Abstract
Introduction
Landmark trials testing immune checkpoint inhibitors (ICIs) in advanced NSCLC are difficult to extrapolate to real-world practice given the exclusion of patients with poor (i.e., ≥2) Eastern Cooperative Oncology Group performance status (ECOG PS). We sought to evaluate the impact of ECOG PS on clinical outcomes and health care utilization in patients with NSCLC treated with ICIs in real-world practice.
Methods
Patients with advanced NSCLC who received at least one dose of pembrolizumab or nivolumab were retrospectively identified from the Alberta Immunotherapy Database. The primary outcome was median overall survival, as stratified by ECOG PS. Secondary outcomes included median time-to-treatment failure and metrics of health care utilization, including emergency department visits, hospitalizations, and death in hospital.
Results
A total of 790 patients were included, with 29.2% having poor ECOG PS at initiation of ICI. These patients had significantly lower median overall survival (3.3 versus 13.4 mo) and median time-to-treatment failure (1.4 versus 4.9 mo) compared with those with favorable ECOG PS (p < 0.0001 for both outcomes). Patients with poor ECOG PS were also more likely to present to the emergency department, be admitted to the hospital, and die in the hospital during their first admission (risk ratio = 1.6, 2.3–2.7, p < 0.001).
Conclusions
Patients with NSCLC with poor ECOG PS treated with ICI had significantly worse survival outcomes and were significantly more likely to use health care services than those with favorable ECOG PS. The large proportion of patients with poor ECOG PS further justifies the urgent need for randomized trials evaluating the efficacy of ICI in this high-risk population.
Keywords: ECOG PS, Non–small lung cancer, Poor performance status, Survival in poor ECOG, Immune checkpoint inhibitors
Introduction
In the past decade immune checkpoint inhibitors (ICIs), including pembrolizumab and nivolumab, have revolutionized the treatment paradigm of nononcogene-driven NSCLC. Recent data updates from the landmark trials of pembrolizumab in first-line treatment of NSCLC with programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) greater than or equal to 50% NSCLC1 and nivolumab in previously treated NSCLC2 reveal superior median overall survival (mOS) compared with controls at 26.3 months and 11.1 months, respectively. Nevertheless, these data are difficult to extrapolate to real-world patient populations due to the high proportion of patients who would not be trial eligible, most often on the basis of poor (i.e., ≥2) Eastern Cooperative Oncology Group performance status (ECOG PS).3
The limited data from prospective studies of ICIs in patients with NSCLC and poor ECOG PS have offered incongruent conclusions,4, 5, 6 but multiple modest-sized retrospective studies all provide data to suggest that survival outcomes in this population are poor.10, 7, 8, 9 Despite the valuable insights garnered from these works, modest sample sizes and lack of robust data on the downstream impacts of how these patients use health care resources impose limitations to real-world integration.
In the present study, we sought to evaluate the impact of ECOG PS on clinical outcomes and health care utilization in a large cohort of patients with NSCLC treated with ICIs. We hypothesized that patients with poor ECOG PS would have worse clinical outcomes and utilize more health care resources after treatment initiation than those with favorable ECOG PS.
Materials and Methods
Study Design and Data Collection
We conducted a retrospective cohort study using the Alberta Immunotherapy Database, as previously described.7 For the present study, analyses were limited to patients with NSCLC who received at least a single dose of pembrolizumab or nivolumab between January 1, 2010, and December 31, 2019. Baseline clinical, pathologic, and laboratory-based data were collected for each patient. If data were not recorded in the 30 days leading up to initiation of ICI, they were considered unavailable. Poor ECOG PS was defined as greater than or equal to 2. Data to capture preidentified metrics of health care utilization, including emergency department (ED) visits, hospital admissions, and vital status on hospital discharge, were acquired from a linked provincial administrative database. Data collection and chart review were initiated on July 1, 2017, with October 1, 2020, being the cutoff date.
This study was approved by the Health Research Ethics Board of Alberta—Cancer Committee (HREBA.CC-19-0380). The need for individual patient consent was waived due to the retrospective nature of the study.
