Abstract
Aims/Introduction
Glucagon, a peptide hormone produced from proglucagon, is involved in the pathophysiology of diabetes. Plasma glucagon levels are currently measured by sandwich enzyme‐linked immunosorbent assay (ELISA), but the currently used sandwich ELISA cross‐reacts with proglucagon‐derived peptides, thereby providing incorrect results in subjects with elevated plasma proglucagon‐derived peptide levels. We aimed to develop a more broadly reliable ELISA for measuring plasma glucagon levels.
Materials and Methods
A new sandwich ELISA was developed using newly generated monoclonal antibodies against glucagon. After its validation, plasma glucagon levels were measured with the new ELISA and the currently used ELISA in subjects who underwent laparoscopic sleeve gastrectomy (LSG) and in outpatients with suspected glucose intolerance. The ELISA results were compared with those from liquid chromatography‐high resolution mass (LC‐HRMS) analysis, which we previously established as the most accurate measuring system.
Results
The new ELISA has high specificity (<1% cross‐reactivities) and high sensitivity (a lower range of 0.31 pmol/L). Plasma glucagon values in the subjects who underwent laparoscopic sleeve gastrectomy and some outpatients with suspected glucose intolerance differed between the new ELISA and the currently used ELISA. These subjects also showed markedly high plasma glicentin levels. Despite the elevated plasma glicentin levels, the new ELISA showed better positive correlation with LC‐HRMS than did the currently used ELISA.
Conclusions
The new ELISA enables more accurate measurement of plasma glucagon than the currently used ELISA, even in subjects with elevated proglucagon‐derived peptide levels. It should be clinically useful in elucidating the pathophysiology of individual diabetic patients.
Keywords: Glicentin, Glucagon, Sandwich ELISA
A new glucagon sandwich enzyme‐linked immunosorbent assay (ELISA) has been developed that enables highly reliable measurement. The new ELISA provided much more reliable glucagon values than the currently used ELISA even in the subjects with elevated plasma proglucagon‐derived peptide levels. This new ELISA should be clinically useful in elucidating the pathophysiology of individual diabetic patients.
INTRODUCTION
Glucagon is produced and secreted mainly in pancreatic α cells. Its main physiological function is to promote hepatic glucose production by enhancing glycogenolysis and gluconeogenesis. Because glucagon is reported to be involved in the pathophysiology of diabetes, 1 , 2 , 3 it is expected to be a new target in the treatment of diabetes. 4 , 5 Recently, it has become known that glucagon also plays important roles in the regulation of amino acid metabolism. 6 , 7 , 8 Moreover, the agonists of glucagon have been shown to ameliorate obesity and diabetes. 9 , 10 Therefore, detailed analyses of the relationship between plasma glucagon levels and the condition of patients are important in understanding the pathophysiology of individual patients with obesity and diabetes.
One of the factors contributing the most to the progress of glucagon research in recent years is that the reliability of plasma glucagon measurements has been improved by the development of more accurate glucagon assays. Since glucagon is produced by the processing of proglucagon, multiple peptides that share a common sequence with glucagon exist in the circulation. In addition, the concentration of plasma glucagon is in the low picomolar range. Therefore, a reliable glucagon assay requires both high specificity and high sensitivity. 11 Recently, a sandwich ELISA that uses antibodies to both the C‐terminus and N‐terminus of glucagon has been developed to increase the specificity of measurement. 12 We also developed a method that can quantitatively measure plasma glucagon in the picomolar concentration range using LC‐HRMS. 13 This system has not only facilitated the accurate measurement of plasma glucagon levels, but has also enabled the evaluation of the reliability of existing glucagon immunoassays. Comparative analyses of plasma glucagon measurements have revealed that the sandwich ELISA is much more reliable than conventional RIA in both healthy subjects and in patients with type 2 diabetes. 13 , 14
However, the currently used ELISA still has cross‐reactivities with proglucagon‐derived peptides due to their remaining non‐specific reaction with the antibodies used in this system. 15 , 16 Thus, it has been reported that in the case of elevated proglucagon‐derived peptide levels, such as after bariatric surgery or pancreatectomy, the currently used ELISA shows falsely high glucagon values due to cross‐reactivity with these peptides. 16 , 17 , 18 In order to solve this problem, a modified protocol of the currently used ELISA has been reported. 16 , 17
As an alternative, we have developed a new sandwich ELISA using newly developed monoclonal antibodies against glucagon. Here we show that the new ELISA provides reliable glucagon measurements, even in the plasma of morbidly obese patients who have undergone laparoscopic sleeve gastrectomy, where markedly elevated levels of proglucagon‐derived peptides have been reported. 19 We conclude that the new ELISA provides more accurate plasma glucagon measurements than the currently used ELISA in all subjects, regardless of their plasma proglucagon‐related peptide levels.
MATERIALS AND METHODS
Development of a new glucagon sandwich ELISA
To develop the new glucagon sandwich ELISA, monoclonal antibodies were raised that recognize either the N‐terminal or C‐terminal sequence of glucagon. Peptide fragments having N‐ or C‐terminal sequences of glucagon conjugated to keyhole limpet hemocyanin were emulsified with Freund's complete adjuvant and used to immunize Wistar rats, B6D2F1 mice (purchased from CLEA Japan, Inc., Tokyo, Japan), or glucagon KO mice 20 which are expected to produce anti‐glucagon antibodies with high efficiency due to the absence of endogenous glucagon. Following a boost with immunogen, polyethylene glycol 1,500 was used to fuse spleen cells with myeloma cells, and we screened for hybridomas reactive only to glucagon.
