally at a starting dose of 80 mg/day for 3 weeks on/1 week off, with nivolumab 480 mg given intravenously every 4 weeks in 4-week cycles. If the starting dose of 80 mg once daily was well tolerated (i.e., absence of any-grade rash/hand–foot skin reaction [HFSR] or other grade ≥2 clinically significant toxicity), it was increased to 120 mg once daily on day 1 of cycle 2 (C2D1). Protocol-defined dose modifications to manage toxicity included interruption of regorafenib and nivolumab, and reducing the regorafenib dose from 120 mg to 80 mg daily or further to 80 mg every other day. No dose adjustments were allowed for nivolumab.
Assessment
All participants who received at least one dose of study intervention were included in the efficacy and safety analyses. Patients underwent evaluations for safety and efficacy, and tissue and blood samples for biomarker and pharmacokinetic analyses were collected. Tumour assessments (computed tomography/magnetic resonance imaging) were obtained at baseline, every 8 weeks for the first year, and every 12 weeks thereafter until progressive disease (PD) or the end of the study. Tumour response was evaluated by the investigators per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. ORR was defined as the proportion of patients with the best objective response of complete response (CR) or partial response (PR). ORR was analysed after all patients had met the criteria for primary completion of five post-baseline scans and either 10-months’ follow-up or drop out. Disease control rate was defined as the proportion of patients with the best objective response of CR, PR, or SD. For patients with a best objective response of SD who had an evaluable tumour assessment post baseline, duration of SD was the time from first treatment to the earliest date of PD or death. Duration of response was defined as the time from first documentation of CR/PR until PD/death. PFS was defined as the time from the first treatment until PD/death. OS was defined as the time from the first treatment until death.
AEs were summarised according to the Medical Dictionary for Regulatory Activities v23.0, and the severity was categorised by the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Treatment continued until unacceptable toxicity, withdrawal of consent, the investigator's decision to stop treatment, or death. A patient with initial radiological PD (i.e., no clinical deterioration) could continue treatment if judged to be beneficial by the investigator, and further imaging was assessed using immune criteria from RECIST v1.1. The primary completion date was defined as the time when all patients had been assessed for tumour response by at least five post-baseline scans unless they discontinued earlier. In pre-planned analyses, treatment outcomes according to the presence of liver metastasis were assessed.
Pharmacokinetics
Pharmacokinetic evaluations of regorafenib, its metabolites (M-2 and M-5), and nivolumab were performed at protocol-defined time intervals over the first two cycles of treatment. Regorafenib area under the curve and maximum concentration were estimated by population pharmacokinetic analysis.
Biomarker sample collection, assessments, and data analyses
Tumour samples, including both fresh and archival biopsies and resections, were obtained at baseline, day 8 of cycle 2 (C2D8), and end of treatment (optional). Pre- and on-treatment blood samples were collected from all patients to measure circulating tumour DNA (ctDNA), soluble biomarkers, and immune phenotypes. Selected immune-related biomarkers were assessed in formalin-fixed paraffin-embedded tumour samples using immunohistochemistry staining (Mosaic, Lake Forest, CA, USA). PD-L1 expression was determined using 28-8 (Agilent, Santa Clara, CA, USA) with combined positive score. Whole-exome sequencing was performed using formalin-fixed paraffin-embedded tumour samples and matched blood samples (Q Squared Solutions, Morrisville, NC, USA). Gene expression profiling was performed using RNA sequencing in formalin-fixed paraffin-embedded tumour samples (Q Squared Solutions, Morrisville, NC, USA) (Appendix methods [genomic assessments]). Concentrations of circulating proteins from baseline serum samples were quantified using either a multiplex immunoassay (DiscoveryMAP® v4.0) or a single molecule array (Simoa®, Quanterix); both from Myriad RBM, Austin, TX, USA. Immune cell subtypes were assessed by flow cytometry in whole blood (Covance, Indianapolis, IN, USA). A next-generation sequencing-based targeted panel (Guardant OMNI) was used for ctDNA extracted from baseline samples to characterise somatic genomic alterations in 500 genes, as well as tumour mutational burden and microsatellite instability. Another next-generation sequencing-based targeted panel (Guardant360) was used in ctDNA extracted from on-treatment samples to characterise somatic genomic alterations in 73 genes.
Statistical analysis
A one-sided exact binomial test was used to demonstrate an ORR of >5%, which was based on the ORR associated with available treatment options in this population.15,21 With a target enrolment of 70 treated patients within 3 months (first patient first visit [planned]: 30 October 2019; last patient first visit [planned]: 14 January 2020) and an expected ORR of 17% at a type I error of approximately 2.5%, the power was calculated as 92.6%. Assuming a baseline ORR of 5%, eight responders were needed to achieve statistical significance. ORR and disease control rate were reported as percentage with confidence intervals (CIs) calculated using the Clopper–Pearson method. Time-to-event endpoints, such as duration of response, duration of SD, OS, PFS, and time to recurrence were analysed using the Kaplan–Meier method. Statistical analyses were performed using SAS v9.4 software (SAS Institute, Cary, NC, USA) and R software (R Core Team 2019) v3.6.1. The cut-offs for biomarker analyses were set at the median, except for PD-L1 expression assessed via immunohistochemistry, which had a cut-off of combined positive score ≥1. No post hoc analyses were carried out. This trial is registered with ClinicalTrials.gov number NCT04126733.
Ethics
The study protocol (Appendix—study protocol) and all revisions, and the informed consent form, received master approval from the Independent Ethics Committee or Institutional Review Board of the 13 study sites (see Appendix—list of participating study sites) before the start of the study. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines after approval by the institutional review board at each institution. All patients provided written informed consent for participation in the study.
Role of the funding source
The study funders were involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
Patient characteristics
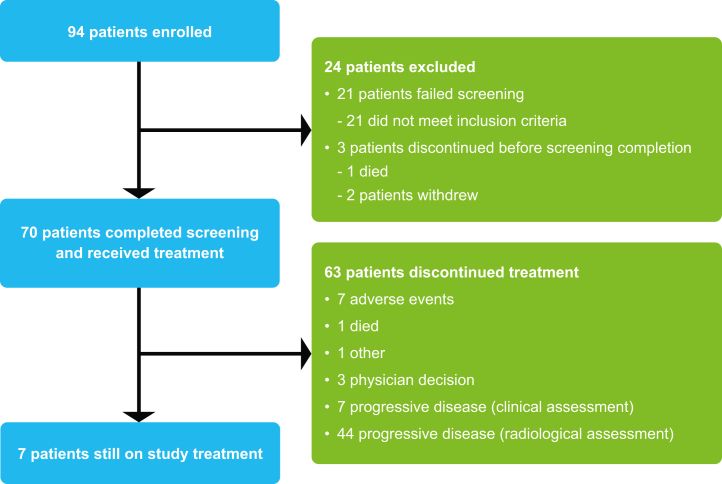
Between 14 October 2019 and 14 January 2020, 94 patients were enrolled; 70 of whom received treatment. The primary cancer site was the right colon in 36% and the left colon and rectum in 64%; 61% of patients had a RAS mutation (Table 1). Median age was 57 years; 49% of patients had ECOG PS 1; 67% had LM; and 73% had lung metastases. On the analysis cut-off date of 11 November 2020, treatment was ongoing in seven patients (10%); most treatment discontinuations were due to PD (n = 51/70; 73%; Fig. 1).
