Abstract
Tissue factor pathway inhibitor (TFPI) is an important regulator of coagulation and a link between inflammation and thrombosis. Here we investigated whether endothelial cell-driven oxidative post-translational modifications could have an impact on TFPI activity. We focused on S-sulfhydration, which is a hydrogen sulfide-dependent post-translational modification that, in endothelial cells, is regulated by the enzyme cystathionine γ-lyase (CSE). The study made use of human primary endothelial cells and blood from healthy individuals or subjects with atherosclerosis as well as from mice lacking endothelial CSE. TFPI was S-sulfhydrated in endothelial cells from healthy individuals and mice, while the loss of endothelial CSE expression/activity reduced its modification. Non-S-sulfhydrated TFPI was no longer able to interact with factor Xa, which facilitated the activation of tissue factor. Similarly, non-S-sulfhydratable TFPI mutants bound less protein S, while supplementation with hydrogen sulfide donors, preserved TFPI activity. Phenotypically, loss of TFPI S-sulfhydration increased clot retraction, suggesting that this post-translational modification is a new endothelial cell-dependent mechanism that contributes to the regulation of blood coagulation.
Keywords: TFPI, Endothelial CSE, S-sulfhydration, Coagulation
1. Introduction
Vascular disease is frequently associated with an enhanced risk of thrombosis, partly because under pathophysiological conditions cells within the vasculature release tissue factor (TF) [1]. TF activation initiates coagulation by binding to factor (F) VIIa to activate FX, which in turn, leads to thrombin generation and the formation of fibrin. The potential detrimental effects of the intravascular FVIIa/TF/FXa complex are partially counteracted by the protein tissue factor pathway inhibitor (TFPI) [2]. The latter is an anticoagulant glycoprotein of 276 amino acids that is organized into an acidic N-terminal sequence, 3 tandem Kunitz (K)-type inhibitory domains and a basic C-terminal tail that plays an important role in regulating the initiation of blood clotting [3,4]. The microvasculature is the main site of TFPI production [5], and TFPIα is secreted by endothelial cells in response to stimulation with thrombin, heparin or shear stress.
While pro-thrombotic events have been partly attributed to a lack of endothelial cell-derived nitric oxide or prostacyclin, it is clear that other mechanisms are likely to also play a role. One endothelial cell pathway of potential relevance, that is directly impaired by vascular inflammation, is the generation of hydrogen sulfide (H2S) and polysulfides (H2Sn) by the enzyme cystathionine γ-lyase (CSE) [6]. Indeed, we recently described the biological role of CSE-derived polysulfides in vascular homeostasis [6] and how the S-sulfhydration of selected proteins contributes to the maintenance of endothelial cell fitness [[7], [8], [9]]. This protective modification is lost in conditions in which the enzyme CSE is inhibited, such as in advanced human vascular disease [8]. In a screen of S-sulfhydrated proteins in endothelial cells, TFPI was found to be modified on Cys250 under basal conditions but the modification was no longer detectable during atherogenesis. This particular cysteine residue is located in one of the 3 tandem K-type inhibitory domains of TFPI, i.e. K3 [10], and is required for the interaction of TFPI with protein S (PS), which is required to enhance its activation [10]. TFPI is a heavily post-translationally modified protein (summarized in Ref. [11]), with reported modifications including the proteolysis of its COOH-terminal, partial phosphorylation of Ser2, N-glycosylation, sulfation of N-linked sugar chains, O-glycosylation and most recently citrullination [12]. While the redox-dependent regulation of TFPI has not been reported to date, reactive oxygen species have been shown to induce a pro-coagulant state in endothelial cells by altering TFPI structure and preventing the binding of TFPI to FX [13]. Herein we sought to determine the impact of S-sulfhydration on the function of TFPI, its interactions with PS and its ability to interact with FXa to inhibit TF. Moreover, we assessed a potential link between the loss of endothelial cell CSE activity, subsequent decrease in TFPI S-sulfhydration and elevated coagulation.
2. Materials and methods
Details are described in the Supplementary Data. All tools and data are available upon request.
