Abstract
Puel and Casanova and Kisand et al. challenge our conclusions that interferonopathy and not IL-17/IL-22 autoantibodies promote candidiasis in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. We acknowledge that conclusive evidence for causation is difficult to obtain in complex human diseases. However, our studies clearly document interferonopathy driving mucosal candidiasis with intact IL-17/IL-22 responses in Aire-deficient mice with strong corroborative evidence in patients.
We recently reported Type II interferonopathy as an unexpected mechanism of oropharyngeal candidiasis (OPC) in mice lacking Aire, the disease gene in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) (1). This finding emerged from an unbiased discovery strategy after finding that mucosal IL-17 and IL-22 production and type 17 responses were intact in Aire−/− mice and that <10% of Aire−/− animals had IL-17 autoantibodies, which are common in APECED patients. Then, in all adult APECED patients whom we examined with chronic mucocutaneous candidiasis (CMC), we also found evidence of excessive IFN-γ responses in oral mucosal tissue, without evidence of impaired IL-17 signaling. Moreover, IFN-γ was toxic to human oral epithelial cells. We thus proposed that excessive IFN-γ produced in the oral mucosa of APECED patients may be an important determinant of CMC susceptibility, a hypothesis now being tested using FDA-approved agents.
Our results led us to re-examine the strength of evidence supporting the IL-17 autoantibody hypothesis promoted by both Technical Comments (2, 3). Puel and Casanova highlight the firmly established causal link between genetic IL-17 receptor (IL-17R) deficiencies and fully penetrant, severe, treatment-refractory CMC in humans (4). Increased OPC susceptibility also occurs in mice with genetic deficiencies of IL-17R signaling (5). However, here signals from all related IL-17-family ligands (IL-17A, IL-17A/F, IL-17F, IL-17B, IL-17C, and IL-17E/IL-25) are completely blocked. By contrast, patients receiving IL-17 pathway-targeted monoclonal antibodies (mAbs) do not develop CMC. They occasionally develop OPC (mean frequency, ~3 to 10% with greater frequencies when targeting IL-17RA or combined IL-17A, IL-17F, and IL-17A/F, compared to IL-17A), but it is invariably mild, non-refractory, and readily controllable with antifungal therapy (6). In this setting, resistance to CMC may simply reflect incomplete mucocutaneous IL-17 pathway blockade by the administered mAbs (7). Likewise, in mice OPC susceptibility is far greater with genetic Il17ra deficiency than with mAb blockade of IL-17RA or the combination of IL-17A and IL-17F than with mAb blockade of IL-17A (5). Increased OPC susceptibility does not occur with mAb blockade of IL-17F alone (5), consistent with the known agonist rank order of IL-17A>IL-17A/F>IL-17F (8). Thus, complete absence of IL-17R responses causes CMC, whereas a blockade regimen that spares a fraction of mucosal IL-17 immunity does not.
Regarding APECED, ~80 to 90% of patients will develop CMC, and there is an established association with serum IL-17 autoantibodies. However, the association is incompletely penetrant (the reported anti-IL-17F and anti-IL-17A incidence is ~20 to 85% and ~35%, respectively) and its pathogenic significance is undefined (9-13). Thus, many patients with persistent IL-17 autoantibodies lack CMC, and many patients with CMC lack IL-17 autoantibodies (10-13). In some APECED cohorts, the frequencies of IL-17 autoantibodies in patients with or without CMC are similar (10, 12). Furthermore, IL-17 autoantibodies are less frequently detected in patient saliva, and when present there, titers are close to background levels (14). Moreover, although IL-17 autoantibodies correlate with decreased IL-17F and normal or increased IL-17A production in circulating T cells (9, 11), we found intact expression of IL17A and IL17F in patient oral mucosa (1), where CMC occurs. Thus, although some APECED patients have IL-17 autoantibodies, this is unlikely to be the only factor that might contribute to their CMC. Our data suggest that interferonopathy may also contribute.
