Significance
The limited understanding of the molecular mechanisms governing cerebral microvascular dysfunction in stroke has been a major obstacle in the development of novel therapeutic approaches. Herein, we elucidated the stroke-induced transcriptomic changes in the mouse cerebral microvasculature and compared them with the alterations observed in human brain stroke lesions. Our study revealed the presence of shared alterations in microvessel-enriched, vascular disease-associated, druggable targets, highlighting the relevance of cerebral microvascular dysfunction in human stroke pathophysiology. We have also identified molecular alterations in the sphingolipid metabolism and signaling pathway in the cerebral microvasculature, which were significantly altered in human stroke. Our work provides a knowledge platform for future investigation of novel endothelial-enriched therapeutic candidates in stroke.
Keywords: cerebrovascular diseases, vascular biology, blood brain barrier, cardiovascular diseases, sphingolipids
Abstract
Stroke-induced cerebral microvascular dysfunction contributes to aggravation of neuronal injury and compromises the efficacy of current reperfusion therapies. Understanding the molecular alterations in cerebral microvessels in stroke will provide original opportunities for scientific investigation of novel therapeutic strategies. Toward this goal, using a recently optimized method which minimizes cell activation and preserves endothelial cell interactions and RNA integrity, we conducted a genome-wide transcriptomic analysis of cerebral microvessels in a mouse model of stroke and compared these transcriptomic alterations with the ones observed in human, nonfatal, brain stroke lesions. Results from these unbiased comparative analyses have revealed the common alterations in mouse stroke microvessels and human stroke lesions and identified shared molecular features associated with vascular disease (e.g., Serpine1/Plasminogen Activator Inhibitor-1, Hemoxygenase-1), endothelial activation (e.g., Angiopoietin-2), and alterations in sphingolipid metabolism and signaling (e.g., Sphigosine-1-Phosphate Receptor 2). Sphingolipid profiling of mouse cerebral microvessels validated the transcript data and revealed the enrichment of sphingomyelin and sphingoid species in the cerebral microvasculature compared to brain and the stroke-induced increase in ceramide species. In summary, our study has identified novel molecular alterations in several microvessel-enriched, translationally relevant, and druggable targets, which are potent modulators of endothelial function. Our comparative analyses have revealed the presence of molecular features associated with cerebral microvascular dysfunction in human chronic stroke lesions. The results shared here provide a detailed resource for therapeutic discovery of candidates for neurovascular protection in stroke and potentially, other pathologies exhibiting cerebral microvascular dysfunction.
Despite many decades of research, stroke is still a leading cause of mortality (1) and long-term disability worldwide (2, 3) with ischemic stroke being the most common type. Therapeutic options for stroke patients are restricted to enzymatic (thrombolysis) or mechanical recanalization (endovascular thrombectomy) of the obstructed blood vessels (4, 5). However, their use and efficacy are very limited, and currently, there are no therapies available to mitigate or repair the extensive neurovascular injury (6, 7) caused by stroke, causing devastating motor and cognitive sequela in survivors. Hence, there is an urgent need to develop novel therapeutic strategies.
The cerebrovascular endothelium plays a critical role in maintaining the blood–brain barrier (BBB), the anticoagulation, and antiadhesion properties of the cerebral microvasculature, regulating microcirculatory flow, and providing trophic factors to maintain neuronal health. These endothelial cell roles are central to comprehensive microvascular function to support neurovascular homeostasis. Cerebral endothelial cells have a distinctive phenotype conferred by their unique molecular signature (8–11) and their interactions with mural (e.g., pericytes) and parenchymal cells (e.g., neurons and glia), which constitute the neurovascular unit (12–16). Compared to other organs, cerebral endothelial cells express very low levels of molecules driving nonspecific transcytosis and leukocyte adhesion and high levels of tight junction proteins, specific nutrient transporters, metabolic enzymes, and neuronal trophic factors, among others (8, 13). In disease states, this unique molecular signature and phenotype become profoundly altered, hindering the ability of the endothelium to maintain or restore neurovascular homeostasis, which compromises neuronal health leading to irreversible injury and exacerbated pathological outcomes.
During ischemic stroke, reductions in blood flow due to arterial occlusion cause not only neuronal death and glial activation but also extensive injury at the level of the cerebral microvasculature. Stroke-induced cerebral microvascular dysfunction is marked by endothelial activation, barrier leakage, leukocyte and platelet adhesion, and microvascular thrombosis culminating in progressive neuronal death, infarct expansion, and compromising blood flow restoration after successful recanalization of the obstructed cerebral artery/ies (17, 18) (nonreflow phenomenon). As the primary barrier between systemic blood supply and the central nervous system (CNS) and key regulator of cerebral blood flow, thromboinflammation, and neurovascular repair, the cerebrovascular endothelium constitutes an incompletely understood yet novel therapeutic opportunity (10, 19–22). Its amenability to genetic or pharmacological manipulation could be harnessed to mitigate secondary neuronal injury and improve the efficiency of reperfusion therapies. The pathways leading to and resulting from cerebral microvascular dysfunction during stroke remain a key knowledge gap and require extensive investigation to broaden our understanding of the molecular mechanisms governing disease progression and identify targetable pathways.
Recent studies have reported a comprehensive molecular atlas of the mouse brain vasculature at a single-cell level providing a characterization of the transcriptomic signatures of distinct vascular cell populations in naive mice (23). In addition, changes in the CNS endothelial cell transcriptome in disease models have been analyzed (24). However, the resultant molecular signatures using these approaches are conceivably affected due to dissociation methodologies, loss of cellular interactions, and cell activation (25, 26), which could mask changes in cellular messenger ribonucleic acid (mRNA). Given these technical challenges, we sought to capture the stroke-induced transcriptomic and molecular alterations in the mouse cerebral microvasculature using a recently optimized method for intact microvasculature isolation, which minimizes cell activation and preserves cellular interactions, RNA, and molecular integrity (27). In addition, we aimed to discover which specific transcriptomic alterations were potentially more relevant to human stroke pathology by comparing these transcriptomic changes with the ones observed in human stroke lesions (28). We conducted comparative analyses of the common alterations in mouse and human stroke, in combination with k-means clustering, pathway, cell function, and druggable gene analyses using several platforms [Ingenuity Pathway Analysis (IPA), Molecular Signature Data Base (MSigDB), and WebGestalt]. Results from these unbiased analyses revealed shared alterations in genes associated with vascular disease, endothelial dysfunction, sphingolipid metabolism, and signaling, highlighting the significance of these pathways in human stroke pathophysiology. In vivo enrichment and validation studies using qRT-PCR and fluorescence in situ hybridization (FISH) approaches validated the RNA seq data and identify promising microvessel-enriched druggable targets in these categories, including Serpine1/Plasminogen Activator Inhibitor-1 (PAI-1), Hemoxygenase-1, Angiopoietin-2, Alkaline Ceramidase 2, and Sphingosine-1-Phosphate receptor 2. Sphingolipid profiling of mouse cerebral microvessels further validated the transcript data and revealed the enrichment of sphingomyelin species and sphingoid bases in cerebral microvessels compared to brain and the increase in C18 ceramide in cerebral microvessels after ischemic stroke. Our study has identified shared alterations in novel, microvessel-enriched, targetable pathways, indicating the presence of molecular features associated with cerebral microvascular dysfunction in human stroke lesions and providing support for future preclinical studies to investigate novel neurovascular protective therapies pertinent to human disease.
Results
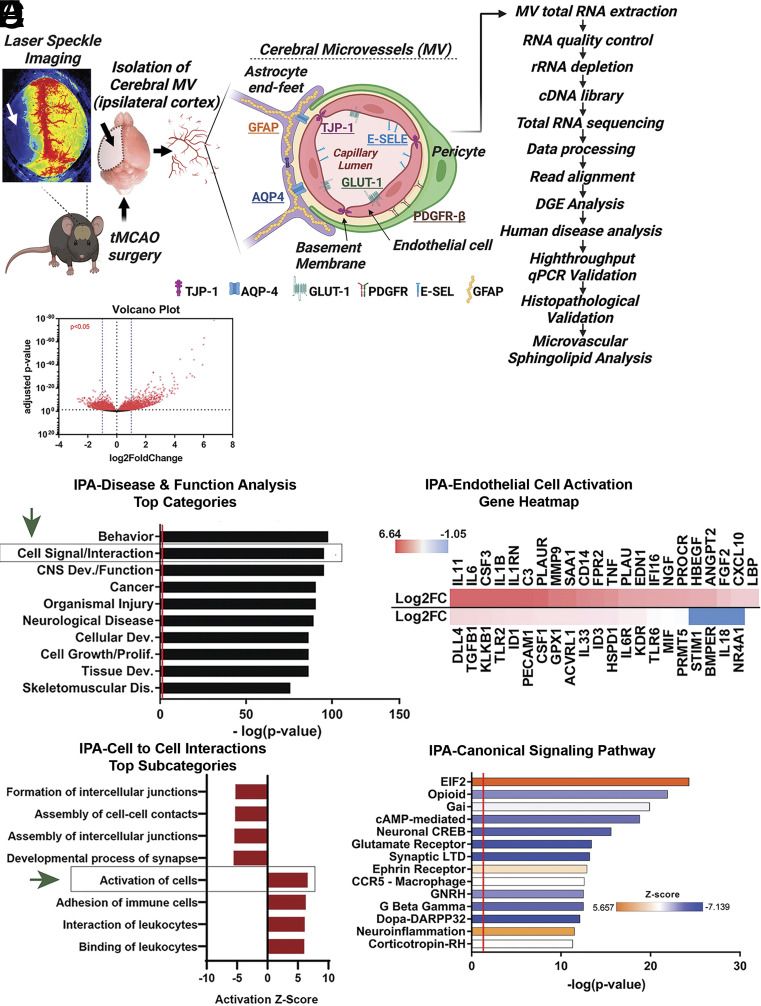
Transcriptomic Analyses of Mouse Cerebral Microvessels after Experimental Stroke Highlight Alterations in Targets Governing Cell-to-Cell Contacts and Endothelial Cell Activation.