Table 1.
Baseline demographics and disease characteristics.
| Without liver metastases (n = 23) | With liver metastases (n = 47) | All patients (N = 70) | |
|---|---|---|---|
| Baseline demographics | |||
| Male sex, n (%) | 9 (39) | 32 (68) | 41 (59) |
| Female sex, n (%) | 14 (61) | 15 (32) | 29 (41) |
| Median age, years (IQR) | 57 (51–66) | 56 (50–66) | 57 (50–66) |
| Race, n (%) | |||
| White | 16 (70) | 34 (72) | 50 (71) |
| Black or African American | 2 (9) | 8 (17) | 10 (14) |
| Asian | 4 (17) | 3 (6) | 7 (10) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (2) | 1 (1) |
| Not reported | 1 (4) | 1 (2) | 2 (3) |
| ECOG PS, n (%) | |||
| 0 | 14 (61) | 22 (47) | 36 (51) |
| 1 | 9 (39) | 25 (53) | 34 (49) |
| Primary cancer site, n (%) | |||
| Right colon | 6 (26) | 19 (40) | 25 (36) |
| Left colon and rectum | 17 (74) | 28 (60) | 45 (64) |
| Disease characteristics | |||
| Liver metastases at baseline, n (%) | 0 | 47 (100) | 47 (67) |
| Lung metastases at baseline, n (%) | 16 (70) | 35 (74) | 51 (73) |
| Peritoneal metastases at baseline, n (%) | 2 (9) | 4 (9) | 6 (9) |
| Mutation status, n (%) | |||
| BRAF, KRAS, and NRAS wild type | 7 (30) | 15 (32) | 22 (31) |
| KRAS or NRAS mutation | 14 (61) | 29 (62) | 43 (61) |
| BRAF mutation | 2 (9) | 1 (2) | 3 (4) |
| Could not be evaluated for BRAF, KRAS, or NRAS | 0 | 2 (4) | 2 (3) |
| Histology, n (%) | |||
| Adenocarcinoma, not otherwise specified | 22 (96) | 43 (91) | 65 (93) |
| Mucinous adenocarcinoma | 1 (4) | 4 (9) | 5 (7) |
| Number of previous anti-cancer regimens,a n (%) | |||
| 1 | 1 (4) | 2 (4) | 3 (4) |
| 2 | 8 (35) | 22 (47) | 30 (43) |
| 3 | 6 (26) | 12 (26) | 18 (26) |
| ≥4 | 8 (35) | 11 (23) | 19 (27) |
| Previous anti-cancer therapy, n (%) | 23 (100) | 47 (100) | 70 (100) |
| Fluoropyrimidines | 23 (100) | 47 (100) | 70 (100) |
| Oxaliplatin | 22 (96) | 47 (100) | 69 (99) |
| Irinotecan | 23 (100) | 47 (100) | 70 (100) |
| Anti-VEGF | 23 (100) | 43 (91) | 66 (94) |
| EGFR inhibitors | 10 (43) | 13 (28) | 23 (33) |
| Napabucasin | 1 (4) | 0 | 1 (1) |
| Prior radiotherapy, n (%) | 8 (35) | 9 (19) | 17 (24) |
| Median time from diagnosis of metastases, months (IQR) | 33 (23–47) | 21 (13–32) | 24 (15–36) |
ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR = epidermal growth factor receptor; IQR = interquartile range; VEGF = vascular endothelial growth factor.
All regimens are counted, including neoadjuvant, adjuvant, and repeated regimens. Neoadjuvant and adjuvant regimens are not considered a previous line of therapy unless there was recurrence within 6 months. Repetition of a regimen is not considered a different line of therapy.
Fig. 1.
Trial profile.
Treatment duration and dosing
Patients had a median treatment duration of 2.2 months (interquartile range 1.6–7.0) for regorafenib and 1.9 months (interquartile range 1.0–6.5) for nivolumab. Patients received a median of three cycles (interquartile range 2–8) of regorafenib and three cycles (interquartile range 2–8) of nivolumab. The mean percentage of planned regorafenib dose (i.e., total amount of actual dose/total amount of planned dose based on either an 80 mg/day starting dose or 120 mg/day escalated dose) received was 82% (standard deviation 21%). A relative nivolumab dose intensity of at least 90% was achieved by 93% of patients. Overall, 41% of patients (n = 29) escalated from regorafenib 80 mg/day to 120 mg/day on C2D1. One patient escalated after C2D1 (protocol deviation). After escalation, half of patients maintained the 120 mg dose level and half required at least one dose modification due to an AE (dose interruption [n = 5], dose reduction [n = 9], and drug withdrawal [n = 3]). During the study, patients required regorafenib dose reductions (n = 23; 33%) and/or regorafenib treatment interruptions or delays (n = 44; 63%) due to AEs. Nivolumab treatment interruptions due to AEs were required in 14 patients (20%). In total, seven patients (10%) discontinued both regorafenib and nivolumab due to AEs. Treatment-related AEs led to treatment discontinuation in four patients (6%). One was related to regorafenib (drug-induced liver injury) and three were related to nivolumab (hepatic enzyme increased, infusion-related reaction, and sepsis).
Tolerability and adverse events
Most patients (97%) experienced at least one treatment-related AE and the majority were grade 1 or 2 (51%) (Table 2; Appendix Tables S1 and S2). The most common treatment-related AEs (≥20%) were fatigue (n = 26; 37%), HFSR/palmar-plantar erythrodysesthesia syndrome (n = 19; 27%), maculopapular rash (n = 17; 24%), increased blood bilirubin (n = 14; 20%), and decreased appetite (n = 14; 20%). Grade 3 or higher treatment-related AEs were experienced by 32 patients (46% [grade 3: n = 28, 40%]; [grade 4: n = 2, 3%]; [grade 5: n = 2, 3%]; Table 2; Appendix Table S2); the most common being maculopapular rash (n = 10; 14%) and fatigue (n = 4; 6%). Three treatment-emergent AEs resulted in death during the trial. One patient had respiratory failure unrelated to treatment and two patients had sepsis (one related to the combination treatment and the other related to nivolumab treatment only).
Table 2.