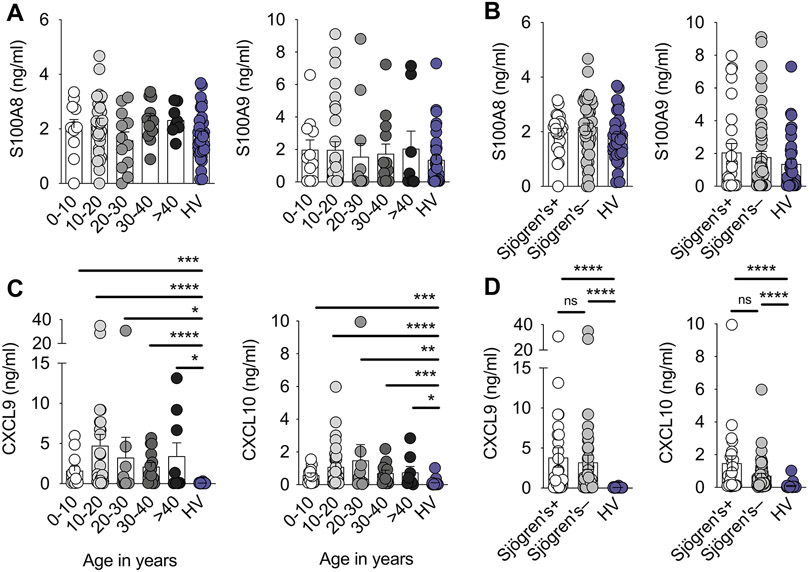
Kisand et al. challenge this (2). First, they assert that IL-17 and IL-22 autoantibodies are implicated in CMC as the first sign of APECED. They reinterpret heightened mucosal type 1 inflammation in patients as being a normal reaction to repeated damage and incidental mucosal breaches instigated by IL-22 neutralization or deficiency, rather than as an “interferonopathy”. However, although IL-22 autoantibodies are detected in ~70 to 90% of APECED patients and correlate with decreased IL-22 production in circulating and cutaneous T cells (9-13), we found intact expression of IL22 and IL-22-dependent genes in oral mucosal tissue (1) and intact salivary levels of IL-17- and IL-22-dependent S100A8 and S100A9 regardless of the presence of Sjögren’s syndrome and across all ages of APECED patients (Fig. 1, A and B). More directly, children with loss-of-function IL10RB (an IL-22 receptor subunit) mutations, whose cells are completely unresponsive to IL-22, do not develop CMC (15).
Figure 1. Enhanced mucosal type 1 responses and intact mucosal type 17 responses in APECED patients are evident across all age groups and regardless of the presence of Sjögren’s syndrome.
(A) Concentrations of IL-17- and IL-22-dependent S100A8 and S100A9 in saliva of healthy volunteers (HV) (n=33) and APECED patients of the indicated age groups (n=11, 30, 12, 13, and 8 for 0-10, 10-20, 20-30, 30-40, and >40-year-old APECED patients, respectively). (B) Concentrations of IL-17- and IL-22-dependent S100A8 and S100A9 in saliva of HV (n=33) and APECED patients with (n=25) or without Sjögren’s syndrome (n=49). (C) Concentrations of IFN-γ-inducible CXCL9 and CXCL10 in saliva of HV (n=28-31) and APECED patients of the indicated age groups (n=12, 30-32, 10-12, 13-14, and 8-9 for 0-10, 10-20, 20-30, 30-40, and >40-year-old APECED patients, respectively). (D) Concentrations of IFN-γ-inducible CXCL9 and CXCL10 in saliva of HV (n=28-31) and APECED patients with (n=23-28) or without Sjögren’s syndrome (n=50-51). Data are excerpted from Figures 2D, 5E, and 5F of Break et al (1). All quantitative data are means ± SEM. ns, not significant, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as calculated using Kruskal–Wallis H test with Dunn’s multiple-comparisons test.
Second, Kisand et al. criticize the mouse model we used by stating that “Candida is not a mouse commensal”, that only NOD Aire−/− mice were susceptible to OPC, and that NOD Aire−/− mice are an inappropriate model of APECED because they rapidly develop multiorgan infiltrates and most die prematurely, unlike APECED children. Although all mouse models of human disease have limitations and mice are indeed not normally Candida-colonized, murine responses to Candida in many other respects mirror those of humans, and the OPC model faithfully recapitulates key immune perturbations underlying human susceptibility. Indeed, the role of IL-17 in mucosal anti-Candida defense was originally discovered in mice. We found that Aire−/− mice were susceptible to experimental OPC in all three examined backgrounds, albeit with differential severity and age of onset (NOD>BALB/c>C57BL/6) (1). Similar variation characterizes all endocrine and non-endocrine manifestations of Aire deficiency (16). NOD Aire−/− mice feature severe early-onset multiorgan lymphocytic infiltration, which differs from the milder disease of BALB/c and C57BL/6 Aire−/− mice. In our prospective cohort of >160 APECED patients, tissue infiltrates develop in early childhood (10). Patient mortality can exceed 30% despite optimal care. Thus, Aire deficiency on the NOD background more closely models human APECED.