3. Results
3.1. Endothelial CSE preserves TFPI secretion and S-sulfhydration
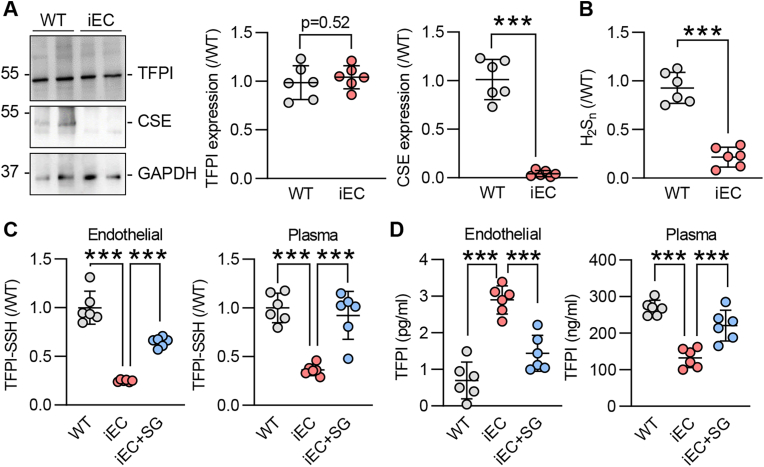
TFPI protein was expressed at comparable levels in cultured pulmonary microvascular endothelial cells from wild-type mice and their littermates that lacked endothelial cell CSE (CSEiEC mice) (Fig. 1A). Consistent with the deletion of CSE, H2Sn availability was attenuated in cells from CSEiEC mice (Fig. 1B) and there was a marked reduction in the S-sulfhydration of endothelial cell TFPI as well as circulating TFPI (Fig. 1C). Importantly, the S-sulfhydration of TFPI in endothelial cells and plasma could be rescued by feeding mice with the H2Sn donor SG1002 for 2 weeks (Fig. 1C), indicating that TFPI is an acceptor protein of endothelial H2Sn. TFPI is a secreted protein and we hypothesized that S-sulfhydration could act as a secretion or retention signal. Therefore, levels of TFPI were assessed in microvascular lung endothelial cells and plasma from the same animals. This revealed that TFPI levels were greater in CSE-deficient endothelial cells and lowest in plasma from CSEiEC mice (Fig. 1D). Both, the endothelial cell retention and the decreased plasma levels of TFPI were reversed by giving SG1002 to CSEiEC mice (Fig. 1D).
Fig. 1.
Loss of murine endothelial CSE reduces TFPI secretion and S-sulfhydration.
(A) TFPI, CSE and GAPDH levels in pulmonary endothelial cells from wild-type (WT) and endothelial cell-specific CSE knockout (iEC) littermates at passage 5; n = 6 independent cell batches/group. (B) Polysulfide levels (H2Sn) in cells as in panel A; n = 6 independent cell batches/group. (C) S-sulfhydration of TFPI (TFPI-SSH) detected as the relative fluorescence intensity of Daz2:Cy5/NBF in endothelial cells and plasma from WT and iEC mice; n = 6 mice/group. (D) Endothelial cell (left panel) and plasma (right panel) levels of TFPI in WT and iEC mice treated with vehicle or SG1002 (40 mg/kg/day in diet) for 2 weeks; n = 6 mice/group. ***P < 0.001, Student's t-test (A, B), one-way ANOVA and Bonferroni multiple comparisons test (C, D).
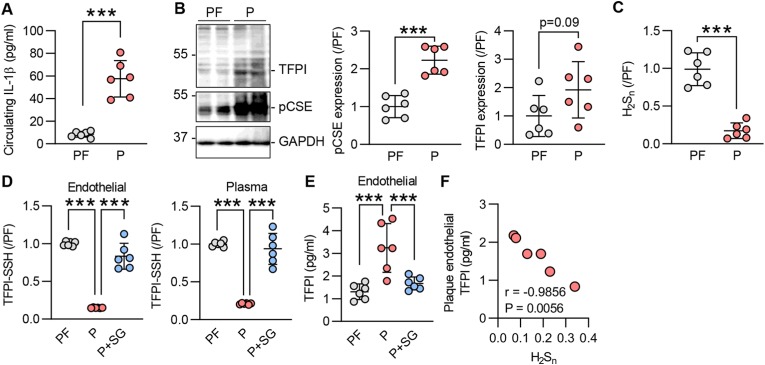
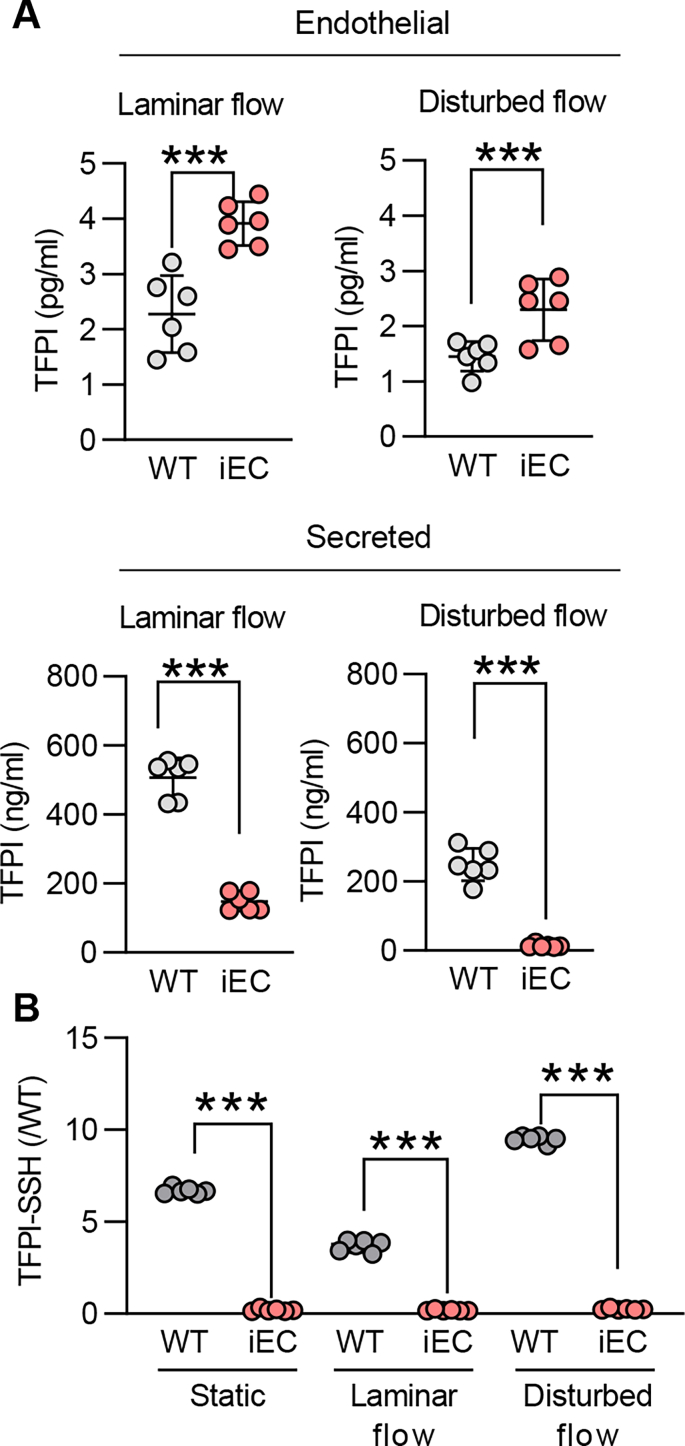
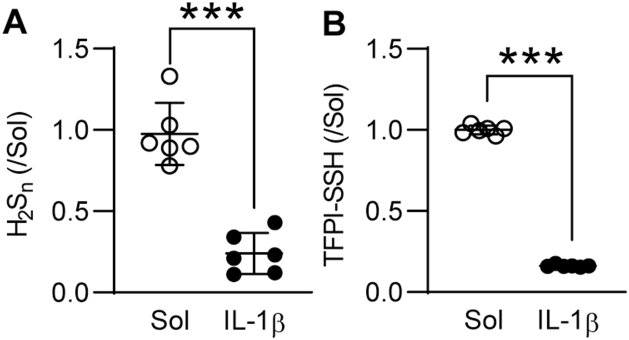
