Abstract
Background
Recently, there has been an uptick in reported cases of monkeypox (Mpox) in Africa and across the globe. This prompted us to investigate the efficacy of the two vaccines that can prevent Mpox, the modified vaccinia Ankara virus (MVA) vaccine and ACAM2000 vaccine. We analyzed them to determine their rates of humoral cell responses, adverse events, and rash reactions and used these factors as the primary indicators.
Methods
This study adapted primary data obtained from the Medline, Google Scholar, and Cochrane Library databases. We included a total of eight studies, three of which explored the ACAM2000 vaccine and five of which explored the JYNNEOS MVA vaccine.
Results
There were significant differences in the rates of humoral responses after inoculation by the two vaccines. JYNNEOS MVA vaccine immunization resulted in a statistically significant increased humoral immune response with an effect size of 81.00 (42.80, 119.21) at a 95% CI and a rash reaction with an effect size of 96.50 (42.09, 235.09.21) at a 95% CI. ACAM2000 resulted in a lesser increase in neutralizing antibodies than JYNNEOS MVA vaccine. Similar findings were identified for the rates of adverse reactions, but the difference was not statistically significant. The differences in rash reaction rates in the two vaccination groups were also not statistically significant.
Conclusion
ACAM2000 and JYNNEOS vaccines have proven to be efficient in preventing Mpox even though variations exist in their modes of action and associated significant effects. The nonreplicating nature of JYNNEOS prevents the occurrence of the adverse effects seen with other vaccines.
Keywords: Monkeypox, Vaccine, Protection, ACAM2000, JYNNEOS
1. Introduction
The Mpox virus is an Orthopoxvirus with zoonotic potential. It was first discovered in 1958 in crab-eating monkeys with vesiculopustular skin lesions. In 1970, the first human case of Mpox was isolated in a child with similar skin lesions, fever, and lymphadenopathy in the Democratic Republic of Congo (DRC) [1]. Since then, Mpox has been reported in several other African countries, including Cameroon, Central African Republic, Ivory Coast, Liberia, Nigeria, Republic of Congo, Sierra Leone, and Sudan [2]. In 2003, the first cases of Mpox outside of Africa were reported in the United States, when a group of individuals who had recently traveled from West Africa were diagnosed with the disease. Since then, there have been sporadic outbreaks of Mpox in various parts of the world [3], [4], [5]. In 1980, the World Health Organization (WHO) declared that smallpox had been eliminated from the earth. However, viruses such as smallpox, cowpox, and Mpox continue to be maintained in small amounts in research facilities worldwide. This makes concerns about bioterrorist attacks valid, especially for unvaccinated humans younger than 45 years of age [6].
Since widespread smallpox vaccination has not been offered for a long time, there is a legitimate cause for fear about the resurgence of the aforementioned viruses today. Routine smallpox immunizations ended in 1984, and this left more than half of the global population without protection from the disease [7]. As of July 1, 2022, the Centers for Disease Control confirmed the spread of the Mpox virus in 52 countries globally, with 5783 confirmed cases of Mpox. Demographic and individual factors associated with increased susceptibility to Mpox include a median age of 31 years and male gender. The discontinuation of the smallpox vaccination after the disease was eradicated means that individuals younger than 50 years of age are more susceptible to infection with a virus than older people, since they were most likely not to have been vaccinated against it [8], [9].
The Mpox virus is from the family Poxviridae, genus Orthopoxvirus, and species Mpox virus. It is a DNA virus with a brick-like shape and usually contains the proteins necessary for mRNA translation, replication, transcription, and assembly. The virus is in the same family as the variola viruses, and the signs of Mpox are milder than those of smallpox. The virus is endemic to the western and central parts of the African continent, with most cases noted in the DRC [10]. The infection rarely occurs outside of the African continent. Still, cases have been reported around the world, and research has pointed to the contribution of the importation of exotic animals from Africa and travels from the continent to other countries [11]. The viral infection was first identified by the presence of pock-like lesions in monkeys. Primary data suggest that African rodents are the main natural reservoirs of the virus [12]. Other species, including prairie dogs, squirrels, rats, mice, and monkeys, are implicated as agents of transition and carriers of the viral load [13], [14]. In addition to the 1970 case identified in Africa, another case was isolated in the Midwestern United States in 2003. It was linked to the importation of giant rats from Gambia, and a total of 53 confirmed cases was found later [15]. More recently, cases in men who traveled from Nigeria to Israel in 2018 and from Nigeria to Singapore in 2019 were associated with minor viral infection outbreaks. European countries such as the United Kingdom have also reported Mpox cases that occurred in recent travelers from Africa (Monkeypox in the U.S. 2022, September 9th, Centers for Disease Control and Prevention (CDC); https://www.cdc.gov/poxvirus/monkeypox/if-sick/treatment.html).
