Abstract
Suprarenal or supraceliac aortic clamping during repair of infrarenal abdominal aortic aneurysms can be complicated by renal, hepatic, and intestinal ischemia. To determine whether suprarenal or supraceliac clamping increases morbidity and mortality, we retrospectively reviewed our recent nonrandomized experience. Between January 1993 and December 1998, 716 patients underwent elective (n=682) or urgent (n=34) infrarenal abdominal aortic aneurysm repair. Infrarenal clamping was used in 516 (72.1%) and suprarenal or supraceliac clamping in 200 (27.9%). The suprarenal/supraceliac group had significantly more older patients (≥70 years of age) (65.5% vs 47.7%) and a higher incidence of preoperative renal insufficiency (7.5% vs 5.5%). Suprarenal or supraceliac clamping was used during repair of ruptured (n=25), juxtarenal (n=7), or inflammatory abdominal aortic aneurysms (n=4); during concomitant renal or visceral revascularization (n = 43); in other difficult settings (n=13); or at the surgeon's discretion (n=108). The decision for such clamping was always made during surgery. In treating ruptured aneurysms, suprarenal/supraceliac clamping (25/200) was used more often than infrarenal clamping (9/516) (12.5% vs 1.74%). Operative times were similar in both groups, but transfusion requirements and length of hospital stay were slightly greater in the suprarenal/supraceliac group. Perioperative mortality was 3.1% overall, but higher in the suprarenal/supraceliac group than in the infrarenal (7.5% vs 1.4%). Postoperative complications developed in 26 (13%) of patients who underwent suprarenal/supraceliac clamping. Abdominal re-exploration was required in 9 other patients. We conclude that, despite associated comorbidities, elective suprarenal/supraceliac clamping during infrarenal abdominal aortic aneurysm repair is safe, facilitates repair, and does not significantly increase mortality. (Tex Heart Inst J 2001;28:254–64)
Key words: Aortic aneurysm, abdominal; supraceliac clamping; suprarenal clamping
Most infrarenal (IR) abdominal aortic aneurysms (AAAs) can be repaired safely under IR aortic cross-clamping. However, unusual lesions and other patient-related factors can occasionally make IR clamping difficult or impossible. In such circumstances, several technical variations can be used to control the IR aorta, 1,2 including clamping of the suprarenal (SR), visceral, or supraceliac (SC) aortic segments.
Deciding on the optimal level of proximal aortic control in the treatment of aortic aneurysmal disease has been controversial. Some researchers say that IR clamping minimizes distal embolization but may cause renal embolization 3 and can result in the later development of para-anastomotic pseudoaneurysms. 4 Interrenal clamping, on the other hand, is associated with more renal and gastrointestinal complications than either SR or SC clamping. 5 Others have argued that visceral clamping (that is, between the renal arteries and the superior mesenteric artery) is associated with a disturbingly high rate of complications and therefore should be avoided entirely. 6 The SC aortic segment is less likely than the IR and visceral aortic segments to have significant atherosclerotic disease. 7 Supraceliac clamping also avoids the need for retraction and manipulation of large aneurysms and might reduce the risk of embolization during dissection.
Still, some authors have expressed reluctance to clamp the aorta at the SR or SC level, since such clamping requires more extensive dissection and can be attended by excessive cardiac morbidity, paraplegia, acute renal failure, and hepatic or intestinal ischemia. 8,9 In a study by Green and colleagues, 10 patients whose aortas were clamped immediately above the renal arteries had higher perioperative mortality rates and a higher incidence of kidney failure requiring dialysis than did patients whose aortas were clamped at the SC or IR level. However, some researchers have noted no differences in mortality rates with regard to the site of aortic clamping and comparable or even favorable cardiac morbidity rates with more proximal clamping. 11,12
To clarify the advantages and disadvantages of SR and SC clamping in comparison with IR clamping in the setting of IR AAA repair—including their comparative effects on morbidity and mortality—we retrospectively reviewed our recent 6-year experience with these approaches in a tertiary referral center. We have found some differences in early mortality and complications and, despite the nonrandomized nature of our patient series, have made a number of useful observations about SR and SC clamping in this setting.
Patients and Methods
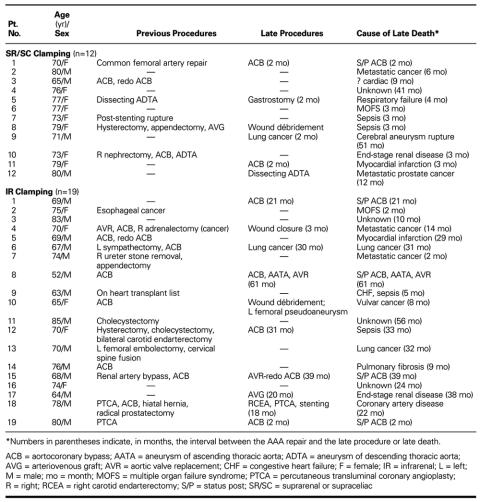
Between January 1993 and December 1998, 716 patients underwent surgical repair of IR AAAs at our institution (Tables IA and IB). Patients fell into 2 groups in accordance with the level of aortic clamping: patients whose aortas were clamped at the SR or the SC level (SR/SC group) and those whose aortas were clamped at the IR level (IR group). Patients were examined clinically by members of our vascular surgery service before and every 2 to 3 months after surgery. The data gathered for analysis were patients' demographic information, including aneurysm status (ruptured or intact) and preoperative risk factors (e.g., prior myocardial revascularization, peripheral or cerebrovascular disease, obesity, chronic obstructive pulmonary disease, and level of renal function). We also analyzed operative data, such as the level and duration of clamping, concomitant renal revascularization, inferior mesenteric artery reimplantation, estimated blood loss, and amount of blood replaced. Outcome analysis included early (30-day) mortality and incidence of complications such as renal dysfunction, mesenteric ischemia, distal thromboembolism, and cardiac events.