Given that the expression and secretion of TFPI depends on blood flow [14] and the expression of CSE is regulated by flow [7,15], we evaluated the impact of CSE deletion on TFPI secretion and S-sulfhydration in response to shear stress. Murine endothelial cells exposed to laminar or disturbed flow exhibited distinct intracellular levels of TFPI, with laminar flow inducing the most marked increase in intracellular and secreted TFPI levels. However, in both cases the loss of endothelial CSE increased intracellular TFPI levels and reduced that detected in the cell supernatant (Supplementary Fig. 1A), indicating an effect of CSE activity on protein retention/secretion. The deletion of CSE was linked to reduced S-sulfhydration of TFPI in all of the tested conditions (Supplementary Fig. 1B). Although the expression of CSE is upregulated by disturbed flow in atheroprone regions [7,15], its activity is inhibited by inflammatory stimuli in early stages of atherosclerosis [9]. In particular, inflammatory cytokines have been shown to inhibit CSE through phosphorylation on Ser377 [9]. To link CSE activity with TFPI S-sulfhydration in the context of vascular disease, we evaluated the impact of IL-1β on the S-sulfhydration of TFPI. Indeed, when human umbilical vein endothelial cells were exposed to IL-1β, which reduced polysulfide levels (Supplementary Fig. 2A), TFPI S-sulfhydration was reduced (Supplementary Fig. 2B). To determine whether similar events occurred in atherosclerosis in humans and to evaluate if there is a link between CSE and TFPI, experiments were repeated using endothelial cells isolated from healthy plaque-free arteries (PF) or arteries containing atherosclerotic plaques (P). Indeed, in endothelial cells isolated from plaque-containing arteries of patients with high circulating levels of IL-1β (Fig. 2A), CSE was phosphorylated on Ser377 and contained more cellular TFPI (Fig. 2B). H2Sn levels were also lower in the latter cells (Fig. 2C). TFPI S-sulfhydration was reduced in endothelial cells and plasma from subjects with atherosclerosis (Fig. 2D). The in vitro addition of SG1002 to plaque-derived endothelial cells placed in culture and to plasma samples restored TFPI S-sulfhydration and H2Sn also reduced TFPI retention by the endothelium (Fig. 2E). Interestingly, a strong correlation between endothelial TFPI levels and H2Sn bioavailability was observed (Fig. 2F), indicating a direct consequence of S-sulfhydration on TFPI secretion.
Fig. 2.
Vascular disease inactivates CSE and reduces TFPI S-sulfhydration.