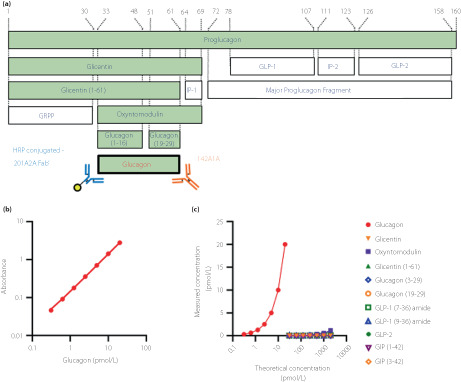
The resulting two monoclonal antibodies (201A2A, 142A1A) were used to establish a sandwich ELISA (Figure 1a). A detailed assay protocol is provided in Appendix S1.
Figure 1.
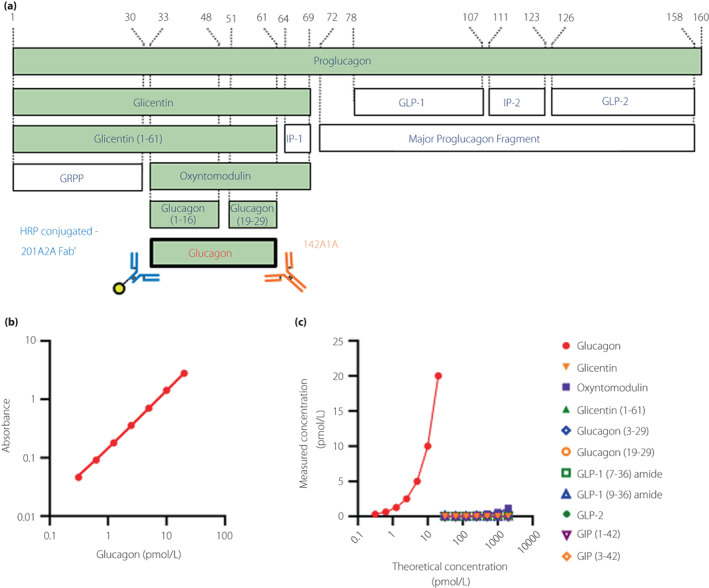
The newly developed glucagon sandwich ELISA. (a) Schematic diagram of proglucagon‐derived peptide and monoclonal antibodies used in the new ELISA. The peptides that share a common sequence with glucagon are shown as the green columns. The numbers of the amino acid residues corresponding to proglucagon are shown at the top. GRPP, glicentin‐related polypeptide; HRP, horseradish peroxidase; IP, intervening peptide. (b) Standard curve and (c) glucagon‐related peptide cross‐reactivity test for the new ELISA.
Validation of the new ELISA
To test the intra‐assay coefficient, control samples of three different concentrations were measured in 24 wells each in the same assay. To test the inter‐assay coefficient, control samples of three different concentrations were measured on seven different days. To test the dilutional linearity of each plasma sample, three plasma samples having different concentrations were serially diluted 2–32 times with an assay buffer, and the glucagon concentration was measured. For the spike and recovery test, a known amount of synthetic glucagon was spiked into three plasma samples and an assay was performed to calculate the recovery rate. To test cross‐reactivity, oxyntomodulin, glicentin, glicentin (1–61), glucagon (3–29), glucagon (19–29) (BEX, Tokyo, Japan), glucagon‐like peptide (GLP)‐1 (7–36) amide (Peptide Institute, Osaka, Japan), GLP‐1 (9–36) amide, GLP‐2 (Bachem, Torrance, CA, USA), glucose‐dependent insulinotropic polypeptide (GIP) (1–42), and GIP (3–42) (Phoenix Pharmaceuticals, Burlingame, CA, USA) were measured with the new ELISA. Peptides were measured in a dilution series. Cross‐reactivity was calculated from the measured values of each peptide at a concentration of 2000 pmol/L. If the cross‐reactivity was less than 0.01%, it was considered ‘not detected’ (ND). Except in the intra‐assay variability test, the tests were measured in duplicate.
Subjects
Patients with morbid obesity, before and after laparoscopic sleeve gastrectomy
Samples were collected from 23 patients with morbid obesity. These patients met the criteria for bariatric surgery in Japan 21 and were recruited from the outpatient obesity clinic at Omi Medical Center for medical and surgical management of morbidly obese patients. The surgical procedure was performed as described previously. 22 An oral glucose tolerance test (OGTT) was performed before and 6 months after laparoscopic sleeve gastrectomy (LSG), and blood samples were collected. Table S1 provides information regarding the patients.
Outpatients with suspected glucose intolerance
The samples were collected from 104 subjects recruited at Kitada Internal Medicine Clinic. Outpatients who were suspected to have impaired glucose tolerance by their attending physicians underwent an oral glucose tolerance test after obtaining informed consent. The exclusion criteria for the subjects were as follows: (1) minors; (2) taking oral hypoglycemic drugs; (3) abnormal liver or renal function test results; (4) cardiopulmonary dysfunction; (5) symptoms of anemia; (6) pregnant woman; (7) judgment of ineligibility for other reasons by the doctor. Table S2 provides information regarding the patients.
Oral glucose tolerance test
Subjects from whom consent had been obtained underwent glucose loading after overnight fasting. Blood samples were collected over time before and after glucose loading (Trelan‐G75 225 mL × 1 bottle containing 75 g of glucose; Yoshindo Inc., Toyama, Japan). For the measurement of glucagon, glicentin, and GLP‐1, blood was collected by using a BD P800 blood collection tube (Becton Dickinson, Tokyo, Japan). Plasma was separated by centrifugation at 1200 g for 20 min, dispensed, and stored at −80°C.
Measurements of hormones and biochemical parameters
Glucagon was measured with the new ELISA, the currently used ELISA (Mercodia Glucagon ELISA, Mercodia AB, Uppsala, Sweden), and LC‐HRMS. 13 In the currently used ELISA, the modified sequential protocol, with additional washing steps to improve reaction specificity 16 , 17 was also used for some subjects. Glicentin and GLP‐1 were measured by use of a Glicentin ELISA kit (Mercodia) and GLP‐1 (9‐36/37) assay kit (Immuno‐Biological Laboratories, Fujioka, Japan), respectively. The other biochemical parameters were measured at the respective institutions. When the concentration of the measured sample was lower than that of the lowest concentration of the standards, it was expressed as being below the lower limit of quantification (LLOQ), and the subsequent calculation was performed using the value of the lowest concentration of the standards.
