Abstract
In the United States, the Centers for Disease Control and Prevention (CDC) terms HIV and tobacco use among the ten most important public health challenges we face today. In the last decade, there has been a remarkable decrease in the number of deaths due to HIV/AIDS, especially after the widespread availability and use of combination antiretroviral therapy (cART). However, people living with HIV/AIDS have a heightened risk of chronic complications and comorbidities, including neurological disorders. Around 40–60 % of HIV-infected individuals progress to NeuroAIDS, a group of disorders caused primarily by HIV-mediated damage to the central and peripheral nervous systems, despite receiving cART. The detrimental effects of chronic smoking on the cerebrovascular system are also well studied and reported. Addictive behavior, such as smoking, is more common in HIV patients compared to the general population. In this context, given the existing immune suppression, smoking can pose a significant risk for the progression of the disease to NeuroAIDS by disrupting the integrity of the blood-brain barrier (BBB). Here we show that co-treatment with Tobacco Smoke Extract (TSE) and HIV-1 gp120 (HIV envelope glycoprotein) in primary cultures of human brain microvascular endothelial cells promoted heightened cellular stress responses compared to control and individual treatments. Our findings suggest that a potential synergistic effect between smoke exposure and gp120 can worsen the loss of BBB viability, possibly exacerbating NeuroAIDS progression.
Keywords: Vaping, Smoking, Alternative, Ischemia, Cerebrovascular, Oxidative stress, Inflammation, Chronic, Exposure, Toxicity
1. Introduction
The Human Immunodeficiency Virus (HIV) causes Acquired Immune Deficiency Syndrome (AIDS) in humans. AIDS is a disease in which there is a critical depletion of the body’s immune cells, thereby considerably increasing the chances of infection and malignancy (Singh et al., 2003; Burkhalter et al., 2018). HIV can also invade the Central Nervous System (CNS) and cause persistent infection and inflammation (Yadav and Collman, 2009; Boissé et al., 2008). The advent of antiretroviral therapy has significantly reduced the burden of the global HIV epidemic in recent decades. However, due to increased life expectancy, people living with HIV have an amplified risk of chronic complications and comorbidities, such as cardiovascular disease (CVD), disease of the kidney, liver, and also neurological disorders (Deeks et al., 2013).
Chronic HIV-1 infection in the CNS can result in neurodegenerative disease overall termed NeuroAIDS (Minagar et al., 2008). HIV can cross to the brain through adsorptive endocytosis across endothelium (Banks et al., 1998) or by hijacking T-cells and macrophages that migrate to the brain (Letendre, 2011). HIV does not directly infect neurons but indirectly causes neuronal damage by infecting other central nervous system cells (CNS). Microglia and astrocytes (in low levels) are the most infected cell types within the CNS (Zayyad and Spudich, 2015; Rock et al., 2004; Gorry et al., 2003).
The immune-privileged nature of the brain is enforced by the BBB, which plays a critical role in the normal physiology of the CNS by regulating what reaches the brain from the periphery (Abbott et al., 2010). Compactly arranged brain microvascular endothelial cells (BMECs) are the main constituents of the BBB. Astrocytes, pericytes, and microglia also support the BBB structurally & biochemically. Tight junctions (TJ) between adjacent BMECs play a major role in forming a gating barrier, one of the most crucial properties of the BBB (Daneman and Prat, 2015).
Once inside, HIV and its toxic components can induce persistent oxidative stress and inflammation, cause cerebrovascular damage, and eventually lead to neuronal death (Mollace et al., 2001). The virus consists of a capsid, envelope, single-stranded RNA genome, and various enzymes (Blood, 2016). The HIV genome codes for various proteins that interfere with CNS homeostasis; the two more important viral proteins that were shown to be cytotoxic are gp120, the virus’s envelope protein, and the trans-activator transcription (Tat) protein present in the capsid (Nath et al., 2000).