In order to examine gene expression changes at the neurovascular level, mice were subjected to transient middle cerebral artery occlusion (tMCAO) or sham surgery (Fig. 1A). Twenty-four hours after reperfusion, microvessels were isolated from mouse cerebral cortices. We followed our recently optimized method (27) to minimize cell activation and preserve cellular interactions with mural cells as well as RNA integrity. RNA was isolated from these microvessels and sequenced on an Illumina platform (n = 4, each group). Expression outliers were identified by measured Cook’s distances and were excluded similarly across all samples (SI Appendix, Fig. S1A). Differentially expressed genes between sham and tMCAO subjects exhibited a uniform distribution pattern as represented by MA (ratio intensity) plot (SI Appendix, Fig. S1B). General examination of mRNA transcript expression alterations revealed significant changes in 6,291 transcripts (SI Appendix, Table S1; P < 0.05), from which, 854 had a log fold change of greater than 1 while 837 had a log fold change of less than −1 (volcano plot shown in Fig. 1B). We confirmed that the cellular composition of microvessel preparations was not significantly altered between the sham and tMCAO groups, by examining the levels of cell-identity markers (SI Appendix, Fig. S2) as we previously described (27).
Fig. 1.
Transcriptomic changes in mouse cortical microvessels after tMCAO. (A) Graphical summary of RNA-sequencing workflow and microvessel cell composition. (B) Volcano plot of differential gene expression in microvessels after tMCAO. Red dots represent significantly altered genes (P < 0.05). (C) Bar plot of Disease and Function analysis generated by IPA. (D) Bar plot of the cell-to-cell interaction subcategories which are an expansion of the cell signaling and interaction IPA category in section C (marked by a box and green arrow). (E) Heatmap of the genes involved in the activation of cells IPA subcategory in section D (marked by a box and a green arrow). log2FC are shown. (F) Bar plot of Canonical Signaling Pathway analysis generated by IPA. Additional analysis is shown in Dataset S1. Prediction Z-score is overlaid on the bars with orange representing predicted pathway activation and blue representing predicted inhibition.
To identify the functional consequences of gene expression alterations after tMCAO, we performed downstream effects (DE) analysis on a subset of the 6,291 significantly altered genes with a log2 fold change, (FC) cut off of <−0.5 and >0.5, using Ingenuity Pathway Analysis (IPA). This DE analysis included Disease and Function (Fig. 1 C–E), Canonical Pathway (Fig. 1F), and Upstream regulators (SI Appendix, Fig. S3A) analyses that were used to identify predicted alterations in various functional and molecular categories based on gene expression changes. Disease and Function analysis predicted alterations in various biological processes such as behavior, cell-to-cell signaling and interaction, CNS development and function, cancer, organ injury, and neurological diseases among others (Fig. 1C). The second most significant functional category, cell-to-cell signaling and interaction, contained several subcategories of significantly altered functions (Fig. 1D): The top four inhibited subpathways emphasized the downregulation of genes responsible for assembly and formation of cellular junctions and contacts, while the top four activated pathways highlighted cellular activation and immune cell trafficking. Further exploration of the cell activation subcategory allowed us to more closely observe specific activation of endothelial cells. This final category contained a list of endothelial activation-related genes such as cytokines (Colony-Stimulating Factor 3, CSF3), interleukins, (IL-11, IL-6, or IL-1β), Angiopoietin-2 (Angpt2), endothelin 1 (Edn1), urokinase-type plasminogen activator (PLAU/uPA), matrix metalloproteinase-9, or Kinase insert domain receptor (KDR)/Vascular endothelial growth factor receptor 2 (VEGFR2) (Fig. 1E), highlighting these significant alterations in cortical microvessels after stroke. A heatmap of the Z-scores of the changes in these genes in our dataset is shown in Fig. 1E. Next, IPA Canonical Pathway analysis most robustly predicted positive activation of neuroinflammation and eukaryotic translation initiation factor 2 (EIF2) pathways, indicating a potential halt in protein translation (Fig. 1F). In contrast, the predicted most robustly inhibited pathways were those with shared downstream alterations related to cyclic adenosine monophosphate (cAMP)-mediated and calcium signaling, including dopamine and cAMP-regulated phosphoprotein of 32 kDa, (Dopa-DARPP32) and cAMP response element binding protein (CREB), which have important implications for endothelial barrier dysfunction (29–32). In another examination to further investigate which biological pathways were differentially enriched in sham or tMCAO microvessels, we performed gene set enrichment analyses (GSEA) of the gene counts per million from each sample of sham and tMCAO microvessels using the Molecular Signature Data Base (MsigDB) platform (33) and the built-in pathway-specific data bases: Pathway Interaction Database (PID), Kyoto Encyclopedia of Genes and Genomes Pathway Database (KEGG), and Reactome Pathway Database (Dataset S1). The most robust of these results came from the PID analysis, in which the top enriched pathway in tMCAO microvessels was nuclear β-catenin signaling, which has been recently reported to be activated in the endothelium during neuroinflammation (34) (SI Appendix, Fig. S3 B and C).
Altogether, the results above highlight the variety of functional and molecular pathway alterations in the cerebral microvasculature after tMCAO. Multiple identified pathways emphasize alterations in cell-to-cell signaling and contacts, including decreased junctional interactions, increased immune cell trafficking, and endothelial cell activation within our stroke cerebral microvessel model. Predicted activation of neurovascular inflammation and EIF2 signaling with inhibition of cAMP and CREB signaling was associated with enrichment of nuclear β-catenin signaling and transcriptional activity in stroke cerebral microvessels. The predicted alterations in these cellular functions and signaling pathways have relevant implications for the understanding of the mechanisms underlying blood–brain barrier leakage, thromboinflammation, and cerebral microvascular dysfunction after transient cerebral ischemia.
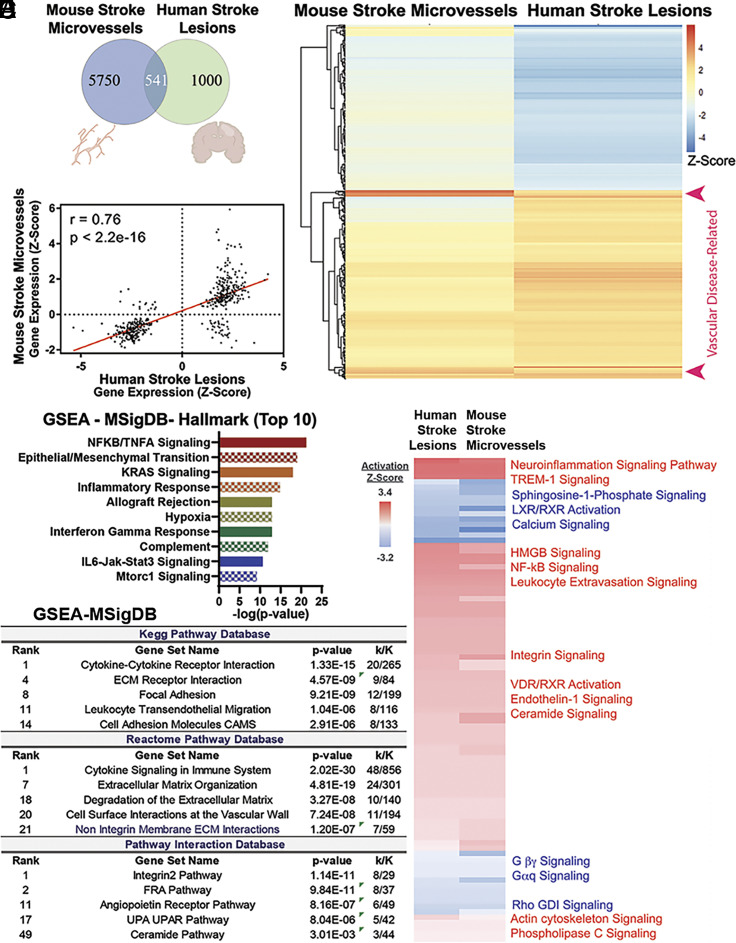
Analyses of Shared Transcriptomic Alterations between Mouse Ischemic Cerebral Microvessels and Human Stroke Lesions Identifies Molecular Features of Vascular Disease with Predicted Alterations in Endothelial Dysfunction Pathways, Sphingolipid Metabolism and Signaling.
In a direct effort to determine which specific alterations in our microvessel model were potentially more relevant to human disease, we compared our microvessel dataset with a publicly available dataset containing RNA-sequencing data from lesion-site samples of stroke patients and the corresponding contralateral brain tissue, not affected by the stroke (28) (SI Appendix, Table S2). Human samples in this dataset included tissue from cortical lesion sites of patients with a history of nonfatal ischemic stroke within the last 5 y before death (GSE56267).
Initial comparative analysis of the mouse stroke (tMCAO) microvessel and the human stroke lesion datasets revealed 541 shared genes (Fig. 2A). A heatmap of the Z-scores of the log2FC for these genes is represented in Fig. 2B. Correlative analysis using Pearson correlation of the 541 shared genes between the human stroke and mouse ischemic cerebral microvessel datasets revealed that they were altered in a highly similar manner (Fig. 2C; r = 0.76, P = 2.2e−16). To further investigate how these 541 genes may relate to another, we performed k-means clustering, which divided these shared genes into five distinct clusters (SI Appendix, Fig. S4 A and B; Dataset S3, “Cluster-SeedSet25” sheet). Notably, GSEA of these five distinct clusters using the WebGestalt platform revealed that they were associated with distinct signaling pathways and disease groups. For example, cluster 3 contained genes related to ubiquitous inflammation pathways (including Tumor Necrosis Factor, TNF, family members, NF-kb, and chemokines) and were associated with cancer and inflammation while cluster 4 contained 18 vascular disease- and sepsis-related genes (including heme oxygenase 1, Hmox1, and the procoagulant serpin-e family member 1, Serpine1/PAI-1, SI Appendix, Fig. S4 C and D; Dataset S3, “Vascular disease genes 18” tab). These 18 genes, robustly upregulated in both datasets, are indicated by the red arrows in Fig. 2B. These clustering analyses support the functional compartmentalization of the shared gene alterations between our mouse and human stroke datasets and highlight the vascular inflammatory component of human stroke chronic lesions, which is shared with other vascular pathologies.
Fig. 2.