The Mpox infection can be transmitted through direct contact with contaminated body fluids, respiratory droplets, skin injuries, and fomites [16]. The WHO also reported that people who have ingested inadequately cooked animal products and people who live near forested areas, especially near animals implicated as carriers or sources of infection, are more at risk of contracting the viral infection. Transmission via respiratory droplets following contact with an infected individual is rarer than for other sources of infection. It appears that the face-to-face contact must be prolonged for the infection to be transmitted [17]. Since the isolation of the first case in 1970, spread of the Mpox virus has increased from person to person, with the longest reported person-to-person chain of transmission being nine; this was an increase from the previously documented person-to-person chain of six. Placental transmission from mother to fetus has also been documented, and possible transmission during parturition is suspected. Because Mpox virus infection is such a new disease, it remains unclear whether sexual transmission is possible. It is known that transmission requires prolonged contact between a naive and an infected person [18].
After contact with the viral load through one of the above transmission modes and contact via the oral, nasal, or dermatological route, the virus first replicates at the primary site of inoculation. It then spreads into the nearby lymph nodes, where it causes primary viremia. Once in the bloodstream, the virus causes secondary viremia, which speeds up the infection’s spread to other organs. The incubation period is defined as the time it takes for the virus to spread throughout the body from the time of infection or exposure to the viral load up to the time it is detected in various organs and systems. This is typically 7 to 14 days, although it can be as short as 7 days or as long as 21 days [19]. The animal does not have clinical signs during incubation. In the prodromal phase, secondary viremia occurs in organs such as the skin, lungs, eyes, and gut lymphoid organs. This prodromal phase is associated with nonspecific clinical signs such as fever, myalgia, headache, chills, fatigue, mouth ulcers, throat ulcers, and lymphadenopathy [10], [16]. Skin rashes begin to develop on the face and extremities first and on the trunk and abdomen later. The typical appearance is vesiculopustular rashes that become transformed into scabs (the progression is exanthems, macules, papules, vesicular pustules, crusts, and scabs). Secondary bacterial infections, such as cellulitis, encephalitis, sepsis, and corneal infections, which can cause scarring or lead to dehydration, commonly occur after infection. Usually, the sloughing off of scabs to reveal new skin underneath is associated with potential risk of infecting others [20], [21]. Diagnosis of the Mpox virus can be made using various assays, such as those for the presence of Mpox DNA in a specimen collected from a suspected patient. For a polymerase chain reaction testing, swabs of blisters or throat tissues are taken and stored in viral transport media while in transit to a laboratory. Serological tests and those for antigen detection are not recommended for the Mpox virus due to their tendency to cross-react and consequently provide inaccurate information about the virus [10].
Since no effective treatments for Mpox exist, approaches for dealing with the disease have been adapted from what is known about dealing with smallpox [10]. The vaccinia vaccine and the medications tecovirimat and cidofovir are management options for Mpox. Tecovirimat, which was originally developed for smallpox, works by inhibiting the viral wrapping protein and is effective against a wide spectrum of Orthopoxviruses, including variola virus, smallpox, cowpox, Mpox, and rabbit pox. The modified vaccinia Ankara virus (MVA; JYNNEOS and IMVAMUNE vaccines) and the replication-competent vaccine ACAM2000 are the two vaccines approved for the Mpox virus [22], [23]. The MVA vaccine is administered in two doses 4 weeks apart and is a nonreplicating attenuated vaccine with a large safety margin [24]. The safety margin means that patients with skin problems and those who are immunocompromised can receive it. The MVA vaccine can be administered in a dosage of 0.5 ml subcutaneously or 0.1 ml intramurally. It is also commonly used for postexposure prophylaxis [25]. This vaccine has a wide safety margin, and it is also a nonreplicating vaccine, unlike many vaccinia vaccines, so it does not have the typical profile of components of these vaccines. The vaccine is administered by a skin prick injection using a bifurcated needle, and it elicits an immune response without the concurrent postvaccination complications common to vaccinia vaccines [26].
The ACAM2000 is recommended for healthier subjects due to its high rate of adverse events [27]. The vaccine can be given in a single dose via several skin pricks using a specialized needle, and vaccination status is achieved 28 days later [28]. It contains a live vaccinia virus, unlike other vaccines, which have dead or attenuated viruses. The vaccine is given with a two-pronged needle soaked in the vaccine and jabbed into the skin on the upper arm. Within 3 days of inoculation, red spots, often known as the red rash, appear on the skin; this suggests a successful uptake of the vaccine and is usually a positive sign. Once in the body, the vaccine acts as a mild form of the original virus by stimulating a similar immune response characterized by an increase in neutralizing antibodies and a T-cell response. Frequently associated side effects include pericarditis, myocarditis, dermatitis, itchiness, erythema, and induration after the vaccine has been administered. The vaccine is not recommended for immunocompromised patients, including those with human immunodeficiency virus (HIV) or cancer, or for those undergoing the concurrent administration of steroids and anticancer drugs [29].
The study question that guided this analysis was, What is the efficacy of the available vaccines (JYNNEOS and ACAM2000) in preventing Mpox? In response to this question, we addressed the following objectives: (1) We wanted to explore the differences in the general efficacy of the vaccines in the prevention of Mpox. (2) We wanted to analyze the differences in the humoral responses of the two vaccines. (3) We wanted to analyze the differences between the two vaccines in terms of the adverse effects associated with their administration and the associated rash reactions.
