In this issue of JEM, Xiaozheng Xu et al. report that the inhibitory protein CTLA4 internalizes in cis the B7 stimulatory molecules previously “gnawed” by T cells from APCs and in doing so prevents stimulatory T–T interactions.
Abstract
In this issue of JEM, Xiaozheng Xu et al. (2023. J. Exp. Med. https://doi.org/10.1084/jem.20221391) report that the inhibitory protein CTLA4 internalizes in cis the B7 stimulatory molecules previously “gnawed” by T cells from antigen-presenting cells (APCs) and in doing so prevents stimulatory T–T interactions.
CTLA4 is an essential negative regulator induced upon T cell activation that controls immune homeostasis. Whereas a lack of CTLA4 expression induces autoimmunity, its high expression is associated with immunosuppression. Thus, CTLA4 is a major target for immunotherapy, and its blockade by monoclonal antibodies has been used with success to treat cancer patients and overcome tumor-induced immune tolerance (Baumeister et al., 2016). CTLA4 inhibits T cell responses by competing with the CD28 co-stimulatory molecule for their shared ligands of the B7 family, CD80 and CD86. In addition to this direct competition, CTLA4 has also been shown to deplete B7 ligands from the surface of APCs through trans-endocytosis (Baumeister et al., 2016; Qureshi et al., 2011). So far, the proposed mechanism was cell extrinsic, with CTLA4 depleting costimulatory ligands from APCs via trans-endocytosis. Once endocytosed, CTLA4 and its ligands are routed to lysosomes where they are degraded. This is related to the high affinity of CTLA4 for its ligands and the rapid endocytic and recycling behavior of CTLA4 (Qureshi et al., 2012). CD28 has also been shown to induce the depletion of B7 ligands from the APC membranes (Hwang et al., 2000). Although they remained partially resolved, the mechanisms used by CTLA4 and CD28 to deplete B7 ligands seem different. CD28 induces trogocytosis of chunks of plasma membranes containing B7 ligands as well as other molecules such as MHC molecules that are shuttled and exposed to the T cell surface, whereas CTLA4 endocytoses the ligands, which are then degraded (Zenke et al., 2022). Trogocytosis is an active process allowing the transfer of plasma membrane proteins from one cell to another in a cell–cell contact-dependent manner. This applies to immune cells, which form cell–cell interactions called immune synapses where membrane-associated proteins are transferred between cells (Joly and Hudrisier, 2003). This transfer of molecules from the APC to the T cells leads to the cross-dressing of T cells that become proficient to present peptides and co-stimulatory molecules to other T cells.

Insights from Noémie Paillon and Claire Hivroz.
In their study, Xiaozheng Xu and co-workers addressed the questions of the precise role of CD28 and CTLA4 in T cell cross-dressing and of how CTLA4 regulates T–T communications (Xu et al, 2023). They used CTLA4-expressing Jurkat leukemic T cells or human primary regulatory T cells (Tregs), which naturally express high amounts of CTLA4. The APCs were CD80−/CD86− Raji B cells expressing fluorescently tagged CD80 or CD86, and the transfer of “material” from the APC to T cells was revealed by confocal and correlative light electron microscopy, as well as by flow cytometry.
Using these models, they showed that T cells capture plasma membrane fragments of the APC containing CD80, CD86, and MHC molecules. This capture was more sensitive to inhibition by anti-CD28 than anti-CTLA4 blocking antibodies. These results suggested that the rapid acquisition of the CD80/86 co-stimulatory molecules by T cells was primarily mediated by CD28 rather than CTLA4 binding during initial contact with APCs. Then, they tested if the cross-dressed T cells could induce T–T activation. Indeed, T cells pre-conditioned with Raji B cells expressing CD80/CD86 were more potent at activating T cells than T cells preconditioned with CD80−/CD86− Raji B cells. In this assay anti-CTLA4 blocking antibodies enhanced T–T stimulation, suggesting that CTLA4 may control the CD80/86 availability within a T cell population. Indeed, anti-CTLA4 antibodies could block the capture of B7 molecules both in trans, during a T–T interaction, or in cis, by depleting the B7 molecules present on the same cell surface. The authors designed an experiment to test the last hypothesis and showed that CTLA4 induced cis depletion of B7 ligands. They preconditioned Tregs with CD80-GFP+ Raji APCs, purified the GFP+ Tregs, and cultured these cells in the presence of an excess of CD28−/− “filler” cells, to block T–T contacts and CTLA4 trans interactions. Cross-dressed Tregs were depleted of CD80-GFP in the presence of filler cells, but they were not depleted of HLA-DR. This depletion was blocked by anti-CTLA4 but not by anti-CD28 antibodies, revealing that CTLA4-driven depletion of CD80 occurs in cis. This depletion was promoted by TCR stimulation, which induces CTLA4 expression and was also efficient to deplete endogenous CD80 molecules. But what was the fate of the molecules captured in cis by CTLA4? Using confocal and electronic microscopy, the authors revealed a progressive accumulation of CTLA4 at sites of acquired APC-derived fragments and a concomitant accumulation of CD80-GFP within CTLA4+ vesicles. Of note, the authors also showed that the endolysosomal trafficking domain of CTLA4 was necessary for the cis depletion of CD80. See summary in figure.