Incidence of treatment-emergent adverse events (≥20% of patients) and treatment-related adverse events.a
| AE, n (%) | All patients (N = 70) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All AEs | Treatment-related AEs |
|||||||||
| Regorafenibb |
Nivolumab |
Both regorafenib and nivolumab |
Relationship to any study drug |
|||||||
| Any grade | Grade 1/2 | Grade ≥3 | Grade 1/2 | Grade ≥3 | Grade 1/2 | Grade ≥3 | Any grade | Grade 1/2 | Grade ≥3 | |
| Total | 69 (99) | 33 (47) | 21 (30) | 22 (31) | 5 (7) | 19 (27) | 13 (19) | 68 (97) | 36 (51) | 32 (46) |
| Fatigue | 30 (43) | 9 (13) | 1 (1) | 2 (3) | 0 | 11 (16) | 3 (4) | 26 (37) | 22 (31) | 4 (6) |
| Decreased appetite | 21 (30) | 8 (11) | 0 | 0 | 0 | 6 (9) | 0 | 14 (20) | 14 (20) | 0 |
| Maculopapular rash | 20 (29) | 3 (4) | 8 (11) | 2 (3) | 0 | 2 (3) | 2 (3) | 17 (24) | 7 (10) | 10 (14) |
| Pyrexia/fever | 20 (29) | 3 (4) | 1 (1) | 2 (3) | 0 | 4 (6) | 0 | 10 (14) | 9 (13) | 1 (1) |
| HFSR/PPES | 19 (27) | 15 (21) | 3 (4) | 1 (1) | 0 | 0 | 0 | 19 (27) | 16 (23) | 3 (4) |
| Blood bilirubin increased | 18 (26) | 11 (16) | 1 (1) | 0 | 0 | 1 (1) | 1 (1) | 14 (20) | 12 (17) | 2 (3) |
| Dysphonia | 18 (26) | 10 (14) | 0 | 0 | 0 | 1 (1) | 0 | 11 (16) | 11 (16) | 0 |
| Nausea | 16 (23) | 6 (9) | 0 | 0 | 0 | 1 (1) | 0 | 7 (10) | 7 (10) | 0 |
| Abdominal pain | 15 (21) | 1 (1) | 0 | 0 | 0 | 3 (4) | 0 | 4 (6) | 4 (6) | 0 |
| Diarrhoea | 14 (20) | 6 (9) | 1 (1) | 0 | 0 | 5 (7) | 0 | 12 (17) | 11 (16) | 1 (1) |
| AST increased | 14 (20) | 7 (10) | 1 (1) | 0 | 1 (1) | 2 (3) | 1 (1) | 12 (17) | 9 (13) | 3 (4) |
AEs throughout the treatment period were summarised according to MedDRA v23.0 and the severity was categorised by the NCI-CTCAE v5.0.
AE = adverse event; AST = aspartate aminotransferase; HFSR = hand–foot skin reaction; MedDRA = Medical Dictionary for Regulatory Activities; NCI-CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events; PPES = palmar-plantar erythrodysesthesia syndrome.
Events listed are those that occurred in ≥20% of patients regardless of relation to treatment (all AEs), listed in order of descending frequency of AEs of any grade.
No corresponding grade 4 regorafenib-related AEs were reported.
Treatment-related AEs deemed by the investigator to be immune related occurred in 24 patients (34%) and were serious in six patients (9%) (Table 3). The most frequent AEs were skin toxicities (16%; most commonly maculopapular rash [7%] and rash, pruritis, and exfoliation [3% each]) and immune/treatment-related diarrhoea occurred in 6% of patients. Other relevant immune-related AEs, such as pneumonitis and colitis, occurred in one patient each (1%), and hypothyroidism and infusion-related reactions were reported in two patients each (3%).
Table 3.
Immune-related treatment-emergent adverse events.
| AE, n (%) | All patients (N = 70) |
||||
|---|---|---|---|---|---|
| All immune-related treatment-emergent AEs | Immune- and treatment-related AEs |
||||
| All | Regorafenib | Nivolumab | Both regorafenib and nivolumab | ||
| Patients with any AE | 25 (36) | 24 (34) | 3 (4) | 13 (19) | 13 (19) |
| Patients with any serious AE | 7 (10) | 6 (9) | 0 | 4 (6) | 3 (4) |
| Hypothyroidism | 2 (3) | 2 (3) | 0 | 2 (3) | 0 |
| Colitis | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Diarrhoea | 4 (6) | 4 (6) | 0 | 0 | 4 (6) |
| Fatigue | 2 (3) | 2 (3) | 0 | 2 (3) | 0 |
| Pyrexia | 2 (3) | 2 (3) | 0 | 1 (1) | 1 (1) |
| Infusion-related reaction | 2 (3) | 2 (3) | 0 | 2 (3) | 0 |
| ALT increased | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| AST increased | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Hypertriglyceridaemia | 1 (1) | 1 (1) | 0 | 0 | 1 (1) |
| Hyponatraemia | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Arthralgia | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Myalgia | 2 (3) | 1 (1) | 0 | 0 | 1 (1) |
| Myositis | 1 (1) | 1 (1) | 0 | 0 | 1 (1) |
| Pneumonitis | 1 (1) | 0 | 0 | 0 | 0 |
| Dry skin | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Erythema | 1 (1) | 1 (1) | 0 | 1 (1) | 0 |
| Nail discolouration | 1 (1) | 1 (1) | 1 (1) | 0 | 0 |
| Pemphigoid | 1 (1) | 1 (1) | 0 | 0 | 1 (1) |
| Pruritus | 2 (3) | 2 (3) | 0 | 2 (3) | 0 |
| Rash | 2 (3) | 2 (3) | 0 | 0 | 2 (3) |
| Maculopapular rash | 6 (9) | 5 (7) | 1 (1) | 1 (1) | 3 (4) |
| Skin exfoliation | 2 (3) | 2 (3) | 0 | 2 (3) | 0 |
| Hypertension | 1 (1) | 1 (1) | 1 (1) | 0 | 0 |
AEs throughout the treatment period were summarised according to MedDRA v23.0 and the severity was categorised by the NCI-CTCAE v5.0.
AE = adverse event; ALT = alanine aminotransferase; AST = aspartate aminotransferase; MedDRA = Medical Dictionary for Regulatory Activities; NCI-CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events.
A marginal difference in the incidence of rash was observed between males and females in our study. Overall, 20/70 patients (29%) had treatment-emergent maculopapular rash (of those, 15 patients were female). Ten patients (14%) had grade 3 treatment-emergent maculopapular rash (of those, eight patients were female). There were no incidences of grade 4 or 5 treatment-emergent maculopapular rash. Only three patients (4%; one female) had maculopapular rash, which was not considered related to any treatment.
The incidence of increased alanine aminotransferase/aspartate aminotransferase/blood bilirubin was higher in patients with LM compared with those without LM (Appendix Table S3). Grade 3 treatment-related rash occurred in 17% of patients (n = 12; rash [n = 2]; maculopapular rash [n = 10], including pemphigoid [n = 1]) during cycle 1 and was manageable with systemic steroids and dose interruptions; no patient discontinued treatment. Treatment-related HFSR/palmar-plantar erythrodysesthesia syndrome and maculopapular rash (any grade) occurred more frequently in patients without LM (48% and 35%, respectively) versus patients with LM (17% and 19%, respectively) (Appendix Table S3).