The envelope glycoprotein gp120 is exposed on the surface of the HIV envelope and is essential for virus entry into cells by its attachment to the CD4 (cluster of differentiation 4) receptor and co-receptors CCR5 (C-C chemokine receptor type 5) and CXCR4 (C-X-C chemokine receptor type 4) (Wilen et al., 2012). It comprises two domains, the exterior envelope spike glycoprotein gp120 and the transmembrane glycoprotein gp41 (Wyatt et al., 1998). In the serum of HIV-infected individuals, it can be found as soluble gp120, cell-associated gp120, and virion-associated gp120. HIV gp120 levels in serum and CSF – 500 ng/ml and 5 μg/ml. Each HIV virion can have up to 300 gp120 molecules on its surface (Cummins et al., 2010). HIV gp120 is a potent neurotoxin and is also known to cause oxidative stress.
Looking into the link between HIV/AIDS & high-risk behaviors, it is observed that drug abuse, alcohol consumption, and smoking are highly implicated in reduced response & adherence to the cART regimen and their roles in disease progression (Winstanley et al., 2006). A study published in 2013 reported that around 45 % of HIV-infected participants who smoked showed signs of cognitive impairment (Cross et al., 2013). The prevalence of cigarette smoking among adults in the United States is 14 %, whereas it is between 50 % and 70 % in HIV-positive adults (Reynolds, 2009). Furthermore, continued smoking in HIV-infected persons causes them to lose more life years than the disease itself (Helleberg et al., 2015).
Previously, in our lab, we have demonstrated that tobacco smoke is a potent oxidative and inflammatory stress-inducing agent that can facilitate the loss of BBB function and mitochondrial redox balance leading to a host of comorbidities (Prasad et al., 2015; Naik et al., 2014). Similarly, other groups have carried out studies that show HIV-1 envelope glycoprotein (gp120) being responsible for causing oxidative stress with direct implications for BBB impairment (Kanmogne et al., 2007; Price et al., 2005). However, whether a synergism exists between the two in compromising the BBB is unknown. This study aims to investigate whether tobacco smoke and HIV-1 envelope protein gp120, collectively, may have a contributory effect on aggravating BBB impairment.
2. Materials and methods
2.1. TSE preparation
For the preparation of tobacco smoke extract, we used the standardized ISO/FTC smoking protocol of 35 ml draw, 2-second puff duration, one puff per 60 s using 3R4F (University of Kentucky) standard reference cigarettes in the Single Cigarette Smoking Machine (SCSM, CH Technologies Inc., Westwood, NJ, USA). Smoke from one cigarette comprising eight puffs was collected in PBS to obtain stock TSE solution. The stock was diluted to the desired concentration as per the study published by our lab previously (Naik et al., 2015).
2.2. HIV-1 gp120
HIV-1 gp120, recombinant (Sigma Aldrich # SAE0071), was obtained, and a stock concentration of 100 μg/ml was prepared by reconstituting in ultrapure water. It is estimated that gp120 levels may range from 500 ng/ml to 5 μg/ml in the serum of HIV-infected patients (Cummins et al., 2010). We performed an MTT assay with varying gp120 concentrations (0, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 and 5 μg/ml) over a period of 24 h and used 1.6 μg/ml concentration for our treatments.
2.3. Cell culture
Primary Human Brain Microvascular Endothelial Cells (Cell Systems # ACBRI 376) were expanded in 75 cm2 flasks precoated with Cell Systems Attachment Factor™ (4Z0–210) in the recommended growth medium (Complete Classic Medium – 4Z0–500). Cells were incubated at 37 °C with 5 % CO2 and 95 % air. The primary cultures used in the current study were between passages 3–8. Before treatment, the growth medium was replaced by a medium without serum (4Z3–500) for 24 h.
2.4. Cell viability
HBMECs were cultured in a 96 well plate for 24 h and were later assessed for cell viability as per the protocol for Cell Growth Determination Kit MTT based (Sigma Aldrich # CGD-1). After completion of the assay, absorbance was measured at 570 nm in a plate reader.
2.5. Treatment
HBMECs in cell culture flasks, plates, and transwells were then exposed to 5 % TSE (model of mainstream smoke exposure previously described by our group (Prasad et al., 2017)) for 24 h with and without 1.6 μg/ml gp120. HIV-1 gp120 treatment doses for in vitro studies were selected based on an MTT cell viability study.