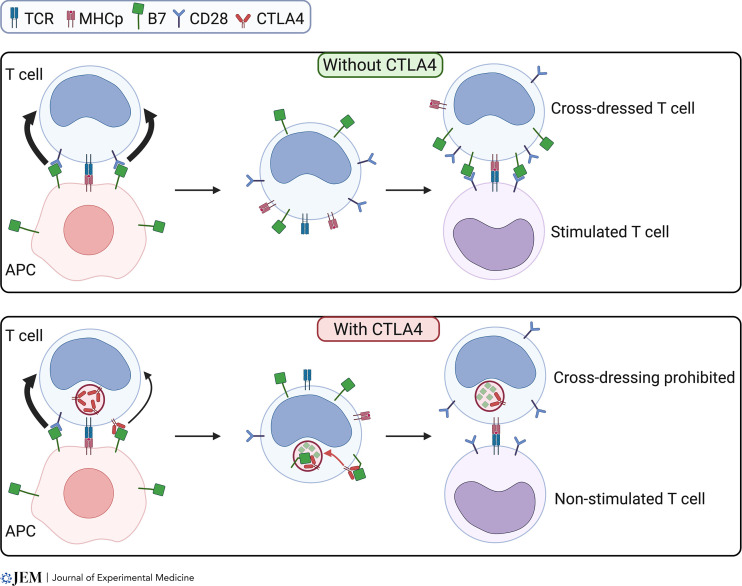
CTLA4-mediated cis-endocytosis of B7 ligands inhibits T–T stimulating interactions. Upper panel: CTLA4-negative T lymphocyte forming an immune synapse with an APC captures B7 and MHC-peptide (MHCp) molecules through CD28 (black bold arrow). This capture is stimulated by TCR triggering. The T cell is cross-dressed with B7 ligands and MHCp and can activate other T cells through T–T interactions. Lower panel: When the T lymphocyte expresses CTLA4, B7 ligands and MHCp are still captured by CD28 (black bold arrow) with a smaller contribution of CTLA4 to B7 capture (small black arrow). The B7 ligands are then cis-endocytosed by CTLA4 and degraded in lysosomal compartments. T–T interactions with these T cells presenting MHCp without CD28 ligands do not induce T cell stimulation. Figure created using Biorender.com.
Altogether, Xu et al. (2023) reveal a new mechanism by which CTLA4-mediated cis-endocytosis depletes CD80/CD86 previously acquired in trans by CD28-mediated trogocytosis.
In this study, no difference was made between CTLA4 targeting of CD80 or CD86. Yet, a recent study has shown that this process results in separate fates for CTLA4 itself. For CD80, CTLA4 remains ligand-bound and traffics via late endosomes and lysosomes. In contrast, once endocytosed, CTLA4 detaches from CD86 and recycles back to the cell surface to allow further trans-endocytosis (Kennedy et al., 2022). Although Xu et al. (2023) looked carefully at the presence of CD80 and CD86 in the endocytic compartments labeled by CTLA4, these compartments were not characterized in terms of intracellular identity. It would be interesting to study if the separate fates reported for the CTLA4-mediated CD80 and CD86 trans-endocytosis also happen for cis-endocytosis of CD80 and CD86. Indeed, recycling of CTLA4 would allow several cycles of depletion and more efficiently support the inhibitory signaling required to maintain tolerance. Moreover, a physical interaction between CD80 and PD-L1 has recently been shown (Sugiura et al., 2019).This interaction takes place predominantly in cis between CD80 and PD-L1 on the same cell (Sugiura et al., 2019), blocking PD-L1 binding to PD-1 but still permitting CD80 interaction with CD28 and CTLA4. It was recently shown that CTLA4-mediated trans-endocytosis of CD80 results in a recovery of PD-L1 availability that is now free to interact with PD-1 and inhibits T cell activation (Kennedy et al., 2023; Tekguc et al., 2021). This CTLA4-mediated regulation of PD-L1 availability might also happen on CD80 cross-dressed PD-L1+ T cells, switching from stimulatory antigen-presenting T cells into inhibitory T cells.