Outcomes
Outcomes of interest were compared between poor and favorable ECOG PS groups. The primary outcome assessed was OS, with secondary outcomes including time-to-treatment failure (TTF) and objective response rate (ORR). These outcomes were defined in the typical fashion.7 Additional clinical outcomes of interest included survival rates at 3-, 12-, and 24-month landmarks and the proportion of patients receiving therapy after cessation of ICI. Finally, to evaluate the impact of ECOG PS on health care service utilization, we compared rates of presentation to the ED and admissions to hospital within the first month of ICI initiation and at any time during ICI treatment. ICI treatment was defined as the time from the date of first dose to a period 14 days after the receipt of the final dose. We also assessed the rate of in-hospital mortality during the first admission after initiation of ICI and at any point during ICI treatment.
Statistical Analysis
Baseline patient characteristics at the time of ICI initiation were stratified according to ECOG PS. Group differences were assessed using Wilcoxon signed rank test and chi-square tests for median age and categorical variables, respectively. The chi-square test was used to evaluate group differences in ORR and subsequent treatment after cessation of ICI therapy. Kaplan-Meier curves for mOS and median TTF were constructed and differences across ECOG PS group were represented by log-rank statistic and hazard ratios (HRs) estimated using the Cox proportional-hazards model. Landmark analyses of OS were compared across ECOG PS category using log-rank test for 3, 12, and 24 months. The association between ECOG PS categories and health care utilization was represented with risk ratios (RRs) and evaluated using chi-square tests. A Cox proportional-hazards model was used to estimate HRs for subgroup analysis. Data were analyzed between November 1, 2021, and December 30, 2021. All analyses were conducted using R (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
A total of 790 patients were included in the analysis. Median follow-up time was 20.6 months. Baseline demographic, clinical, and pathologic characteristics, as stratified by ECOG PS, are found in Table 1. Among the cohort, 559 (70.7%) had ECOG PS less than 2, 401 (50.8%) were of female sex, 571 (72.3%) had adenocarcinoma as the histologic subtype, and 499 (63.2%) had metastatic disease at the time of diagnosis. Moreover, most patients were treated with pembrolizumab (65.1%) and received an ICI in the first-line setting (54.1%). Among the patients with adenocarcinoma, 27 (4.7%) had EGFR mutations identified—none of whom received ICI in the first line. Further breakdown of ICI received by line of treatment can be found in Table 2.
Table 1.
Baseline Clinical and Pathologic Characteristics, According to ECOG PS
| Characteristics | ECOG PS |
p Value | |
|---|---|---|---|
| <2 (n = 559) | ≥2 (n = 231) | ||
| Age, median (range) | 68 (33–88) | 68 (32–86) | 0.626 |
| Sex, n (%) | 0.141 | ||
| Female | 293 (52.5) | 108 (46.8) | |
| Male | 265 (47.5) | 123 (53.2) | |
| Smoking status, n (%) | 0.639 | ||
| Never | 42 (7.5) | 22 (9.5) | |
| Ever | 499 (89.3) | 202 (87.4) | |
| Unknown | 18 (3.2) | 7 (3.0) | |
| Histology, n (%) | 0.145 | ||
| Adenocarcinoma | 416 (74.6) | 155 (67.1) | |
| Squamous | 105 (18.8) | 61 (26.4) | |
| Other | 37 (6.6) | 15 (6.5) | |
| Stage at diagnosis, n (%) | 0.168 | ||
| I/II | 72 (12.9) | 19 (8.2) | |
| III | 141 (25.2) | 59 (25.5) | |
| IV | 346 (61.9) | 153 (66.2) | |
| Site of metastases, n (%) | |||
| Brain | 0.715 | ||
| No | 474 (84.9) | 193 (83.9) | |
| Yes | 84 (15.1) | 37 (16.1) | |
| Liver | 0.003 | ||
| No | 467 (83.5) | 171 (74.3) | |
| Yes | 92 (16.5) | 59 (25.7) | |
| Bone | 0.040 | ||
| No | 392 (70.1) | 144 (62.6) | |
| Yes | 167 (29.9) | 86 (37.4) | |
| Line of therapy, n (%) | <0.001 | ||
| 1 | 325 (58.1) | 102 (44.2) | |
| 2+ | 234 (41.9) | 129 (55.8) | |
| Treatment agent, n (%) | 0.127 | ||
| Nivolumab | 186 (33.3) | 90 (39.0) | |
| Pembrolizumab | 373 (66.7) | 141 (61.0) | |
| PD-L1 expression, n (%) | 0.671 | ||
| <1% | 86 (15.4) | 30 (13.0) | |
| 1%–49% | 86 (15.4) | 42 (18.2) | |
| ≥50% | 277 (49.7) | 112 (48.5) | |
| Unknown | 108 (19.4) | 47 (20.3) | |
ECOG PS, Eastern Cooperative Group Performance Status; PD-L1, programmed death-ligand 1.