(A) Circulating levels of IL-1β in plasma from individuals used to isolate endothelial cells from atherosclerotic plaque-containing carotid arteries (P) or healthy arteries (PF); n = 6 independent cell batches/group. (B) TFPI, phosphorylated CSE (on Ser377) and GAPDH in endothelial cells from atherosclerotic plaque-containing carotid arteries (P) or healthy arteries (PF); n = 6 independent cell batches/group. (C) H2Sn levels in cells as in panel B. (D) TFPI-SSH detected as the relative fluorescence intensity of Daz2:Cy5/NBF in endothelial cells and plasma from P or PF arteries; SG1002 (1 μmol/L) was added for 15 min in both isolated cells or plasma from the respective arteries. n = 6 independent cell batches/group. (E) TFPI in endothelial cells from P or PF arteries, treated with solvent or with SG1002 (1 μmol/L, 24 h); n = 6 independent cell batches/group. (F) Linear correlation of endothelial TFPI and H2Sn levels in plaque-containing endothelial cells (Pearson). ***P < 0.001, Student's t-test (A, B, C), one-way ANOVA and Bonferroni multiple comparisons test (D, E).
3.2. Loss of TFPI S-sulfhydration induces tissue factor activity and reduces its binding to protein S and FXa
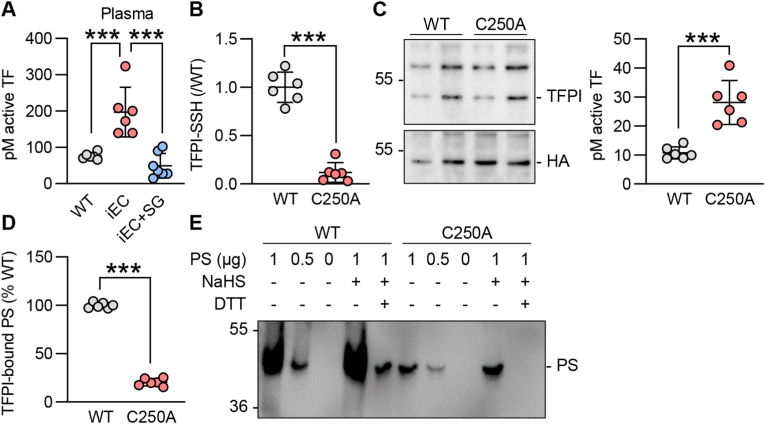
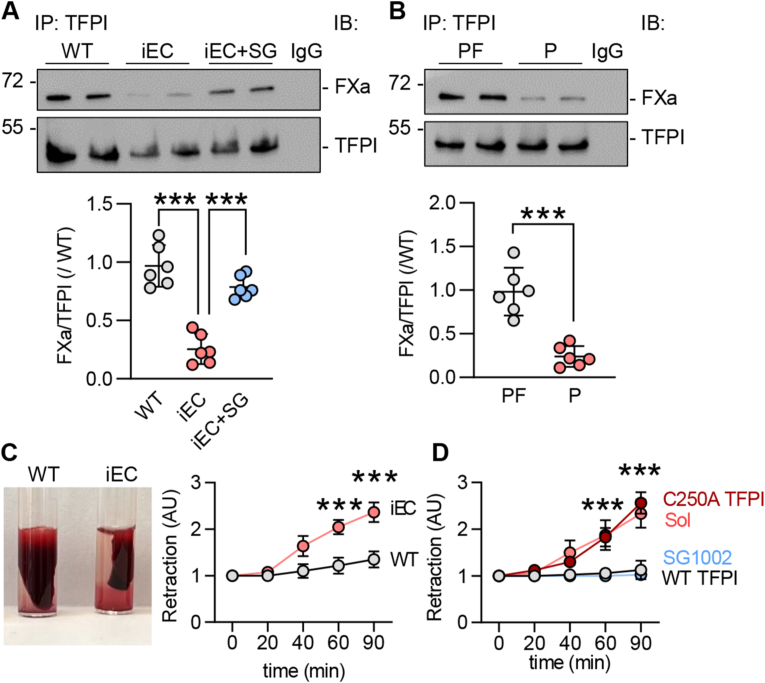
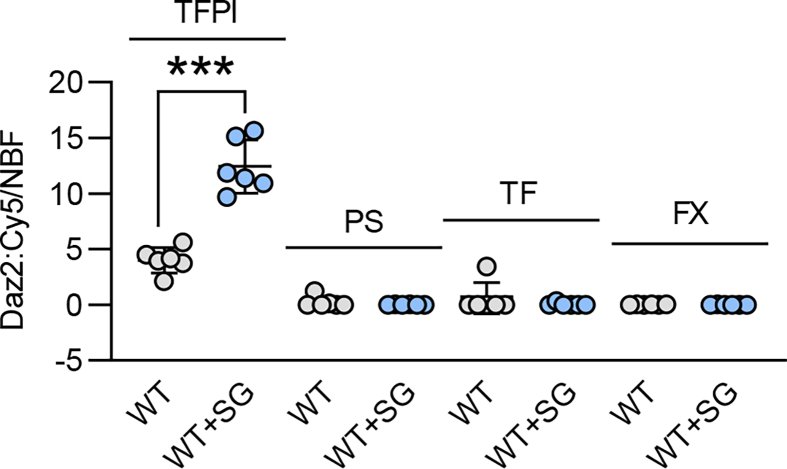
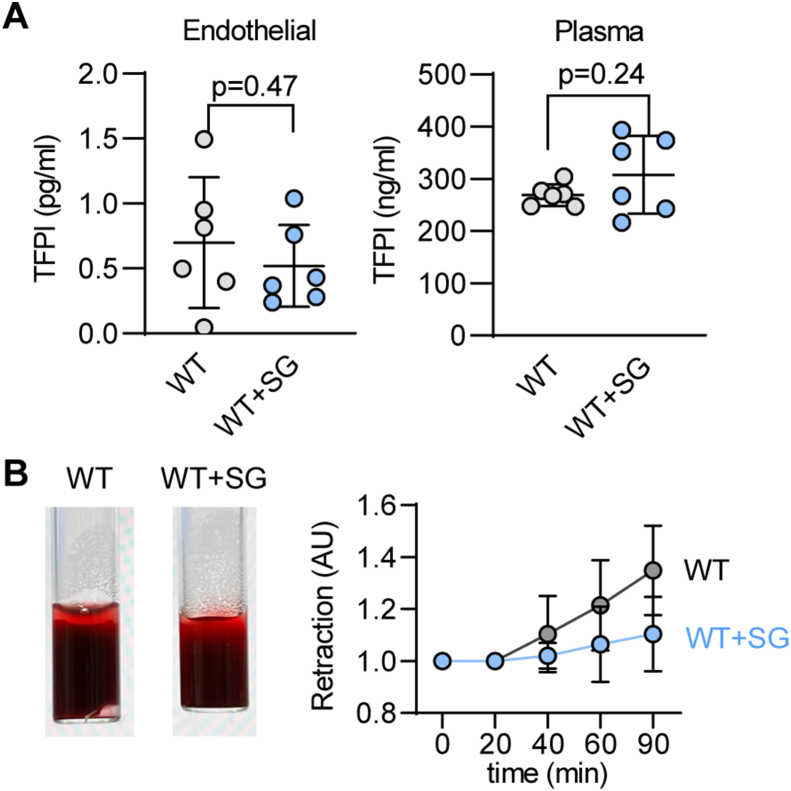
Next, we evaluated the impact of S-sulfhydration on TFPI activity. Given that TFPI directly binds to TF to inhibit it [2], we assessed TF activity in endothelial cells from wild-type and CSEiEC littermates as well as CSEiEC mice that received SG1002 for 2 weeks. There was a 2-fold increase in TF activity in endothelial cells that lacked CSE, an effect that was reduced by treatment with SG1002 to the levels detected in wild-type mice (Fig. 3A). As the S-sulfhydrome dataset identified Cys250 as the site of S-sulfhydration [8], we mutated Cys250 to alanine to generate a non-S-sulfhydratable C250A TFPI mutant. Cys250 was indeed S-sulfhydrated in the wild-type protein and, since no signal was detected in cells expressing the C250A mutant, we concluded that Cys250 is the predominant S-sulfhydrated residue in TFPI (Fig. 3B). Next, we assessed the impact of the lack of S-sulfhydration on TF activity. The cellular expression of the wild-type TFPI and the C250A mutant was comparable but the introduction of the TFPI mutant markedly increased TF activity (Fig. 3C). Since TF activity is determined by PS binding [10], the impact of S-sulfhydration of Cys250 on the ability of TFPI to bind PS was assessed. This revealed that the physical association of the two