TABLE IA. Summary of Patients' Characteristics by Clamping Type
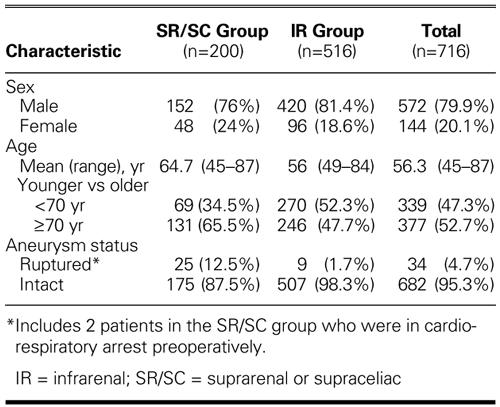
TABLE IB. Summary of Patients' Characteristics by Aneurysm Status (n = 716)
Results
Preoperative and Operative Data
SR/SC Group.
A total of 200 patients (152 men and 48 women; mean age, 64.7 years; age range, 45–87 years; 65.5% ≥70 years) underwent SR or SC clamping during repair of an AAA (Tables IA and IB). Indications for SR or SC clamping included ruptured aneurysm in 25 cases (including 2 cases in which the patient suffered preoperative cardiorespiratory arrest), concomitant renal revascularization in 40, visceral revascularization in 3, juxtarenal aneurysm in 7, and inflammatory aneurysm in 4. In 121 cases, SR or SC clamping was indicated by difficult exposure of the IR aorta (n=13) or was the surgeon's preference (n=108).
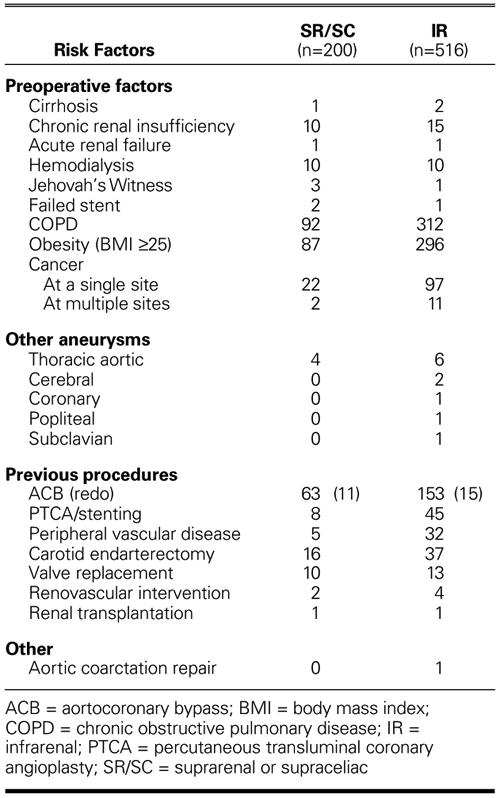
Most patients in the SR/SC group had multiple comorbid risk factors (Table II). Seventy-one of the 200 patients (35.5%) had undergone previous coronary revascularization; 10 (5%), cardiac valve replacement; and 16 (8%), carotid endarterectomy. Preoperative acute or chronic renal insufficiency was present in 15 patients (7.5%), including 4 in whom the patient required chronic hemodialysis. The average aneurysm size (± SD) was 6.5 ± 1.46 cm.
TABLE II. Comorbid Risk Factors
The mean SR/SC clamping time was 22 min (range, 12–69 min). The clamping time was less than 15 min in 58 patients, 15 to 30 min in 59, 30 to 45 min in 10, 45 to 60 min in 4, and more than 60 min in 1. The average blood loss was 1684 ± 1328 mL, and the average volume of packed red blood cells transfused was 1063 ± 868 mL. Autotransfusion was used in 74 cases; in those cases, the average volume of returned blood was 914 ± 747 mL.
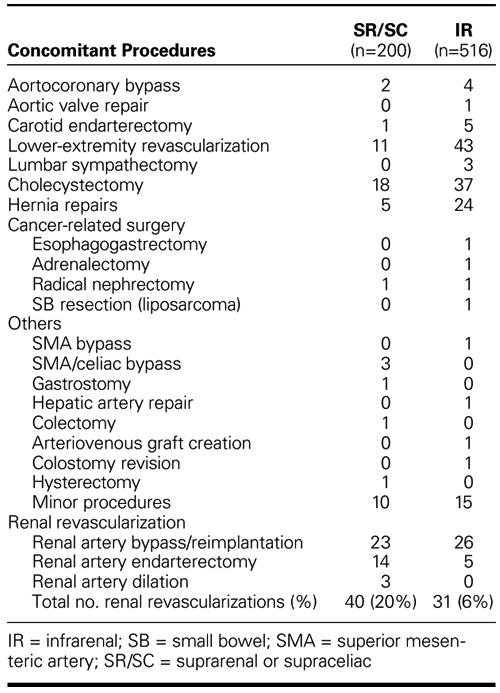
Concomitant procedures performed in the SR/SC group are listed in Table III. Tube grafts were used in 103 patients; bifurcated grafts to the iliac (n=51) or the femoral arteries (n=46) were used in the remaining 97 patients. The inferior mesenteric artery was reimplanted in 46 patients (23%).
TABLE III. Concomitant Procedures
IR Group.
A total of 516 patients (420 men and 96 women; mean age, 56 years; age range, 49–84 years; 47.7% ≥70 years old) underwent IR clamping (Tables IA and IB). Nine of them (1.7%) presented with ruptured aneurysms. Comorbid factors included acute or chronic renal insufficiency in 26 patients, including 10 patients who required chronic hemodialysis (Table II). One hundred ninety-eight patients (38.4%) had undergone previous myocardial revascularization; 37 (7.2%), carotid endarterectomy; and 32 (6.2%), lower-extremity revascularization. Six patients had undergone previous repairs of aneurysms of the thoracic aorta.