2. Materials and methods
2.1. Registration and database sources
Before starting this work, the protocol of this study was registered in the PROSPERO database (registration ID CRD42023398238). Data for this meta-analysis were obtained from databases such as PubMed, Cochrane Library, and Google Scholar and the reference lists of other studies.
2.2. Database search
The databases used keyword combinations such as ACAM2000, MVA-BN, MVA, JYNNEOS, IMVAMUNE, Mpox vaccine efficiency, and smallpox prevention and smallpox to limit the sources to the relevant ones. Boolean operators were used in the PubMed search with medical subject headings (MeSH), which helped narrow the findings to the relevant ones.
Participants in the authorized studies could be human or animal subjects for whom the efficacy of the vaccinations in the prevention of Mpox was assessed. The participants could be in any age category and the gender of the populations had to be specified. In addition, the participants had to be in generally good health and not taking any drugs that could have an adverse effect. The exposures in the accepted studies were the vaccines ACAM2000 and JYNNEOS, which were given different names in different studies (e.g., MVA, MVA-BN, IMVAMUNE). Comparators included other vaccines such as Dryvax, Elstree-RIVM, and Elstree-BN or placebo groups with total buffered saline. Outcomes of interest included the T-cell responses, B-cell responses, adverse events, and rash reactions to the vaccine. Experimental randomized controlled trials were acceptable designs for the studies used in the analysis. Indicators of vaccine efficacy for this study were the humoral response, rash reaction, and the occurrence of adverse events following inoculation.
2.3. Quality appraisal
The Joanna Briggs Institute critical assessment tool was used for our evaluation. Potential biases in study reporting, design, and analysis can be uncovered with the use of this tool. Studies were evaluated using a 12-item checklist from the John Abrams Institute’s appraisal tool, for which the possible answers were yes (scored as 1), no (minus 1), or maybe (0). Each study’s data were reported as a percentage of the whole, and only those with an overall rating score of more than 50% were considered for the analysis.
2.4. Data extraction
Data extraction was conducted using a data extraction sheet. The study designs (experiential, cross-sectional, longitudinal, or randomized controlled studies), participants (number, age, and gender), intervention and control group exposure characteristics, types of diagnostic tests used, measures, and outcomes were extracted from all the studies.
2.5. Statistical analysis
Statistical analysis was conducted on STATA Version 17.0. The heterogeneity of the studies was determined using the Q and I2 statistics, where a level of heterogeneity between 0.25 < I2 < 0.5 and below was acceptable to ensure homogeneity. Reliance was assured using a Q-statistic value higher than or equal to 5, and the meta-analysis used a fixed effect model. The odds ratio was used to determine the size effect of the outcomes.
3. Results
3.1. Search strategy and outcomes
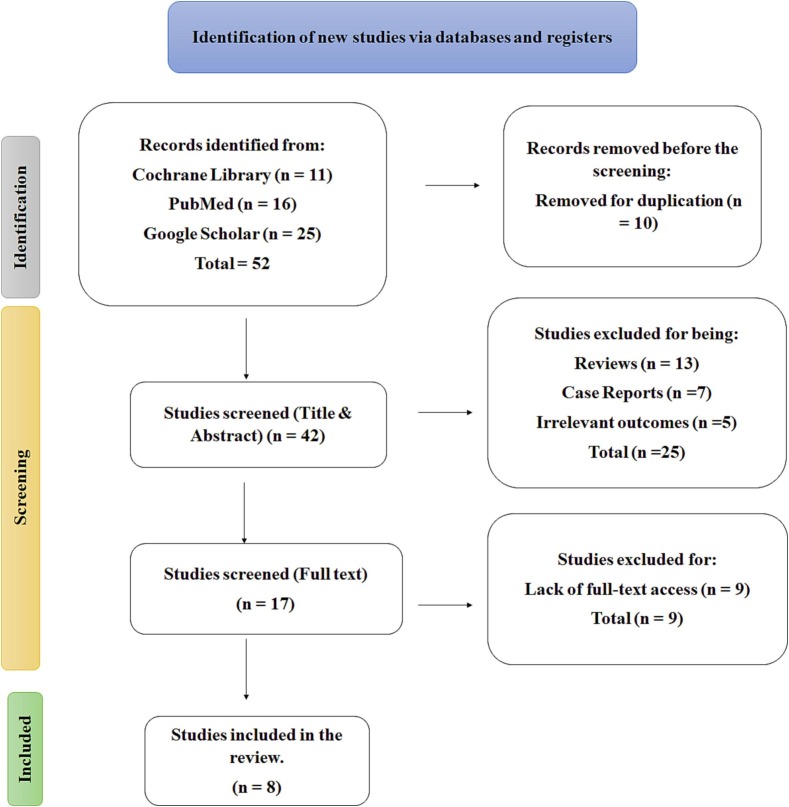
A search of the PubMed, Cochrane Library, and Google Scholar databases yielded 52 results, 10 of which were eliminated due to duplication. The research studies were initially screened for titles and abstracts by two separate reviewers, with studies that scored in the “yes” or “maybe” range ultimately being approved and those that scored lower being discarded. To reduce conflicts, all reviewers were made aware of which studies would be acceptable before the screening procedure. In cases where disagreements developed about which studies should be included or excluded, we held discussions to make the final decisions. After titles and abstracts had been excluded, the remaining studies were read to find those that covered the maximum amount of relevant material required for the inquiry. Twenty-five articles were eliminated because they had irrelevant outcomes, study designs, study participants, or themes. The full-text screening eliminated nine further studies, three of which lacked full-text access, bringing the total number of studies accepted to eight (Fig. 1 ).