2.6. Intracellular ROS detection assay
HBMECs were seeded on 96 well plates and treated for 24 h. Intracellular ROS was assessed by employing ROS Detection Cell-Based Assay Kit – DCFDA (Cayman Chemical # 601520) as per the protocol mentioned therein. At the end of the assay, fluorescence was measured in a fluorescent imaging plate reader at excitation (480–500 nm) and emission (510–550 nm) wavelengths.
2.7. Western blotting
HBMECs were plated on T75 flasks and treated for 24 h. At the end of the treatment period, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and harvested into lysis buffer (Thermo Fisher # 89900) containing protease inhibitors (Thermo Fisher # 78442). The protein content in each sample was determined using BCA Assay (Thermo Fisher # 23225). Equal amounts of protein (30 μg) were loaded in each well and run on SDS–PAGE. The separated proteins were then transferred onto nitrocellulose membranes using Power Blotter XL (Thermo Fisher # PB0013). 5 % nonfat milk in Tris-buffered saline containing 0.1 % Tween-20 was used to block the membranes for 45 min at room temperature. The membranes were then incubated with anti-NRF2 antibody (1:500) (Santa Cruz # sc-365949), anti-NF-κB (1:1000) (Cell Signaling # 8242S), anti-ZO-1 (1:1000) (Invitrogen # 61–7300), anti-Occludin (Invitrogen # 33–1500) (1:1000) anti-β-Actin antibody (1:1000), anti-GAPDH antibody (1:5000) (Cell Signaling # sc-47724) at 4 °C overnight, followed by washing and incubation in HRP-conjugated secondary antibodies for one hour (1:10,000). Immuno-reactive bands were visualized using a chemiluminescence system according to the manufacturer’s instructions. Band densities were analyzed using ImageJ and expressed as fold change over controls after normalizing them against β-actin and GAPDH.
2.8. Immunostaining
HBMECs were plated on 6 well plates for immunocytochemistry and treated for 24 h. Cells were then washed twice with ice-cold PBS before being fixed in methanol (HPLC grade) for 5 min. Next, the cells were permeabilized with 0.2 % Triton-X in PBS for 5 min. After another round of washing with PBS, cells were blocked with 5 % bovine serum albumin (BSA) in PBS for one hour at room temperature (RT). Samples were then incubated overnight at 4 °C with anti-NRF2 antibody (Santa Cruz # sc-365949), anti-ZO-1 (Invitrogen # 61–7300), anti-Occludin (Invitrogen # 33–1500) antibodies (1:100) in 10 % goat serum in PBS. The following day, cells were washed, stained with Alexa Fluor 488 or 555 conjugated goat anti-rabbit or anti-mouse antibodies, and mounted with DAPI in prolonged gold anti-fade mounting media (Thermo Fisher # P36935). Cells were observed at 20 × using an inverted fluorescence microscope. ImageJ software was used to quantify mean fluorescence intensity.
2.9. Real-time quantitative polymerase chain reaction (qPCR)
HBMECs were plated on T75 flasks and treated for 24 h. In brief, total RNA was extracted from treated cells using the RNeasy Mini Kit (Qiagen # 74104). The RNA concentration and purity were confirmed using the Nanodrop ND-1000 system. High-Capacity RNA-to-cDNA Kit (Thermo Fisher # 4387406) was used to prepare cDNA. The reaction mixture of 12 μl consisted 1 μl of cDNA, 0.5 μl of each primer pair, NRF2 forward primer (5′-GTT GCC CAC ATT CCC AAA TC-3′), reverse primer (5′-CGT AGC CGA AGA AAC CTC AT-3′), and NFκB-p65 forward primer (5′-TGA GCC CAC AAA GCC TTA TC-3′) reverse primer (5′-ACA ATG CCA GTG CCA TAC A-3′). The reaction was carried out using a Bio-Rad CFX96 Touch Real-Time PCR detection system. The relative gene expression was calculated by the ΔΔCt method by normalizing gene of interest expression (Ct value) against house-keeping genes (β-actin or GAPDH).