Concomitant procedures performed in the IR group included those listed in Table III. Inferior mesenteric artery reimplantation was performed in 136 cases (26.4%). A total of 31 renal revascularization procedures were performed, which included renal artery bypass or reimplantation (or both) in 26 cases and renal artery endarterectomy in 5.
Early Postoperative Mortality and Complications
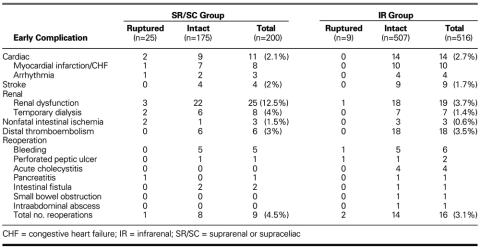
Overall, 21 of 716 patients (2.9%) died in the early postoperative period (within 30 days) (Table IV). The early postoperative mortality rate was higher in patients with ruptured aneurysms (5/34, 14.7%) than in those with intact aneurysms (16/682, 2.35%). Most early postoperative deaths (9/21, 43%) were cardiac related. Intestinal ischemia complicated AAA repair in 12 cases, 6 of which ended in death. Preoperative renal function temporarily worsened in 19 patients, 5 of whom required temporary dialysis support.
TABLE IV. Early Postoperative Mortality (n = 21)
SR/SC Group.
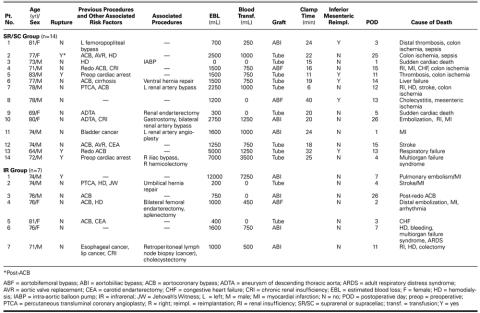
Fourteen patients in the SR/SC group (7%) died in the early postoperative period (Table IV). These included 4 of 25 patients with ruptured aneurysm (16% early mortality) and 10 of 175 patients with intact aneurysm (5.7% early mortality). Causes of early death included sepsis due to mesenteric ischemia in 6 cases, sudden death after an uneventful early recovery in 2, liver failure in 1, stroke in 1, myocardial infarction in 2, respiratory failure in 1, multiorgan failure syndrome in 1, and preoperative cardiopulmonary arrest (as previously mentioned) in 2.
Early postoperative complications in this group included nonfatal myocardial infarction or congestive heart failure in 8 cases, nonfatal arrhythmia in 3, and stroke in 4 (Table V). Abdominal reexploration was required for bleeding in 5 cases, perforated chronic peptic ulcer in 1, small bowel fistula in 2, and pancreatic débridement in 1. Transient renal dysfunction developed in 25 patients (12.5%), 8 of whom required temporary dialysis. In addition, intestinal ischemia requiring reoperation developed in 3 patients (1.5%). The rate of postoperative complications was markedly higher after repair of ruptured aneurysms, in comparison with intact aneurysms (8% vs 4.6%). Early graft-related complications (graft thrombosis and distal atheroembolism) developed in 6 patients (3%).
TABLE V. Early Postoperative Complications in Accordance with Aneurysm Status
IR Group.
Seven patients in the IR group (1.4%) died in the early postoperative period (Table IV). These included 1 of 9 patients with ruptured aneurysm (11.1% early mortality) and 6 of 507 patients with intact aneurysm (1.2% early mortality). Causes of early death included cardiac-related causes in 4 cases, multiorgan failure syndrome in 1, colon ischemia in 1, and complication of redo aortocoronary bypass in 1.
Early postoperative complications in the IR group are listed in Table V. Early renal dysfunction complicated repair in 19 patients (3.7%), 7 of whom required temporary dialysis (1.4%). Nonfatal intestinal ischemia complicated repair in 3 patients (0.6%), all after repair of intact aneurysms. Nonfatal myocardial infarction or congestive heart failure complicated repairs in 10 patients (1.9%), 3 of whom required aortocoronary bypass. Four patients developed nonfatal arrhythmias. Nine patients (1.7%) suffered strokes of varying degrees postoperatively. Distal thromboembolic complications requiring graft thrombectomy or revision developed in 18 patients (3.5%). Abdominal reexploration was indicated by bleeding in 6 patients, acute cholecystitis in 4, perforated chronic peptic ulcer in 2, gastric fistula following esophagogastrectomy for esophageal cancer in 1, and other causes in 3.
Late Postoperative Mortality
Overall, during a mean follow-up period of 27 months (range, 2 months to 7 years), 31 patients died of various causes (Table VI). Of these deaths, 10 were cardiac related, 7 were due to complications of cancer, and 5 were due to sepsis and multiorgan failure syndrome.
TABLE VI. Late Postoperative Mortality (n = 31)
Discussion
In a retrospective review of our recent 6-year nonrandomized experience with SR/SC clamping during IR AAA repair in which the decision to use SR/SC clamping was always made during surgery, we found that patients who underwent SR/SC clamping were disproportionately older and had a higher incidence of preoperative renal insufficiency. We also found that SR/SC clamping was used more often than IR clamping in repairing ruptured aneurysms. These factors were reflected in a higher postoperative mortality rate for patients who underwent SR/SC clamping, in comparison with IR clamping.
These findings are important because, as endovascular 13 and other minimally invasive techniques 14 for IR aortic aneurysm repair become more widely applied, an increasing proportion of patients with AAAs for whom conventional open repair is indicated will have aneurysms deemed unsuitable for IR clamping. Such lesions will include juxtarenal aneurysms with short IR necks, aneurysms associated with renal or visceral arterial disease, aneurysms associated with excessive calcification and atherosclerotic disease of the juxtarenal aorta, and inflammatory aneurysms. Consequently, alternatives to IR clamping during AAA repair (such as SR, SC, and visceral clamping) will have to be used.