Statistical analysis
Data are expressed as the mean ± SD, except for data with unequal variance on clinical characteristics of the participants, which are expressed as the medians with the first and third quartile. Differences between the two groups were assessed by using Student's or Welch's t‐test. Comparison of three or more groups was performed by Bonferroni post hoc testing. Relationships between parameters were assessed using Pearson's correlation coefficient. In the correlation analysis, samples indicating below the LLOQ were excluded from the analysis. The relationship between categorical data was analyzed by Fisher's exact test or chi‐square test. A P‐value of less than 0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics version 28 software.
RESULTS
Development of the new glucagon sandwich ELISA
Using Wistar rats, B6D2F1 mice, and glucagon KO mice, 17 hybridoma clones producing anti‐glucagon N‐terminal monoclonal antibodies and 18 clones producing C‐terminal monoclonal antibodies were established. Sandwich ELISA systems were constructed by the combination of these antibodies and assay validation tests performed for each ELISA. As a result, the combination of 201A2A (rat‐derived anti‐N‐terminal antibody) and 142A1A (glucagon KO mouse‐derived anti‐C‐terminal antibody) (Figure 1a) showed the highest specificity and sensitivity in the validation test (data not shown). In the sandwich ELISA measurement system using these new monoclonal antibodies, quantification of glucagon was possible in the range of 0.31–20 pmol/L (Figure 1b). Since the results of the intra‐/inter‐assay variability tests (Table S3), dilutional linearity test (Figure S1), and spike and recovery test (Table S4) were acceptable, this new ELISA was considered able to measure glucagon levels in plasma at a low picomolar range quantitatively. Notably, the cross‐reactivities with the related peptides other than glucagon were very low, at <1% (Figure 1c, Table 1). The new ELISA was expected to have very high specificity and sensitivity.
Table 1.
Cross‐reactivities of glucagon‐related peptides in the new ELISA
| Peptide | Cross reactivity |
|---|---|
| Glicentin | ND |
| Oxyntomodulin | 0.06% |
| Glicentin (1–61) | 0.05% |
| Glucagon (3–29) | ND |
| Glucagon (19–29) | ND |
| GLP‐1 (7–36) amide | 0.02% |
| GLP‐1 (9–36) amide | 0.01% |
| GLP‐2 | ND |
| GIP (1–42) | ND |
| GIP (3–42) | ND |
The reactivity with glucagon is calculated as 100%.
ND, not detected.
Plasma glucagon levels measured by the new ELISA better positively correlated with those measured by LC‐HRMS than did those from the currently used ELISA, in the subjects after LSG
To compare the reliability of the new ELISA with that of the currently used ELISA, plasma glucagon levels were measured in the patients after laparoscopic sleeve gastrectomy, in whom the plasma proglucagon‐derived peptides were considered to be elevated, and the results verified with LC‐HRMS. As expected, plasma glicentin and GLP‐1 were markedly elevated during OGTT after LSG (Figure S2a,b). Also, glicentin and GLP‐1 showed significant positive correlations before and after laparoscopic sleeve gastrectomy (Figure S2c,d). Before LSG, the patterns of glucagon levels during OGTT were similar among these three assays (Figure 2a) and the results measured by both the new ELISA and the currently used ELISA were well correlated with the results of LC‐HRMS (Figure 2b,c). However, after LSG, although the new ELISA and LC‐HRMS showed similar patterns (Figure 2d) and glucagon levels were well correlated (Figure 2e), the currently used sandwich ELISA showed a paradoxically elevated glucagon pattern during OGTT (Figure 2d), and therefore its correlation with LC‐HRMS was diminished (Figure 2f). Because glicentin, which may cause cross‐reaction with sandwich ELISA, was markedly elevated after LSG (Figure S2a), we investigated the correlation between the levels of glucagon and glicentin. Before LSG, glucagon showed a weak and negative correlation with glicentin in all three assays (Figure 2g–i). However, after LSG, only the glucagon values measured by the currently used ELISA positively correlated with glicentin (Figure 2j–l), suggesting that cross‐reaction with glicentin interferes with glucagon measurement in the currently used ELISA.
Figure 2.