Shared transcriptomic and signaling alterations in mouse ischemic cerebral microvessels and human stroke lesions. (A) Venn diagram of gene expression comparisons between mouse stroke microvessels and human stroke lesions. (B) Heatmap of the differential gene expression Z-scores of 541 shared genes ranked by k-means clustering (seed = 25). (C) Pearson correlation of transcript expression Z-scores between mouse stroke microvessels and human stroke lesion-sites (r = 0.76; P < 2.2e−16). (D) Bar plot of the top 10 significant signaling pathways associated with the shared upregulated genes between human stroke lesion sites and mouse ischemic cerebral microvessels. GSEA using the Hallmark database was conducted. (E) Table describing the results from GSEA in Kegg, Reactome, and Pathway Interaction Database (PID) databases using shared upregulated genes between human stroke lesion sites and mouse ischemic cerebral microvessels. Consistent with the results obtained with IPA analysis, these analyses revealed enrichment in pathways involved in cytokine signaling, cell adhesion (Kegg pathway database), and vascular-specific cell surface interactions (Reactome pathway database). The PID indicated alterations in integrin, activator protein-1 FRA, angiopoietin, uPA/uPA receptor, and ceramide pathways. Representative results from these analyses, within the top 50 categories, are shown (full results are shown in Dataset S4, “subset human shared with MV” tab). (F) Leading-edge analysis was completed by comparing all the genes that had two or more occurrences within each database output (PID, Kegg, and Reactome, shown in Dataset S4). Results of IPA Canonical Pathway Analysis using these 53 leading edge genes shared between the three databases are shown. Results include an activation Z-score that predicts an activated (red) or inhibited (blue) pathway. Full results are shown in Dataset S5.
To investigate the signaling pathways associated with the 541 shared genes, we conducted DE analysis using IPA. This analysis predicted a significant upregulation of multiple canonical pathways related to neuroinflammation, while cAMP-protein kinase A (PKA) signaling was predicted as the most significantly suppressed pathway (SI Appendix, Fig. S5A and Dataset S3, “IPA Canonical pathway 541 shared” tab). Pearson correlation of the top predicted activated (neuroinflammation) and inhibited (protein kinase A) categories revealed that they were strongly concordant (neuroinflammation: r = 0.86, P < 0.0001, n = 27; PKA: r = 0.87, P < 0.00011, n = 25) (SI Appendix, Fig. S5A and Dataset S3, “Neuroinflammation and Protein kinase A” tabs), indicating that the gene alterations in these two categories are highly similar across both datasets.
Next, we conducted further GSEA with the 541 shared genes using the MSigDB platform and several built-in databases. We used the Hallmark database (35), to obtain an initial overview of enrichments and Kegg pathway, Reactome pathway, and PID databases (33, 36), to conduct further analysis (Dataset S4). Hallmark analysis indicated the enrichment of inflammation-related pathways such as TNF-α-NFκB, epithelial to mesenchymal transition, hypoxia, and interferon-gamma response (Fig. 2D). Then, in order to reveal the most robust shared changes between human lesion sites and mouse microvessels, we conducted deeper analysis using Kegg pathway, Reactome pathway, and PID databases with a subset of these 541 genes (249 shared upregulated genes). These analyses also revealed the enrichment of both ubiquitous inflammation pathways [e.g., fos related antigen (FRA) pathway, cytokine signaling] as well as vascular-specific pathways (e.g., angiopoietin, cell surface interactions at the vascular wall, Fig. 2E and Dataset S4). To distill the most robust pathway enrichments, a leading-edge analysis across the results from all three databases was performed revealing 53 genes which were shared between the three pathway database results (Dataset S4, “Leading Edge across 3 upreg db” tab). These 53 genes with their accompanying expression data in both mouse microvessels and human lesion sites were inputted into Canonical Pathway analysis in IPA to capture any conserved and significant changes in molecular pathways (Fig. 2F and Dataset S5). Interestingly, these distilled results also illuminated several pathways of interest including ubiquitous inflammation signaling pathways highly relevant to neuro-/vascular inflammation (e.g., triggering receptor expressed on myeloid cells-1, TREM-1, High mobility group box 1, HMGB1, nuclear factor kappa-B (NF-κB), and ceramide signaling), as well as endothelial activation signaling pathways (e.g., endothelin pathway). Taken together, results from these unbiased analyses align with the salient findings in our mouse model (Fig. 1 and SI Appendix, Fig. S3) and further highlight the importance of investigating these pathways in human stroke.
In sum, these findings highlight the conserved transcriptomic and signaling alterations in mouse stroke cerebral microvessels and human stroke lesions. Analyses of these shared transcriptomic changes identified a cluster of robustly altered genes associated with vascular disease (e.g., Hmox1 and Serpine1/PAI-1). Pathway analyses predicted the activation of ubiquitous inflammation pathways (e.g., TREM-1 and ceramide) together with endothelial activation pathways (e.g., angiopoietin signaling, endothelin), highlighting the presence of molecular features of microvascular dysfunction in human stroke.
Stroke Induces Alterations in Endothelial Activation and Blood–Brain Barrier Dysfunction-Related Transcripts in Mouse Cerebral Microvessels and Human Brain.
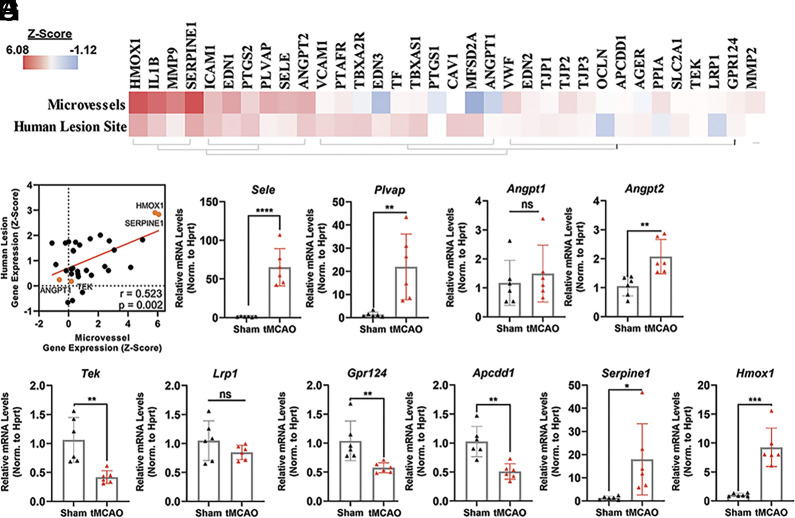
Since our analysis revealed that transcriptomic changes in genes related to vascular disease and endothelial dysfunction (Fig. 2 B and F and SI Appendix, Fig. S4) were shared in stroke (tMCAO) cerebral microvessels and human brain stroke lesions, we set out to more closely investigate a carefully curated subset of 34 genes previously reported to modulate endothelial cell activation and BBB dysfunction (Fig. 3A) (13, 15, 16, 37–40). Interestingly, overall changes in expression of these genes were significantly correlated between mouse ischemic cerebral microvessels and human stroke lesion sites (Fig. 3B, r = 0.52, P = 0.002, n = 34).
Fig. 3.
Validation of shared transcriptomic alterations in targets associated with endothelial activation and BBB function in mouse stroke microvessels and human stroke lesions. (A) Heatmap of a curated subset of 34 genes relevant to endothelial cell activation and BBB integrity ordered by hierarchical clustering. (B) Pearson correlation of transcripts represented in 3A between RNA-sequencing data of mouse microvessels after tMCAO and human stroke lesion-sites (r = 0.52; P < 0.01). (C–I) qRT-PCR validation of the changes in mRNA levels of selected BBB and endothelial activation-related target genes in cerebral microvessels (tMCAO relative to sham) including (C) Sele, (D) Plvap, (E) angiopoietin ligands (Angpt1/2), and (F) their receptor, Tie2 (Tek), (G and H) WNT signaling components Lrp1, Gpr124, and Apcdd1, and (I) druggable targets Serpine1 and Hmox1. (*P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Tukey. ns = not significant). mRNA levels were normalized by Hprt mRNA. The individual values and the mean ± SEM are shown. N = 6.
Subsequent validation by qRT-PCR of selected shared targets confirmed significant alterations in the expression of markers of endothelial inflammation, such as leukocyte adhesion molecules (e.g., robust upregulation of e-Selectin, Sele, 64.08 ± 9.84-fold) and caveolar molecules governing endothelial transcytosis (e.g., plasmalemma vesicle-associated protein, PLVAP; 21.91 ± 5.81-fold) (Fig. 3 C and D). We also validated novel transcript alterations in ischemic cerebral microvessel in the family of angiopoietins (Angpt 1 and 2) and their receptor, endothelial tyrosine kinase receptor (Tek, Tie2). Angpt2, which is induced upon endothelial activation and causes barrier leakage (41–43), was induced in the microvessel preparations post-tMCAO (2.07 ± 0.28-fold), while Angpt1 was not (Fig. 3E). Notably, Tek, the gene that transcribes the Tie2 receptor for Angpt1/2 and mediates the vasoprotective effects of Ang1 (44–47) was dramatically downregulated in cerebral microvessels after tMCAO (0.42 ± 0.16-fold, Fig. 3F). Among these gene alterations observed in mice, there was a robust trend for upregulation of Angpt2 in human stroke lesions (Fig. 3A and SI Appendix, Table S3, P = 0.058).
As one of the top predicted pathways enriched in tMCAO microvessels (nuclear β-catenin, SI Appendix, Fig. S3B) and key regulator of central nervous system angiogenesis, BBB formation, and maintenance (13, 16, 48–52), we analyzed changes in the expression of key Wingless-related integration site (WNT)-β-catenin molecular modulators. While this pathway plays a critical role in BBB function, the alterations in the expression of Wnt-β-catenin genes during stroke are not known. qPCR-based validation revealed that the Wnt activator and critical for BBB maintenance G-protein coupled receptor 124 (Gpr124) (49, 50, 53) and the Wnt downstream target adenomatosis polyposis coli downregulated 1 (Apcdd1) (54–56) were dramatically downregulated in cerebral microvessels after stroke (Gpr124; 0.57 ± 0.14-fold, Apcdd1; 0.51 ± 0.12-fold, Fig. 3H). In contrast, the levels of the multifunctional endocytic receptor, Wnt modulator (57–59), lipoprotein receptor-related protein 1 (Lrp1), were not significantly changed (Fig. 3G). Altogether, these data are consistent with recent studies showing downregulation of Wnt targets in the endothelium after stroke (53) and the critical role of the Wnt-β-catenin pathway in the maintenance of the BBB molecular signature and phenotype by restricting the expression of leukocyte adhesion molecules and caveolar proteins governing transcytosis (16, 34). While these molecular features were very pronounced in our mouse stroke microvessels, no significant alterations were observed in human stroke (SI Appendix, Table S3).