Table 2.
Treatment Received, According to ECOG PS
| Characteristics | ECOG PS |
|
|---|---|---|
| <2 (n = 559) | ≥2 (n = 231) | |
| Nivolumab: line of therapy, n (%) | ||
| 1 | 23 (12.4) | 6 (6.7) |
| 2 | 94 (50.5) | 48 (53.3) |
| 3 | 48 (25.8) | 26 (28.9) |
| 4 | 17 (9.1) | 6 (6.7) |
| 5 | 3 (1.6) | 4 (4.4) |
| 6 | 1 (0.5) | 0 (0) |
| Pembrolizumab: line of therapy, n (%) | ||
| 1 | 302 (81) | 96 (68.1) |
| 2 | 61 (16.4) | 39 (27.7) |
| 3 | 9 (2.4) | 4 (2.8) |
| 4 | 1 (0.3) | 1 (0.7) |
| 5 | 0 (0) | 0 (0) |
| 6 | 0 (0) | 1 (0.7) |
| 1L pembrolizumab + chemotherapy | ||
| Yes | 55 | 1 |
1L, first line; ECOG PS, Eastern Cooperative Group Performance Status.
When comparing baseline characteristics by ECOG PS, those with poor ECOG PS were more likely to have liver or bone metastases, but not brain metastases at the time of ICI initiation (p = 0.003, 0.040, and 0.72, respectively). Patients with poor ECOG PS were also more likely to have received initial ICI treatment in the second (or later)-line setting (p < 0.001). Other baseline characteristics were not significantly different between groups.
Clinical Outcomes
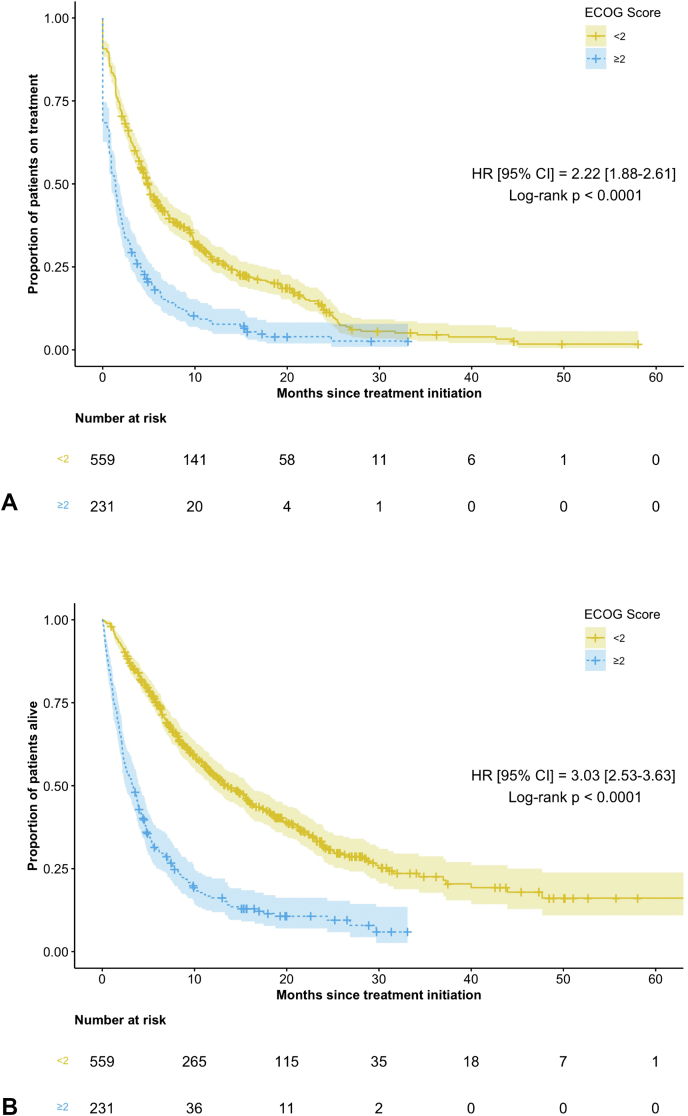
Patients with poor ECOG PS had inferior clinical outcomes across all points of interest (Table 3 and Fig. 1A and B). Compared with the favorable ECOG PS group, those with poor ECOG PS had inferior mOS (3.3 mo [95% confidence interval or CI: 2.5–4.0] versus 13.4 mo [95% CI: 11.7–16.0] [HR = 3.0, 95% CI: 2.5–3.6, p < 0.0001]), median TTF (1.4 mo [95% CI: 0.9–1.8] versus 4.9 mo [95% CI: 4.4–5.6] [HR = 2.2, 95% CI: 1.9–2.6, p < 0.0001]), and ORR (10.8% versus 24.3%, p < 0.0001).
Table 3.
Selected Clinical Outcomes, According to ECOG PS
| Clinical Outcomes | ECOG PS |
p Value | |
|---|---|---|---|
| <2 (n = 559) | ≥2 (n = 231) | ||
| ORR, n (%) | 136 (24.3) | 25 (10.8) | <0.0001 |