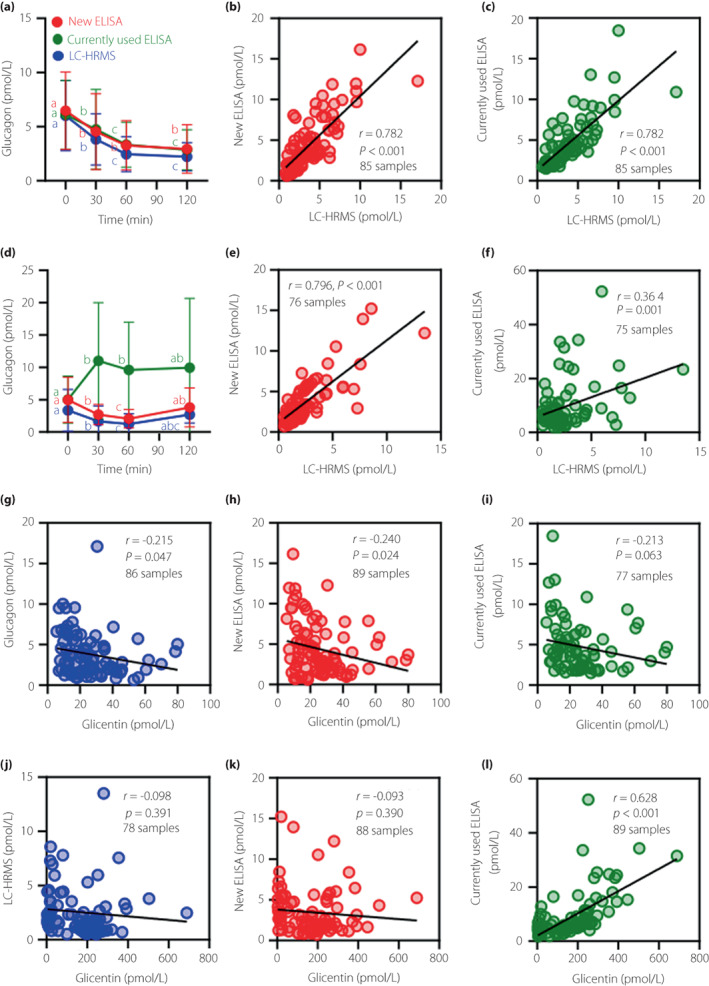
Plasma glucagon levels measured by the new ELISA, the currently used ELISA, and LC‐HRMS during oral glucose tolerance test in patients with morbid obesity before and after laparoscopic sleeve gastrectomy. (a) Changes in plasma glucagon levels during OGTT in the patients before LSG. (b, c) Correlations of plasma glucagon levels between the new ELISA (b) or the currently used ELISA (c) and LC‐HRMS. (d) Changes in plasma glucagon levels during OGTT in the patients 6 months after LSG. (e, f) Correlations of plasma glucagon levels between the new ELISA (e) or the currently used ELISA (f) and LC‐HRMS. (g–i) Correlations between plasma glucagon levels measured by LC‐HRMS (g), the new ELISA (h), or the currently used ELISA (i) and plasma glicentin levels in patients with morbid obesity before LSG. (j–l) Correlations between plasma glucagon levels measured by LC‐HRMS (j), the new ELISA (k), or the currently used ELISA (l) and plasma glicentin levels in patients with morbid obesity 6 months after LSG. Data are mean ± SD. The different letters in glucagon levels during OGTT indicate statistical significance in the changes of glucagon in each measurement method (P < 0.05). In the correlation analysis, the number of samples in each panel is shown, in addition to the correlation coefficient (r) and P‐value, because samples with values below the LLOQ were excluded from the analysis.
We also performed glucagon measurements by using the sequential protocol of the currently used ELISA on the samples from patients who underwent laparoscopic sleeve gastrectomy (Figure S3a,d). Even after LSG, glucagon measured by the sequential protocol showed improved positive correlation with glucagon measured by LC‐HRMS and no significant correlation with glicentin (Figure S3e,f). However, in the sequential protocol, many samples had values below the LLOQ (in total, 64 out of 92 samples) (Figure S3j,n). In contrast, in the new ELISA, only 5 out of 92 samples had values below the LLOQ (Figure S3h,l), suggesting that the new ELISA has sufficient sensitivity to evaluate plasma glucagon levels in the low picomolar concentration range.
Plasma glucagon levels in 30 out of 104 outpatients with suspected glucose intolerance differed between the new ELISA and the currently used ELISA
Next, to test whether the new ELISA is useful for evaluating plasma glucagon levels in patients who have not undergone bariatric surgery, we conducted OGTT on 104 outpatients with suspected glucose intolerance and measured plasma glucagon levels using the new ELISA and the currently used ELISA. We found that the patterns of glucagon decline during OGTT differed between the two ELISAs (Figure S4a,b). Furthermore, although in most samples the glucagon values were consistent between the two ELISAs, in some samples they were apparently different: low by the new ELISA, but high by the currently used ELISA (Figure 3a). Therefore, we investigated the correlation between the glucagon values measured by the two ELISAs for each subject and found that the values from 74 subjects had a high (r > 0.8), while those from 30 subjects had a low (r ≤ 0.8) correlation coefficient (Figure 3b). We then divided these subjects into two groups, a comparable‐result (r > 0.8) group and a different‐result (r ≤ 0.8) group. The comparable‐result group showed similar patterns of glucagon values during OGTT between the two ELISAs (Figure 3c,d), while the different‐result group showed the opposite pattern of glucagon during OGTT: glucagon decreased according to the new ELISA, whereas it increased when measured by the currently used ELISA (Figure 3f,g). Next, the glucagon values of the two ELISAs were compared with those of LC‐HRMS in some subjects (nine subjects in the comparable‐result group and 15 subjects in the different‐result group). The new ELISA showed a positive correlation with LC‐HRMS in both groups, but the currently used ELISA did not correlate with LC‐HRMS in the different‐result group (Figure 3e,h). Importantly, we found that plasma glicentin levels were significantly higher in the different‐result group than in the comparable‐result group (Figure 3i), and glucagon values of the currently used ELISA showed significantly positive correlation with plasma glicentin levels only in the different‐result group (Figure 3k), suggesting that cross‐reaction with glicentin was interfering with glucagon measurement in the currently used ELISA. On the other hand, the plasma glucagon levels in 72 subjects (49 subjects in the comparable‐result group and 23 subjects in the different‐result group) measured by the sequential protocol of the currently used ELISA decreased during OGTT in both groups (Figure S5a) and correlated well with the results of LC‐HRMS, while being weakly negatively correlated with glicentin even in the different‐result group (Figure S5c,e), suggesting the improved specificity of the sequential protocol. However, many samples (102 of 360 samples) during OGTT had values below the LLOQ (Figure S5f). In contrast, only 10 of 360 samples were detected as being below the LLOQ in the new ELISA (Figure S5g), confirming that the new ELISA has sufficient sensitivity to analyze low levels of plasma glucagon.
Figure 3.