Antitumour activity
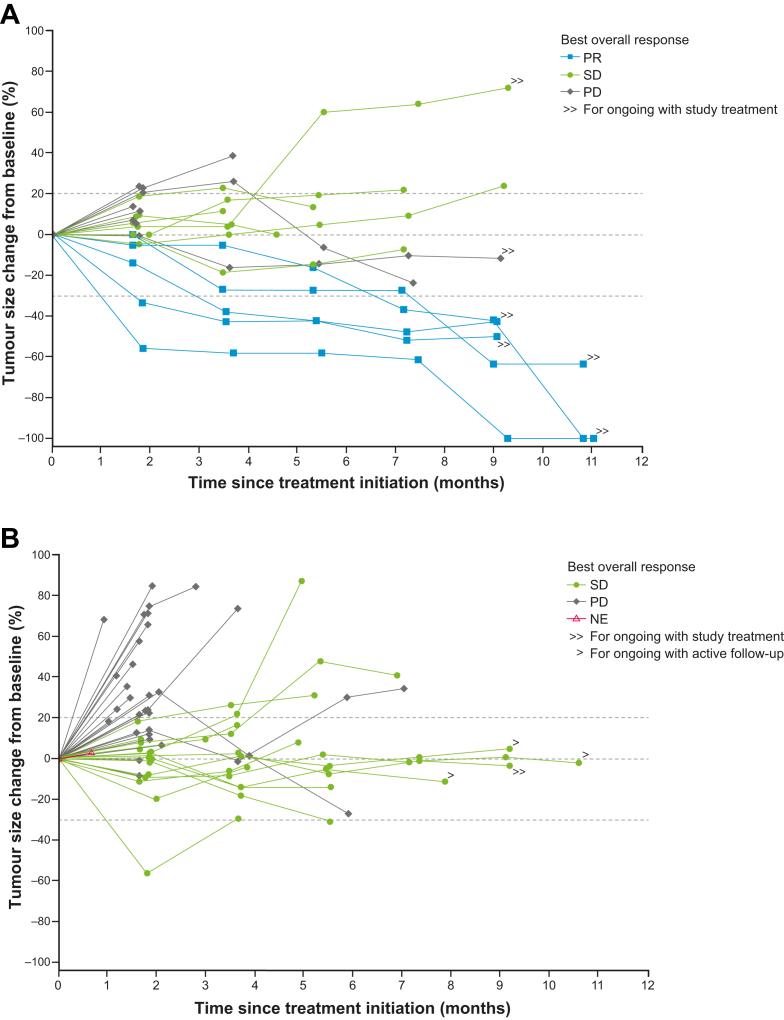
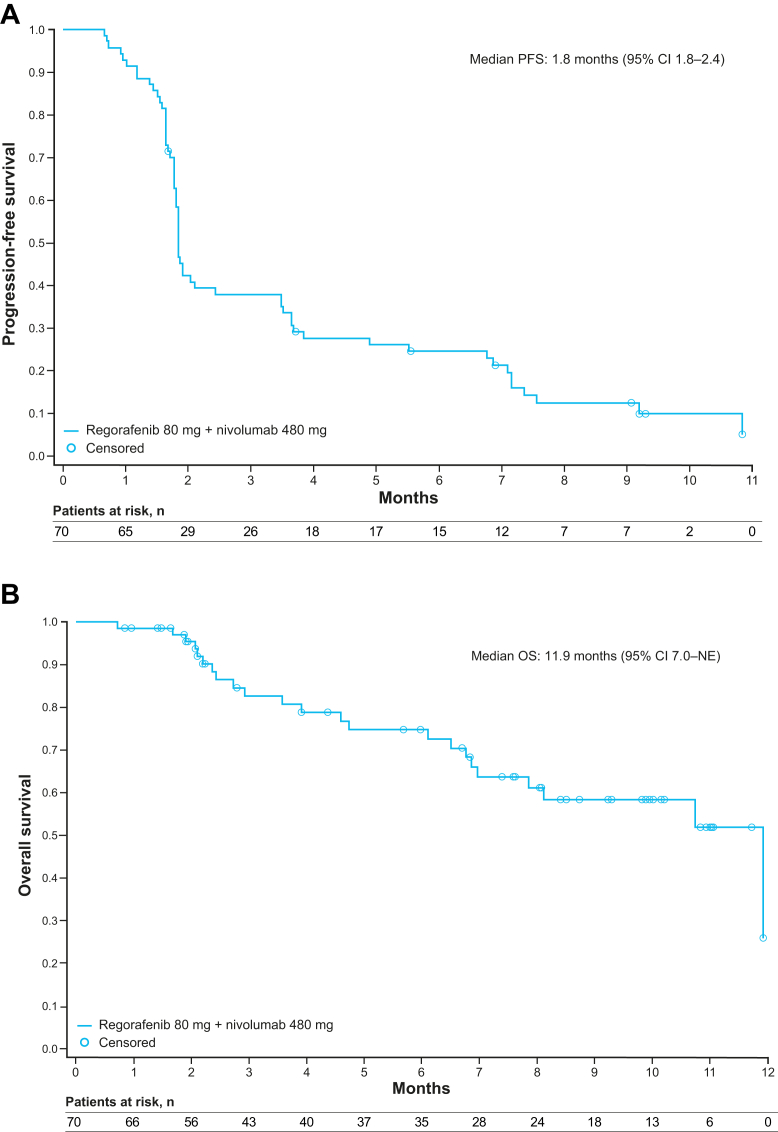
Overall, five patients in the entire treated population (5/70 patients) had a PR according to investigator assessment per RECIST v1.1, yielding an ORR of 7% (95% CI 2.4–15.9; p = 0.27) (Table 4; Fig. 2; Appendix Fig. S1). All five responders did not have LM at baseline and had treatment-emergent AEs of any-grade HFSR and/or rash. In total, 31% of patients (n = 22) had SD, providing a disease control rate of 39%. In the 23 patients without LM, the ORR was 22%. One patient had a CR that was confirmed after the primary completion analysis date. Antitumour activity was observed regardless of regorafenib dose level at C2D1. Median duration of response is not available as only one responder had PD (duration of response was 16.1 weeks). The remaining four responders were censored at 1.9–7.5 months from confirmed response. Median PFS and OS were 1.8 months (95% CI 1.8–2.4) and 11.9 months (95% CI 7.0–not evaluable), respectively (Fig. 3); however, OS data were immature.
Table 4.
Best overall tumour responsea (RECIST v1.1) by presence of liver metastases at baseline.
| Response, n (%) | Without liver metastases (n = 23) | With liver metastases (n = 47) | All patients (N = 70) |
|---|---|---|---|
| Complete response | 0 | 0 | 0 |
| Partial response | 5 (22) | 0 | 5 (7) |
| Stable disease | 8 (35) | 14 (30) | 22 (31) |
| Progressive disease | 9 (39) | 27 (57) | 36 (51) |
| Not evaluable | 1 (4) | 6 (13) | 7 (10) |
| Objective response rate | 5 (22) | 0 | 5 (7) |
| Disease control rate ≥8 weeks | 13 (57) | 14 (30) | 27 (39) |
| Median duration of stable disease, weeks | 30 | 21 | 30 |
Duration of response results are not available for responders since only one patient had progression and the remaining four patients were still classified as responders at the time of analysis.
RECIST = Response Evaluation Criteria in Solid Tumors.
Only confirmed responses are shown: an additional two patients (with liver metastases) had an unconfirmed partial response and one patient with a partial response had a complete response that was confirmed after primary completion.
Fig. 2.
Change in the sum of target lesions by presence of liver metastases at baseline. (A) Patients without liver metastases at baseline. (B) Patients with liver metastases at baseline. NE = not evaluable; PD = progressive disease; PR = partial response; SD = stable disease.
Fig. 3.
Kaplan–Meier survival plots. (A) Progression-free survival in all patients. (B) Overall survival in all patients. CI = confidence interval; NE = not evaluable; OS = overall survival; PFS = progression-free survival. For PFS, 9 patients were censored, and for OS, 47 patients were censored.