| mTTF, mo (95% CI) | 4.9 (4.4–5.6) | 1.4 (0.9–1.8) | <0.0001 |
| mOS, mo (95% CI) | 13.4 (11.7–16.0) | 3.3 (2.5–4.0) | <0.0001 |
| Landmark analyses, n (%) | |||
| 3 mo | 483 (86.4) | 122 (52.8) | <0.0001 |
| 12 mo | 229 (41.0) | 31 (13.4) | <0.0001 |
| 24 mo | 75 (13.4) | 9 (3.9) | <0.0001 |
| Subsequent treatment, n (%) | 125 (22.4) | 21 (9.1) | <0.001 |
CI, confidence interval; ECOG PS, Eastern Cooperative Group Performance Status; mOS, median overall survival; mTTF, median time-to-treatment failure; ORR, objective response rate.
Figure 1.
(A) Median time-to-treatment failure in the poor ECOG PS versus favorable ECOG PS. (B) Overall survival in the poor ECOG PS versus favorable ECOG PS. CI, confidence interval; ECOG PS, Eastern Cooperative Group Performance Status; HR, hazard ratio.
Analysis of landmark survival times revealed significant differences at the 3- (52.8% versus 86.4%), 12- (13.4% versus 41.0%), and 24-month (3.9% versus 13.4%) cutoffs when comparing poor and favorable ECOG PS groups (p < 0.0001 for all outcomes).
After the cessation of ICI therapy, patients with poor ECOG PS were also less likely to receive subsequent treatment (9.1% versus 22.4%, p < 0.001).
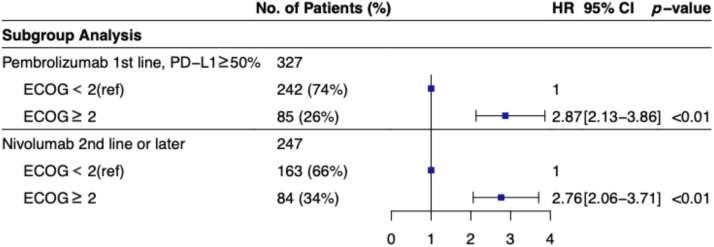
Subgroup analyses were conducted in patient cohorts comparable with relevant pivotal clinical trials (Fig. 2).1,2 In patients treated with first-line pembrolizumab who had PD-L1 TPS greater than or equal to 50% (n = 327), mOS was 2.8 months (95% CI: 2.1–4.8) versus 15.5 months (95% CI: 11.6–22.2) for poor ECOG PS compared with favorable ECOG PS (HR = 2.9, 95% CI: 2.1–3.9, p < 0.001). In those treated with nivolumab in the second line or later without PD-L1 stratification (n = 247), mOS was 3.7 months (95% CI: 2.7–4.6) versus 11.1 months (95% CI: 9.0–15.4) for poor ECOG PS compared with favorable ECOG PS (HR = 2.8, 95% CI: 2.1–3.7, p < 0.001).