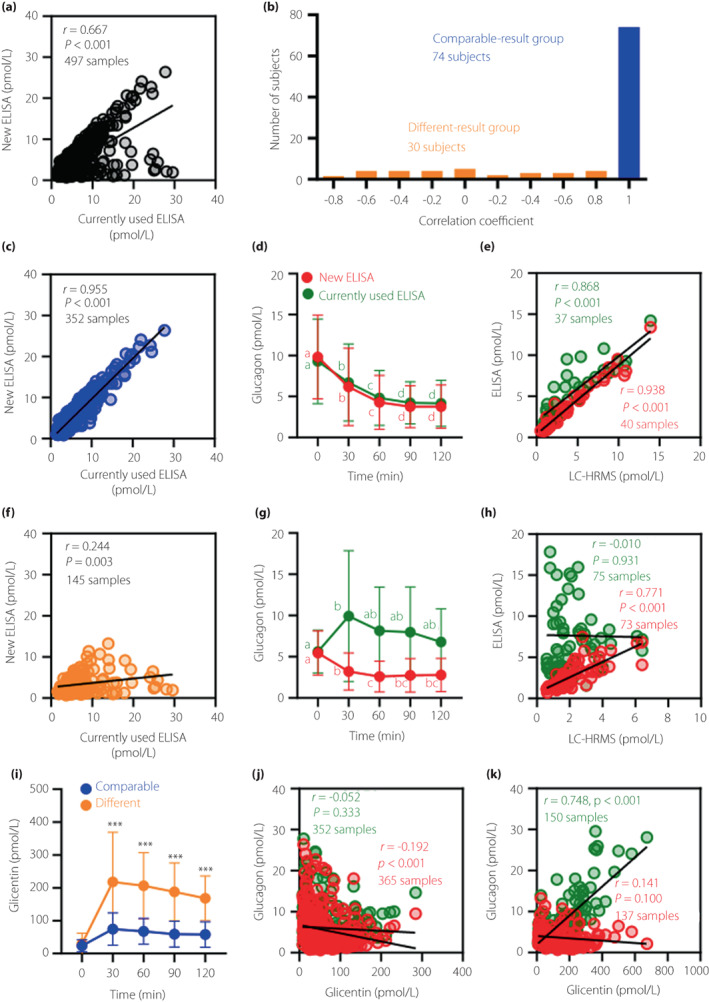
Comparison of plasma glucagon levels between the new ELISA and the currently used ELISA during OGTT in outpatients with suspected glucose intolerance. (a) Correlation between plasma glucagon levels measured by the new ELISA and the currently used ELISA. (b) A histogram of the correlation coefficient for the new ELISA and the currently used ELISA for each subject. (c, d) Comparison of plasma glucagon levels between the new ELISA and the currently used ELISA in the comparable‐result group. (e) Correlations of plasma glucagon levels between the new ELISA (red) or the currently used ELISA (green) and LC‐HRMS in the comparable‐result group. (f, g) Comparison of plasma glucagon levels between the new ELISA and the currently used ELISA in the different‐result group. (h) Correlations of plasma glucagon levels between the new ELISA (red) or the currently used ELISA (green) and LC‐HRMS in the different‐result group. (i) Changes in plasma glicentin levels during OGTT in the comparable‐result group (blue) and different‐result group (orange). (j, k) Correlations of plasma glucagon levels between the new ELISA (red) or the currently used ELISA (green) and plasma glicentin levels in the comparable‐result group (j) and different‐result group (k). Data are mean ± SD. ***P < 0.001, comparable‐result group vs. different‐result group at the same time point. Other statistical explanations are the same as in Figure 2.
We then examined the factors associated with either high or low correlation between the two ELISAs. When plasma glicentin levels were grouped by the quartile of maximum concentration, we found a strong association between the plasma glicentin levels and the correlation coefficient (Table 2). Since the subjects in this study were prescribed various drugs (Table S2), we also investigated the relationship between the prescribed drugs and the correlation coefficient. A significant association was found between eicosapentaenoic acid (EPA) and the correlation coefficient; that is, more subjects prescribed EPA showed low correlation between the two ELISAs (Table 2). Interestingly, the subjects prescribed EPA had higher plasma glicentin and GLP‐1 levels during OGTT (Figure S6c,d). Furthermore, compared with male subjects, more female subjects had a low correlation between the two ELISAs (Table 2). Plasma glicentin and GLP‐1 levels were significantly higher (Figure S7c,d), and plasma glucagon levels were lower, in female subjects compared with male subjects (Figure S7a). Plasma glicentin and GLP‐1 also showed a significant positive correlation in these subjects, consistent with the results from the patients who underwent LSG (Figures S6e and S7e).
Table 2.
Association factors with the differences in the correlation between the new ELISA and the currently used ELISA
| n | Correlation coefficient between two ELISAs | P‐value | ||
|---|---|---|---|---|
| r > 0.8 | r ≤ 0.8 | |||
| Plasma glicentin (maximum value) | ||||
| <Q1 | 26 | 26 | 0 | <0.001*** |
| Q1–Q2 | 26 | 26 | 0 | |
| Q2–Q3 | 26 | 17 | 9 | |
| >Q3 | 26 | 5 | 21 | |
| Eicosapentaenoic acid | ||||
| Prescribed | 20 | 9 | 11 | 0.006** |
| Unprescribed | 84 | 65 | 19 | |
| Sex | ||||
| Female | 62 | 38 | 24 | <0.001*** |
| Male | 42 | 36 | 6 | |
Q1: first quartile (56.4 pmol/L); Q2: second quartile (90.8 pmol/L); Q3: third quartile (172.1 pmol/L).
**P < 0.01, ***P < 0.001.
n, number of subjects; r, correlation coefficient.
DISCUSSION
Plasma glucagon levels are regulated by blood glucose and nutrients, and the secretory response of glucagon is altered in diabetes mellitus. 23 , 24 , 25 The concentration of glucagon in plasma is at low picomolar levels. Therefore, in order to analyze the changes in plasma glucagon levels, a reliable assay having both high specificity and high sensitivity is required. In addition, an assay for use in clinical examinations requires not only high reliability but also high throughput measurement with simple operation. Sandwich ELISA is suitable for use in clinical examinations because it can handle multiple samples at once with multi‐well plates. Therefore, the development of an accurate glucagon sandwich ELISA should help to elucidate the pathophysiology of individual patients and to promote personalized medicine for diabetes.