Controversy continues to surround the relative merits of IR in comparison with SR/SC clamping. In a study by Green and colleagues, 10 the rates of operative mortality (32% vs 3%) and renal failure requiring dialysis (23% vs 3%) after AAA repair were much higher when clamping was done between the superior mesenteric artery and the renal arteries, rather than proximal to the celiac artery. This difference was attributed to the greater likelihood of dislodging atherosclerotic débris in the pararenal aorta than in the SC aorta, which is usually less diseased. Clamping-related complications included atheroembolization to the kidneys, legs, and intestines, and injury to the aorta or renal arteries.
Some surgeons are reluctant to clamp the aorta above the level of the renal arteries during elective aneurysm repair because of the risks of death, renal failure, and atheroembolization. Surgical mortality rates of 15% or more after SR or SC clamping, usually due to cardiac complications and visceral ischemic syndromes, have been reported. 15 However, many of the studies that argue against SR and SC clamping have included patients with SR aneurysms. More recent studies that were restricted to IR and juxtarenal aneurysms have shown more encouraging results, including mortality rates for SR and SC clamping that are indistinguishable from those associated with conventional IR clamping. 12,16
Breckwoldt and associates 11 noted the relative safety of clamping the SC aorta, which can be easily accessed by dividing the gastrohepatic ligament and the diaphragmatic crus. In patients with juxtarenal aneurysms, hiatal clamping enables safe and easy anastomosis to the healthy aorta, which in turn helps prevent the formation of late anastomotic aneurysms. Giulini and coworkers 12 compared the outcomes of treatment of juxtarenal AAAs after SR clamping (56 patients) with those of IR clamping (634 patients) and found no significant difference between the 2 groups in terms of 30-day mortality (3.6% vs 1.9%), the incidence of acute myocardial infarction (3.6% vs 2.3%), or the need for homologous blood transfusion. On the other hand, when they compared the incidence of renal dysfunction in the 2 groups, they found that the SR group had higher rates of renal dysfunction (14% vs 0) and that one of the patients in the SR group who developed renal dysfunction required permanent dialysis. The rate of ischemic colitis was also higher after SR clamping (7% vs 2%).
In our experience, accurate preoperative evaluation of the extent of disease in the aorta at the planned level of cross-clamping is not always possible. We have found that the midline approach provides adequate exposure of the SR and subdiaphragmatic portions of the aorta and affords fast exposure in cases of ruptured aneurysms. Careful dissection around the aorta in the presence of a complex aneurysm is always important. Adherence of the aorta to surrounding tissues in the region of the left renal vein should contraindicate further exploration of the juxtarenal segment. Left renal vein ligation is associated with notable morbidity rates 17,18 and is rarely necessary.
Perioperative Mortality
The overall perioperative mortality rate of 2.9% in our current series compares favorably with rates cited in other recent reports. 19–21 The early mortality rate of 14.7% for patients with ruptured aneurysms (including 2 patients who suffered preoperative cardiac arrest and were operated on while undergoing continuous cardiopulmonary resuscitation) is remarkable. Similarly, our 2.49% mortality rate for patients with intact aneurysms is remarkable when one considers this subgroup's high rates of preoperative renal insufficiency (4.1%), renal revascularization (10%), and other concomitant procedures (27%).
At first, our direct comparison of early mortality after SR/SC clamping with IR clamping appeared to reveal a strikingly higher mortality rate after SR/SC clamping (7.5% vs 1.4% overall and 6.3% vs 1.19% in patients with intact aneurysms). However, in-depth analysis revealed that most patients in the SR/SC group had considerably more risk factors and underwent more extensive procedures (such as renal revascularization) than did those in the IR group. A higher death rate was to be expected, since poor preoperative renal function is strongly associated with postoperative death, 22 and since the mortality rate is high for patients who need postoperative renal replacement therapy. 23 Moreover, due to the very nature of their illness, sicker patients more often underwent SR/SC clamping.
Cardiac Complications
In a prospective review of surgical results after elective AAA repair, Blankensteijn 21 found a 30-day hospital-based mortality rate of 3.0% to 4.8% and a cardiac complication rate of 0.5% to 13.9%. The most frequent complications in that series were cardiac related.
Several authors have described the various physiologic and cardiac consequences of clamping the aorta above the level of the renal arteries. 24–26 These consequences are more severe in patients with coronary artery disease (CAD) 27–29 and increase in severity with higher levels of clamping. In a study by Bush's group, 30 cardiac depression was minimal after aortic clamping and appropriate preoperative volume loading of the left ventricle. In a more recent study by Hafez and colleagues, 31 SC clamping was associated with significant release of the myocardial injury marker troponin-T. However, this finding corresponded with the severity of oxidative rather than hemodynamic stresses, which suggests that ameliorating oxidative injury during AAA surgery may be cardioprotective.
Our current experience confirms that cardiac-related death is a serious problem in the setting of IR AAA repair. Clinical predictors of perioperative cardiac death include a history of heart failure, angina pectoris, or myocardial infarction; an abnormal resting electrocardiogram; a history of stroke or other vascular disease; previous vascular operation; hypertension; and diabetes mellitus. Patients with angina, CAD, or a history of myocardial infarction should undergo coronary angiography. Patients whose clinical findings are equivocal should be evaluated by stress testing and, if the results are positive, by subsequent coronary angiography. Aggressive correction of significant CAD, either before or during AAA repair, substantially decreases the risk of postoperative cardiac-related death. 24,27,28,32–39
In our current experience, left ventricular failure complicated repair in only 1 case and necessitated temporary placement of an intra-aortic balloon pump. Myocardial infarction or congestive heart failure in the early postoperative period developed more often after SR/SC clamping than after IR clamping (4% vs 2%) and was more often fatal to affected patients in the SR/SC group (63% vs 40%) (Table V). The difference, however, is partly explained by the higher average age of patients (a large proportion of whom had undergone prior or concomitant myocardial revascularization) and partly by the higher incidence of ruptured aneurysms in the SR/SC group.