2.10. Mitochondrial Stress Assay
HBMECs were seeded as per optimal guidelines suggested in the Agilent Seahorse XF Cell Mito Stress Test Kit User Guide (Agilent # 103015–100). Cells were allowed to attain the desired confluency and then treated for 24 h. After treatment, the cell culture medium was replaced by an assay medium (Agilent # 103575-100) supplemented with 10 mM glucose, 10 mM pyruvic acid, and 1 mM L-glutamine. Subsequently, using a Seahorse Bioscience DfES 24 flux analyzer, we measured the basal oxygen consumption rate (OCR) without the addition of stressors.
2.11. Mitochondrial membrane potential
Mitochondrial membrane potential was evaluated using JC-1 Mitochondrial Membrane Potential Assay Kit (Cayman # 10009172). HBMECs were plated in a 96-well plate, ensuring 5 × 104 cells/well. 24 h following treatment, cells were stained with JC-1 reagent and incubated at 37 °C for 30 min. Subsequently, assay buffer was added to each well, and fluorescence intensity was determined using a plate reader (excitation – 485 nm and emission – 535 nm).
2.12. BBB integrity
HBMECs were seeded on transwells (Corning # 3460) and coated as described before. Following treatments for 24 h, BBB integrity was assessed by measuring TEER and paracellular permeability to sodium fluorescein. TEER values were obtained using EVOM 2 (World Precision Instruments). Paracellular permeability was assessed by adding sodium fluorescein (10 μm) to the apical chamber and sampling 100 μl from the basolateral chamber at 15 min intervals for 60 min while replenishing media therein. Permeability was calculated by using 1/(Pe ×S) = 1/(Pt ×S) −1/(Pf ×S) where, Pt = clearance slope of sample, Ps = clearance slope of blank and S = surface area of insert (cm2).