Fig. 1.
PRISMA flow chart and the retrieved data.
3.2. Description of studies
Following the screening process, we had a total of eight studies, five of which covered the efficacy of the JYNNEOS vaccine, also known as the MVA/IMVAMUNE vaccine, and three of which examined the efficacy of the ACAM2000 vaccine [21], [23], [29], [30], [31], [32], [33], [34] (Table 1 and Supplementary Tables 1 and 2). In accordance with our eligibility criteria, we identified two studies that compared the efficacy of the IMVAMUNE vaccine in terms of the occurrence of rash reactions and humoral responses with other vaccines such as the ACAM2000, Dryvax, Lister, and Elstree-RIVM and with placebo control groups using Sharma vaccines [30], [31], [35]. The rate of vaccine-associated rash reactions of the ACAM2000 vaccines and others had been compared in two studies [29], [34].
Table 1.
The selected studies characteristics for MVA and ACAM2000 vaccines.
|
MVA vaccine | ||||||
|---|---|---|---|---|---|---|
| Study | Participants | Vaccines | Humoral Response | Adverse Events | Rash Reaction | T-cell responses |
| Frey, 2009 | 90 (75 Dryvax + IMVAMUNE and 15 in placebo + Dryvax) | IMVAMUNE, Dryvax and Saline Placebo | IMVAMUNE = 100 Dryvax = 87% | – | IMVAMUNE = 93% Dryvax = 100% |
– |
| Walsh, 2013 | 24 patients | 10 High MVA, 10 Low dose MVA, 4 placebo | Low = 90%, High = 90%, Placebo = 0% | Low = 90%, High = 90%, Placebo = 100% | Low = 20%, High = 10%, Placebo = 0% | – |
| Stittelaar, 2005 | 24 monkeys in groups | Elstree-RIVM = 12 MVA-BN = 8 Placebo = 4 | – | NA | – | – |
| Earl, 2007 | 30 Rhesus macaques | MVA -KB9-5 = 16 Placebo = 14 |
– | 53 MVA 18 Placebo |
– | – |
| Phelps, 2006 | 6 to 8 week old Balb/c mice | Losyer, MVA | – | MVA = 92%, Placebo = 94% | – | – |
| Parrino, 2007 | 76 Vaccinia naive and 75 immune patients | IMVAMUNE Dryvax | IMVAMUNE = 80 Dryvax = 20 | – | IMVAMUNE = 100 Dryvax = 100 | IMVAMUNE = 85 Dryvax = 80 |
| ACAM2000 vaccine | ||||||
| Marriott, 2007 | 24 (12 cynomolgus macaques in 3 study groups) | ACAM2000 = 8, Dryvax = 8 Control = 8 | – | – | ACAM2000 = 0, Dryvax = 0 Placebo = 100 | ACAM2000 = 50%, Dryvax = 48% Placebo = 8% |
| Keckler, 2020 | 86 live trapped black-tailed prairie dogs (50 animals for the 170 × LD50 study and 36 for the 2 × LD50 study) |
MVA ACAM2000 | MVA = 99%, ACAM2000 = 99% | MVA = 62%, ACAM2000 = 38% | MVA = 10 ACAM2000 = 10 | – |
| Hatch, 2013 | 24 captive cynomolgus macaques in 4 treatment groups of 6 monkeys each | IMVAMUNE = 12 ACAM2000 = 6 Placebo = 6 | ACAM2000 = 24% MVA = 84%, TBS = 69% | ACAM2000 = 0% TBS = 2%, IMVAMUNE = 2% | ACAM2000 = 100 MVA = 100 PLACEBO = 100 | ACAM2000 = 98% IMVAMUNE = 100% TBS = 97% |
3.3. Quality appraisal results
Table 2 shows the 12-item checklist as provided by the JBI critical appraisal tool. The scale was yes, no, or unclear.
Table 2.
Tabular representation of the 12 item checklist as provided by the JBI critical appraisal tool.
| Study | True randomisation to treatment groups | Similar Baseline Treatments | Participants Blinding | Treatment Deliverer Blinding | Assessors Blinding | Identical treatment of groups | Complete follow up | Randomised Analysis |
Identical measurement of Outcomes |
Reliable Outcomes Measurements |
Appropriate statistical analysis |
Appropriately study design |
Overall Rating Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frey, 2000 | Y | Y | Y | N | Y | U | U | Y | Y | U | Y | Y | 66.7% |
| ssParrino, 2007 | Y | Y | U | Y | U | Y | U | U | U | Y | U | Y | 50% |
| Earl, 2007 | Y | U | U | Y | Y | U | N | U | Y | Y | Y | Y | 50% |
| Stittelaar, 2005 | U | Y | U | Y | Y | Y | Y | Y | Y | Y | U | Y | 75% |
| Phelps, 2006 | Y | Y | Y | U | Y | Y | U | U | Y | U | Y | Y | 66.7% |
| Keckler, 2020 | N | Y | U | N | U | Y | Y | Y | Y | Y | Y | Y | 50% |
| Marriott, 2007 | N | U | U | Y | Y | Y | Y | U | Y | U | Y | Y | 50% |
| Hatch, 2013 | U | Y | Y | Y | U | Y | N | U | Y | Y | Y | Y | 58.3% |
Y = Yes = 1; N = No = -1; U = Unclear = 0.