Third, Kisand et al. state that “the connection between OPC susceptibility in NOD Aire−/− mice and exuberant mucosal IFN-γ responses is hard to sustain”. In fact, our data lead inescapably to this connection. NOD Aire−/− mice rarely have IL-17 autoantibodies and mount normal type 17 mucosal responses, yet are still highly susceptible to OPC (1). Thus, there is no evidence in the model for impaired type 17 immunity being the primary driver of fungal susceptibility. Importantly, Aire−/− T cells were both necessary and sufficient to promote OPC (1), revealing that OPC is a T cell-driven autoimmune manifestation, similar to all endocrine and non-endocrine disease components, and consistent with the impaired central tolerance of AIRE deficiency (16). Not only did exaggerated IFN-γ produced by mucosal Aire−/− T cells impair epithelial integrity and promote OPC, but IFN-γ or JAK–STAT inhibition ameliorated this, establishing a causative link (1). Enhanced type 1 responses were similarly observed and temporally correlated with the age of onset of OPC susceptibility in BALB/c Aire−/− mice (1).
Both Technical Comments stipulate that the intact IL-17R-regulated genes that we reported in patient oral mucosal tissues were “inferred from mouse studies” and examined in “patients that did not display candidiasis”. Indeed, the patients who underwent mucosal biopsies were in CMC remission when sampled. However, (a) IL-17-regulated genes are highly concordant between murine OPC mucosa and Candida-infected human oral epithelial cells (17); (b) these genes are established as IL-17-regulated in human epithelial cells even without Candida stimulation (18); and (c) these genes are also IL-17-regulated in human skin in the absence of candidiasis (7). Consistent with our own findings, salivary levels of IL-17-regulated β-defensins were intact or even increased in European APECED patients (19). Our data point to residual type 17 cytokine activity in the mucosa of APECED patients, which may also explain the lack of mucocutaneous bacterial infections, which often occur in genetic complete IL-17RA deficiency (4).
The assertions of both Technical Comments that patients’ exacerbated type 1 mucosal responses, characterized by enrichment of IFN-γ–induced genes (e.g., CXCL9, IDO1, and GBP4), may be secondary to Sjögren’s syndrome or delayed complications of prior candidiasis or periodontitis are not supported by the data. Exaggerated type 1 mucosal responses were prominent across all ages, including <10-year-old children (Fig. 1C). Thus, they cannot reflect adult-specific sequelae. Moreover, it is noteworthy that (a) gingivae do not contain salivary glands; (b) enhanced type 1 transcriptional responses in mucosal tissue were prominent in patients with and without Sjögren’s syndrome (1); (c) increased salivary IFN-γ levels were independently reported in European APECED patients without Sjögren’s syndrome (14); and (d) increased salivary CXCL9 and CXCL10 levels were present in patients with and without Sjögren’s syndrome (Fig. 1D). Congruently, salivary gland dysfunction did not underlie OPC in Aire−/− mice (1).
Finally, Puel and Casanova indicate that intermittent IFN-γ administration and certain immune disorders with enhanced IFN-γ responses are not associated with CMC. Although this is true, it is unknown whether mucosal type 1 responses are exaggerated in these settings. By contrast, excessive type 1 mucosal inflammation may explain CMC in trisomy 21, which features intact circulating Th17 cells (20), and STAT1 gain-of-function, where many patients have intact circulating Th17 cells and IL-17 secretion by T cells and where JAK–STAT inhibitors ameliorate CMC (21). Circulating Th17 cells may poorly mirror mucosal IL-17 produced by αβ and γδ T cells and innate lymphoid cells (ILCs), as STAT3 deficiency, which diminishes circulating Th17 cells, does not alter IL-17 in skin blisters (22). Decreased circulating Th17 cells may not be a reliable immunological biomarker for CMC risk assessment, since a reduction is seen in multiple clinical conditions that do not manifest with CMC including idiopathic CD4 lymphocytopenia (23). Because immune responses are cell type-, context-, and tissue-specific, direct evaluation of mucosal responses may be required to decipher CMC mechanisms.