3. Results
3.1. TSE and HIV-1 gp120 impact cell viability
Endothelial cell viability was evaluated using an MTT assay. We observed a marked reduction in cell viability in the TSE-gp120 co-exposure group compared to control and individual treatments (Fig. 1A).
Fig. 1.
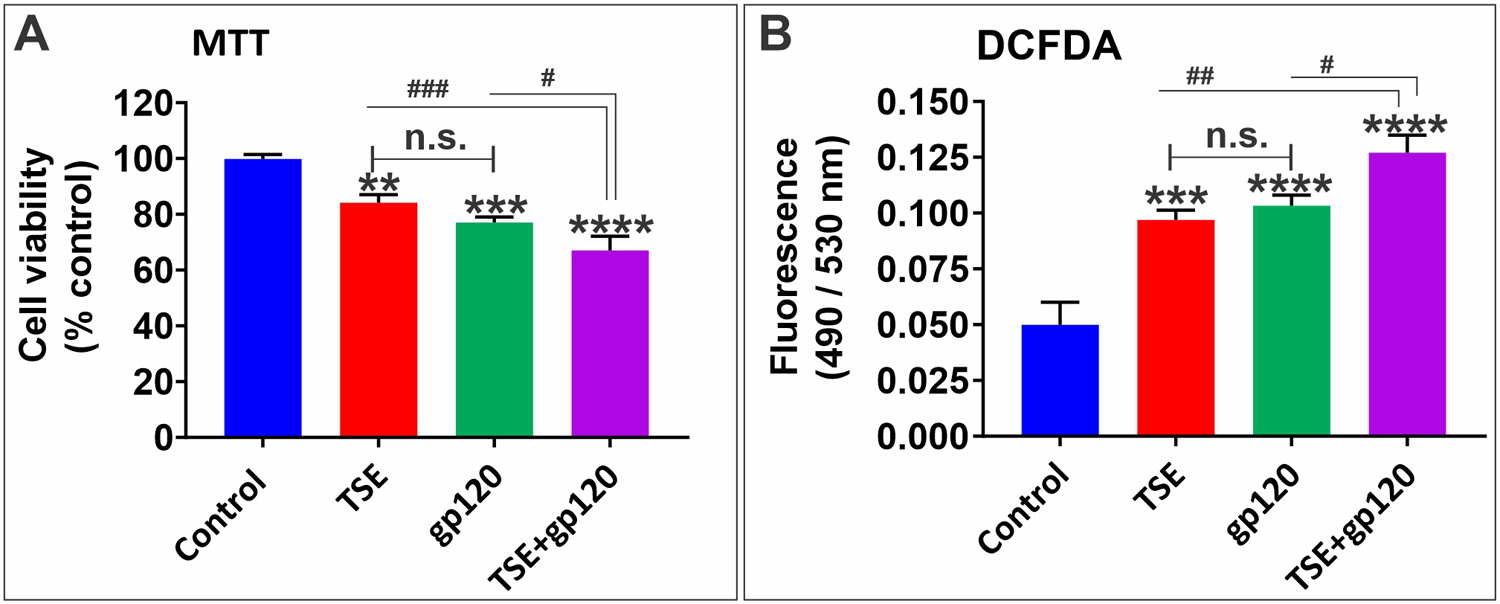
Effects of TS and gp120 exposure on cell viability and oxidative stress. A: MTT cytotoxicity assay for cell viability following TS - gp120 treatments for 24 h. B: DCFDA assay as an indicator for reactive oxygen species (ROS) in cells. n = 3 biological replicates/group. *p < 0.05, ** p < 0.01, **p < 0.001, ****p < 0.0001 vs. control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. TS + gp120 co-treatment. n.s. = non statistical significance.
3.2. TSE and HIV-1 gp120 increase the intracellular levels of reactive oxygen species (ROS)
To examine the role of oxidative stress caused by combination treatment of TSE and gp120, intracellular ROS was assessed. Our results show that the combination treatment distinctly increased ROS (Fig. 1B) when compared to individual treatments and control.
3.3. TSE and HIV-1 gp120 down-regulates NRF2 expression
TSE and gp120 are known to cause oxidative stress individually. In response to such stimuli, the cellular antioxidant defense is activated. Nuclear factor-E2 related Factor-2 (NRF2) is a transcription factor that is responsible for cellular redox