Figure 2.
Subgroup analyses of patient cohort compared with pivotal clinical trials separated in ECOG PS. CI, confidence interval; ECOG PS, Eastern Cooperative Group Performance Status; HR, hazard ratio; ref, reference.
Health Care Utilization
Table 4 displays the patterns of health care utilization in the study population. When compared with those with favorable ECOG PS, patients with poor ECOG PS were significantly more likely to use ED services within the first month of treatment (RR = 1.6, 95% CI: 1.3–2.0, p < 0.001) and had a significantly shorter median time to presentation to ED after treatment initiation (19.0 versus 43.0 d). Utilization of ED services did not significantly differ between groups when considering the entire treatment duration with ICI.
Table 4.
Health Care Utilization, According to ECOG PS
| Metrics of Health Care Utilization | ECOG PS |
RR [95% CI] | |
|---|---|---|---|
| ≥2 (n = 231) | <2 (n = 559) | ||
| ED visits, n (%) | |||
| First mo from treatment start | 92 (39.8) | 139 (24.9) | 1.6 [1.3–2.0]a |
| During treatment | 153 (66.2) | 339 (60.6) | 1.1 [1.0–1.2] |
| Median time to ED (IQR) | 19.0 (48.0)b | 43.0 (115.0) | |
| Hospital admission, n (%) | |||
| First mo from treatment start | 72 (31.2) | 77 (13.8) | 2.3 [1.7–3.0]b |
| During treatment | 129 (55.8) | 240 (42.9) | 1.3 [1.1–1.5]b |
| Median time to admission (IQR) | 26 (42.0)b | 62 (126.0) | |
| In-hospital mortality, n (%) | |||
| During first admission | 44 (19.0) | 39 (7.0) | 2.7 [1.8–4.1]b |
| During treatment | 58 (25.1) | 64 (11.4) | 2.2 [1.6–3.0]b |
| Any | 95 (41.1) | 182 (32.6) | 1.3 [1.0–1.5]c |
CI, confidence interval; ECOG PS, Eastern Cooperative Group Performance Status; ED, emergency department; IQR, interquartile range; RR, risk ratio.
p < 0.01.
p < 0.001.
p < 0.05.
When compared with those with favorable ECOG PS, patients with poor ECOG PS were significantly more likely to be admitted within the first month after treatment initiation (RR = 2.3, 95% CI: 1.7–3.0, p < 0.0001) and at any point during (RR = 1.3, 95% CI: 1.1–1.5, p < 0.0001). The median time to admission was also significantly shorter in patients with poor ECOG PS (26 versus 62 d).
Finally, patients with poor ECOG PS were significantly more likely to die in the hospital during their first admission after treatment initiation (RR = 2.7, 95% CI: 1.8–4.1, p < 0.0001) and at any point during treatment (RR = 2.2, 95% CI: 1.60–3.0, p < 0.0001). These patients were also significantly more likely to die in the hospital at any point after treatment initiation; 41.1% versus 32.6% (RR = 1.3, 95% CI: 1.0–1.5, p < 0.05).