proteins was abolished by the mutation of Cys250 (Fig. 3D). A separate ligand blotting approach was used to confirm the association of the two proteins. To this end, purified wild-type TFPI or the C250A mutant were incubated with increasing concentrations of PS in the presence of the H2S donor NaHS or in the presence of DTT to remove any potential S-sulfhydration. While the wild-type TFPI protein bound PS in a concentration-dependent manner that was increased in the presence of NaHS, the binding of PS to the C250A mutant was less pronounced and unaffected by the H2S donor (Fig. 3E). As FXa forms a complex with PS and TFPI, we determined whether or not the loss of TFPI S-sulfhydration also interfered with its ability to bind FXa. TFPI immunoprecipitated from CSE-deficient murine endothelial cells physically associated with less FXa than the TFPI immunoprecipitated from CSE-expressing endothelial cells (Fig. 4A). The latter effect was largely normalized by treating the cells with SG1002 (Fig. 4A). A comparable effect on FXa binding to TFPI was observed in human endothelial cells as the association of the two proteins was markedly impaired in endothelial cells from subjects with atherosclerosis (Fig. 4B). To address the functional impact of our observations, we performed a clot retraction assay using thrombin-stimulated whole blood. This revealed that clots from CSEiEC mice retracted faster than clots generated using blood from their wild-type littermates (Fig. 4C). When S-sulfhydration of TFPI and its associated proteins was evaluated in whole blood from wild-type mice receiving vehicle or SG1002, only TFPI was found to be modified (Supplementary Fig. 3). There was no detectable S-sulfhydration either on PS, TF or FX. To demonstrate the impact of S-sulfhydration on this phenomenon, blood from CSEiEC was incubated with SG1002, which abrogated clot retraction. Similarly, when an excess of NaHS-treated wild-type TFPI was added to the assay, clot retraction failed to occur over 90 min, while retraction in the presence of C250A-TFPI mutant was comparable to the effect observed in the absence of an H2Sn donor (Fig. 4D). Wild-type mice treated with SG1002 for two weeks did not show a clear significant effect in endothelial and plasma TFPI levels (Supplementary Fig. 4A), neither did the treatment with SG1002 of blood from wild-type mice affect blood clot formation (Supplementary Fig. 4B). These data indicate that protein S-sulfhydration significantly influences TFPI secretion, activity and clot retraction and polysulfide donors would be beneficial in cases that endothelial CSE is inhibited.