homeostasis and transcription of various antioxidant genes (Sivandzade et al., 2019). The effects of our treatments on NRF2 mRNA expression levels were assessed by qPCR. We observed a significant decrease in NRF2 transcription across all treatments compared to controls (Fig. 2B).
Fig. 2. Effects of TSE-gp120 exposure on NRF2 levels.
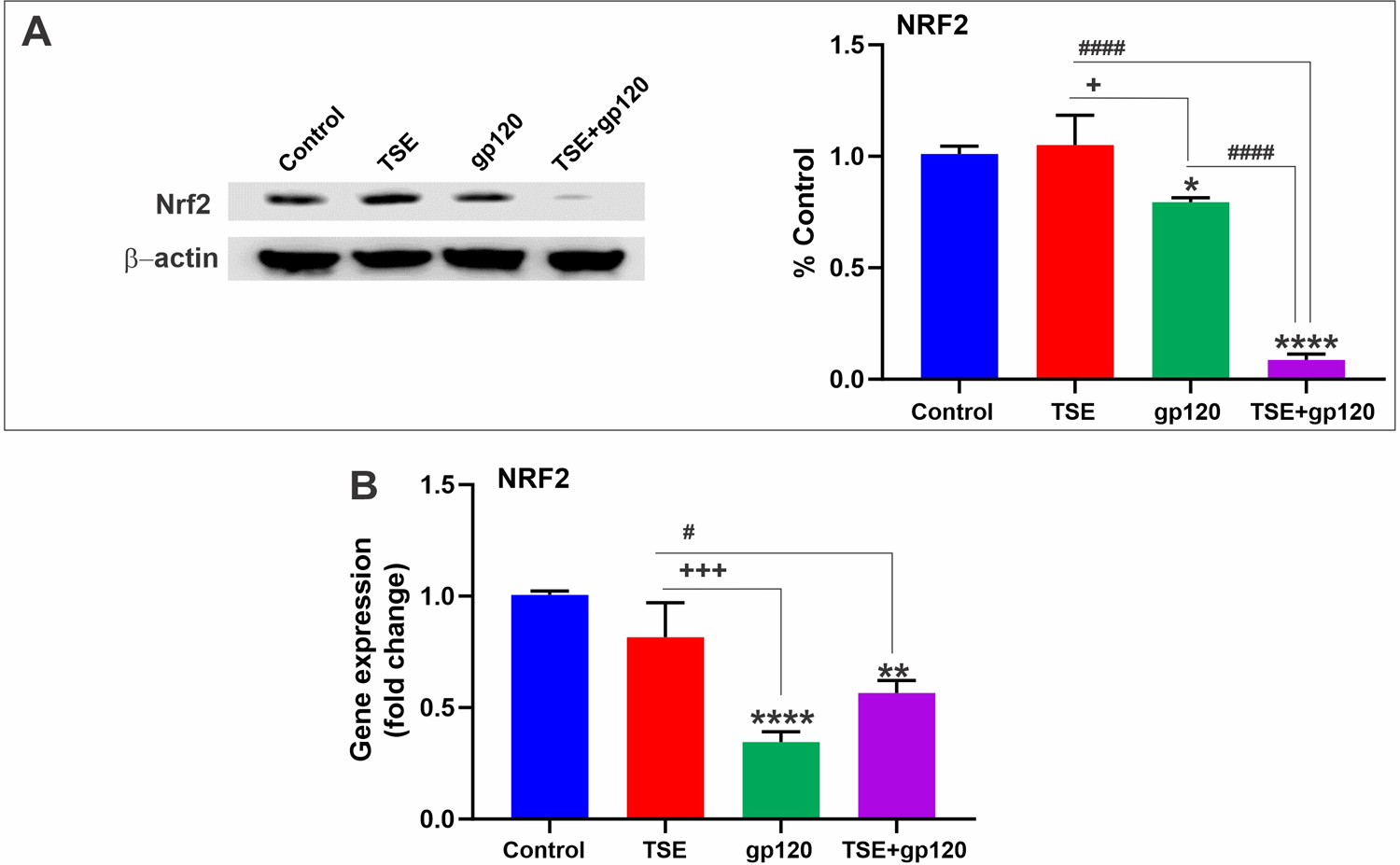
A. Western Blot analysis of NRF2 protein. B. qPCR results show NRF2 mRNA levels. n = 3 biological replicates/group. *p < 0.05, ** p < 0.01, ****p < 0.0001 vs. control; +p < 0.05, +++p < 0.001 vs. gp120; #p < 0.05, ####p < 0.0001 vs. TS + gp120 co-treatment.
On the other hand, exposure to TSE upregulated NRF2 protein expression levels, whereas the standalone exposure to gp120 led to a modest decrease. However, combination treatment of TSE and gp120 reduced NRF2 protein levels, as shown by western blotting (Fig. 2A).
3.4. TSE and HIV-1 gp120 increase NF-kB expression
Another transcription factor implicated in oxidative stress events is the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). This factor is also critical for regulating the immune response of a cell (Liu et al., 2017). The effects of our treatments on NF-kB-p56 sub-unit mRNA expression levels were assessed by qPCR. As shown in Fig. 3A, mRNA expression of NF-kB-p56 was greatly increased in response to co-treatment with TSE and gp120 but remained relatively unchanged with individual treatments. Correspondingly, our western blot data showed elevated NF-κB protein levels in the TSE-gp120 co-treated group (Fig. 3B).
Fig. 3.