In this study, we developed a sandwich ELISA using novel monoclonal antibodies against glucagon by immunizing rats and glucagon KO mice (Figure 1a). Notably, the cross‐reactivities of the new ELISA with proglucagon‐derived peptides are markedly low (Figure 1c, Table 1). Therefore, the new ELISA is expected to provide highly reliable measurements, even in samples in which proglucagon‐derived peptides levels are elevated and in which the currently used ELISA therefore yields falsely high values due to cross‐reaction. As expected, the results from the new ELISA correlated significantly with those of LC‐HRMS, even in the samples in which glicentin levels were markedly elevated after LSG (Figure 2d,e). Thus, the new ELISA is a highly reliable assay, having much less cross‐reaction than the currently used ELISA.
In the previous reports, the glucagon levels measured by the currently used ELISA showed good correlations with the levels measured by LC‐HRMS in both healthy subjects and in patients with type 2 diabetes mellitus. 13 , 14 Thus, in subjects who have not undergone bariatric surgery or pancreatectomy, plasma glucagon levels measured by the currently used ELISA and the new ELISA should be comparable, because plasma levels of proglucagon‐derived peptides such as glicentin are low. However, in the present study, among 104 outpatients with suspected glucose intolerance, 30 subjects returned different plasma glucagon levels from the two ELISAs (Figure 3a,b). Interestingly, plasma glucagon levels measured by the currently used ELISA in the different‐result group showed a paradoxical increase during OGTT (Figure 3g) and no significant correlation with LC‐HRMS (Figure 3h). Furthermore, the plasma glucagon levels measured by the currently used ELISA showed a significant positive correlation with plasma glicentin levels, which were markedly increased during OGTT (Figure 3i,k). Therefore, the paradoxical increase in the plasma glucagon levels indicated by the currently used ELISA was thought to be caused by cross‐reaction with glicentin. On the other hand, the plasma glucagon levels measured by the new ELISA, even in the different‐result group, showed a significantly positive correlation with those measured by LC‐HRMS (Figure 3h). Thus, consistent with the results in the subjects after LSG, the new ELISA provided accurate plasma glucagon levels in most outpatients with suspected glucose intolerance.
Since cross‐reactivity affects the results of the currently used ELISA in plasma samples in which proglucagon‐derived peptides are significantly elevated, the modified sequential protocol was recently established. 16 , 17 In the present study, plasma glucagon levels measured by the sequential protocol showed a significant positive correlation with those obtained by LC‐HRMS, even in the samples in which plasma glicentin levels were markedly increased (Figures S3d,e and S5c). Therefore, the reaction specificity has been significantly improved by the sequential protocol. However, we also found that many of the values measured by the sequential protocol were below the LLOQ (Figures S3j,n and S5f). Because circulating glucagon is at low picomolar concentrations and the absolute magnitude of the decrease in glucagon is particularly important for the regulation of glucose tolerance, 26 not only high specificity but also high sensitivity is required for the reliability of the measurement. Therefore, it seems inappropriate to draw conclusions from data that include many values below the LLOQ. In contrast, when measured by the new ELISA, only a few samples had values below the LLOQ (Figures S3h,l and S5g), indicating that the new ELISA has sufficient sensitivity to evaluate plasma glucagon levels.
This study revealed that the currently used ELISA may have mismeasured plasma glucagon levels in some subjects with elevated plasma glicentin levels. Increased nutrient flow into the distal gut after bariatric surgery, including LSG, enhances not only GLP‐1 secretion 19 but also glicentin secretion, because glicentin and oxyntomodulin are secreted along with GLP‐1 from L cells. 27 , 28 On the other hand, the higher plasma glicentin levels in the different‐result group should be caused by a different mechanism. Eicosapentaenoic acid has been reported to stimulate GLP‐1 secretion via GPR120 in L cells, 29 and indeed glicentin as well as GLP‐1 levels were increased in the EPA‐prescribed subjects (Figure S6e), which may have led to the lower correlation coefficient between the two ELISAs (Table 2), due to increased cross‐reaction in the currently used ELISA. In female subjects, plasma glicentin levels were higher and glucagon levels were lower than in male subjects (Figure S7a,c). Not only higher glicentin levels but also lower glucagon levels in female subjects could have accounted for a higher influence of cross‐reaction in the currently used ELISA and led to the lower correlation coefficient between the two ELISAs compared with that from male subjects (Table 2). However, the detailed mechanism by which plasma glicentin was increased in some subjects in this study is unknown. Furthermore, in order to confirm that the new ELISA is useful not only for the specific diabetic patients, but for most patients with or without diabetes, analysis of plasma glucagon levels using the new ELISA in healthy subjects and in type 2 diabetic patients is currently ongoing. This study has some limitations, as conditions such as age, sex, health, and medications differed among the subjects. Future prospective interventional trials are required to clarify this issue.
In conclusion, we have developed a new glucagon sandwich ELISA with greatly improved measurement accuracy. This assay should be suitable for clinical examinations because it can measure a large number of samples with high reliability and efficiency. This new ELISA is expected to contribute to the understanding of the pathophysiology of individual patients with diabetes and the development of future personalized medicine.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: This study was approved by the Ethics Committee of Omi Medical Center and Gunma University (approval number: 2017–261) and the Ethics Committee of Kitada Internal Medicine Clinic and Gunma University (approval number: HS2020‐212). These protocols conform to the provisions of the Declaration of Helsinki.
Informed consent: All individual participants included in the study gave their informed consent.
Registry and the registration no. of the study/trial: N/A.
Animal studies: All animal care and experimental procedures were approved by the Institutional Animal Care and Experimentation Committee at Gunma University (approval number: 19‐080).
Supporting information
Figure S1 | Linearity of the glucagon levels measured by the new ELISA in the serially diluted plasma samples.
Figure S2 | (a, b) Plasma glicentin (a) and GLP‐1 (b) levels during OGTT in patients with morbid obesity, before and after LSG. (c, d) Correlations between plasma glicentin and GLP‐1 levels before (c) and after LSG (d).