Fig. 3.
Loss of TFPI S-sulfhydration induces tissue factor activity and inhibits protein S binding.
(A) Active tissue factor (TF) in pmol/L (pM) in plasma of wild-type (WT) and endothelial-cell specific CSE knockout mice (iEC) littermates treated with solvent or SG1002 (40 mg/kg/day) in the chow diet for 2 weeks; n = 6/group. (B) S-sulfhydration of TFPI (TFPI-SSH) detected as the relative fluorescence intensity of Daz2:Cy5/NBF in HEK293 cells overexpressing CSE as well as WT or C250A-mutated TFPI; n = 6 independent experiments/group. (C) Representative immunoblot of TFPI and HA (left panel) and TF activity in cells as in panel B. (D) Percentage of bound TFPI to PS identified by a microtiter plate assay in cells as in panel B; n = 6 independent experiments/group. (E) Ligand blotting of PS to TFPI. Representative immunoblot showing binding of increasing concentrations of PS to WT or C250A-mutated TFPI. Experiments were also performed in the presence of 10 μmol/L NaHS to increase in vitro TFPI S-sulfhydration. As a negative control of S-sulfhydration, samples were co-treated with 1 mmol/L DTT. Similar results were obtained in 3 independent experiments. ***P < 0.001. Student's t-test (B, C, D), one-way ANOVA and Bonferroni multiple comparisons test (A).
Fig. 4.
TFPI S-sulfhydration preserves FX binding and delays blood clot formation.
(A) Representative immunoblotting of factor Xa (FXa) and TFPI after TFPI immunoprecipitation in pulmonary endothelial cells from WT and iEC littermates at passage 5, iEC cells were treated with SG1002 (1 μmol/L, 24 h); n = 6 independent cell batches/group. IgG was used as a negative control. (B) Representative immunoblotting of FXa and TFPI after TFPI immunoprecipitation in endothelial cells isolated from atherosclerotic plaque-containing carotid arteries (P) or healthy arteries (PF); n = 6 independent cell batches/group. IgG was used as a negative control. (C) Clot retraction in response to thrombin in whole blood in response to thrombin, isolated from WT and iEC littermates; n = 6 mice/group. (D) Clot retraction in whole blood from iEC mice. Samples were treated with solvent, SG1002 (1 μmol/L), TFPI C250A (1 μg) or WT TFPI (1 μg) for 5 min at 37 °C prior to thrombin addition; n = 6 mice/group. ***P < 0.001 one-way ANOVA and Bonferroni multiple comparisons test (A, B), two-way ANOVA followed by Tukey's multiple comparisons test (C, D).
4. Discussion
The results of the current study highlight that the loss of H2Sn generation by endothelial cells decreases the S-sulfhydration of TFPI on Cys250. The lack of S-sulfhydration had marked consequences on TFPI activity and its ability to bind both PS and FXa and, thus, inhibit TF activity. Functionally, the loss of TFPI S-sulfhydration resulted in increased/accelerated clot retraction.
Endothelial dysfunction is the earliest phenotypic change detectable in the vasculature following exposure to atherothrombotic factors. Endothelial cell damage induces thrombotic complications as a consequence of the reduced bioavailability of anti-thrombotic substances, e.g. nitric oxide, and exposing sub-endothelial matrix proteins that stimulate coagulation [16]. Vascular inflammation and endothelial cell damage have also been shown to increase the release of pro-coagulants by endothelial cells [17]. Recently, we proposed that, in response to excessive vascular inflammation, endothelial cell dysfunction is initiated by impaired activity of the enzyme CSE which impacts on the ability of the endothelium to generate H2S and H2Sn. Such loss of endothelial H2Sn was found to be indispensable for the post-translational modification of proteins by S-sulfhydration [8,9]. S-sulfhydration is considered to be the main mechanism by which H2Sn exert their biological effects. Indeed, we identified that vascular disease is associated with marked changes in the S-sulfhydration of endothelial cell proteins, to alter mechanosensing [8], redox balance [7] and atherosclerosis [9]. By screening for S-sulfhydrated proteins in endothelial cells we were able to detect the modification of TFPI on Cys250 and show its reduction in endothelial cells isolated from plaque-containing arteries.