Next, we validated the expression changes of some of the vascular disease-associated genes identified in our cluster analysis of the shared alterations between mouse microvessels and human lesion sites (SI Appendix, Fig. S4 and Dataset S3) such as the procoagulant Serpine1/PAI-1 and the antioxidant Hmox1. Serpine1 and Hmox1 were robustly shared between the two datasets and recurred as strong driving genes throughout the study. They were also identified in a list of 68 currently druggable genes using additional analysis in IPA of the shared transcript alterations in the mouse and human datasets (SI Appendix, Fig. S5B). The strong induction of these two specific transcripts was validated in mouse cortical microvessels after tMCAO by qPCR (Fig. 3I, 17.91 ± 6.28, and 9.25 ± 1.36-fold, respectively). Furthermore, Serpine1 and Hmox1 were significantly induced in human lesion sites post stroke (2.01 and 2.04-fold, respectively, SI Appendix, Table S3). The antioxidant and anti-inflammatory Hmox1 (60–63) and the procoagulant Serpine1 (64, 65) are of high interest as emerging therapeutic targets for the microvasculature and have presented promising experimental and clinical outcomes for stroke in preclinical studies and clinical trials.
We also investigated the relative enrichment profile of these selected targets in mouse cortical microvessel preparations compared to the whole brain (WB) and cortex in naive mice. We grouped the genes into four categories based on the relative enrichment (fold difference) in microvessels compared to total brain: highly enriched (>10-fold), moderately enriched (fivefold to 10-fold), modestly enriched (twofold to fourfold), or not enriched (SI Appendix, Fig. S6). Gpr124, Apcdd1, Plvap, and Tek were highly enriched while Hmox1, Angpt1 and 2, and Lrp1 were modestly enriched. Serpine1 was not enriched in cortical microvessels compared to brain but enriched compared to cortex. These results are in agreement with previously published bulk (66, 67) and single-cell (23) RNA seq data sets (summarized in SI Appendix, Fig. S7).
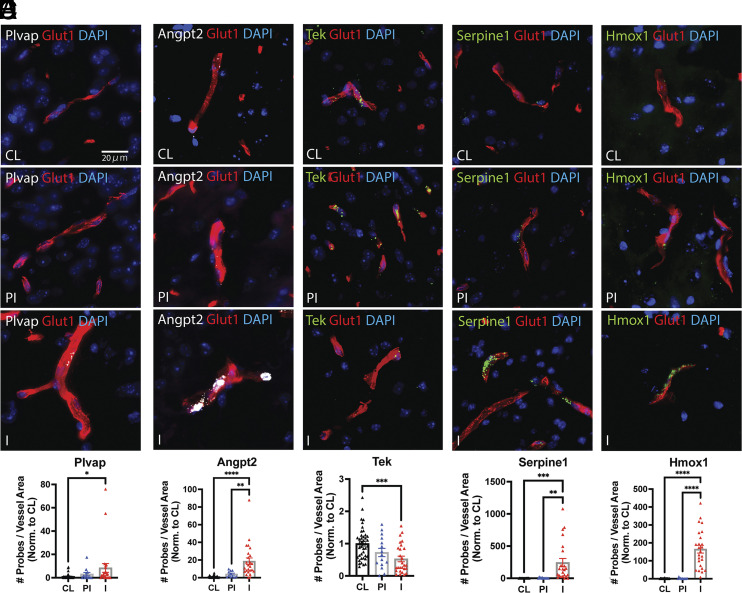
Given these promising findings in our stroke cerebral microvessels, we next carried out histopathological validation of some of the most highly enriched or robustly altered targets in the mouse and human stroke datasets by FISH (Fig. 4 and SI Appendix, Fig. S8). Between bregma +0.5 and −2.6mm, 24 regions of interest (ROI), comprising cortex, striatum, thalamus, and hippocampus, were quantified per brain (12 in ipsilateral, ischemic hemisphere, IL, and 12 in the contralateral hemisphere, CL) (SI Appendix, Fig. S8F). At the histopathological level, ROIs in the IL hemisphere were grouped into infarct (I) or periinfarct (PI) areas based on microtubule-associated protein-2 and ionized calcium-binding adapter molecule (IBA)-1 immunofluorescence (IF) staining. These analyses showed a robust induction of Plvap, Angpt2, Serpine1, and Hmox1 transcripts and a profound downregulation of Tek in cerebral microvessels in the infarcted areas of cortex, basal ganglia, thalamus, and hippocampus. The levels of Plvap mRNA in parenchymal vessels of the contralateral hemisphere were very low but significantly increased in the infarct area. While this induction was robust and significant, the mRNA levels of Plvap in the choroid plexus were markedly higher than in the parenchymal vessels in the infarct area (SI Appendix, Fig. S8). Representative images from the I, PI, and CL regions are shown in Fig. 4 and SI Appendix, Fig. S8, together with the quantitative data. These data confirm and further validate our RNA Seq and qRT-PCR results by providing spatial and histopathological resolution of the stroke-induced alterations in the selected targets in the cerebral microvasculature.
Fig. 4.
Histopathological validation of selected targets of the endothelial activation and BBB function module. FISH analysis of key microvessel-enriched, druggable and robustly altered targets in the stroke datasets. Integrated Glut1 IF and FISH analysis for the target genes (A) Plvap, (B) Angpt2, (C) Tek, (D) Serpine1, and (E) Hmox1 was conducted as described in the Materials and Methods. Glut1 IF is shown in the red channel and the target gene FISH signals are shown in the white (Plvap and Angpt2) or green (Tek, Serpine1, and Hmox1) channels. Nuclear stain is shown in the blue channel (DAPI). Images were acquired with a 40x/0.95NA objective (3DHistech). Representative images of contralateral (CL), periinfarct (PI) and infarct (I) regions are shown (80× magnification). (Scale bar: 20µm.) (F) Quantitative analysis of probe signals. A total of 24 ROIs were quantified per brain (N = 4 to 6 animals), as described in the Materials and Methods. QuPath software was used to quantify the number of probes (transcripts) per unit of Glut1 positive vessel area in each ROI. The number of probes per ROI in PI and I areas (ipsilateral hemisphere) were normalized to CL hemisphere. Values are fold induction per ROI in PI and I areas versus CL. The individual values and the mean ± SEM are shown. *P < 0.05; **P < 0.01; ***P < 0.001, **** P < 0.0001 one-way ANOVA followed by Tukey.
Altogether, these analyses indicate that stroke induces robust changes in the expression of microvessel-enriched, endothelial activation and BBB-related transcripts in mouse cerebral microvessels and in the human brain, emphasizing the relevance of cerebral microvascular dysfunction in ischemic stroke pathophysiology. We highlight the downregulation of Tek and upregulation of Plvap, Angpt2, Hmox1, and Serpine1/PAI-1 in cerebral microvessels in the infarcted region. Upregulation of druggable targets associated with vascular dysfunction such as Hmox1 and Serpine1 was robust and statistically significant both in mouse ischemic cerebral microvessels and in human stroke chronic lesions. These studies present a scope for investigating the therapeutic potential of the cerebral microvasculature in human stroke.
Stroke Alters the Expression of Microvessel-Enriched, Sphingolipid Synthesis, Metabolism and Signaling Transcripts in Mouse Cerebral Microvessels and the Human Brain.
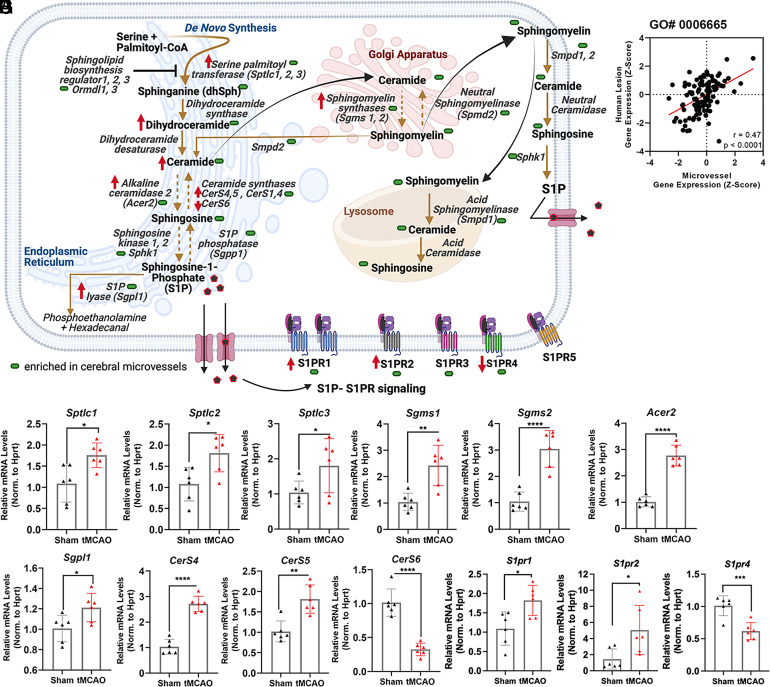
Given that our comparative analysis between our mouse ischemic cerebral microvessels and human stroke lesion sites (28) datasets (Fig. 2 E and F) revealed predicted significant overlapping changes in the sphingolipid metabolism and signaling pathway and as emerging modulators of endothelial dysfunction (68, 69), we aimed to further examine transcriptome changes in this category (Gene ontology, GO# 0006665, mediators of sphingolipid metabolism) in our experimental stroke model. A succinct summary of this pathway is represented in Fig. 5A. Correlation analysis of the differential gene expression of these 125 genes (GO# 0006665) between our mouse ischemic cerebral microvessels and human stroke lesion sites revealed a moderate, but statistically significant correlation (r = 0.47, P < 0.0001, n = 125, Fig. 5B), indicating that the genes in the sphingolipid pathway were similarly altered in these two datasets. A full heatmap comparing the changes in gene expression of the genes in this category between both datasets is available in SI Appendix, Fig. S9.
Fig. 5.