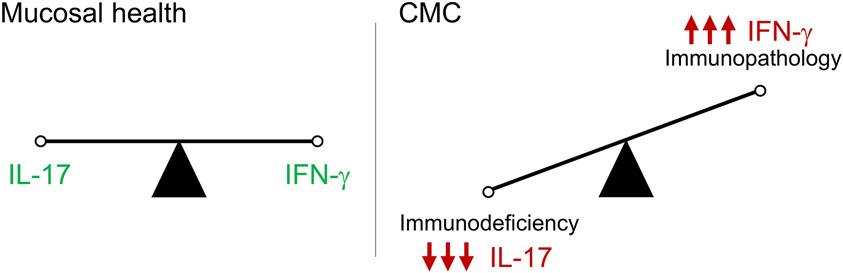
Collectively, our data support a molecular framework to classify CMC subtypes across a spectrum of impaired type 17 immunity and/or immunopathology-promoting excessive type 1 inflammation (Fig. 2). These mechanisms are not mutually exclusive and could act combinatorially in APECED and other CMC conditions. Importantly, APECED is fundamentally a disease of impaired thymic education causing broad lymphocyte defects and autoantibody production. Many organs are damaged and multiple immune mechanisms may contribute, even beyond IFN-γ and IL-17 signaling. Our study highlights this point, expands our fundamental understanding of tissue-specific immunity, and may enable the development of targeted immunotherapies.
Figure 2. A conceptual framework for classifying molecular subtypes of chronic mucocutaneous candidiasis.
Susceptibility to chronic mucocutaneous candidiasis can be explained on the basis of either impaired antifungal resistance caused by type 17 mucosal immune defects and/or immunopathology promoted by excessive type 1 mucosal inflammation. CMC, chronic mucocutaneous candidiasis.
Acknowledgments:
This work was supported by the Division of Intramural Research of the NIAID, NIDCR, NCI, NIDDK, and NIDCD, NIH, and NIH grants R01DE022600, R01AI124566 (S.G.F.), and R00DE026856 (M.S.).
References
- 1.Break TJ et al. , Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisand K, Meager A, Hayday A, Willcox N, Comment on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science, (2021). [DOI] [PubMed] [Google Scholar]
- 3.Puel A, Casanova JL, Comment on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puel A et al. , Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whibley N et al. , Antibody blockade of IL-17 family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol 99, 1153–1164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunte DM, Mrowietz U, Puig L, Zachariae C, Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol 177, 47–62 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Krueger JG et al. , IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 144, 750–763 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Wright JF et al. , The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 181, 2799–2805 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Ahlgren KM et al. , Increased IL-17A secretion in response to Candida albicans in autoimmune polyendocrine syndrome type 1 and its animal model. Eur J Immunol 41, 235–245 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Ferre EM et al. , Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisand K et al. , Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207, 299–308 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlova EM et al. , Expanding the Phenotypic and Genotypic Landscape of Autoimmune Polyendocrine Syndrome Type 1. J Clin Endocrinol Metab 102, 3546–3556 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Puel A et al. , Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207, 291–297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaleviste E et al. , IL-22 Paucity in APECED Is Associated With Mucosal and Microbial Alterations in Oral Cavity. Front Immunol 11, 838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glocker EO et al. , Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361, 2033–2045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathis D, Benoist C, Aire. Annu Rev Immunol 27, 287–312 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Conti HR et al. , IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe 20, 606–617 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao CY et al. , IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 173, 3482–3491 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Oftedal BE et al. , Impaired salivary gland activity in patients with autoimmune polyendocrine syndrome type I. Autoimmunity 50, 211–222 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Kong XF et al. , Three Copies of Four Interferon Receptor Genes Underlie a Mild Type I Interferonopathy in Down Syndrome. J Clin Immunol 40, 807–819 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toubiana J et al. , Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 127, 3154–3164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myles IA et al. , TNF overproduction impairs epithelial staphylococcal response in hyper IgE syndrome. J Clin Invest 128, 3595–3604 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu UI et al. , Patients with Idiopathic Pulmonary Nontuberculous Mycobacterial Disease Have Normal Th1/Th2 Cytokine Responses but Diminished Th17 Cytokine and Enhanced Granulocyte-Macrophage Colony-Stimulating Factor Production. Open Forum Infect Dis 6, ofz484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]