3.4. Humoral response
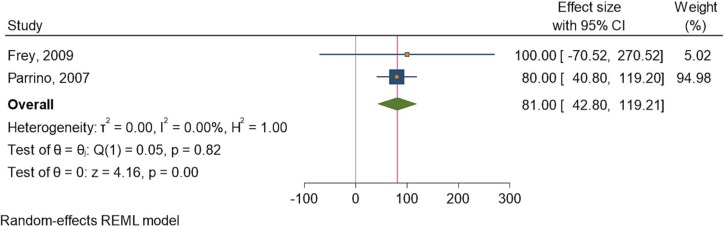
Two studies [30], [35] assessed the humoral response rates after administration of the IMVAMUNE vaccine. The heterogeneity of the studies was low, with a similarly low significance between the control and intervention cohorts. The I2 statistic was 0.00%, and the Q statistic was 0.05. The odds ratio showed that the effect size was 81.00 (42.80, 119.21) at a 95% confidence interval (CI) (Fig. 2 ).
Fig. 2.
Forest plot of summary analysis of the effect size with 95% CI of IMVAMUNE vaccine on humoral response against Mpox. The size of the blue squares corresponds to the statistical significance of each experiment. The pooled point estimate is shown by the green diamond. The placement of diamonds and squares (together with the 95% CIs) beyond the vertical line (unit value) indicates a noteworthy result.
3.5. Rash reaction
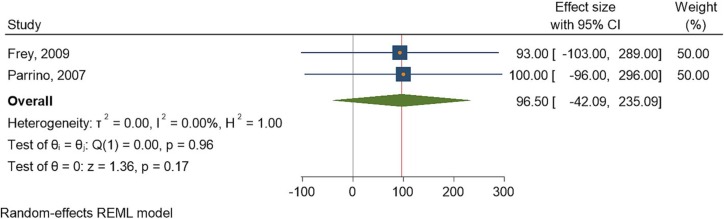
Two studies [30], [35] assessed the rash response rates after administration of the IMVAMUNE vaccine. The heterogeneity of the studies was low, with a similarly high significance between the control and intervention cohorts. The I2 statistic was 0.00%, and the Q statistic was 0.00. The effect size, as depicted by the odds ratio, was 96.50 (42.09, 235.09.21) at a 95% CI (Fig. 3 ).
Fig. 3.
Forest plot of summary analysis of the effect size with 95% CI of IMVAMUNE vaccine on rash reaction. The size of the blue squares corresponds to the statistical significance of each experiment. The pooled point estimate is shown by the green diamond. The placement of diamonds and squares (together with the 95% CIs) beyond the vertical line (unit value) indicates a noteworthy result.
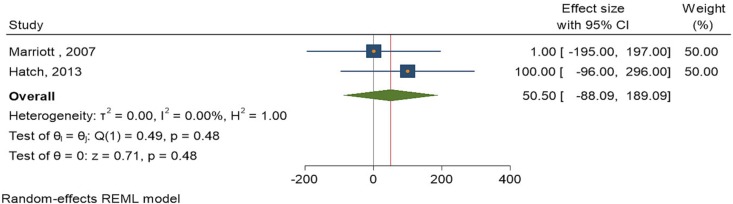
Two studies [29], [34] assessed the rash reaction rates after administration of the ACAM2000 vaccine. The heterogeneity of the studies was low, with a similarly low significance between the control and intervention cohorts. The I2 statistic was 0.00%, and the Q statistic was 1. The odds ratio showed that the effect size was 50.50 (-88.09, 189.00) at a 95% CI (Fig. 4 ).
Fig. 4.
Forest plot of summary analysis of the effect size with 95% CI of ACAM2000 vaccine on rash reaction. The size of the blue squares corresponds to the statistical significance of each experiment. The pooled point estimate is shown by the green diamond. The placement of diamonds and squares (together with 95% CIs) beyond the vertical line (unit value) indicates a noteworthy result.
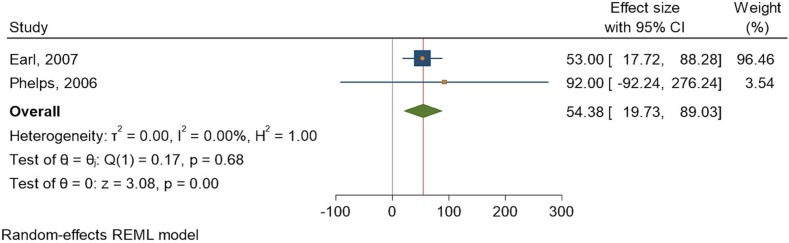
3.6. Adverse events
Two studies [31], [32] assessed the rates of adverse events after administration of the IMVAMUNE vaccine. The heterogeneity of the studies was low, with a similarly low significance between the control and intervention cohorts. The I2 statistic was 0.00%, and the Q statistic was 1. The odds ratio showed that the effect size was 54.38 (19.730, 89.03) at a 95% CI (Fig. 5 ).