Of the 8/23 patients without LM who had treatment-related maculopapular rash, two had SD for at least 7 months and four had a PR. In addition, 10/23 patients without LM had treatment-related HFSR/palmar-plantar erythrodysesthesia syndrome, of whom three had SD for 5.5–9.2 months and four had a PR. Most cases (91%; n = 20/22) of grade 2/3 rashes occurred in the first 2 months of therapy. Median PFS in patients without LM was 3.5 months (95% CI 1.8–7.2) versus 1.8 months (95% CI 1.6–1.9) in patients with LM. Median OS in patients without LM was 11.9 months (95% CI 7.9–11.9) versus 10.7 months (95% CI 6.1–not evaluable) in patients with LM (Appendix Fig. S2).
Biomarkers
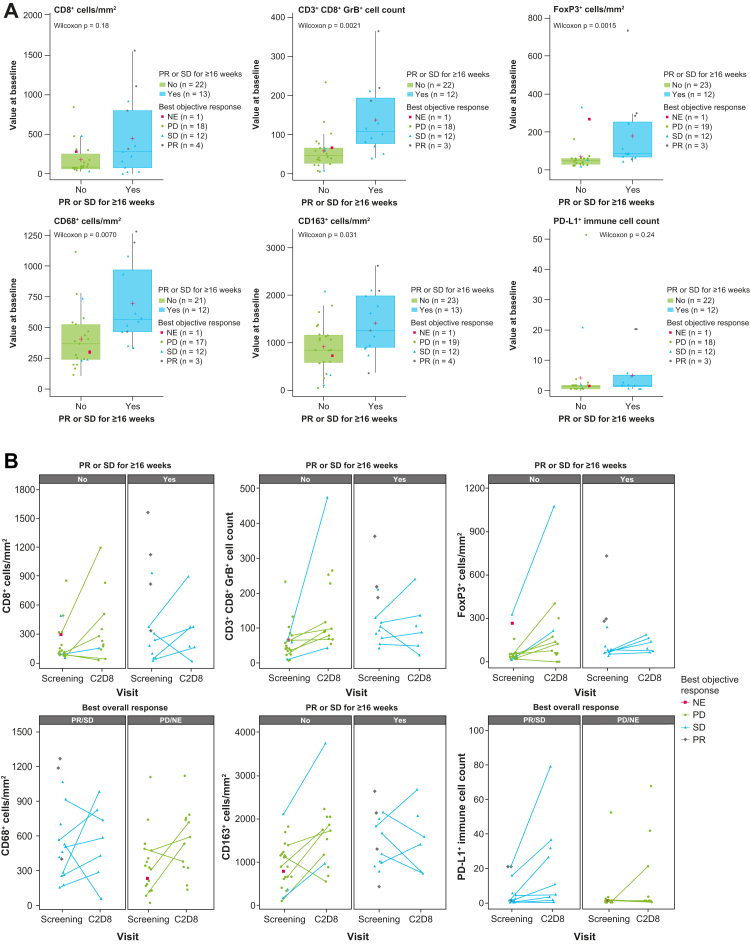
Baseline characteristics and tumour response data in the biomarker subgroups were generally comparable to the overall cohort (Appendix Table S4). Tumour samples from 40 patients were analysed by immunohistochemistry. A higher baseline density of cytotoxic T cells (CD3+ CD8+ GranzymeB+ [GrB], p = 0.0021), regulatory T cells (FoxP3+, p = 0.0015), and macrophages (CD68+, p = 0.0070; CD163+, p = 0.031) in the tumour was significantly associated with a PR or SD for at least 16 weeks (Fig. 4A). The p value was calculated based on a Wilcoxon signed-rank test. A higher baseline density of CD8 (p = 0.031), CD3/CD8/GrB (p = 0.0064), FoxP3 (p = 0.0018), CD68 (p = 0.0095), and higher baseline PD-L1 combined positive score (p = 0.004) in the tumour was significantly associated with prolonged PFS when considering a log-rank test (Appendix Fig. S3). Patients with LM had significantly lower expression of CD8 (p = 0.00024), CD3+CD8+GrB+ (p = 0.0061), and FoxP3 (p = 0.0024) compared with patients without LM (Appendix Fig. S4). The comparison was made using a Wilcoxon signed-rank test. The caveat of such an analysis is potential confounding by the differential sites of biopsy collection (Appendix Table S5), as the tumour microenvironment may differ by organ. In 14 patients with paired tumour samples (baseline and C2D8), increases in the density of CD8+ T cells and CD8+ GrB+ T cells were observed at C2D8, which did not result in a clinical response. There was no evidence of a decrease in regulatory T cells (FoxP3) (Fig. 4B).
Fig. 4.
Association between baseline expression of T cell/macrophage surface proteins and partial response or stable disease for ≥16 weeks. (A) Immunohistochemistry results. (B) Immunohistochemistry results for paired samples. C2D8, day 8 of cycle 2; GrB = GranzymeB; NE = not evaluable; PD = progressive disease; PD-L1 = programmed cell death-ligand 1; PR = partial response; SD = stable disease.
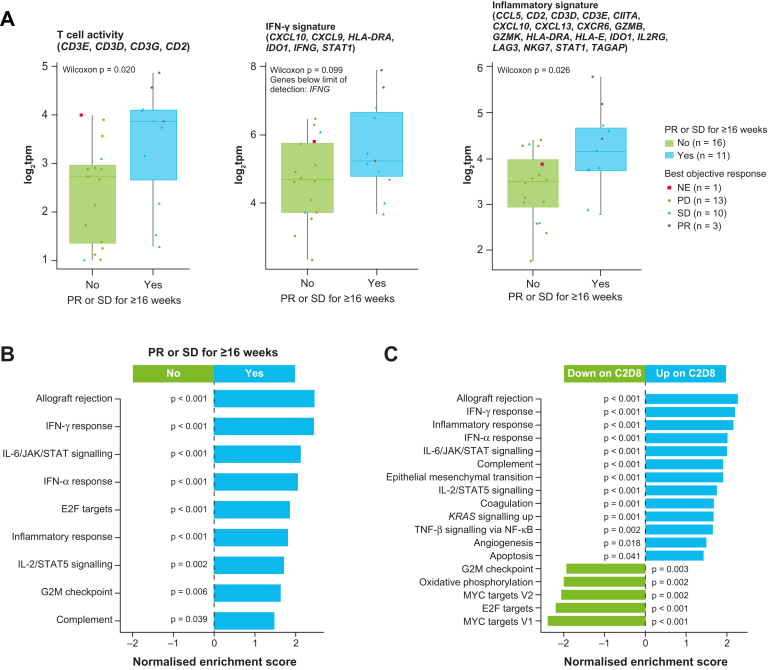
RNA sequencing data showed that higher baseline mRNA expression of gene sets associated (using a Wilcoxon signed-rank test) with immune sensitivity (T cell activity, p = 0.02; interferon [IFN]-γ signature, p = 0.099; and expanded inflammatory signature, p = 0.026)22 were associated with a PR or SD for at least 16 weeks (Fig. 5A). Baseline expression of gene sets that were significantly associated with a PR or SD for at least 16 weeks were mostly related to immune sensitivity, including allograft rejection (p < 0.001), IFN-γ response (p < 0.001), interleukin (IL)-6/JAK/STAT signalling (p < 0.001), and IFN-α response (p < 0.001) (Fig. 5B). Most of these gene sets were upregulated during treatment (Fig. 5C). Analysis of five patients with paired samples (none with a PR) showed immune activation (e.g., T cell, IFN-γ, and immune signatures) in the tumour microenvironment at C2D8, yet there was no evidence of a decrease in suppressive signals (e.g., regulatory T cells) (Appendix Fig. S5).