Discussion
Previous works from our group have revealed that poor ECOG PS is the strongest independent clinicopathologic factor associated with inferior OS in patients with advanced NSCLC treated with ICI.7,11 In this real-world retrospective cohort study, we specifically evaluated the impact of ECOG PS on the clinical outcomes and health care utilization of patients in this setting. To our knowledge, the present study represents the largest comparative cohort of these outcomes in the NSCLC literature. Our data help to reinforce and contextualize preceding studies exploring the relationship between ECOG PS and ICI in advanced NSCLC.8,9,12
We found that among all clinical metrics of interest—including OS, TTF, and ORR—patients with poor ECOG PS had significantly worse outcomes compared with patients with favorable ECOG PS. Importantly, patients with poor ECOG PS made up 30% of our cohort. The significant differences in mOS were retained in subgroup analyses of populations mirroring two landmark clinical trials, with the HR for the poor ECOG PS group being 2.9 (95% CI: 2.7–4.6) in first-line pembrolizumab with PD-L1 TPS greater than or equal to 50% and 2.8 (95% CI: 2.1–3.7) in second (or later)-line nivolumab without stratification by PD-L1. The mOS for the poor ECOG PS cohorts in the subgroups was 2.8 months (95% CI: 2.1–4.8) and 3.7 months (95% CI: 2.7–4.6), respectively. These data are all consistent with findings from retrospective cohort studies by other groups.8,9,12,13 Moreover, in landmark survival analyses, we found that nearly 50% of patients with poor ECOG PS were deceased by 3 months and approximately 90% by 12 months.
Unfortunately, there are limited prospective data defining the efficacy of ICI in patients with NSCLC with poor ECOG PS, making extrapolation to a real-world treatment setting difficult.14,15 In the industry-sponsored, single-arm PePS2 study from Middleton et al.,5 60 patients with NSCLC with poor ECOG PS treated with pembrolizumab were enrolled. They reported a mOS of 9.8 months with an “acceptable” toxicity profile, concluding these outcomes were “… at least as good as those in patients with PS0-1.” In addition, the CheckMate 1536 (phase 3b-4) and CheckMate 1714 (phase 2) trials enrolled patients with NSCLC treated with nivolumab in the second line or later. For the poor ECOG PS populations, the median OS was 4.0 and 5.2 months, respectively. Overall, these data do not impart providers with the confidence that using ICI in patients with poor ECOG PS and advanced NSCLC will lead to similar outcomes as those expected from the landmark randomized trials.
Finally, our data suggest that patients with poor ECOG PS are more likely to use health care services after treatment initiation than those with favorable ECOG PS. Specifically, they had higher rates of presentation to the ED, admission to the hospital, and death in the hospital. Notably, of the 231 patients with poor ECOG PS initiated on ICI, 58 (25.1%) died in the hospital during treatment, compared with 64 (11.4%) with favorable ECOG PS. These data are supported by recent findings from Krishnan et al.16 and Petrillo et al.12 Krishnan et al.16 found higher rates of in-hospital mortality among patients with poor ECOG PS treated with ICI across a number of solid tumor types. Similarly, Petrillo et al.12 revealed an association between poor ECOG PS and ICI initiation at end-of-life. Although we did not study the reason for hospitalization, previously published data do not suggest a difference in high-grade immune-related adverse events between patients with poor and favorable ECOG PS.6,8 As such, the differences in rates of hospitalization and death in hospital may be driven by lack of treatment response and resultant disease progression. There may also be interactions between disease and treatment-related factors, such as ICI-related hyperprogression.17 Nevertheless, the rate and manifestations of this diagnostic entity are still uncertain.
Especially in light of the poor survival outcomes we have outlined, the impact of increased rates of ED visits, hospitalization, and death in the hospital cannot be overstated. In addition to being able to counsel patients on the expected survival gains and side effects associated with a treatment, it is important to discuss how patients will spend their time while on treatment—especially in the palliative setting. It is well known that cancer care is associated with significant time burdens,18 and the concept of “time toxicity” has recently been conceptualized and explored by Gupta et al.19 in a commentary published in the Journal of Clinical Oncology. Although not quantified specifically in our work, it would seem that the use of ICI in patients with advanced NSCLC and poor ECOG PS may be associated with a high degree of time toxicity. This is an area that would be of high priority for future study.