Renal Complications and Outcomes
Several factors influence renal outcome after open repair of IR AAAs. 40 These include preoperative renal function, the presence of renovascular disease, the site and duration of aortic clamping, and intraoperative and major postoperative complications. 41,42 Preoperative renal insufficiency is a significant risk factor for death after elective repair of IR AAAs. 43 Although the indications for AAA repair in patients with chronic renal failure are the same as those for patients with normal renal function, the operative mortality rate for those with renal failure is 5 times higher (2.0% vs 0.4%). 44 However, the 5-year survival rate for patients with renal dysfunction and treated aneurysms is still significantly higher than it is for patients with renal dysfunction and untreated aneurysms (44% vs 20%). 45 In a study by Sasaki and associates, 46 postoperative renal dysfunction (defined by a serum creatinine level >2.0 mg/dL) developed in 50% of patients after bilateral SR clamping, in comparison with only 8.4% after IR clamping. In that study, the postoperative peak BUN level was significantly higher after a longer (>30 min), as opposed to shorter, SR clamping time, although no patient required either temporary or permanent hemodialysis.
In our present study, chronic renal insufficiency was present preoperatively in 3.5% of our patients, including 14 patients (2%) who required hemodialysis support. The risk of postoperative renal dysfunction was occasionally greater with SR/SC clamping, although this trend was transient and eventual outcomes after SR/SC clamping were otherwise indistinguishable from outcomes after IR clamping. The rates of transient postoperative renal dysfunction after elective SR/SC clamping were 12.6% as opposed to 3.35% after IR clamping, which rates are significantly lower than those observed in other comparative series. 47
Several measures can be implemented to lower the risk of renal damage during SR/SC clamping. One is to place the aortic clamp in a manner that preserves flow into the renal parenchyma. However, this placement is anatomically possible in only 22% of patients with AAA. Another measure is to create a beveled proximal aortic anastomosis that incorporates one of the renal artery orifices in order to expeditiously restore perfusion to one of the kidneys and leave only one renal artery to be reimplanted or grafted. However, this technique is restricted to patients with juxtarenal aneurysms. A 3rd measure, used only occasionally, is interrenal clamping of the aorta. 5 However, this increases the risk of renal damage.
Irrigation of the aortic lumen with saline while keeping the renal arteries occluded minimizes the risk of renal embolization. Topical renal cooling, however, appears to confer no clear benefit; it may even be associated with increased morbidity rates and can be made cumbersome by the hypothermic perfusion systems needed for such cooling. 48–50 We find intravenous volume loading and induction of diuresis with mannitol to be necessary before SC clamping. 51 In our current series, expeditious and meticulous proximal anastomosis decreased the mean clamping time in the SR/SC group. In general, we have found that the renal dysfunction attendant upon SR or SC clamping can be minimized by meticulously monitoring and rapidly treating intraoperative and postoperative hypotension.
Poorer outcomes, including a higher mortality rate, are often cited as reasons for avoiding concomitant renal revascularization during AAA surgery, 52 and there are no well established indications for the procedure in this circumstance. However, our experience and those of others 15,53,54 suggest that, despite adding to the surgical complexity, concomitant renal revascularization does not substantially increase the risk of death. Selected patients with associated renovascular disease should undergo full renal revascularization. In addition to the preservation of renal function, the indications for renal revascularization in patients with renal artery stenosis include poorly controlled hypertension, ischemic nephropathy, recurrent episodes of “flash” pulmonary edema, and congestive heart failure. 55 The added benefit of ameliorating renovascular hypertension and preventing further deterioration in renal function outweighs the added complexity of the combined procedure.
Postoperative acute renal failure after repair of intact or ruptured AAAs has a high mortality rate. 47,56 To avert acute failure, various therapeutic maneuvers for accelerating the recovery of glomerular filtration have been introduced. Modern dialysis—performed without anticoagulation and with the use of biocompatible membranes—reduces recovery time by activating fewer neutrophils and less complement. Using bicarbonate (rather than acetate) as a buffer reduces cardiovascular instability and provides more precise regulation of volume removal.
Intestinal and Other Visceral Complications
Intestinal and other visceral ischemia is a significant risk factor for early postoperative death, especially when complicated by bowel infarction and perforation. Several prospective studies have shown an incidence of 1% to 2% and associated mortality rates of 40% to 100%. 57,58 In our current series, mesenteric ischemia developed in 6 patients (3%) in the SR/SC group (2 with ruptured and 4 with intact AAAs), in comparison with 3 patients (0.6%) in the IR group (all with intact AAAs). Moreover, 2 of the 4 patients with intact aneurysms in the SR/SC group died of their aneurysms, versus none in the IR group. The mechanism for intestinal ischemia after AAA repair is atheroembolization or temporary or permanent interruption of blood flow by clamping or ligation. Recent studies using multivariate regression analysis have shown no statistically significant correlation between clamping level and the development of visceral ischemia or infarction. 11,12,59,60 However, the fact that the incidence of intestinal and other visceral ischemia is higher after operation for ruptured AAAs underscores the important causative role of poor hemodynamics in mesenteric vasoconstriction, hypoperfusion, and nonocclusive intestinal ischemia. 61
Reimplantation of the inferior mesenteric artery is warranted when a preoperative angiogram shows occlusion of both internal iliac arteries, or when back-flow from the patent inferior mesenteric artery is absent or poor. In at least 1 report, a stump pressure of less than 40 mmHg was cited as an indication for reimplantation, 62 although in that instance higher pressure failed to avert colonic necrosis.