Stroke-associated changes in sphingolipid synthesis, metabolism and signaling genes in mouse microvessels and human stroke lesion sites. (A) Simplified summary of the sphingolipid pathway: de novo synthesis, sphingomyelin synthesis/degradation, ceramide and S1P synthesis/degradation and S1P signaling. Summary of qPCR results (Figs. 5, 10S, 12S, and 6) and sphingolipid profiling (SI Appendix, Fig. S15) is included (green shape: microvessel-enriched; red arrow: altered measurement in tMCAO samples compared to sham). (B) Pearson correlation of transcript changes in sphingolipid metabolic processes (GO: 0006665) between mouse stroke microvessels and human stroke lesion sites. (C–H) qRT-PCR validation of significantly altered transcripts governing sphingolipid metabolism and signaling separated into functional groups of genes for (C) de novo metabolism: Sptlc1, 2, and 3; (D) sphingomyelin synthesis/degradation: Sgms1 and 2; (E–G) ceramide and S1P synthesis/degradation: Acer2, Sgpl1, CerS4, 5, and 6; (H) S1P signaling: S1PR1, 2, and 4. mRNA levels were normalized by Hprt mRNA. The individual values and the mean ± SEM are shown. N = 6. *P < 0.05; **P < 0.01; *** P < 0.001; ****P < 0.0001, one-way ANOVA followed by Tukey.
To confirm the changes observed in our analysis of sphingolipid metabolism, we conducted qRT-PCR validation of selected key targets of this pathway. Beginning with the de novo sphingolipid synthesis pathway, we determined the changes in expression of the serine palmitoyltransferase long-chain base subunit 1, 2, and 3 (Sptlc; Sptlc1/2/3), the first enzyme in the de novo sphingolipid synthetic pathway, along with the Sptlc complex inhibitors, the sphingolipid biosynthesis regulators orosomucoid-like proteins (Ormdl) 1, 2, and 3. These enzymes play a critical role in the synthesis of ceramide, a key signaling molecule and bioactive intermediate in the synthesis of complex sphingolipids, such as sphingomyelins, as well as sphingosine and S1P. We found a modest but significant upregulation in Sptlc1, 2, and 3 (1.76 ± 0.21, 1.81 ± 0.24, and 1.80 ± 0.34-fold, respectively) transcripts in mouse tMCAO microvessels compared to sham (Fig. 5C), while the levels of Ormdl1-3 mRNAs remained unchanged (SI Appendix, Fig. S10A). Sphingomyelin, an abundant membrane component that is highly enriched in lipid rafts and caveolae, is generated from ceramide via the actions of sphingomyelin synthases (Sgms) and reversibly converted to ceramide by sphingomyelin phosphodiesterases (Smpd1 and 2). Transcript levels of sphingomyelin synthase 1 (Sgms1) and 2 (Sgms2) were significantly induced upon ischemia in mouse microvessels (Fig. 5D; 2.42 ± 0.34 and 3.04 ± 0.32-fold, respectively), while Smpd1,2 were not (SI Appendix, Fig. S10B). The bioactive sphingolipid S1P, a potent modulator of endothelial function, is generated from ceramide through the activity of ceramidases (Cer), which convert ceramide to sphingosine, and sphingosine kinases (Sphk1, 2), which phosphorylate sphingosine to give rise to S1P (Fig. 5A). Conversely, S1P can be converted to sphingosine by S1P phosphatases (Sgpp) and subsequently to ceramide via ceramide synthases (CerS) or irreversibly degraded by S1P lyase (Sgpl) giving rise to phosphoethanolamine and hexadecanal. After tMCAO, there was a significant elevation of alkaline ceramidase 2 transcript (Acer2) in microvessels, compared to sham, (Fig. 5E, 2.77 ± 0.18-fold), a modest increase in S1P lyase 1 (Sgpl1, 1.21 ± 0.08-fold, Fig. 5F), an induction in Cers4 and Cers5 (Fig. 5G, 2.71 ± 0.17 and 1.82 ± 0.18-fold, respectively) and downregulation of Cers6 mRNA (Fig. 5G, 0.33 ± 0.09-fold). In contrast, there were no observed differences in the transcripts encoding for Sphk1/2 or Sgpp1 (SI Appendix, Fig. S10 C and D). These data point to a potential activation of the de novo synthesis of sphingolipids and a putative increase in ceramide and/or sphingosine levels in ischemic cerebral microvessels with multiple regulators induced at the transcript level. Among all these transcript alterations in genes governing sphingolipid synthesis and metabolism, there were significant alterations of Sgms1 and CerS6 in human stroke lesions (SI Appendix, Table S4).
As a shared altered pathway between human lesion sites and mouse tMCAO microvessels (Fig. 2F), next, we sought to examine the S1P signaling pathway after tMCAO. A heat map to compare the shared changes in the S1P signaling pathway in the mouse and human stroke lesion sites is shown in SI Appendix, Fig. S11. Validation of cognate S1P receptor (S1PR) levels by qRT-PCR showed significant stroke-induced upregulation of S1PR1 (1.82 ± 0.24-fold), S1PR2 (5.06 ± 1.35-fold), and downregulation of S1PR4 (0.62 ± 0.08-fold) in mouse microvessels (Fig. 5H), while no significant changes in S1PR3 or S1PR5 were observed (SI Appendix, Fig. S10F). The most salient and robust S1PR induction in mouse tMCAO microvessels was S1PR2, consistent with our previously published studies (27, 70). Interestingly, among all S1PR isotypes, a significant upregulation of S1PR2 was observed in human stroke lesions (SI Appendix, Table S4).
The tissue compartmentalization of these sphingolipid metabolism and signaling genes is shown in SI Appendix, Fig. S12. These analyses revealed that Acer2 was the most highly enriched transcript (>10-fold) in cortical microvessels compared to the whole brain (SI Appendix, Fig. S12A) and cortex, and Sgms1 and S1PR2 were moderately and modestly enriched, respectively (SI Appendix, Fig. S12 B and I), which was consistent with available RNA sequencing data [SI Appendix, Fig. S13 (66, 67)].
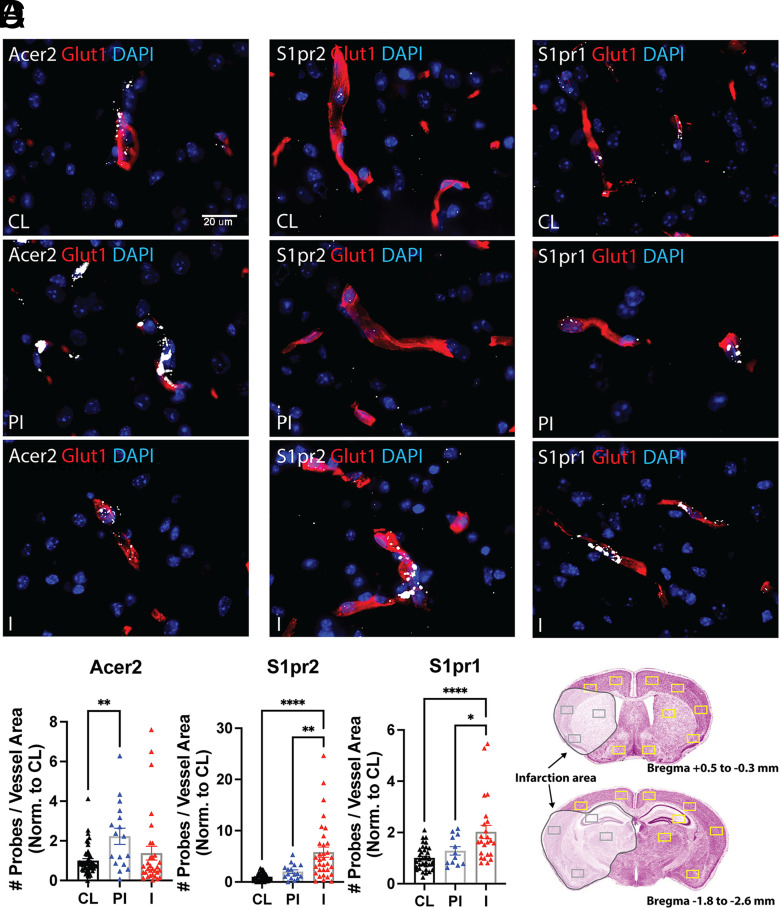
Next, we used FISH analysis to validate at the histopathological level the stroke-induced alterations of key highly enriched and robustly altered targets of the pathway. Representative pictures of in situ hybridization (ISH) analyses for Acer2, S1PR2, and S1PR1 are shown in Fig. 6 and SI Appendix, Fig. S14. FISH analyses confirmed the high enrichment of Acer2 transcripts in cerebral microvessels in all regions of the brain and revealed its potent upregulation in the periinfarct regions of cortex and hypothalamus compared to CL (Fig. 6 A and D). S1PR2 mRNA was detected at low levels in the cerebrovascular endothelium in CL and PI regions (cortex and hypothalamus) but robustly induced in infarcted areas of cortex, basal ganglia, thalamus, and hippocampus (Fig. 6 B and D). In contrast, S1PR1 mRNA expression (Fig. 6 C and D) was very abundant in all vessels throughout the brain and significantly upregulated in the damaged areas of cortex, striatum, hippocampus, and thalamus. Low-magnification representative pictures of the whole-brain sections are shown in SI Appendix, Fig. S14 together with a scheme of the quantified ROIs. These data further validate our qRT-PCR results at the histopathological level, by providing quantification of the alterations in transcripts levels in areas of the brain differentially affected by the stroke.
Fig. 6.
Histopathological validation of selected targets of the sphingolipid metabolism and signaling pathway. FISH analysis of key microvessel-enriched, druggable targets of the sphingolipid pathway altered in the stroke datasets. Integrated Glut1 IF and FISH analyses for the target genes (A) Acer2, (B) S1pr2, and (C) S1pr1 were conducted as described in the Materials and Methods. Glut1 IF is shown in the red channel and the target gene FISH signals are shown in the white channel. Nuclear stain is shown in the blue channel (DAPI). Images were acquired with a 40×/0.95NA objective (3DHistech). Representative images of contralateral (CL), periinfarct (PI), and infarct (I) regions are shown (80× magnification). Scale bar: 20 µm. (D) Quantitative analysis. A total of 24 ROIs were quantified per brain (N = 4 to 6), as described in Materials and Methods. QuPath software was used to quantify the number of target probes (Acer2, S1pr2, and S1pr1 transcripts) per unit of Glut1 positive vessel area in each ROI. The number of probes per unit of vessel area per ROI in the PI and I areas (ipsilateral hemisphere) was normalized to CL hemisphere. Values are fold induction per ROI in PI and I areas versus CL. The individual values and the mean ± SEM are shown. *P < 0.05; **P < 0.01; **** P < 0.0001; one-way ANOVA followed by Tukey.