TFPI synthesis is inflammation-independent [18], however, blood flow is able to increase TFPI secretion [14], an effect that was abrogated when endothelial CSE was deleted or inhibited. Perhaps the most interesting observation was that the lack of TFPI S-sulfhydration correlated with its increased retention in endothelial cells and a clear decrease in its appearance in the cell supernatant. Although TFPI has been reported to be continuously synthetized and secreted from the vascular wall [19], our data indicate that this process of secretion can be regulated by the oxidative post-translational modification of the protein, i.e. by S-sulfhydration. Given that CSE expression and H2Sn generation is highest in atherpoprone regions of the vasculature that are exposed to disturbed flow [7,15], the S-sulfhydration of TFPI would be expected to enhance the anti-thrombotic functions of the endothelium in these areas at risk. However, the situation would be very different when vascular inflammation occurs, which results in the phosphorylation and inactivation of CSE and thus a decrease in protein S-sulfhydration.
Although a TFPI-deficiency syndrome has not been identified so far in humans, the deletion of TFPI in mice results in embryonic lethality, which highlights its vital importance for embryogenesis and organ growth [20]. A low plasma level of TFPI is associated with an increased risk of venous thrombosis [20]. Recent preclinical and clinical data demonstrated that, despite the natural anticoagulant property of thrombomodulin/protein C pathway, the TFPI/PS system functions as a potent natural anticoagulant [10]. As the interaction of PS with the K3 domain of TFPI is essential for the potentiation of anticoagulant activity and only the TFPIα isoform possess the K3 domain it has been proposed that TFPIα possesses the most potent anticoagulant activity of all the isoforms. The major site of TFPIα synthesis is the endothelium [5], and previous studies have shown that optimal binding of endogenously produced TFPIα at the cell surface requires the K3 domain. TFPI is a heavily post-translationally modified protein and it has been proposed that post-translational modifications including glycosylation between the K2 and K3 domains may protect TFPI from proteolysis [11]. Interestingly, redox regulation of TFPI has also been proposed as reactive oxygen species induce a pro-coagulant state in endothelial cells by altering TFPI structure, resulting in inhibition of TFPI binding to FXa and loss of activity [13]. Our findings suggest that S-sulfhydration of the K3 domain alters TFPI to PS binding and directly affects its anticoagulant activity. Taken together our data highlight a previously unexplored mechanism of endothelial redox-dependent TFPI regulation. Maintaining endothelial homeostasis and CSE activity is expected to preserve S-sulfhydration of endothelial TFPI and might serve as a mechanism against thrombotic events.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB1366, Project B1 to I.F. and S.-I.B., Project ID:394046768; SFB1531, Project A2 to S.-I.B. and Project A9 to K.S., Project ID: 456687919; the Emmy Noether Programme BI 2163/1-1 to S.-I.B.; the Johanna Quandt Young Academy at Goethe to S.-I.B.; the Cardio-Pulmonary Institute, EXC 2026, Project ID: 390649896). M.-K.D. was supported by a scholarship from Onassis Foundation.
Author contributions
Conceptualization: S.-I.B. and J.W. Study design: S.-I.B., J.W., I.F., and K.S. Writing: S.-I.B. and J.W. Methodology: A.K., J.W., M.B., M.-K.D., F.D-L., J.H., S.-I.B. and K.S. All authors read the manuscript and provided feedback before submission.
Declaration of competing interest
There is no conflict of interest to declare.
Acknowledgements
The authors are indebted to Katharina Herbig for expert technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102694.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
figs4.
Data availability
Data will be made available on request.
References
- 1.im D., Flaumenhaft R., Furie B., Furie B. Interactions of platelets, blood-borne tissue factor, and fibrin during arteriolar thrombus formation in vivo. Microcirculation. 2005;12:301–311. doi: 10.1080/10739680590925682. [DOI] [PubMed] [Google Scholar]
- 2.Girard T.J., Warren L.A., Novotny W.F., Likert K.M., Brown S.G., Miletich J.P., Broze G.J. Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 3.Cao X., Su Y., Zhang W., Zhao H., Wen M., Lu S., Zhao Y., Chen Y., Liu L., Zang X., Wu J. The impact of anticoagulant activity of tissue factor pathway inhibitor measured by a novel functional assay for predicting deep venous thrombosis in trauma patients: a prospective nested case-control study. Clin. Appl. Thromb. Hemost. 2021;27 doi: 10.1177/10760296211063877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehic D., Tolios A., Hofer S., Ay C., Haslacher H., Rejtö J., Ouwehand W.H., Downes K., Haimel M., Pabinger I., Gebhart J. Elevated levels of tissue factor pathway inhibitor in patients with mild to moderate bleeding tendency. Blood Adv. 2021;5:391–398. doi: 10.1182/bloodadvances.2020003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajaj M.S., Kuppuswamy M.N., Saito H., Spitzer S.G., Bajaj S.P. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. Proc. Natl. Acad. Sci. U. S. A. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibli S.-I., Fleming I. Oxidative Post-Translational Modifications: a focus on cysteine S-sulfhydration and the regulation of endothelial fitness, Antioxid. Redox Signal. 2021;35:1494–1514. doi: 10.1089/ars.2021.0162. [DOI] [PubMed] [Google Scholar]