Fig. 5.
Association between the expression of T cells, interferon-γ, and inflammatory signature with partial response or stable disease for ≥16 weeks (A), gene sets at baseline that were associated with partial response or stable disease for ≥16 weeks (B), and gene sets with expression that changed from baseline today 8 ofcycle 2(C)∗. ∗Analyses included the 27 patients in the RNA sequencing subgroup. C2D8 = day 8 of cycle 2; IFN = interferon; IL = interleukin; JAK = Janus kinase; NF = nuclear factor; PR = partial response; SD = stable disease; STAT = signal transducer and activator of transcription; TNF = tumour necrosis factor.
In a longitudinal analysis of blood samples, statistical significance was assumed if the CIs of the estimated marginal means from a linear mixed model did not contain zero at a visit. We observed statistically significant decreases in the proportion of CD8+ central memory T cells (Appendix Fig. S6A) and statistically significant increases in CD8+ terminally differentiated effector memory T cells (Appendix Fig. S6B). The proportion of CD4+ T cells significantly decreased throughout the study (Appendix Fig. S6C), whereas the proportion of CD4+ regulatory T cells significantly increased (Appendix Fig. S6D). Plasma levels of tumour necrosis factor-α, IFN-γ, IL-2, and IL-6 significantly increased after 1 week of treatment, and soluble vascular endothelial growth factor receptor 2 significantly decreased during cycle 2 (Appendix Fig. S7).
Analysis of KRAS mutation status in tumour samples by next-generation sequencing (n = 22), in ctDNA (n = 57), and from historical data (i.e., at initial diagnosis or from another pre-trial time point in the disease course) showed a higher percentage of PR or SD for at least 16 weeks in KRAS wild type compared with KRAS mutant, although the differences were not statistically significant according to Fisher's exact test (Appendix Table S6). Changes in ctDNA from day 1 of cycle 1 to C2D1 (mean mutation allele frequency/maximum mean mutation allele frequency) were not significantly associated with a PR or SD for at least 16 weeks (p = 0.86/p = 0.51) when using a Wilcoxon signed-rank test (Appendix Fig. S8). The one patient with a CR had ctDNA clearance at C2D1; most patients with increased ctDNA had PD. A numerical trend was observed between a higher clonal tumour mutational burden in baseline ctDNA and prolonged PFS (p = 0.072, log-rank test) (Appendix Fig. S9A) and tumour shrinkage (Rho = −0.25, p = 0.070, Spearman correlation) (Appendix Fig. S9B and S9C). A cut-off of 10 mutations/Mb was applied to define tumour mutational burden ‘high’ versus ‘low’.
Low baseline serum levels of markers related to angiogenesis (IL-8, p = 0.048; cadherin-1, p = 0.00048; vascular endothelial growth factor -D, p = 0.00010; angiopoietin-2, p = 0.022; von Willebrand factor, p = 0.00058; and platelet endothelial cell adhesion molecule-1 [CD31], p = 0.041) were significantly associated with prolonged PFS (Appendix Fig. S10).
Pharmacokinetics
Regorafenib and nivolumab exposures were consistent with previous monotherapy studies, confirming the expected lack of drug–drug interaction between them. Regorafenib exposure increased in a dose proportional manner from 80 mg to 120 mg. There was a trend for higher regorafenib clearance (lower area under the curve) in patients who were escalated to 120 mg in cycle 2 versus those who remained at 80 mg (Appendix Fig. S11). There was no apparent relationship between regorafenib exposure and increases in alanine aminotransferase/aspartate aminotransferase/blood bilirubin, HFSR/rash or treatment-related AEs, or AEs leading to dose reduction/interruption (data on file).
Discussion
In this study, which is, to our knowledge, the largest prospective trial to evaluate PD-1 plus tyrosine kinase inhibitor combination in MSS/pMMR mCRC, treatment with regorafenib plus nivolumab in patients from the USA resulted in five PRs yielding an ORR of 7%; all responses were observed in patients without LM at baseline. In a similar population, Kim et al. demonstrated three confirmed PRs in 40 radiographically evaluable patients (ORR 8%), which supports the response data presented in our study.23 Overall, 73% of patients (n = 38/52) had LM at baseline (compared with 67% in this study). Neither study (ours nor that of Kim et al.) reached the response rate observed in a study of Japanese patients (ORR 33% [n = 8/24]) that included 52% (n = 13/25) with LM at baseline, including one patient with MMR-deficient/microsatellite instability—high CRC.14
In our study, median PFS was 1.8 months and was longer in patients without LM than those with LM (3.5 vs. 1.8 months). In the Japanese study, patients with CRC had a longer median PFS of 7.9 months,14 and the Kim et al. study demonstrated a median PFS of 4.3 months.23 Our median OS was 11.9 months. The OS data were immature in the Japanese study,14 and OS was similar at 11.1 months in the Kim et al. study.23 The baseline characteristics of the study populations (i.e., ECOG PS, tumour sidedness, and presence of LM) were, in general, more favourable in the Japanese study, which may explain the differences in survival outcomes. Notably, the Japanese study had a lower proportion of patients with CRC and LM at baseline (52%) compared with our study (67%).14 Only one of the nine responding patients with CRC in the Japanese trial had LM as target lesions.14 Other factors (genetic, epigenetic, prognostic, environmental) may have contributed to the differences in the results from these trials of patients with MSS/pMMR mCRC. Since the publication of the Japanese trial, several studies of multikinase inhibitors report a low ORR consistent with our study; therefore, we conclude that the Japanese REGONIVO outcomes reflect a select patient population that does not reflect the general MSS refractory population.
The combination of tyrosine kinase inhibitors and PD-1/PD-L1-targeting agents has been investigated in the setting of MSS CRC with varying success17, 18, 19, 20,24, 25, 26, 27; although consistently, patients without LM at treatment initiation seem to derive more benefit, with higher response rates (20–58%) compared with patients with LM (0–5%),14,28,29 as supported by our study (22% vs. 0%). Translational research data have shown that tumour biomarkers for immune sensitivity (e.g., CD8+ T cells) have significantly lower expression in patients with LM compared with patients without LM,30 which may contribute to the lack of clinical benefit in patients with LM. The tumour microenvironment of the liver is known to be immunosuppressive, and LM are associated with resistance to immunotherapy.31,32 Unfortunately, LM are eventually present in around 70% of patients and are hypothesised to influence the systemic immune response. In our study population, 81% had a history of LM, and from the 47 patients (67%) with LM at baseline, none achieved a confirmed response. Of note, three of the five responders had a history of treated LM (surgery or radiotherapy). Local treatment of LM might favourably affect the response to a tyrosine kinase inhibitor/immune checkpoint inhibitors combination, and this approach is being investigated.33
This study confirms that the combination of regorafenib and nivolumab has a manageable safety profile.14,23 Of note, in the Japanese study, regorafenib 160 mg plus nivolumab was considered too toxic; therefore, subsequent studies combined nivolumab with regorafenib at the 120 mg dose level or lower since dose-limiting toxicities occurred at 160 mg but not at the 80 or 120 mg dose levels. The maximum tolerated dose was 120 mg/day, but most patients in the dose expansion cohort required dose reduction to 80 mg/day, and patients who were treated with a starting dose of 80 mg tolerated treatment well. Moreover, there was no difference in efficacy between these two starting doses.14 Our pharmacokinetic analyses support a customised dosing approach for regorafenib. The safety profile in this study was consistent with the known safety profile of regorafenib and/or nivolumab, although we observed a higher incidence of grade 3 rash. No association between regorafenib exposure and rash was observed. Immune-related skin toxicities have been associated with response to immune checkpoint inhibitors and are hypothesised to be indicative of immune activation.34 Likewise, the early occurrence of HFSR is described for regorafenib monotherapy35 and may be associated with treatment priming that results in a robust tumour-mediated immune response.36 In patients without LM and prevalent skin toxicity, we observed better disease control, suggesting an interplay between the pattern of metastatic disease and ability to mount an effective immune response.