As it has long been known that approximately 30% of patients with advanced NSCLC in real-world practice have poor ECOG PS,20 it is troubling that these patients are consistently excluded from the practice-defining randomized trials. Despite not having adequate prospective data to counsel these patients on expected outcomes in the ICI era, they continue to account for approximately 15% to 40% of patients receiving these therapies.8,9,21 As we, and others, have revealed, the survival outcomes relative to patients with favorable ECOG PS are dismal. Notably, our observed mOS of 3.3 months is comparable with the mOS of 4.0 months in the best-supportive-care arm of a randomized trial in advanced NSCLC by Cartei et al.22 These data further strengthen the rationale to provide early referral to palliative care services to manage symptoms and optimize survival outcomes.23 It is possible, but unlikely, that patients with poor ECOG PS would be as willing to accept ICI if our findings were corroborated in a well-designed prospective trial. To that end, clinicians would also seemingly be less likely to prescribe ICI to patients with poor ECOG PS if randomized data confirmed a lack of efficacy, or even an association with harm. Above all, high-quality randomized data are crucial to ensure our therapies provide meaningful benefit to patients, especially given the rising cost of cancer medicines and the associated exponential rise in revenues generated by industry.24
Although the present study represents the largest to evaluate the impacts of poor ECOG PS on ICI outcomes in advanced NSCLC in terms of specific clinical outcomes and impacts on health care utilization, the findings should be interpreted in the context of methodological limitations. First, although our data set is derived from multiple tertiary cancer centers, they all fall within one geographic area within a single-payer health system. As such, aspects of the data may reflect practice patterns specific to these factors. Second, the assignment of ECOG PS is known to be associated with a high degree of interobserver variability.25 Therefore, patient stratification using ECOG PS may be susceptible to associated biases. Third, in light of our previously published works,7,11 we did not re-study the association of ECOG PS with other clinicopathologic factors. Future studies should seek to derive a readily available and simple prognostic tool to help risk stratify patients with advanced NSCLC being treated with ICI.
In summary, we investigated the effect of ECOG PS on clinical outcomes and metrics of health care utilization among patients with advanced NSCLC being treated with ICI. We found that patients with poor ECOG PS had significantly worse outcomes among all clinical end points of interest and notably had a 50% mortality rate within 3 months of treatment initiation. Furthermore, poor ECOG PS was associated with significantly higher rates of presentation to ED, admission to hospital, and death in hospital. Given the high proportion of patients with poor ECOG PS treated with ICI in real-world practice, randomized trials in this space are urgently needed. These data are crucial to ensure our practices, above all else, benefit our patients.
CRediT Authorship Contribution Statement
Daniel Meyers: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing—original draft, Writing—review and editing.
Meghann Pasternak: Methodology, Formal analysis, Visualization, Writing—review and editing.
Samantha Dolter, Heidi Grosjean, Chloe Lim, Igor Stukalin, Siddhartha Goutam: Investigation, Data curation, Writing—review and editing.
Vishal Navani, Daniel Heng, Winson Cheung, Don Morris, Aliyah Pabani: Conceptualization, Supervision, Project administration, Writing—review and editing.
Footnotes
Disclosure: Dr. Navani reports receiving personal consulting fees from Novotech, Pfizer, EMD Serono, and AstraZeneca. Dr. Heng reports receiving personal consulting fees and/or honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Seagen, Janssen, Merck, Eisai, Ipsen, Exelixis, and Bayer. Dr. Pabani declares receiving a research grant from Bristol-Myers Squibb unrelated to the current work, and having participation in advisory boards for Pfizer, Merck, AstraZeneca, and Bristol-Myers Squibb. The remaining authors declare no conflict of interest.
Cite this article as: Meyers DE, Pasternak M, Dolter S, et al. Impact of performance status on survival outcomes and health care utilization in patients with advanced NSCLC treated with immune checkpoint inhibitors. JTO Clin Res Rep. XXXX;X:XXXXXX.