With these data, we highlight stroke-induced transcriptomic alterations in key players of the sphingolipid pathway and their potential relevance in stroke-induced cerebral microvascular dysfunction. Our findings suggest an imbalance in sphingolipid synthesis, metabolism, and signaling in cerebral microvessels after stroke. Noteworthily, aCer2 was the top enriched transcript in the cerebral microvasculature compared to brain. Among S1PR, S1PR2 was modestly enriched in microvessels in naive mice, but it was the most robustly altered S1PR in mouse and human stroke, indicating a shift toward the proinflammatory axis of S1PR signaling.
Sphingolipid Profiling Identifies the Enrichment of Sphingomyelin and Sphingoid Species in the Mouse Cerebral Microvasculature and the Increase in Ceramide Species after Stroke.
As our transcriptomic (Fig. 2F) and qRT-PCR validation data showed stroke-induced alterations in many of the key genes governing sphingolipid synthesis and metabolism in mouse cerebral microvessels (Fig. 5), we next investigated if these changes in gene expression resulted in altered sphingolipid levels. Alterations in sphingolipid levels have been implicated in a wide range of human diseases (71–73) including stroke and other vascular disorders (74, 75, 76, 77). Therefore, we profiled the sphingolipid metabolites in cerebral microvessels and whole brain in mice after stroke (tMCAO) or sham surgeries. The sphingolipids measured included complex sphingolipids, i.e., sphingomyelin, as well as ceramide species, sphingoid bases (dihydro-Sphingosine, dhSph and sphingosine, Sph), and Sphingoid base-1-Phosphates (dihydro-Sphingosine-1-phosphate, dhS1P and S1P). In SI Appendix, Table S5, a summary of the levels of these sphingolipid species (pmol/mg of protein) in cerebral microvessels and whole brain after sham or tMCAO surgeries is shown. In sham animals, C16, C18, and C24:1 sphingomyelin were the most abundant sphingomyelin species in cortical microvessels, while C18 and C18:1 sphingomyelin were the most abundant in brain. Among ceramides and sphingoid species, C18 and C24:1 ceramide, and sphingosine were the most abundant in microvessels while C18 and C18:1 were the most abundant in brain.
We analyzed the tMCAO-induced changes in sphingolipid levels (pmol/mg protein) in mouse cortical microvessels (SI Appendix, Fig. S15 A and B) and in the whole brain (SI Appendix, Fig. S16 A and B) compared to sham. We did not observe statistically significant differences across any sphingomyelin species in microvessel samples (SI Appendix, Fig. S15 A) despite the significant increase in Sptlc and Sgms1 mRNA levels (Fig. 5). Examination of ceramide and sphingoid species revealed significant increases in total ceramides in microvessel samples after tMCAO (SI Appendix, Fig. S15 B, Lower). Species-specific changes in microvessels included significant increases in C18, C18:1, C20:1, and C20:4 ceramide (2.11 ± 0.15, 1.87 ± 0.13, 1.41 ± 0.20, and 1.48 ± 0.09-fold, respectively, SI Appendix, Fig. S15 B). In whole-brain samples, there were significant increases in C22, C24, and C24:1 sphingomyelin, C18 and C20:1 ceramide, dhSph, and Sph, while C26 ceramide levels decreased after tMCAO (SI Appendix, Fig. S16 A and B).
To better understand the tissue compartmentalization of these sphingolipid species within the brain, we compared sphingomyelin, ceramide, and sphingoid species levels in microvessel and brain samples from sham animals (SI Appendix, Fig. S15 C and D). This comparison revealed that almost all detectable sphingomyelin species (except for C26, C14, and lyso sphingomyelin) were differentially enriched between microvessels and whole-brain samples. Of these, eight species including C16, C20, C20:1, C22, C22:1, C24, C24:1, and C26:1 were highly enriched in microvessels over whole-brain samples (fivefold to 10-fold enrichment), while two species including C18 and C18:1 were enriched in the brain over microvessel samples (0.66 and 0.54-fold, respectively, SI Appendix, Fig. S15 D). Overall, the total levels of sphingomyelin species were higher in microvessels compared to brain (SI Appendix, Fig. S15 C, Lower, 1.9-fold, P = 0.05). Out of the ceramide species, there was a modest enrichment of C14, C20:1, C20:4, C22, C22:1, C24, C24:1, C26, and dhC16 in cerebral microvessels (~twofold to sevenfold) compared to whole-brain samples while C18, C18:1, and C20 were enriched in brain (0.65, 0.60, and 0.38-fold, respectively, SI Appendix, Fig. S15 D). The total content of ceramide species was similar in cerebral microvessels and brain (SI Appendix, Fig. S15 D, Lower). In contrast, consistent with the high enrichment of Acer 2 mRNA in microvessels over whole-brain samples (>10-fold), the total levels of sphingoid species were significantly higher in microvessels (6.7-fold, SI Appendix, Fig. S15 D Lower). These data indicate that cerebral microvessels harbor distinct levels of specific sphingomyelin, ceramide, and sphingoid species compared to brain. A graph summarizing the levels of ceramide and sphingoid species in cerebral microvessels and brain, their changes after tMCAO, and the stroke-induced transcript changes in their related enzymes are shown in SI Appendix, Fig. S15 E. An auxiliary literature analysis (SI Appendix, Table S6) revealed that similar alterations in specific ceramide species have been found in cerebro- and cardio-vascular diseases and in several neurological conditions associated with cerebral microvascular dysfunction in humans.
Together, these data indicate that stroke induces alterations in sphingolipid metabolism in cerebral microvessels resulting in an increase in the total content of ceramides and specific ceramide species. While the levels of sphingomyelin and sphingoid species were not significantly altered in cerebral microvessels, they were highly enriched (fivefold to 10-fold) in microvessels compared to brain. These studies indicate the tissue compartmentalization of sphingolipid species within the brain and highlight the cerebral microvasculature as a key compartment for sphingolipid metabolism and signaling with an important therapeutic potential in stroke.
Discussion
While recent advances in enzymatic and neurosurgical clot removal technologies (i.e. endovascular thrombectomy) have facilitated the recanalization of the obstructed cerebral artery/ies after ischemic stroke, currently, there are not efficacious therapies to prevent or repair damage at the level of the cerebral microvasculature. Therefore, stroke survivors face devastating motor and cognitive sequela. The cerebrovascular endothelium, as the primary barrier between systemic blood supply and the central nervous system (CNS) and key regulator of cerebral blood flow and neurovascular homeostasis, presents a promising therapeutic opportunity. However, our limited understanding of the stroke-induced molecular alterations in cerebral microvessels is restricting the investigation of novel therapeutic approaches to mitigate cerebral microvascular dysfunction and thereby improve neuronal health. To bridge this knowledge gap, in this study we elucidated the molecular signature associated with stroke-induced cerebral microvascular injury. Using a recently optimized method which minimizes cell activation and preserves endothelial cell interactions and RNA integrity, we have profiled the stroke-induced transcriptomic alterations in the mouse cerebral microvasculature. We also interrogated whether some of these molecular features associated with stroke-induced cerebral microvascular dysfunction were present in human stroke chronic lesions and focused our investigation on the shared transcriptomic alterations with the most potential relevance to human stroke pathology. Results from these unbiased comparative analyses have identified shared alterations in endothelial-enriched druggable pathways associated with vascular disease, modulators of microvascular dysfunction, and sphingolipid metabolism and signaling, highlighting the relevance of the cerebral microvasculature in human stroke chronic pathophysiology and its therapeutic potential. Our study provides a detailed resource for future investigation of novel neurovascular protective therapies in stroke.
Stroke is a complex multifactorial disease in which a wide array of molecular perturbations occur after blood flow is occluded in the brain. At the level of the cerebral microvasculature, stroke activates inflammatory and thrombotic pathways which contribute to progressive neuronal death and infarct expansion (17). Our comparative analysis of the mouse stroke microvessel and the human stroke datasets in combination with clustering, pathway, and function analyses using several platforms (IPA, MSigDB, and Webgestalt) has captured this large variety of molecular changes and revealed the functional compartmentalization of the shared gene alterations. Our clustering analyses have identified a vascular disease-associated molecular signature in human stroke chronic lesions, pointing to the potential role of cerebral microvascular dysfunction in chronic stroke pathophysiology (SI Appendix, Fig. S4). In addition, further pathway analysis of the shared transcriptomic changes using IPA and MSigDB confirmed predicted alterations in both vascular-specific (e.g., angiopoietin and endothelin signaling) and ubiquitous inflammation pathways (e.g., neuroinflammation, cAMP/PKA, FRA pathway, or ceramide signaling, Fig. 2 D–F and SI Appendix, Fig. S5 and Datasets S3–S5). Pearson analysis of the top predicted activated (neuroinflammation) and inhibited (PKA) categories revealed the high similarity of the gene alterations in these two categories across both datasets. Of interest, the alterations in the druggable cAMP/PKA pathway, key modulator of barrier function, (29–32, 78) and platelet aggregation (79) have important implications in stroke pathophysiology. In sum, these analyses have improved our understanding of the stroke-induced molecular and cellular changes in cerebral microvessels and identified molecular features associated with vascular disease, endothelial activation, and alterations in sphingolipid signaling in human stroke.