Higher expression of biomarkers for pre-existing immune sensitivity (e.g., PD-L1 or CD8) in tumour samples and lower concentration of biomarkers related to angiogenesis, such as IL-8 in peripheral blood samples, trended with better clinical outcome. Elevated serum IL-8 is known to be associated with enhanced intratumour neutrophils and reduced clinical benefit of immune checkpoint inhibitors.37 Tumour biomarkers that were associated with clinical benefit were expressed at a significantly lower level in patients with LM compared with patients without LM. Increased immune activity during treatment was observed in both tumour and blood samples, but was not correlated with clinical outcome. Owing to the small sample size and the absence of a paired tumour sample from a patient with a PR, the results should be considered as hypothesis-generating only.
The main limitations of this study are inherent to its design, including the single arm nature that impedes a direct comparison of the combination versus regorafenib monotherapy, which is an approved indication for previously treated mCRC. In addition, since this was an open-label study, there is the possibility that bias could be introduced (e.g., through detection bias). Furthermore, the study was not designed or powered to prospectively test the activity of regorafenib plus nivolumab in patients with LM versus those without LM, and our findings are hypothesis-generating. However, the results are corroborated by two recently reported studies on botensilimab (an anti-cytotoxic T-lymphocyte–associated antigen 4 antibody) combined with balstilimab (an anti-PD-1 antibody) and a study of regorafenib, ipilimumab, and nivolumab.38,39 Both studies confirmed that responses were limited to non-LM MSS CRC patients. Lastly, as in the case of other oncology trials, this study excluded patients with ECOG PS 2 on account of a much poorer prognosis and an increased risk of grade 3/4 toxicities compared with patients with ECOG PS 0/1. Including patients with ECOG PS 2 may have negatively impacted the study results, undermining the outcomes of those patients with better PS. The authors acknowledge that more interventional studies are warranted in this patient population.
In conclusion, although our study did not meet its predefined target primary endpoint in an unselected cohort of patients with MSS/pMMR mCRC, the demonstrated level of activity in a subset of patients in our study warrants further investigation to identify subgroups of patients with clinical characteristics and/or biomarkers that would benefit most from treatment with regorafenib plus nivolumab.
Contributors
SC, DDA, HZG, NS, AS, and JF contributed to the design of the study. YAW, BB, CO, DZC, DC, ALC, DDA, MF, HZG, TH, ASP, KPSR, TL, NS, and RT contributed to data collection. YAW, BP, BB, CO, DZC, DC, ALC, DDA, MF, HZG, TH, ASP, KPSR, NS, HS, RT, and UM contributed to data analysis. YAW, BB, CO, ALC, SC, MF, HZG, KPSR, and RT contributed to data interpretation. CO and NS contributed to the literature search. DDA and JF contributed to the supervision of the study. SC contributed to medical data monitoring. AS and TL provided resources necessary for the study. HS contributed to the preparation of the figures. YAW, BP, BB, CO, MF, HZG, KPSR, TL, and RT contributed to the writing of the manuscript. All authors were involved in the reviewing and editing of the manuscript, agree to be accountable for all aspects of the work, and accept responsibility for the decision to submit the publication. All authors had access to all data reported in the study. CO and MF accessed and verified the underlying data in the study and had final responsibility for the decision to submit the publication.
Data sharing statement
Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point, and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after 1 January 2014.
Interested researchers can use www.vivli.org to request access to anonymised patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal.
Data access will be granted to anonymised patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Declaration of interests
YAW, AW, and HS report employment with Bayer. BP and BB report employment and stock ownership with Bayer. CO and SC report previous employment with Bayer. HZG report previous employment and stock or stock options with Bayer. DDA reports employment and stock ownership from Bristol Myers Squibb. MF reports research funding (to institution) from Amgen, Bristol Myers Squibb, Genentech, Verastem, and Novartis, consulting fees from AstraZeneca, Bristol Myers Squibb, Incyte, PsiOxus, Zhuhai Yufan Biotech, and Taiho Pharmaceutical, honoraria from Guardant360, and advisory roles from Amgen, Bayer, Array Biopharma, Eisai, GSK, Merck, Mirati, Nouscom, Roche/Genentech, and Xenthera. NS reports employment, support for attending meetings and stock ownership with Bayer. ASP reports research funding (to institution) from Ipsen, Bristol Myers Squibb, Exelixis, Hutchison MediPharma, Taiho Pharmaceutical, Lilly, AstraZeneca, Incyte, Deciphera, G1 Therapeutics, Zentalis, Tempus, Camurus, Relay Therapeutics, Nucana, Merck, Bayer, Seattle Genetics, Sotio, Innovative Cellular Therapeutics, Camurus, Regenxbio, Gilead Sciences, Gritstone Bio, BioNTech S, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Cardinal Health and Ideo Oncology, leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Amgen, Bristol Myers Squibb, Eisai, Ipsen, Advanced Accelerator Applications, Incyte, Exelixis, Pfizer, QED Therapeutics, Lilly, Mirati Therapeutics, Hutchison MediPharma, Astellas Pharma, AADi, Stromatis Pharma, EMD Serono, AstraZeneca, and Servier, and stock or stock options from Actinium Pharmaceuticals, Aptose Biosciences, Alexion Pharmaceuticals, Lynx Health, and Stromatis Pharma. KPSR reports research funding to institution from Bayer, Daiichi Sankyo, Merck, Hibercell, UCB Biosciences, Janssen, Eisai, AbbVie, Guardant, Innovent, and Xencor, payment or honoraria for educational events from Bayer, Daiichi Sankyo, and Seagen, and participation on advisory board of Daiichi Sankyo, Eisai, Merck, SAGA Diagnostics, Bayer, Seagen, Pfizer, and AstraZeneca. RT reports employment and stock ownership with Bristol Myers Squibb. UM reports consulting fees from Bayer as a statistician for ClinStat. AC, DZC, DC, TH, TL, and JF have nothing to disclose.