Postoperative identification of mesenteric ischemia requires a high index of suspicion, and diagnosis is greatly complicated by the systemic and abdominal changes that typically occur after aortic surgery. However, 95% of ischemic lesions in this setting affect the rectum or the sigmoid or descending colon, and so are within reach of the sigmoidoscope. 63
Other Considerations
In our current series, consequential infrainguinal arterial disease that required concomitant AAA repair and peripheral revascularization arose in 11 patients in the SR/SC group versus 46 in the IR clamp group (5.5 % vs 8.9%). Early graft-related complications that required revision (graft thrombosis and distal atheroembolism) arose in 6 patients in the SR/SC clamp group versus 18 in the IR group (3% vs 3.5%), a statistically insignificant difference. Anastomotic hemorrhage that required abdominal reexploration developed in 3 patients in the SR/SC group versus 5 in the IR group (1.5% vs 1%). No patient in either clamp group developed a pseudoaneurysm, graft-enteric erosion or fistula, or graft infection. In addition, we have found only a slightly greater transfusion requirement with SC clamping and have seen no cases of clinically apparent coagulopathy in patients undergoing either SC or IR clamping.
Several authors have cautioned against performing other procedures concomitantly with AAA repair 64 on the grounds that this will prolong operating time or complicate the primary surgical procedure. While we do not dispute the extra time and complexity, we have found that such combinations add very little to morbidity or mortality, but tend instead to shorten convalescence and thereby to decrease the overall cost of the hospital stay.
Conclusions
In the setting of IR AAA repair, when cuff dissection is a likely hazard, SR or SC clamping is our preferred method of achieving proximal control of the IR aorta. Selection of the best approach to gain the needed arterial exposure, careful dissection of the pararenal and paravisceral aorta, and close adherence to the proper sequence for clamping and unclamping the aorta and visceral branches are paramount and might be even more crucial than the actual cross-clamp position in reducing associated mortality and morbidity.
Footnotes
Address for reprints: George J. Reul, MD, Department of Cardiovascular Surgery, MC 2-114, Texas Heart Institute at St. Luke's Episcopal Hospital, P.O. Box 20345, Houston, TX 77225-0345
References
- 1.Veith FJ, Gupta SK, Daly VR. Technique for occluding the supraceliac aorta through the abdomen. Surg Gynecol Obstet 1980;151:426–8. [PubMed]
- 2.Nypaver TJ, Shepard AD, Reddy DJ, Elliott JP Jr, Ernst CB. Supraceliac aortic cross-clamping: determinants of outcome in elective abdominal aortic reconstruction. J Vasc Surg 1993;17:868–76. [DOI] [PubMed]
- 3.Hosaka S, Kamiya K, Akimoto S, Suzuki O, Kobayashi M, Matsukawa T, Tada Y. Atheromatous embolization as a cause of postoperative renal dysfunction in infrarenal aortic reconstructive surgery [in Japanese]. Nippon Geka Gakkai Zasshi 1994;95:109–15. [PubMed]
- 4.Locati P, Socrate AM, Costantini E. Paraanastomotic aneurysms of the abdominal aorta: a 15-year experience review. Cardiovasc Surg 2000;8:274–9. [DOI] [PubMed]
- 5.Hines GL, Chorost M. Supraceliac aortic occlusion: a safe approach to pararenal aortic aneurysms. Ann Vasc Surg 1998; 12:335–40. [DOI] [PubMed]
- 6.Crawford ES, Becket WC, Greer MS. Juxtarenal infrarenal abdominal aortic aneurysm. Special diagnostic and therapeutic considerations. Ann Surg 1986;203:661–70. [DOI] [PMC free article] [PubMed]
- 7.Frazier OH, Oalmann MC, Strong JP, Cooley DA. Clinical applications of the supraceliac aorta: anatomical and pathologic observations. J Thorac Cardiovasc Surg 1987;93:631–3. [PubMed]
- 8.Gloviczki P, Cross SA, Stanson AW, Carmichael SW, Bower TC, Pairolero PC, et al. Ischemic injury to the spinal cord or lumbosacral plexus after aortoiliac reconstruction. Am J Surg 1991;162:131–6. [DOI] [PubMed]
- 9.Budden J, Hollier LH. Management of aneurysms that involve the juxtarenal or suprarenal aorta. Surg Clin North Am 1989;69:837–44. [DOI] [PubMed]
- 10.Green RM, Ricotta JJ, Ouriel K, DeWeese JA. Results of supraceliac aortic clamping in the difficult elective resection of infrarenal abdominal aortic aneurysm. J Vasc Surg 1989; 9:124–34. [PubMed]
- 11.Breckwoldt WL, Mackey WC, Belkin M, O'Donnell TF Jr. The effect of suprarenal cross-clamping on abdominal aortic aneurysm repair. Arch Surg 1992;127:520–4. [DOI] [PubMed]
- 12.Giulini SM, Bonardelli S, Portolani N, Giovanetti M, Galvani G, Maffeis R, et al. Suprarenal aortic cross-clamping in elective abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2000;20:286–9. [DOI] [PubMed]
- 13.Ohki T, Veith FJ. Abdominal aortic aneurysms. Curr Treat Options Cardiovasc Med 1999;1:19–26. [DOI] [PubMed]