Figure S3 | (a, d) Plasma glucagon levels measured by the sequential protocol of the currently used ELISA before (a) and after LSG (d). Data are mean ± SD. (b, c, e, f) Correlations between plasma glucagon levels measured by the sequential protocol of the currently used ELISA and those by LC‐HRMS (b, e) or plasma glicentin levels (c, f) before (b, c) and after LSG (e, f). (g–n) Dot plots of plasma glucagon levels measured by LC‐HRMS (g, k), the new ELISA (h, l), the currently used ELISA (i, m), and the sequential protocol of the currently used ELISA (j, n) before (g–j) and after LSG (k–n). Black plots indicate values below the LLOQ. The horizontal bars indicate the mean and standard deviation. The numbers above the graph indicate the number of samples below the LLOQ. Statistical explanations are the same as in Figure 2.
Figure S4 | Plasma glucagon levels measured by the new ELISA (a) and the currently used ELISA (b) during OGTT in outpatients with suspected glucose intolerance.
Figure S5 | (a) Plasma glucagon levels during OGTT measured by the sequential protocol of the currently used ELISA in outpatients with suspected glucose intolerance. (b, c) Correlations between plasma glucagon levels measured by the sequential protocol and those by LC‐HRMS in outpatients with suspected glucose intolerance in the comparable‐result group (b) and the different‐result group (c). (d, e) Correlations between plasma glucagon levels measured by the sequential protocol and plasma glicentin levels in the subject outpatients with suspected glucose intolerance in the comparable‐result group (d) and in the different‐result group (e). (f, g) Dot plots of plasma glucagon levels measured by the sequential protocol (f) and the new ELISA (g). Black plots indicate values below the LLOQ. The horizontal bars indicate the mean and standard deviation. The numbers above the graph indicate the number of samples with levels below the LLOQ.
Figure S6 | Plasma glucagon levels measured by the new ELISA (a) or the currently used ELISA (b), and plasma glicentin (c) and GLP‐1 (d) levels, either with or without eicosapentaenoic acid prescription in the outpatients with suspected glucose intolerance. (e) Correlations between plasma glicentin and GLP‐1 levels either with or without eicosapentaenoic acid prescription in the outpatients with suspected glucose intolerance.
Figure S7 | Plasma glucagon levels measured by the new ELISA (a) or the currently used ELISA (b), and plasma glicentin (c) and GLP‐1 (d) levels, in either female or male outpatients with suspected glucose intolerance. (e) Correlations between plasma glicentin and GLP‐1 levels in either female or male outpatients with suspected glucose intolerance.
Appendix S1 | Assay protocol.
Table S1 | Clinical characteristics of the patients with morbid obesity.
Table S2 | Clinical characteristics of the outpatients with suspected glucose intolerance.
Table S3 | The intra‐/inter‐ assay coefficients of the new ELISA.
Table S4 | The spike and recovery tests of the new ELISA.
ACKNOWLEDGMENTS
We thank Ms Mana Shimizu, Ms Satomi Miyashita, and Ms Hiromi Yokota‐Hashimoto for technical assistance.
REFERENCES
- 1. Dobbs R, Sakurai H, Sasaki H, et al. Glucagon: Role in the hyperglycemia of diabetes mellitus. Science 1975; 187: 544–547. [DOI] [PubMed] [Google Scholar]
- 2. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975; 1: 14–16. [DOI] [PubMed] [Google Scholar]
- 3. Del Prato S, Castellino P, Simonson DC, et al. Hyperglucagonemia and insulin‐mediated glucose metabolism. J Clin Invest 1987; 79: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kazda CM, Ding Y, Kelly RP, et al. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12‐ and 24‐week phase 2 studies. Diabetes Care 2016; 39: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 5. Kazierad DJ, Bergman A, Tan B, et al. Effects of multiple ascending doses of the glucagon receptor antagonist PF‐06291874 in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2016; 18: 795–802. [DOI] [PubMed] [Google Scholar]
- 6. Holst JJ, Wewer Albrechtsen NJ, Pedersen J, et al. Glucagon and amino acids are linked in a mutual feedback cycle: the liver‐α‐cell axis. Diabetes 2017; 66: 235–240. [DOI] [PubMed] [Google Scholar]
- 7. Hayashi Y, Seino Y. Regulation of amino acid metabolism and alpha‐cell proliferation by glucagon. J Diabetes Investig 2018; 9: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayashi Y. Glutaminostatin: Another facet of glucagon as a regulator of plasma amino acid concentrations. J Diabetes Investig 2019; 10: 1391–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capozzi ME, DiMarchi RD, Tschöp MH, et al. Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocr Rev 2018; 39: 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP‐1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double‐blind, ascending dose and phase 2a study. Lancet 2018; 391: 2607–2618. [DOI] [PubMed] [Google Scholar]
- 11. Holst JJ, Christensen M, Lund A, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab 2011; 13(Suppl 1): 89–94. [DOI] [PubMed] [Google Scholar]
- 12. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014; 57: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 13. Miyachi A, Kobayashi M, Mieno E, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography‐high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 2017; 409: 5911–5918. [DOI] [PubMed] [Google Scholar]
- 14. Katahira T, Kanazawa A, Shinohara M, et al. Postprandial plasma glucagon kinetics in type 2 diabetes mellitus: comparison of immunoassay and mass spectrometry. J Endocr Soc 2019; 3: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuo T, Miyagawa J, Kusunoki Y, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wewer Albrechtsen NJ, Kjeldsen SAS, Jensen NJ, et al. On measurements of glucagon secretion in healthy, obese, and roux‐en‐Y gastric bypass operated individuals using sandwich ELISA. Scand J Clin Lab Invest 2022; 82: 75–83. [DOI] [PubMed] [Google Scholar]