Fig. 5.
Forest plot of summary analysis of the adverse events associated with IMVAMUNE with effect size with 95% CI. The size of the blue squares corresponds to the statistical significance of each experiment. The pooled point estimate is shown by the green diamond. The placement of diamonds and squares (together with the 95% CIs) beyond the vertical line (unit value) indicates a noteworthy result.
4. Discussion
Vaccination is an important tool in preventing the spread of Mpox, protecting individuals from severe illness, and reducing the risk of transmission to others. Although Mpox is rare, outbreaks can occur and cause significant public health concerns. Vaccination can help to contain outbreaks and prevent the disease from spreading to other regions or countries. While most cases of Mpox are self-limited and resolve without complications, some individuals can develop severe symptoms, including pneumonia, sepsis, and encephalitis. Vaccination can reduce the risk of developing these complications and can also reduce the severity of the disease if infection does occur. In addition, Mpox vaccination is particularly important for individuals who work with animals or who travel to regions where the disease is known to occur. These individuals are at a higher risk of infection and are more likely to be exposed to the virus. Vaccination can help to protect them from the disease and prevent them from transmitting the virus to others.
This investigation analyzed the efficacy of the JYNNEOS and ACAM2000 vaccines in the prevention or management of Mpox in various groups of patients, which were both human and animal subjects. One of the primary responses analyzed was the humoral response after the administration of either vaccine, as mentioned earlier. Our investigations found significant elevations in antibodies, especially neutralizing antibodies to vaccinia vaccines. However, a higher significance was seen after the administration of JYNNEOS than ACAM2000 (p =.00 vs. p =.48). The level of neutralizing antibodies was elevated in the ACAM2000 replication-competent vaccine compared with the control vaccines, such as the MVA and Dryvax, but the difference was not statistically significant. However, analysis of the MVA vaccines showed significant differences in the humoral immune response.
The application of vaccines in the control of Mpox has gained much attention from public health researchers. Ring vaccination is one of the strategies used to control Mpox outbreaks and has been of special interest [36]. This approach involves vaccinating the close contacts of individuals who have been infected with the virus. The strategic implementation of ring vaccination for Mpox containment involves several steps. The first step is to identify cases of Mpox and isolate the infected individuals to prevent further transmission of the virus. Once the cases have been identified, health officials identify the close contacts of the infected individuals, such as family members, friends, and healthcare workers. These individuals are then vaccinated to prevent the spread of the virus. The challenges associated with ring vaccination for Mpox containment include the availability of vaccines and the logistics of vaccine distribution. Mpox vaccines are not widely available, and they are primarily used in Africa. This means that in the event of an outbreak in another part of the world, there may be limited access to vaccines. Another challenge is the identification of close contacts. In some cases, it may be difficult to identify all of the individuals who have been in close contact with an infected person, particularly if the infected individual has traveled to multiple locations or has had contact with many people. Finally, there may be resistance to vaccination in some communities. This may be due to religious or cultural beliefs or to concerns about the safety and efficacy of the vaccine. Health officials must work to address these concerns and build trust within the community to ensure that ring vaccination is successful.
This study categorized adverse events as an umbrella term encompassing consequences associated with Mpox vaccinations, such as pericarditis, myocarditis, and mortality. Inoculation with the MVA vaccine was found to be significant in resulting in lower adverse outcomes between the two cohorts when compared with the controls or comparators utilized in the selected studies. This finding was consistent with previous studies that found the MVA to be safe due to its broad safety margin and absence of replication, which prevents the complications seen with other vaccinia vaccines [8], [33].
Our study also analyzed the rash reaction rates at the site of inoculation, and we noted no significant differences between the MVA group and the placebo group. Other studies reported higher rates of vaccination applications and, therefore, higher rates of rash reactions after administering the MVA and the other placebos. The rate of uptake of the vaccines indicated their efficacy; the reaction began as erythema or redness of the skin and gradually developed into a localized rash that sometimes spread. The study noted variations in the efficacy of the vaccines as analyzed; ACAM2000, for example, when administered in low doses, was associated with less efficacy and lower rates of other responses, including cell-mediated immunity, humoral immunity, and rates of adverse effects and rash reactions.
Although our research had certain merits in terms of the reliability of our assessments, it had some limitations as well. The rates of humoral responses, vaccination responses as shown by the rash reaction, and adverse events were the primary metrics we used to assess the effectiveness of ACAM2000 and JYNNEOS. The T-cell response and the decrease in viral DNA within the host system are also important markers of a vaccine’s efficacy. Accordingly, we recognize the need for additional markers of vaccine efficacy and the limitations of the three basic endpoints used following vaccination. The scope of our research and the number of relevant papers we identified informed our decision to focus on the three outcomes we chose. Another limitation was the low number of available studies for vaccine evaluation against the Mpox virus.