- 14.Ludemann R, Swanstrom LL. Totally laparoscopic abdominal aortic aneurysm repair. Semin Laparosc Surg 1999 Sep; 6(3):153–63. [DOI] [PubMed]
- 15.Qvarfordt PG, Stoney RJ, Reilly LM, Skioldebrand CG, Goldstone J, Ehrenfeld WK. Management of pararenal aneurysms of the abdominal aorta. J Vasc Surg 1986;3:84–93. [DOI] [PubMed]
- 16.Buket S, Atay Y, Islamoglu F, Yagdi T, Posacioglu H, Alat I, et al. Proximal clamping levels in abdominal aortic aneurysm surgery. Tex Heart Inst J 1999;26:264–8. [PMC free article] [PubMed]
- 17.Calligaro KD, Savarese RP, McCombs PR, DeLaurentis DA. Division of the left renal vein during aortic surgery. Am J Surg 1990;160:192–6. [DOI] [PubMed]
- 18.AbuRahma AF, Robinson PA, Boland JP, Lucente FC. The risk of ligation of the left renal vein in resection of the abdominal aortic aneurysm. Surg Gynecol Obstet 1991;173:33–6. [PubMed]
- 19.Kazmers A, Striplin D, Jacobs LA, Welsh DE, Perkins AJ. Outcomes after abdominal aortic aneurysm repair: comparison of mortality defined by centralized VA Patient Treatment File data versus hospital-based chart review. J Surg Res 2000;88:42–6. [DOI] [PubMed]
- 20.Dardik A, Lin JW, Gordon TA, Williams GM, Perler BA. Results of elective abdominal aortic aneurysm repair in the 1990s: a population-based analysis of 2335 cases. J Vasc Surg 1999,30:985–95. [DOI] [PubMed]
- 21.Blankensteijn JD. Mortality and morbidity rates after conventional abdominal aortic aneurysm repair. Semin Interv Cardiol 2000;5:7–13. [PubMed]
- 22.Brady AR, Fowkes FG, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. On behalf of the UK Small Aneurysm Trial participants. Br J Surg 2000;87:742–9. [DOI] [PubMed]
- 23.Braams R, Vossen V, Limsan BA, Eikelboom BC. Outcome in patients requiring renal replacement therapy after surgery for ruptured and non-ruptured aneurysm of the abdominal aorta. Eur J Vasc Endovasc Surg 1999;18:323–7. [DOI] [PubMed]
- 24.Fiser WP, Thompson BW, Thompson AR, Eason C, Read RC. Nuclear cardiac ejection fraction and cardiac index in abdominal aortic surgery. Surgery 1983;94:736–9. [PubMed]
- 25.Dunn E, Prager RL, Fry W, Kirsh MM. The effect of abdominal aortic cross-clamping on myocardial function. J Surg Res 1977;22:463–8. [DOI] [PubMed]
- 26.Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, Hardesty RL. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol 1990;15:1261–9. [DOI] [PubMed]
- 27.Gooding JM, Archie JP Jr, McDowell H. Hemodynamic response to infrarenal cross-clamping in patients with and without coronary artery disease. Crit Care Med 1980;8:382–5. [DOI] [PubMed]
- 28.Attia RR, Murphy JD, Snider M, Lappas DG, Darling RC, Lowenstein E. Myocardial ischemia due to infrarenal cross-clamping during aortic surgery in patients with severe coronary artery disease. Circulation 1976;53:961–5. [DOI] [PubMed]
- 29.Roger VL, Ballard DJ, Hallett JW Jr, Osmundson PJ, Puetz PA, Gersh BJ. Influence of coronary artery disease on morbidity and mortality after abdominal aortic aneurysmectomy: a population-based study, 1971–1987. J Am Coll Cardiol 1989;14:1245–52. [DOI] [PubMed]
- 30.Bush HL Jr, LoGerfo FW, Weisel RD, Mannick JA, Hechtman HB. Assessment of myocardial performance and optimal volume loading during elective abdominal aortic aneurysm resection. Arch Surg 1977;112:1301–5. [DOI] [PubMed]
- 31.Hafez HM, Berwanger CS, McColl A, Richmond W, Wolfe JH, Mansfield AO, Stansby G. Myocardial injury in major aortic surgery. J Vasc Surg 2000;31:742–50. [DOI] [PubMed]
- 32.Toal KW, Jacocks MA, Elkins RC. Preoperative coronary artery bypass grafting in patients undergoing abdominal aortic reconstruction. Am J Surg 1984;148:825–9. [DOI] [PubMed]
- 33.Ruby ST, Whittemore AD, Couch NP, Collins JJ, Cohn L, Shemin R, Mannick JA. Coronary artery disease in patients requiring abdominal aortic aneurysm repair. Selective use of a combined operation. Ann Surg 1985;201:758–64. [DOI] [PMC free article] [PubMed]
- 34.Hertzer NR, Beven EG, Young JR, O'Hara PJ, Ruschhaupt WF 3rd, Graor RA, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 1984;199:223–33. [DOI] [PMC free article] [PubMed]
- 35.McCollum CH, Garcia-Rinaldi R, Graham JM, DeBakey ME. Myocardial revascularization prior to subsequent major surgery in patients with coronary artery disease. Surgery 1977;81:302–4. [PubMed]
- 36.McCann RL, Wolfe WG. Resection of abdominal aortic aneurysm in patients with low ejection fractions. J Vasc Surg 1989;10:240–4. [PubMed]
- 37.Eagle KA, Brundage BH, Chaitman BR, Ewy GA, Fleisher LA, Hertzer NR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Asssociation Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 1996;27:910–48. [DOI] [PubMed]
- 38.Eagle KA, Coley CM, Newell JB, Brewster DC, Darling RC, Strauss HW, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med 1989;110:859–66. [DOI] [PubMed]