References
- 1.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H., Gettinger S., Vokes E.E., et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan C.L., Stukalin I., Meyers D.E., et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–125. doi: 10.1016/j.ejca.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Felip E., Ardizzoni A., Ciuleanu T., et al. CheckMate 171: a phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–172. doi: 10.1016/j.ejca.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Middleton G., Brock K., Savage J., et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med. 2020;8:895–904. doi: 10.1016/S2213-2600(20)30033-3. [DOI] [PubMed] [Google Scholar]
- 6.Spigel D.R., McCleod M., Jotte R.M., et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153) J Thorac Oncol. 2019;14:1628–1639. doi: 10.1016/j.jtho.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Grosjean H.A.I., Dolter S., Meyers D.E., et al. Effectiveness and safety of first-line pembrolizumab in older adults with PD-L1 positive non-small cell lung cancer: a retrospective cohort study of the Alberta immunotherapy database. Curr Oncol. 2021;28:4213–4222. doi: 10.3390/curroncol28050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehgal K., Gill R.R., Widick P., et al. Association of performance status with survival in patients with advanced non–small cell lung cancer treated with pembrolizumab monotherapy. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alessi J.V., Ricciuti B., Jiménez-Aguilar E., et al. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non-small cell lung cancer and a poor performance status. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facchinetti F., Mazzaschi G., Barbieri F., et al. First-line pembrolizumab in advanced non–small cell lung cancer patients with poor performance status. Eur J Cancer. 2020;130:155–167. doi: 10.1016/j.ejca.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Meyers D.E., Stukalin I., Vallerand I.A., et al. The lung immune prognostic index discriminates survival outcomes in patients with solid tumors treated with immune checkpoint inhibitors. Cancers (Basel) 2019;11:1713. doi: 10.3390/cancers11111713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrillo L.A., El-Jawahri A., Nipp R.D., et al. Performance status and end-of-life care among adults with non–small cell lung cancer receiving immune checkpoint inhibitors. Cancer. 2020;126:2288–2295. doi: 10.1002/cncr.32782. [DOI] [PubMed] [Google Scholar]
- 13.Facchinetti F., Di Maio M., Perrone F., Tiseo M. First-line immunotherapy in non-small cell lung cancer patients with poor performance status: a systematic review and meta-analysis. Transl Lung Cancer Res. 2021;10:2917–2936. doi: 10.21037/tlcr-21-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passaro A., Spitaleri G., Gyawali B., De Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37:1863–1867. doi: 10.1200/JCO.18.02118. [DOI] [PubMed] [Google Scholar]
- 15.Gridelli C., Peters S., Mok T., et al. First-line immunotherapy in advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an International Expert Panel Meeting by the Italian Association of Thoracic Oncology. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan M., Kasinath P., High R., Yu F., Teply B.A. Impact of performance status on response and survival among patients receiving checkpoint inhibitors for advanced solid tumors. JCO Oncol Pract. 2022;18:e175–e182. doi: 10.1200/OP.20.01055. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal K. Hyperprogression in patients with cancer receiving immune checkpoint inhibitors. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1839. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Jensen E.H., Virnig B.A., Beg M.S. Time-related burdens of cancer care. JCO Oncol Pract. 2022;18:245–246. doi: 10.1200/OP.21.00662. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A., Eisenhauer E.A., Booth C.M. The time toxicity of cancer treatment. J Clin Oncol. 2022;40:1611–1615. doi: 10.1200/JCO.21.02810. [DOI] [PubMed] [Google Scholar]
- 20.Buccheri G., Ferrigno D., Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 21.Cortellini A., Tiseo M., Banna G.L., et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother. 2020;69:2209–2221. doi: 10.1007/s00262-020-02613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartei G., Cartei F., Cantone A., et al. Cisplatin-cyclophosphamide-mitomycin combination chemotherapy with supportive care versus supportive care alone for treatment of metastatic non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:794–800. doi: 10.1093/jnci/85.10.794. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan D.R., Chan B., Lapidus J.A., et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol. 2019;5:1702–1709. doi: 10.1001/jamaoncol.2019.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers D.E., Meyers B.S., Chisamore T.M., et al. Trends in drug revenue among major pharmaceutical companies: a 2010–2019 cohort study. Cancer. 2022;128:311–316. doi: 10.1002/cncr.33934. [DOI] [PubMed] [Google Scholar]
- 25.Datta S.S., Ghosal N., Daruvala R., et al. How do clinicians rate patient’s performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience. 2019;13:913. doi: 10.3332/ecancer.2019.913. [DOI] [PMC free article] [PubMed] [Google Scholar]