Given the identification of this vascular disease-related module and the predicted activation of endothelial-specific inflammatory pathways in the shared stroke-induced transcriptomic alterations in the mouse and human datasets, we conducted further analysis with the most robustly altered genes in this vascular disease module (Serpine1/PAI-1, Hmox1) and a carefully curated group of genes governing endothelial cell activation and barrier dysfunction (Figs. 3 and 4). We found a significant correlation in the alterations in this group of genes in both datasets. The druggable targets Serpine1 and Hmox1 were robustly and significantly altered in both datasets (Fig. 3B and SI Appendix, Table S3) and recurred as strong driving genes throughout the study. qRT-PCR and histological validation by ISH analysis confirmed the robust induction of Serpine1 and Hmox1 transcripts in cerebral microvessels in the infarct regions of cortex, striatum, thalamus, and hippocampus. The anti-fibrinolytic Serpine1 plays a critical role in vascular senescence (80–82) and its pharmacological inhibition restores vascular function in experimental models of hypertension (65) and stroke (64). In contrast, Hmox1 is an endogenous protective pathway induced in several vascular pathological states including acute coronary syndromes and stroke (60–62 and 83–85) Indeed, polymorphisms in Hmox1 promoter leading to decreased expression of Hmox1 have been associated with atherosclerosis and cardiovascular disease in humans (63). Noteworthily, several modulators of Hmox1 are currently being tested in clinical trials to harness this endogenous protective mechanism for the treatment of cardiovascular diseases (86) (SI Appendix, Table S7). Additional robustly altered genes in our endothelial activation module included members of the angiopoietin pathway (Tek, Angpt2) and the caveolar protein, PLVAP (Figs. 3 and 4). Tek, the gene that transcribes the Tie2 receptor for Angpt1/2, mediates the vasoprotective effects of Ang1 (44–47). In contrast, the Tie2 antagonist, Angpt2, has been associated with vascular leakage (41, 42) in stroke, cytokine release syndrome, and sepsis (43). In cerebral microvessels in the infarct areas, we found that Angpt2 was upregulated while Tek was profoundly downregulated. In the human dataset, there was a robust trend for upregulation of Angpt2 in the stroke lesions (SI Appendix, Table S3, P = 0.058), pointing to the potential role of endothelial activation in human stroke chronic pathophysiology. These data have important therapeutic implications in the investigation of modulators of the pathway as vasoprotective agents (87). Finally, PLVAP, a key driver of endothelial transcytosis whose expression is actively inhibited at the BBB (13), was upregulated in microvessels in the infarct area. In sum, these alterations in gene expression are consistent with the switch of the cerebrovascular endothelium to a procoagulant and permeability phenotype induced by stroke. Our histopathological and qRT-PCR validation of these transcript changes confirms key alterations predicted by our disease, cell function, and pathway analyses using IPA and MSigDB and provides molecular mechanistic insights into stroke-induced cerebral microvascular dysfunction.
Another salient pathway identified in our analyses of the shared stroke-induced transcriptomic alterations between the mouse and human datasets was the sphingolipid pathway (Fig. 2 E and F and Dataset S5). Alterations in the levels of bioactive sphingolipids have been implicated in stroke and a gamut of CNS pathologies. In humans, increased levels of ceramides and decreased levels of sphingoid species in cerebrospinal fluid (CSF) and plasma have been linked to worsened outcomes in cerebrovascular, neurodegenerative, and cardiovascular diseases (71–77, and 88–97), suggesting their role as potential biomarkers and drivers of brain injury and cerebrovascular and cardiovascular dysfunction in humans (SI Appendix, Table S6). However, the upstream molecular players driving these changes, their tissue compartmentalization, and their role in cerebral microvascular dysfunction are not well-understood. Studies with genetically engineered mice and pharmacological inhibitors indicate that ceramides are key drivers of vascular inflammation and aging, while sphingomyelins play a protective role. Inhibition of ceramide metabolizing enzymes, such as aCer2, or increased levels and activity of Smpd1, the enzyme that metabolizes sphingomyelin to give rise to ceramide, results in accumulation of ceramides (68, 69, 98–100) and decreased levels of sphingosine and dihydrosphingosine (98) leading to endothelial dysfunction (99). Ceramide levels in the vasculature are tightly controlled with regulators at multiple levels, being enzymes such aCer2 and Sgms critical checkpoints to limit excessive increase of ceramide levels while Smpd and CerS activities cause a rise in ceramides levels and vascular dysfunction. Our study has provided new knowledge in this area and revealed that in the cerebral microvasculature, sphingomyelin and sphingoid species (Sph and dhSph) along with aCer2 and Sgms transcripts are highly enriched compared to brain and that stroke induced an increase in the total levels of ceramides in cerebral microvessels as well as the levels of Acer2, Sptlc1-3, CerS4, 5, and Sgms1 transcripts. We postulate that Acer2 and Sgms could serve as a major compensatory feedback mechanism to limit the elevation in ceramide levels in cerebral microvessels and, thereby, mitigate cerebral microvascular dysfunction. Noteworthily, there are several modulators of the sphingolipid pathway available (100–106), (SI Appendix, Table S7). In addition to these changes in key mediators of sphingolipid metabolism, our study has also identified the shared alterations in the S1P signaling pathway in human and mouse stroke. S1PR, which are key modulators of vascular and immune function, are becoming attractive targets for drug discovery. Several modulators of S1PR1, which plays a key role in vascular and lymphocyte function (107), are clinically used as immunosuppressors for the treatment of multiple sclerosis (108). While these S1PR1 modulators have showed efficacy in experimental stroke (109) due to their immunosuppressor effects (110), the therapeutic targeting of the S1PR pathway in the vascular wall to restore endothelial function without inducing immunosuppression remains a key challenge in this area (19, 111, 112). Our prior work showed that in contrast to S1PR1, S1PR2 is a key driver of BBB leakage but dispensable for the immune response (19, 70, 113), which highlights the therapeutic potential of S1PR2 to preserve or restore barrier function in stroke. In the present study, we showed the induction of S1PR1 and S1PR2 transcripts in cerebral microvessels in the infarct areas and the upregulation of S1PR2 in both mouse and human stroke, which strengthens the translational relevance in humans of S1PR2 pathway (19, 70, 113). Altogether, our data point to the cerebral microvasculature as a key compartment for sphingolipid metabolism and signaling in stroke. Noteworthily, S1PR2 and Sgms1 were also significantly upregulated in human stroke chronic lesions (SI Appendix, Table S4). Given the emerging role of the sphingolipids in cardiovascular diseases, our study supports the therapeutic potential of this endothelial-enriched pathway to mitigate cerebral microvascular dysfunction in stroke.
There is increasing human evidence that BBB dysfunction is a hallmark of acute and chronic stroke pathophysiology. Gadolinium-based imaging human studies indicating active BBB leakage in patients with subcortical ischemic vascular disease, vascular cognitive impairment, (114) and in chronic cortical ischemic stroke lesions (115, 116) have confirmed earlier histopathological studies in postmortem brain tissue reporting plasma protein extravasation in the brain parenchyma (117, 118). Our work has advanced our knowledge in this area by providing a resource of the shared stroked-induced molecular changes in mouse cerebral microvessels and human stroke chronic lesions and identifying targetable, endothelial-enriched pathways with high therapeutic potential. One limitation of our study is that we compared the changes in acute stroke in mice to chronic human stroke, so additional targets relevant in the acute phase might not be retained in the chronic phase. In addition, the covariate of age and other underlying pathologies may have aggravated the neurovasculature outside of the original stroke. A deeper understanding of the functional relevance of these changes will allow the development of endothelial-targeted therapies to restore microvascular function after stroke. Despite these limitations, our work has identified shared alterations in key endothelial-enriched druggable pathways as promising therapeutic candidates in stroke.
In conclusion, our study provides a knowledge platform of the stroke-induced molecular alterations in mouse cerebral microvessels and the shared molecular changes with human stroke lesions. Our comprehensive analyses and validation have improved our understanding of the pathophysiology of stroke, identified molecular features of cerebral microvascular dysfunction in human chronic stroke lesions, and revealed new promising candidates for future investigation of novel vasoprotective therapies in stroke and other cerebrovascular diseases.
Materials and Methods
Materials and methods are described in detail in the SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Acknowledgments
This work was supported by funds provided by American Heart Association Grant-in-Aid 12GRNT12050110, NIH grant R01NS114561 (to T.S.) and the Department of Pathology and Laboratory Medicine, Weill Cornell Medicine. We thank Jason Pierce (Medical University of South Carolina) for his help with the sphingolipid quantification and Angeles Duran (Weill Cornell Medicine) for her help with QuPath.
Author contributions
K.C., S.D., H.U., Y.S., C.L., Y.L., T.Z., J.X., and T.S. designed research; K.C., S.D., H.U., Y.S., C.L., A.K., Y.L., A.I., T.Z., and T.S. performed research; T.S. contributed new reagents/analytic tools; K.C., S.D., H.U., Y.S., A.K., Y.L., A.L., T.Z., and T.S. analyzed data; and K.C., S.D., H.U., Y.S., C.L., M.J.K., and T.S. wrote the paper.
Competing interests
S.D., Y.L., and T.S. are inventors in US patent application PCT/US2020/045455 (2020).
Footnotes
This article is a PNAS Direct Submission.