- 39.Lalka SG, Sawada SG, Dalsing MC, Cikrit DF, Sawchuk AP, Kovacs RL, et al. Dobutamine stress echocardiography as a predictor of cardiac events associated with aortic surgery. J Vasc Surg 1992;15:831–42. [DOI] [PubMed]
- 40.Bush HL Jr. Renal failure following abdominal aortic reconstruction. Surgery 1983;93(1 Pt 1):107–9. [PubMed]
- 41.Johnston KW. Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg 1989;9:437–47. [DOI] [PubMed]
- 42.Miller DC, Myers BD. Pathophysiology and prevention of acute renal failure associated with thoracoabdominal or abdominal aortic surgery. J Vasc Surg 1987;5:518–23. [PubMed]
- 43.Cohen JR, Mannick JA, Couch NP, Whittemore AD. Abdominal aortic aneurysm repair in patients with preoperative renal failure. J Vasc Surg 1986;3:867–70. [DOI] [PubMed]
- 44.Komori K, Kuma S, Eguchi D, Okazaki J, Kawasaki K, Onohara T, et al. Surgical strategy of abdominal aortic aneurysm with preoperative renal failure. Eur J Vasc Endovasc Surg 1997;14:105–8. [DOI] [PubMed]
- 45.Sugawara Y, Sato O, Miyata T, Deguchi J, Kimura H, Namba T, et al. Surgical results of abdominal aortic aneurysm repair in patients with chronic renal dysfunction. Jpn Circ J 1997;61:762–6. [DOI] [PubMed]
- 46.Sasaki T, Ohsawa S, Ogawa M, Mukaida M, Nakajima T, Komoda K, et al. Postoperative renal function after an abdominal aortic aneurysm repair requiring a suprarenal aortic cross-clamp. Surg Today 2000;30:33–6. [DOI] [PubMed]
- 47.Barratt J, Parajasingam R, Sayers RD, Feehally J. Outcome of acute renal failure following surgical repair of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2000; 20:163–8. [DOI] [PubMed]
- 48.Tandan V, Panos AL, Houck JP, Salerno TA. Renal perfusion with the Biomedicus pump during resection of an abdominal aortic aneurysm. Can J Surg 1992;35:634–6. [PubMed]
- 49.Allen BT, Anderson CB, Rubin BG, Flye MW, Baumann DS, Sicard GA. Preservation of renal function in juxtarenal and suprarenal abdominal aortic aneurysm repair. J Vasc Surg 1993;17:948–59. [DOI] [PubMed]
- 50.Svennson LG, Coselli JS, Safi HJ, Hess KR, Crawford ES. Appraisal of adjuncts to prevent acute renal failure after surgery on the thoracic or thoracoabdominal aorta. J Vasc Surg 1989;10:230–9. [PubMed]
- 51.Bush HL Jr, Huse JB, Johnson WC, O'Hara ET, Nabseth DC. Prevention of renal insufficiency after abdominal aortic aneurysm resection by optimal volume loading. Arch Surg 1981;116:1517–24. [DOI] [PubMed]
- 52.Benjamin ME, Hansen KJ, Craven TE, Keith DR, Plonk GW, Geary RL, Dean RH. Combined aortic and renal artery surgery. A contemporary experience. Ann Surg 1996; 223:555–67. [DOI] [PMC free article] [PubMed]
- 53.Taylor SM, Langan EM 3rd, Snyder BA, Cull DL, Sullivan TM. Concomitant renal revascularization with aortic surgery: are the risks of combined procedures justified? Am Surg 2000;66:768–72. [PubMed]
- 54.Stewart MT, Smith RB 3d, Fulenwider JT, Perdue GD, Wells JO. Concomitant renal revascularization in patients undergoing aortic surgery. J Vasc Surg 1985;2:400–5. [DOI] [PubMed]
- 55.Begelman SM, Olin JW. Renal artery stenosis. Curr Treat Options Cardiovasc Med 1999;1:55–62. [DOI] [PubMed]
- 56.Tarazi RY, Hertzer NR, Beven EG, O'Hara PJ, Anton GE, Krajewski LP. Simultaneous aortic reconstruction and renal revascularization: risk factors and late results in eighty-nine patients. J Vasc Surg 1987;5:707–14. [DOI] [PubMed]
- 57.Bast TJ, Van der Biezen JJ, Scherpenisse J, Eikelboom BC. Ischaemic disease of the colon and rectum after surgery for abdominal aortic aneurysm: a prospective study of the incidence and risk factors. Eur J Vasc Surg 1990;4:253–7. [DOI] [PubMed]
- 58.Longo WE, Lee TC, Barnett MG, Vernava AM, Wade TP, Peterson GJ, et al. Ischemic colitis complicating abdominal aortic aneurysm surgery in the U.S. veteran. J Surg Res 1996; 60:351–4. [DOI] [PubMed]
- 59.Atnip RG, Neumyer MM, Healy DA, Thiele BL. Combined aortic and visceral arterial reconstruction: risks and results. J Vasc Surg 1990;12:705–15. [DOI] [PubMed]
- 60.Nypaver TJ, Shepard AD, Reddy DJ, Elliott JP Jr, Smith RF, Ernst CB. Repair of pararenal abdominal aortic aneurysms. An analysis of operative management. Arch Surg 1993;128:803–13. [DOI] [PubMed]
- 61.Welling RE, Roedersheimer LR, Arbaugh JJ, Cranley JJ. Ischemic colitis following repair of ruptured abdominal aortic aneurysm. Arch Surg 1985;120:1368–70. [DOI] [PubMed]
- 62.Ernst CB, Hagihara PF, Daugherty ME, Griffen WO Jr. Inferior mesenteric artery stump pressure: a reliable index for safe IMA ligation during abdominal aortic aneurysmectomy. Ann Surg 1978;187:641–6. [DOI] [PMC free article] [PubMed]
- 63.Bjorck M, Troeng T, Bergqvist D. Risk factors for intestinal ischaemia after aortoiliac surgery: a combined cohort and case-control study of 2824 operations. Eur J Vasc Endovasc Surg 1997;13:531–9. [DOI] [PubMed]
- 64.Ouriel K, Ricotta JJ, Adams JT, Deweese JA. Management of cholelithiasis in patients with abdominal aortic aneurysm. Ann Surg 1983;198:717–9 [DOI] [PMC free article] [PubMed]