Due to the recent chain of outbreaks of Mpox and the past elimination of smallpox, the urgency of investigations of poxviruses has been low. Even considering the resurgence of cases of Mpox, the disease has been little investigated Current experimental investigations and trials have addressed a wide range of features of the disease, including its epidemiology, pathogenesis, diagnostic tools, clinical symptoms, and possible methods of disease prevention. Medical research has failed to make significant progress in treating viral infections. As a result, we urge that more studies be performed to add to the medical knowledge on specific techniques of preventing and controlling Mpox based on the current knowledge on poxviruses, particularly smallpox.
5. Conclusions
The purpose of the study was to assess the efficacy of the existing vaccines for the Mpox virus, and we recognized ACAM2000 and JYNNEOS as the most generally available vaccines for the virus at the time. JYNNEOS, also known as the modified vaccinia Ankara virus, was employed to prevent smallpox prior to its eradication. Because of the close association between the smallpox and Mpox viruses and their classification in the same family, modifying smallpox vaccines for the management and prevention of Mpox has proven to be underscored. This study assessed and compared the efficacy of both vaccines against each other and against other vaccines on the market. We found that utilizing MVA vaccines resulted in a better humoral response than using ACAM2000. Furthermore, although the data were not statistically significant, we found that ACAM2000 caused more adverse effects, such as myocarditis, than MVA. The rash reaction and response rates were not statistically significant among the groups studied.
CRediT authorship contribution statement
Mahmoud Kandeel: Conceptualization, Methodology, Supervision, Visualization, Data curation, Validation. Mohamed A. Morsy: Conceptualization, Methodology. Hany M. Abd El-Lateef: Conceptualization, Methodology. Mohamed Marzok: Conceptualization, Methodology. Hossam S. El-Beltagi: Conceptualization, Methodology. Khalid M. Al Khodair: Supervision, Visualization. Ibrahim Albokhadaim: Data curation, Validation. Katharigatta N. Venugopala: Data curation, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number INSTR004.
Data availability statement:
All data are within the manuscript and supplementary materials. Further details can be requested from the corrsponding author.
Funding statement:
This project is funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the project number INSTR004.
Ethics approval statement:
Not apply.
Patient consent statement:
Not apply.
Permission to reproduce material from other sources:
Not apply.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2023.110206.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Petersen B.W., Kabamba J., McCollum A.M., Lushima R.S., Wemakoy E.O., Muyembe Tamfum J.J., Nguete B., Hughes C.M., Monroe B.P., Reynolds M.G. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.H. Murphy, H. Ly, The Potential Risks Posed by Inter-and Intraspecies Transmissions of Monkeypox Virus, Taylor & Francis, 2022, pp. 1681–1683. [DOI] [PMC free article] [PubMed]
- 3.Arita I., Jezek Z., Khodakevich L., Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am. J. Trop. Med. Hyg. 1985;34(4):781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 4.Meo S.A., Jawaid S.A. Human monkeypox: fifty-two years based analysis and updates. Pak. J. Med. Sci. 2022;38(6):1416. doi: 10.12669/pjms.38.6.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O.P. Choudhary, Priyanka, H. Chopra, M. Shafaati, M. Dhawan, A.A. Metwally, A.A. Saied, A.A. Rabaan, S. Alhumaid, A. Al Mutair, R. Sarkar, Reverse zoonosis and its relevance to the monkeypox outbreak 2022, New Microbes New Infect. 49-50 (2022) 101049. [DOI] [PMC free article] [PubMed]
- 6.Tiecco G., Degli Antoni M., Storti S., Tomasoni L.R., Castelli F., Quiros-Roldan E. Monkeypox, a literature review: what is new and where does this concerning virus come from? Viruses. 2022;14(9) doi: 10.3390/v14091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezek Z., Khodakevich L., Wickett J.F. Smallpox and its post-eradication surveillance. Bull. World Health Organ. 1987;65(4):425. [PMC free article] [PubMed] [Google Scholar]
- 8.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang C., Qian J., Liu L. Biological characteristics, biosafety prevention and control strategies for the 2022 multi-country outbreak of monkeypox. Biosaf. Health. 2022;4(6):376–385. doi: 10.1016/j.bsheal.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazanfar A. Epidemiology, clinical features, diagnosis and management of monkeypox virus: a clinical review article. Cureus. 2022;14(8):e28598. doi: 10.7759/cureus.28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kmiec D., Kirchhoff F. Monkeypox: a new threat? I J. Mol. Sci. 2022;23(14):7866. doi: 10.3390/ijms23147866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N., The, outbreak and the pathobiology of the monkeypox virus. J. Autoimm. 2022;2022 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seang S., Burrel S., Todesco E., Leducq V., Monsel G., Le Pluart D., Cordevant C., Pourcher V., Palich R. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400(10353):658–659. doi: 10.1016/S0140-6736(22)01487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 15.Ciccozzi M., Petrosillo N. The monkeypox pandemic as a worldwide emergence: much ado? Infect. Dis. Rep. 2022:597–599. doi: 10.3390/idr14040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaler J., Hussain A., Flores G., Kheiri S., Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022;14(7):e26531. doi: 10.7759/cureus.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Shea J. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71 doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., Hardman A., Harper N., Jarvis R., Mawdsley S., McGivern M., Morgan D., Morris G., Nixon G., O'Connor C., Palmer R., Phin N., Price D.A., Russell K., Said B., Schmid M.L., Vivancos R., Walsh A., Welfare W., Wilburn J., Dunning J. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altindis M., Puca E., Shapo L. Diagnosis of monkeypox virus–an overview. Travel Med. Infect. Dis. 2022 doi: 10.1016/j.tmaid.2022.102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastula D.M., Tyler K.L. An overview of monkeypox virus and its neuroinvasive potential. Ann. Neurol. 2022;92(4):527–531. doi: 10.1002/ana.26473. [DOI] [PubMed] [Google Scholar]
- 21.Paparini S., Whitacre R., Smuk M., Thornhill J., Mwendera C., Strachan S., Nutland W., Orkin C. Public understanding and awareness of and response to monkeypox virus outbreak: a cross-sectional survey of the most affected communities in the United Kingdom during the 2022 public health emergency. HIV Med. 2022 doi: 10.1111/hiv.13430. [DOI] [PubMed] [Google Scholar]
- 22.Walsh S.R., Wilck M.B., Dominguez D.J., Zablowsky E., Bajimaya S., Gagne L.S., Verrill K.A., Kleinjan J.A., Patel A., Zhang Y., Hill H., Acharyya A., Fisher D.C., Antin J.H., Seaman M.S., Dolin R., Baden L.R. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J. Infect. Dis. 2013;207(12):1888–1897. doi: 10.1093/infdis/jit105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., Niesters H.G., van Doornum G., van der Zeijst B.A., Mateo L., Chaplin P.J., Osterhaus A.D. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlova O.V., Glazkova D.V., Bogoslovskaya E.V., Shipulin G.A., Yudin S.M. Development of modified vaccinia virus Ankara-based vaccines: advantages and applications. Vaccines. 2022;10(9):1516. doi: 10.3390/vaccines10091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luong Nguyen L.B., Ghosn J., Durier C., Tachot C., Tartour E., Touati A., Simon T., Autran B., Ortega Perez I., Telford E., Ward J.K., Michels D., Meyer L., Rousseau A., Berard L., de Lamballerie X., Launay O. A prospective national cohort evaluating ring MVA vaccination as post-exposure prophylaxis for monkeypox. Nat. Med. 2022;28(10):1983–1984. doi: 10.1038/d41591-022-00077-1. [DOI] [PubMed] [Google Scholar]
- 26.Arbel R., Sagy Y.W., Zucker R., Arieh N.G., Markovits H., Abu-Ahmad W., Battat E., Ramot N., Carmeli G., Mark-Amir A. Effectiveness of a single-dose modified vaccinia Ankara in human monkeypox: an observational study. Res. Square. 2022 [Google Scholar]
- 27.Gruber M.F. Current status of monkeypox vaccines. npj Vaccines. 2022;7(1):1–3. doi: 10.1038/s41541-022-00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., Campos-Outcalt D., Morgan R.L., Damon I., Sánchez P.J. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71(22):734. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., Funnell S.G., Bate S.R., Steeds K., Tipton T. Assessment of the protective effect of Imvamune and Acam 2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey S.E., Newman F.K., Kennedy J.S., Sobek V., Ennis F.A., Hill H., Yan L.K., Chaplin P., Vollmar J., Chaitman B.R. Clinical and immunologic responses to multiple doses of IMVAMUNE®(Modified Vaccinia Ankara) followed by Dryvax® challenge. Vaccine. 2007;25(51):8562–8573. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Montefiori D.C., Byrum R., Piatak M., Lifson J.D., Amara R.R., Robinson H.L., Huggins J.W., Moss B. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366(1):84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelps A.L., Gates A.J., Hillier M., Eastaugh L., Ulaeto D.O. Comparative efficacy of modified vaccinia Ankara (MVA) as a potential replacement smallpox vaccine. Vaccine. 2007;25(1):34–42. doi: 10.1016/j.vaccine.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Keckler M.S., Salzer J.S., Patel N., Townsend M.B., Nakazawa Y.J., Doty J.B., Gallardo-Romero N.F., Satheshkumar P.S., Carroll D.S., Karem K.L., Damon I.K., Imvamune(®), and ACAM2000(®) provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines (Basel) 2020;8(3) doi: 10.3390/vaccines8030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrino J., McCurdy L.H., Larkin B.D., Gordon I.J., Rucker S.E., Enama M.E., Koup R.A., Roederer M., Bailer R.T., Moodie Z., Gu L., Yan L., Graham B.S. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naïve and vaccinia-immune individuals. Vaccine. 2007;25(8):1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhary O.P., Priyanka M.L., Fahrni A.A., Saied H.C. Ring vaccination for monkeypox containment: strategic implementation and challenges. Int. J. Surg. 2022;105 doi: 10.1016/j.ijsu.2022.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.