Another question of interest is whether the CTLA4-mediated cis-endocytosis of CD80/86 only targets molecules that are normally inserted in the plasma membrane or if it can also target CD80/CD86 inserted in a fragment of trogocytosed membrane decorating T cells. If not, the process would first require the fusion of the membranes and then the cis-endocytosis. It would be interesting to study if such fusion events can happen.
This study addresses an important question; it is indeed increasingly appreciated that during activation, T cells acquire APC-derived surface molecules via trogocytosis. One of the important consequences of this is that T cells redisplay APC-derived ligands on their surface and can thereby act as APCs to stimulate other T cells. Such T–T interactions have been implicated in the collective regulation of T cell proliferation and differentiation during immune responses. An antigen-capturing quorum-sensing mechanism has been proposed for CD4+ T cells, whereby as the number of cross-dressed CD4+ T cells increases, they outnumber the APCs, increasing the probability of T–T interactions (Helft et al., 2008). These interactions have been shown to lead to different outcomes such as inhibition of the expansion of antigen-experienced T cells (Helft et al., 2008), differentiation of Th17 (Boccasavia et al., 2021), fratricide killing of CD8+ T cells (Huang et al., 1999), more vigorous restimulation of T helpers, or stronger suppressive activity of Tregs (Zhou et al., 2011). T–T interactions also promote synapse-based cytokine delivery between activating T cells (Sabatos et al., 2008). These trogocytosis-mediated events have also been shown to regulate chimeric antigen receptors T cell response (Hamieh et al., 2019).
Distinct from the trans-endocytosis model, the T cell-intrinsic model reported by Xu et al. (2023) represents a new mechanism by which CTLA4 limits the amount and time that costimulatory molecules are displayed on the T cell surface. It probably mainly concerns Tregs cells that express high levels of CTLA4 but might also apply to effector T cells that express some CTLA4. Changing the ratio between co-stimulatory and co-inhibitory molecules exposed at the surface of T cells might alter the collective regulation within T cell populations.
What remains is to find a good in vivo model that will allow testing the role of cis-endocytosis of co-stimulatory molecules on T cell responses.
Acknowledgments
We want to thank Laurence Bataille for critical reading and discussion.
References
- Baumeister, S.H., et al. 2016. Annu. Rev. Immunol. 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- Boccasavia, V.L., et al. 2021. Cell Rep. 10.1016/j.celrep.2021.108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamieh, M., et al. 2019. Nature. 10.1038/s41586-019-1054-1 [DOI] [Google Scholar]
- Helft, J., et al. 2008. Blood. 10.1182/blood-2007-09-114389 [DOI] [Google Scholar]
- Huang, J.F., et al. 1999. Science. 10.1126/science.286.5441.952 [DOI] [Google Scholar]
- Hwang, I., et al. 2000. J. Exp. Med. 10.1084/jem.191.7.1137 [DOI] [Google Scholar]
- Joly, E., and Hudrisier D.. 2003. Nat. Immunol. 10.1038/ni0903-815 [DOI] [PubMed] [Google Scholar]
- Kennedy, A., et al. 2023. EMBO J. 10.15252/embj.2022111556 [DOI] [Google Scholar]
- Kennedy, A., et al. 2022. Nature immunol. 10.1038/s41590-022-01289-w [DOI] [Google Scholar]
- Qureshi, O.S., et al. 2012. J. Biol. Chem. 10.1074/jbc.M111.304329 [DOI] [Google Scholar]
- Qureshi, O.S., et al. 2011. Science. 10.1126/science.1202947 [DOI] [Google Scholar]
- Sabatos, C.A., et al. 2008. Immunity. 10.1016/j.immuni.2008.05.017 [DOI] [Google Scholar]
- Sugiura, D., et al. 2019. Science. 10.1126/science.aav7062 [DOI] [Google Scholar]
- Tekguc, M., et al. . 2021. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.2023739118 [DOI] [Google Scholar]
- Xu, X., et al. 2023. J. Exp. Med. 10.1084/jem.20221391 [DOI] [Google Scholar]
- Zenke, S., et al. 2022. Nat. Commun. 10.1038/s41467-022-34156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, G., et al. 2011. J. Immunol. 10.4049/jimmunol.1002917 [DOI] [Google Scholar]