- 7.Bibli S.-I., Hu J., Leisegang M.S., Wittig J., Zukunft S., Kapasakalidi A., Fisslthaler B., Tsilimigras D., Zografos G., Filis K., Brandes R.P., Papapetropoulos A., Sigala F., Fleming I. Shear stress regulates cystathionine γ lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibli S.-I., Hu J., Looso M., Weigert A., Ratiu C., Wittig J., Drekolia M.K., Tombor L., Randriamboavonjy V., Leisegang M.S., Goymann P., Delgado Lagos F., Fisslthaler B., Zukunft S., Kyselova A., Justo A.F.O., Heidler J., Tsilimigras D., Brandes R.P., Dimmeler S., Papapetropoulos A., Knapp S., Offermanns S., Wittig I., Nishimura S.L., Sigala F., Fleming I. Mapping the endothelial cell S-sulfhydrome highlights the crucial role of integrin sulfhydration in vascular function. Circulation. 2021;143:935–948. doi: 10.1161/CIRCULATIONAHA.120.051877. [DOI] [PubMed] [Google Scholar]
- 9.Bibli S.-I., Hu J., Sigala F., Wittig I., Heidler J., Zukunft S., Tsilimigras D.I., Randriamboavonjy V., Wittig J., Kojonazarov B., Schürmann C., Siragusa M., Siuda D., Luck B., Abdel Malik R., Filis K.A., Zografos G., Chen C., Wang D.W., Pfeilschifter J., Brandes R.P., Szabo C., Papapetropoulos A., Fleming I. Cystathionine γ lyase sulfhydrates the RNA binding protein human antigen R to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;139:101–114. doi: 10.1161/CIRCULATIONAHA.118.034757. [DOI] [PubMed] [Google Scholar]
- 10.Ndonwi M., Tuley E.A., Broze G.J. The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010;116:1344–1351. doi: 10.1182/blood-2009-10-246686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramaniam S., Kanse S.M., Kothari H., Reinhardt C., Fletcher C. Post-transcriptional, post-translational and pharmacological regulation of tissue factor pathway inhibitor. Blood Coagul. Fibrinolysis. 2018;29:668–682. doi: 10.1097/MBC.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 12.Thomassen M.C.L.G.D., Bouwens B.R.C., Wichapong K., Suylen D.P., Bouwman F.G., Hackeng T.M., Koenen R.R. Protein arginine deiminase 4 inactivates tissue factor pathway inhibitor-alpha by enzymatic modification of functional arginine residues. J. Thromb. Haemostasis. 2023 doi: 10.1016/j.jtha.2023.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Cimmino G., Cirillo P., Ragni M., Conte S., Uccello G., Golino P. Reactive oxygen species induce a procoagulant state in endothelial cells by inhibiting tissue factor pathway inhibitor. J. Thromb. Thrombolysis. 2015;40:186–192. doi: 10.1007/s11239-015-1199-1. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski E.F., Reininger A.J., Petteruti P.G., Tsukurov O., Orkin R.W. Shear stress decreases endothelial cell tissue factor activity by augmenting secretion of tissue factor pathway inhibitor. Arterioscler. Thromb. Vasc. Biol. 2001;21:157–162. doi: 10.1161/01.atv.21.1.157. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S., Yurdagul A., Peretik J.M., Alfaidi M., Al Yafeai Z., Pardue S., Kevil C.G., Orr A.W. Cystathionine γ-lyase modulates flow-dependent vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2018;38:2126–2136. doi: 10.1161/ATVBAHA.118.311402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidya A.R., Wolska N., Vara D., Mailer R.K., Schröder K., Pula G. Diabetes and thrombosis: a central role for vascular oxidative stress. Antioxidants. 2021;10 doi: 10.3390/antiox10050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verkleij C.J.N., Bruijn R.E.d., Meesters E.W., Gerdes V.E.A., Meijers J.C.M., Marx P.F. The hemostatic system in patients with type 2 diabetes with and without cardiovascular disease. Clin. Appl. Thromb. Hemost. 2011;17:E57–E63. doi: 10.1177/1076029610384112. [DOI] [PubMed] [Google Scholar]
- 18.Ameri A., Kuppuswamy M.N., Basu S., Bajaj S.P. Expression of tissue factor pathway inhibitor by cultured endothelial cells in response to inflammatory mediators. Blood. 1992;79:3219–3226. [PubMed] [Google Scholar]
- 19.Warn-Cramer B.J., Almus F.E., Rapaport S.I. Studies of the factor Xa-dependent inhibitor of factor VIIa/tissue factor (extrinsic pathway inhibitor) from cell supernates of cultured human umbilical vein endothelial cells. Thromb. Haemostasis. 1989;61:101–105. [PubMed] [Google Scholar]
- 20.Dennis J., Kassam I., Morange P.-E., Trégouët D.-A., Gagnon F. Genetic determinants of tissue factor pathway inhibitor plasma levels. Thromb. Haemostasis. 2015;114:245–257. doi: 10.1160/TH14-12-1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.