Supporting Information
References
- 1.Team GBoD, Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 385, 117–171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D., et al. , Heart disease and stroke statistics–2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119, 480–486 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Benjamin E. J., et al. , American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Molina C. A., Alvarez-Sabin J., Recanalization and reperfusion therapies for acute ischemic stroke. Cerebrovasc Dis. 27, 162–167 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Saver J. L., Improving reperfusion therapy for acute ischaemic stroke. J. Thromb. Haemost. 9, 333–343 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Savitz S. I., Fisher M., Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann. Neurol. 61, 396–402 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Fisher M., Schaebitz W., An overview of acute stroke therapy: Past, present, and future. Arch Intern. Med. 160, 3196–3206 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Daneman R., et al. , The Mouse Blood-Brain Barrier Transcriptome: A New Resource for Understanding the Development and Function of Brain Endothelial Cells. PloS one. 5, e13741. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S., et al. , The vasculome of the mouse brain. PloS one. 7, e52665. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aird W. C., Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 3, 1392–1406 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Regan E. R., Aird W. C., Dynamical systems approach to endothelial heterogeneity. Circulation Res. 111, 110–130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott N. J., Ronnback L., Hansson E., Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Daneman R., Zhou L., Kebede A. A., Barres B. A., Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armulik A., et al. , Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Obermeier B., Daneman R., Ransohoff R. M., Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman R., et al. , Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 641–646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer S. F. D., et al. , Thromboinflammation in stroke brain damage. Stroke 47, 1165–1172 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Shuaib A., Butcher K., Mohammad A. A., Saqqur M., Liebeskind D. S., Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet Neurol. 10, 909–921 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Sanchez T., Sphingosine-1-Phosphate Signaling in Endothelial Disorders. Curr. Atheroscler Rep. 18, 31 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Saver J., et al. , Final Report of the Stroke Progress Review Group. NINDS report. (2011). [Google Scholar]
- 21.Faraci F. M., Vascular protection. Stroke 34, 327–329 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Hu X., De Silva T. M., Chen J., Faraci F. M., Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circulation Res. 120, 449–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanlandewijck M., et al. , A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Munji R. N., et al. , Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 22, 1892–1902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haimon Z., et al. , Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 19, 636–644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossner M. J., et al. , Global transcriptome analysis of genetically identified neurons in the adult cortex. J. Neurosci. 26, 9956–9966 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y., Uchida H., Smith H., Ito A., Sanchez T., The isolation and molecular characterization of cerebral microvessels. Nat. Protocol. 14, 3059–3081 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huttner H. B., et al. , The age and genomic integrity of neurons after cortical stroke in humans. Nat. Neurosci. 17, 801–813 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Chava K. R., Tauseef M., Sharma T., Mehta D., Cyclic AMP response element-binding protein prevents endothelial permeability increase through transcriptional controlling p190RhoGAP expression. Blood 119, 308–319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya R., et al. , The neurotransmitter dopamine modulates vascular permeability in the endothelium. J. Mol. Signal. 3, 14 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vohra P. K., et al. , Dopamine inhibits pulmonary edema through the VEGF-VEGFR2 axis in a murine model of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L185–L192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarei S., et al. , Dopamine modulates von Willebrand factor secretion in endothelial cells via D2–D4 receptors. J. Thromb. Haemost. 4, 1588–1595 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A., et al. , Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lengfeld J. E., Endothelial Wnt/β-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 114, E1168–E1177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberzon A., et al. , The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberzon A., et al. , Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebner S., et al. , Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathologica. 135, 311–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keep R. F., et al. , Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cerebral Blood Flow Metab. 38, 1255–1275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata M., et al. , Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 103, 1799–1805 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Zhao Z., Nelson A. R., Betsholtz C., Zlokovic B. V., Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163, 1064–1078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurnik S., et al. , Angiopoietin-2-induced blood–brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathologica. 131, 753–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gust J., et al. , Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 7, 1404–1419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh S. M., et al. , Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 3, e46. (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro N. I., et al. , The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care. 14, R182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David S., et al. , Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit. Care Med. 40, 3034–3041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh C. C., et al. , Impaired function of the Tie-2 receptor contributes to vascular leakage and lethality in anthrax. Proc. Natl. Acad. Sci. U.S.A. 109, 10024–10029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David S., et al. , Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L851–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., et al. , Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife 8, e43257. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y., Nathans J., Gpr124 Controls CNS Angiogenesis and Blood-Brain Barrier Integrity by Promoting Ligand-Specific Canonical Wnt Signaling. Dev. Cell. 31, 248–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho C., Smallwood P. M., Nathans J., Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron 95, 1056–1073.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart P. A., Wiley M. J., Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev. Biol. 84, 183–192 (1981). [DOI] [PubMed] [Google Scholar]
- 52.Liebner S., et al. , Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang J., et al. , Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 23, 450–460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimomura Y., et al. , APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 464, 1043–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi M., et al. , Isolation of a novel human gene, APCDD1, as a direct target of the beta-Catenin/T-cell factor 4 complex with probable involvement in colorectal carcinogenesis. Cancer Res. 62, 5651–5656 (2002). [PubMed] [Google Scholar]
- 56.Mazzoni J., et al. , The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron 96, 1055–1069.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herz J., Strickland D. K., LRP: A multifunctional scavenger and signaling receptor. J. Clin. Invest. 108, 779–784 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata M., et al. , Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nikolakopoulou A. M., et al. , Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J. Exp. Med. 218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idriss N. K., Blann A. D., Lip G. Y., Hemoxygenase-1 in cardiovascular disease. J. Am. Coll. Cardiol. 52, 971–978 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Duckers H. J., et al. , Heme oxygenase-1 protects against vascular constriction and proliferation. Nature Med. 7, 693–698 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Kinderlerer A. R., et al. , Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: A role for increased bilirubin and ferritin. Blood. 113, 1598–1607 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Pechlaner R., et al. , Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biol. 35, 229–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan S.-L., Bishop N., Li Z., Cipolla M. J., Inhibition of PAI (Plasminogen Activator Inhibitor)-1 Improves Brain Collateral Perfusion and Injury After Acute Ischemic Stroke in Aged Hypertensive Rats. Stroke 49, 1969–1976 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boe A. E., et al. , Plasminogen Activator Inhibitor-1 Antagonist TM5441 Attenuates Nω-Nitro-l-Arginine Methyl Ester-Induced Hypertension and Vascular Senescence. Circulation 128, 2318–2324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., et al. , An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Official J. Soc. Neurosci. 34, 11929–11947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., et al. , Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 89, 37–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith A. R., et al. , Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: Evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 5, 391–400 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Ohanian J., Liao A., Forman S. P., Ohanian V., Age-related remodeling of small arteries is accompanied by increased sphingomyelinase activity and accumulation of long-chain ceramides. Physiol Rep., 2, e12015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim G. S., et al. , Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat. Commun. 6, 7893 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hannun Y. A., Obeid L. M., Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 175–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hannun Y. A., Obeid L. M., The Ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Dunn T. M., Tifft C. J., Proia R. L., A perilous path: The inborn errors of sphingolipid metabolism. J. Lipid Res. 60, 475–483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Testai F. D., et al. , Changes in the cerebrospinal fluid ceramide profile after subarachnoid hemorrhage. Stroke 43, 2066–70 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Sheth S. A., Iavarone A. T., Liebeskind D. S., Won S. J., Swanson R. A., Targeted lipid profiling discovers plasma biomarkers of acute brain injury. PloS one. 10, e0129735. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Testai F. D., et al. , Changes in the metabolism of sphingolipids after subarachnoid hemorrhage. J. Neurosci. Res. 93, 796–805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mielke Michelle M., et al. , Elevated plasma ceramides are associated with higher white matter hyperintensity volume—brief report. Arteriosclerosis, Thrombosis, and Vascular Biol. 39, 2431–2436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schauer I. E., et al. , CREB downregulation in vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biol. 30, 733–741 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gresele P., Momi S., Falcinelli E., Anti-platelet therapy: Phosphodiesterase inhibitors. Br. J. Clin. Pharmacol. 72, 634–46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eren M., et al. , PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc. Natl. Acad. Sci. U.S.A. 111, 7090–7095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tashiro Y., et al. , Inhibition of PAI-1 induces neutrophil-driven neoangiogenesis and promotes tissue regeneration via production of angiocrine factors in mice. Blood 119, 6382–6393 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Jeong B. Y., et al. , Novel Plasminogen Activator Inhibitor-1 Inhibitors Prevent Diabetic Kidney Injury in a Mouse Model. PloS one. 11, e0157012. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satoh T., et al. , Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophillic phase II inducers. Proc. Natl. Acad. Sci. U.S.A. 103, 768–773 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bereczki D. Jr., Balla J., Bereczki D., Heme Oxygenase-1: Clinical Relevance in Ischemic Stroke. Curr. Pharm. Des. 24, 2229–2235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X., et al. , Higher level of heme oxygenase-1 in patients with stroke than TIA. J. Thorac. Dis. 6, 772–777 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryter S. W., Choi A. M., Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 167, 7–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akwii R. G., Mikelis C. M., Targeting the Angiopoietin/Tie Pathway: Prospects for Treatment of Retinal and Respiratory Disorders. Drugs 81, 1731–1749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Wit N. M., et al. , Altered sphingolipid balance in capillary cerebral amyloid angiopathy. J. Alzheimer’s Dis. 60, 795–807 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Laaksonen R., et al. , Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schulz M. E., Katunaric B., Hockenberry J. C., Gutterman D. D., Freed J. K., Manipulation of the Sphingolipid Rheostat Influences the Mediator of Flow-Induced Dilation in the Human Microvasculature. J. Am. Heart Assoc. 8, e013153. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwedhelm E., et al. , Serum Sphingosine-1-Phosphate Levels Are Associated With Severity and Outcome in Patients With Cerebral Ischemia. Stroke 52, 3901–3907 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Kim M., et al. , Association between Plasma Ceramides and Phosphatidylcholines and Hippocampal Brain Volume in Late Onset Alzheimer’s Disease. J. Alzheimers Dis. 60, 809–817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cutler R. G., et al. , Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 101, 2070–5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Filippov V., et al. , Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J. Alzheimers Dis. 29, 537–47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Wit N. M., et al. , Astrocytic ceramide as possible indicator of neuroinflammation. J.Neuroinflammation 16, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vidaurre O. G., et al. , Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 137, 2271–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meeusen Jeffrey W., et al. , Plasma Ceramides. Arteriosclerosis, Thrombosis, and Vascular Biol. 38, 1933–1939 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Li F., et al. , Alkaline ceramidase 2 is essential for the homeostasis of plasma sphingoid bases and their phosphates. FASEB J. 32, 3058–3069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F., et al. , Maternal and fetal alkaline ceramidase 2 is required for placental vascular integrity in mice. FASEB J. 34, 15252–15268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lallemand T., et al. , nSMase2 (Type 2-Neutral Sphingomyelinase) Deficiency or Inhibition by GW4869 Reduces Inflammation and Atherosclerosis in Apoe(-/-) Mice. Arterioscler Thromb. Vasc. Biol. 38, 1479–1492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mao Z., et al. , Alkaline ceramidase 2 (ACER2) and its product dihydrosphingosine mediate the cytotoxicity of N-(4-hydroxyphenyl)retinamide in tumor cells. J. Biol. Chem. 285, 29078–90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petit C. S., et al. , Inhibition of sphingolipid synthesis improves outcomes and survival in GARP mutant wobbler mice, a model of motor neuron degeneration. Proc. Natl. Acad. Sci. U.S.A. 117, 10565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schiffmann S., et al. , Inhibitors of specific ceramide synthases. Biochimie 94, 558–565 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Turner N., et al. , A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun. 9, 3165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jensen S. A., et al. , Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. U. S. A. 111, 5682–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barcelo-Coblijn G., et al. , Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. U. S. A. 108, 19569–19574 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matloubian M., et al. , Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Brinkmann V., et al. , FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug. Discov. 9, 883–897 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Wei Y., et al. , Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69, 119–129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kraft P., et al. , FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 44, 3202–3210 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Swendeman S. L., et al. , An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal 10, eaal2722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nitzsche A., et al. , Endothelial S1P1 Signaling Counteracts Infarct Expansion in Ischemic Stroke. Circulation Res. 128, 363–382 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang G., et al. , Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood 122, 443–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taheri S., et al. , Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 42, 2158–2163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wardlaw J. M., et al. , Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 65, 194–202 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Topakian R., Barrick T. R., Howe F. A., Markus H. S., Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry. 81, 192–197 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Tomimoto H., et al. , Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke 27, 2069–2074 (1996). [DOI] [PubMed] [Google Scholar]
- 118.Akiguchi I., Tomimoto H., Suenaga T., Wakita H., Budka H., Blood-brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol. 95, 78–84 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)