- 17. Roberts GP, Kay RG, Howard J, et al. Gastrectomy with roux‐en‐Y reconstruction as a lean model of bariatric surgery. Surg Obes Relat Dis 2018; 14: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi M, Waki H, Nakayama H, et al. Pseudo‐hyperglucagonemia was observed in pancreatectomized patients when measured by glucagon sandwich enzyme‐linked immunosorbent assay. J Diabetes Investig 2021; 12: 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larraufie P, Roberts GP, McGavigan AK, et al. Important role of the GLP‐1 Axis for glucose homeostasis after bariatric surgery. Cell Rep 2019; 26: 1399–408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayashi Y, Yamamoto M, Mizoguchi H, et al. Mice deficient for glucagon gene‐derived peptides display normoglycemia and hyperplasia of islet {alpha}‐cells but not of intestinal L‐cells. Mol Endocrinol 2009; 23: 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto H, Kaida S, Yamaguchi T, et al. Potential mechanisms mediating improved glycemic control after bariatric/metabolic surgery. Surg Today 2016; 46: 268–274. [DOI] [PubMed] [Google Scholar]
- 22. Ugi S, Yamamoto H, Kusunoki C, et al. Laparoscopic sleeve gastrectomy leads to rapid improvement of glucose tolerance and insulin secretion with enhanced glucagon‐like peptide (GLP‐1) secretion. Diabetol Int 2010; 1: 99–103. [Google Scholar]
- 23. Færch K, Vistisen D, Pacini G, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 2016; 65: 3473–3481. [DOI] [PubMed] [Google Scholar]
- 24. Ichikawa R, Takano K, Fujimoto K, et al. Basal glucagon hypersecretion and response to oral glucose load in prediabetes and mild type 2 diabetes. Endocr J 2019; 66: 663–675. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi M, Satoh H, Matsuo T, et al. Plasma glucagon levels measured by sandwich ELISA are correlated with impaired glucose tolerance in type 2 diabetes. Endocr J 2020; 67: 903–922. [DOI] [PubMed] [Google Scholar]
- 26. Wewer Albrechtsen NJ, Veedfald S, Plamboeck A, et al. Inability of some commercial assays to measure suppression of glucagon secretion. J Diabetes Res 2016; 2016: 8352957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ørskov C, Holst JJ, Knuhtsen S, et al. Glucagon‐like peptides GLP‐1 and GLP‐2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas*. Endocrinology 1986; 119: 1467–1475. [DOI] [PubMed] [Google Scholar]
- 28. Wewer Albrechtsen NJ, Hornburg D, Albrechtsen R, et al. Oxyntomodulin identified as a marker of type 2 diabetes and gastric bypass surgery by mass‐spectrometry based profiling of human plasma. EBioMedicine 2016; 7: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon‐like peptide‐1 secretion through GPR120. Nat Med 2005; 11: 90–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Linearity of the glucagon levels measured by the new ELISA in the serially diluted plasma samples.
Figure S2 | (a, b) Plasma glicentin (a) and GLP‐1 (b) levels during OGTT in patients with morbid obesity, before and after LSG. (c, d) Correlations between plasma glicentin and GLP‐1 levels before (c) and after LSG (d).
Figure S3 | (a, d) Plasma glucagon levels measured by the sequential protocol of the currently used ELISA before (a) and after LSG (d). Data are mean ± SD. (b, c, e, f) Correlations between plasma glucagon levels measured by the sequential protocol of the currently used ELISA and those by LC‐HRMS (b, e) or plasma glicentin levels (c, f) before (b, c) and after LSG (e, f). (g–n) Dot plots of plasma glucagon levels measured by LC‐HRMS (g, k), the new ELISA (h, l), the currently used ELISA (i, m), and the sequential protocol of the currently used ELISA (j, n) before (g–j) and after LSG (k–n). Black plots indicate values below the LLOQ. The horizontal bars indicate the mean and standard deviation. The numbers above the graph indicate the number of samples below the LLOQ. Statistical explanations are the same as in Figure 2.
Figure S4 | Plasma glucagon levels measured by the new ELISA (a) and the currently used ELISA (b) during OGTT in outpatients with suspected glucose intolerance.
Figure S5 | (a) Plasma glucagon levels during OGTT measured by the sequential protocol of the currently used ELISA in outpatients with suspected glucose intolerance. (b, c) Correlations between plasma glucagon levels measured by the sequential protocol and those by LC‐HRMS in outpatients with suspected glucose intolerance in the comparable‐result group (b) and the different‐result group (c). (d, e) Correlations between plasma glucagon levels measured by the sequential protocol and plasma glicentin levels in the subject outpatients with suspected glucose intolerance in the comparable‐result group (d) and in the different‐result group (e). (f, g) Dot plots of plasma glucagon levels measured by the sequential protocol (f) and the new ELISA (g). Black plots indicate values below the LLOQ. The horizontal bars indicate the mean and standard deviation. The numbers above the graph indicate the number of samples with levels below the LLOQ.
Figure S6 | Plasma glucagon levels measured by the new ELISA (a) or the currently used ELISA (b), and plasma glicentin (c) and GLP‐1 (d) levels, either with or without eicosapentaenoic acid prescription in the outpatients with suspected glucose intolerance. (e) Correlations between plasma glicentin and GLP‐1 levels either with or without eicosapentaenoic acid prescription in the outpatients with suspected glucose intolerance.
Figure S7 | Plasma glucagon levels measured by the new ELISA (a) or the currently used ELISA (b), and plasma glicentin (c) and GLP‐1 (d) levels, in either female or male outpatients with suspected glucose intolerance. (e) Correlations between plasma glicentin and GLP‐1 levels in either female or male outpatients with suspected glucose intolerance.
Appendix S1 | Assay protocol.
Table S1 | Clinical characteristics of the patients with morbid obesity.
Table S2 | Clinical characteristics of the outpatients with suspected glucose intolerance.
Table S3 | The intra‐/inter‐ assay coefficients of the new ELISA.
Table S4 | The spike and recovery tests of the new ELISA.