Acknowledgements
We acknowledge Rebecca Freudling and Laura Schlieker (Staburo GmbH, Munich, Germany) for their contributions to the statistical analyses. We acknowledge Jochen Zisowsky (Bayer AG, Germany) and Blesson Chacko, Michael Watts, and Jon Moss (BAST Inc Ltd, UK) for their contribution to the pharmacokinetic and exposure–response analyses. We acknowledge Johanna C. Bendell (Roche, Basel, Switzerland) and Amy Hammell (Bristol Myers Squibb, Lawrenceville, NJ, USA) for their contributions to this study. Editorial assistance in the preparation of this manuscript was provided by Matthew Reynolds, Matthew Naylor, and Robyn Fowler of OPEN Health Communications (London, UK), with financial support from Bayer.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101917.
Appendix A. Supplementary data
References
- 1.Bayer A.G. 2022. Regorafenib (Stivarga). European Medicines Agency: summary of product characteristics. [Google Scholar]
- 2.Bayer Healthcare Pharmaceuticals Inc . 2020. Regorafenib (Stivarga) U.S Food and Drug Administration prescribing information. [Google Scholar]
- 3.Cervantes A., Adam R., Rosello S., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Bristol Myers Squibb Company . 2021. Nivolumab (Opdivo). U.S. Food and Drug Administration prescribing information. [Google Scholar]
- 5.Merck Sharp & Dohme Corp . 2020. Pembrolizumab (Keytruda) U.S Food and Drug Administration prescribing information. [Google Scholar]
- 6.Borelli B., Antoniotti C., Carullo M., Germani M.M., Conca V., Masi G. Immune-checkpoint inhibitors (ICIs) in metastatic colorectal cancer (mCRC) patients beyond microsatellite instability. Cancers. 2022;14(20):4974. doi: 10.3390/cancers14204974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemohammad H., Najafzadeh B., Asadzadeh Z., et al. The importance of immune checkpoints in immune monitoring: a future paradigm shift in the treatment of cancer. Biomed Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112516. [DOI] [PubMed] [Google Scholar]
- 8.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Makker V., Rasco D., Vogelzang N.J., et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PubMed] [Google Scholar]
- 11.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 12.Keeler M., Kessler E., Bernard B., et al. Pembrolizumab (pembro) and cabozantinib (cabo) in patients (pts) with metastatic renal cell carcinoma (mRCC): phase I results. J Clin Oncol. 2017;37(Suppl 7S):600. [Google Scholar]
- 13.Doleschel D., Hoff S., Koletnik S., et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res. 2021;40(1):288. doi: 10.1186/s13046-021-02043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuoka S., Hara H., Takahashi N., et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion Phase Ib trial (REGONIVO, EPOC1603) J Clin Oncol. 2020;38(18):2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A., Van Cutsem E., Sobrero A., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 16.Ooki A., Shinozaki E., Yamaguchi K. Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon. 2021;5(1):11–24. doi: 10.23922/jarc.2020-064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R.D., Kovari B.P., Martinez M., et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer. 2022;169:93–102. doi: 10.1016/j.ejca.2022.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Barzi A., Azad N., Yang Y., et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractorymicrosatellite stable colorectalcancer (MSSCRC) J Clin Oncol. 2022;40(4_suppl):15. [Google Scholar]
- 19.Saeed A., Park R., Dai J., et al. Phase II trial of cabozantinib (Cabo) plus durvalumab (Durva) in chemotherapy refractory patients with advanced mismatch repair proficient/microsatellite stable (pMMR/MSS) colorectal cancer (CRC): CAMILLA CRC cohort results. J Clin Oncol. 2022;40(4_suppl):135. [Google Scholar]
- 20.Abrams T., Kazmi S., Winer I., et al. A phase 1b multitumor cohort study of cabozantinib plus atezolizumab in advanced solid tumors (COSMIC-021): results of the colorectal cancer cohort. J Clin Oncol. 2022;40(4_suppl):121. [Google Scholar]
- 21.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 22.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim R., Imanirad I., Strosberg J., Carballido E., Kim D. PD-2 Final result of phase IB study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Ann Oncol. 2021;32(Suppl 3):S199. [Google Scholar]
- 24.Cousin S., Bellera C.A., Guégan J.P., et al. REGOMUNE: a phase II study of regorafenib plus avelumab in solid tumors—results of the non-MSI-H metastatic colorectal cancer (mCRC) cohort. J Clin Oncol. 2020;38(15 Suppl):4019. [Google Scholar]
- 25.Wang F., He M.M., Yao Y.C., et al. 433P A phase Ib/II clinical trial of tolerability, safety and efficacy of regorafenib in combination with toripalimab (a PD-1 antibody) in patients with relapsed or metastatic colorectal cancer. Ann Oncol. 2020;31(4 Suppl):S425. [Google Scholar]
- 26.Gomez-Roca C., Yanez E., Im S.-A., et al. LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—results from the colorectal cancer cohort. J Clin Oncol. 2021;39(3 Suppl):94. [Google Scholar]
- 27.Xiao L., Zhang Y., Lin Q. 442P Camrelizumab combined with apatinib in the treatment of patients with advanced gastric cancer and colorectal cancer: one-arm exploratory clinical trial. Ann Oncol. 2020;31(4 Suppl):S429. [Google Scholar]
- 28.Wang C., Sandhu J., Ouyang C., Ye J., Lee P.P., Fakih M. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.18416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C., Fakih M. Targeting MSS colorectal cancer with immunotherapy: are we turning the corner? Expert Opin Biol Ther. 2021;21(10):1347–1357. doi: 10.1080/14712598.2021.1933940. [DOI] [PubMed] [Google Scholar]
- 30.Fakih M., Raghav K.P.S., Chang D.Z., et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC) J Clin Oncol. 2021;39(15 Suppl):3560. [Google Scholar]
- 31.Yu J., Green M.D., Li S., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botticelli A., Cirillo A., Scagnoli S., et al. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines (Basel) 2020;8(2):203. doi: 10.3390/vaccines8020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim C.W., Chon H.J., Kim C. Combination immunotherapies to overcome intrinsic resistance to checkpoint blockade in microsatellite stable colorectal cancer. Cancers. 2021;13(19):4906. doi: 10.3390/cancers13194906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y., Xie W., Huang H., et al. Association of immune related adverse events with efficacy of immune checkpoint inhibitors and overall survival in cancers: a systemic review and meta-analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.633032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grothey A., Sobrero A.F., Siena S., et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol. 2013;31(15 Suppl):3637. [Google Scholar]
- 36.Kobayashi K., Kawakami K., Yokokawa T., et al. Association of hand-foot skin reaction with regorafenib efficacy in the treatment of metastatic colorectal cancer. Oncology. 2019;96(4):200–206. doi: 10.1159/000495989. [DOI] [PubMed] [Google Scholar]
- 37.Schalper K.A., Carleton M., Zhou M., et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullock A., Grossman J., Fakih M., et al. LBA O-9: botensilimab, a novel innate/adaptive immune activator, plus balstilimab (anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer. Ann Oncol. 2022;33(4 Suppl):S376. [Google Scholar]
- 39.Fakih M., Sandhu J., Lim D., Li S., Wang C. 320MO–a phase I clinical trial of regorafenib, ipilimumab, and nivolumab (RIN) in chemotherapy resistant MSS metastatic colorectal cancer (mCRC) Ann Oncol. 2022;33(suppl_7):S136–S196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.