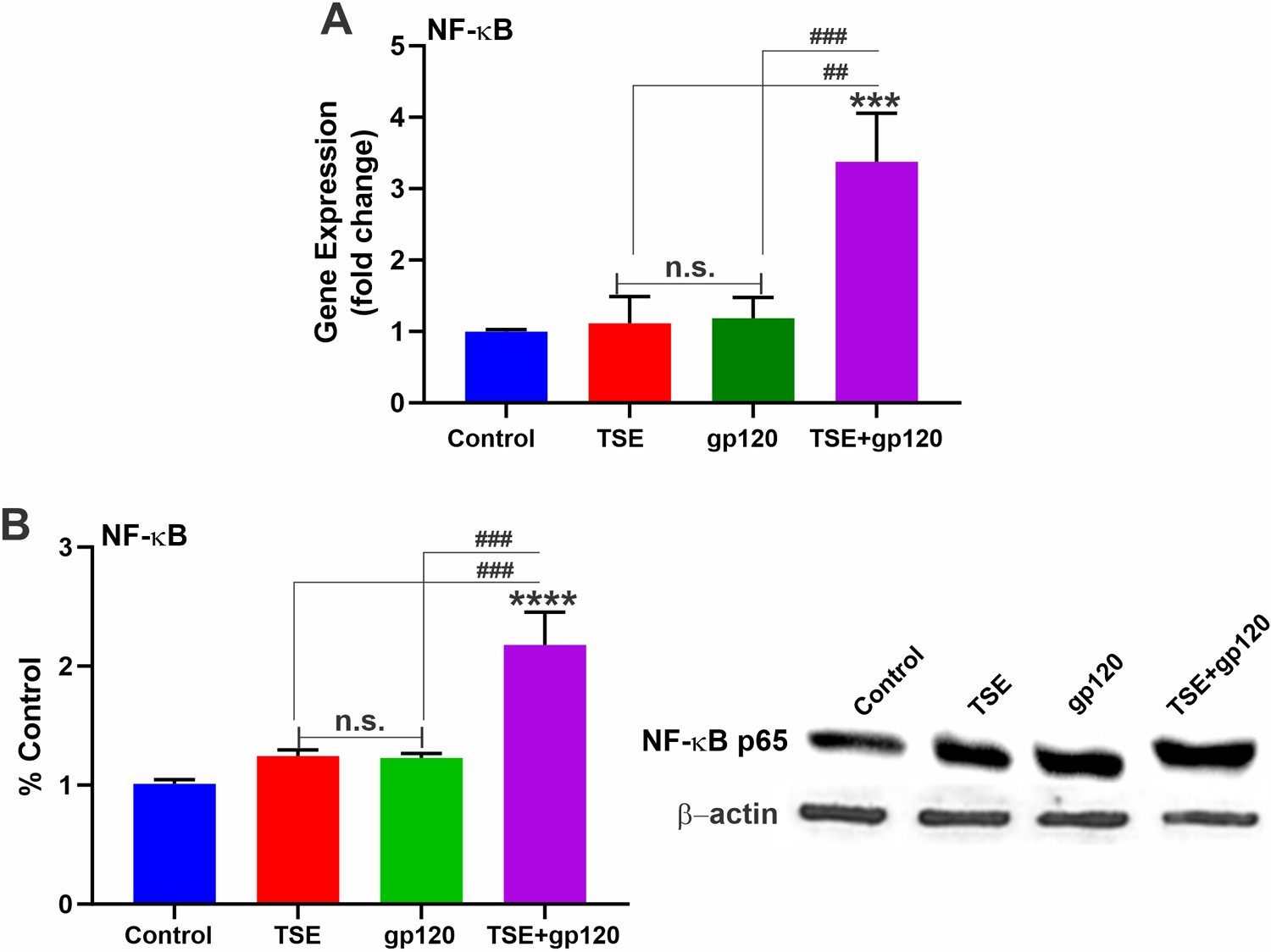
A: NF-κB transcription assessed by qPCR showed substantial increase in NF-κB mRNA levels. B: Western Blot analysis showed elevated levels of NF-κB protein in the TSE-gp120 treated group. n = 3 biological replicates/group. ***p < 0.001, ****p < 0.0001 vs. control); ##p < 0.01, ###p < 0.001 vs. TS + gp120 co-treatment. n.s. = non statistical significance.
3.5. TSE and HIV-1 gp120 disrupt mitochondrial function
Oxidative stress can adversely impact mitochondrial equilibrium, which may give rise to pro-inflammatory signaling within the cell. Basal oxygen consumption rate (OCR), an indicator of mitochondrial respiration, was assessed using a seahorse flux analyzer. Our results showed that the TSE-gp120 co-treated group had a much-diminished basal oxygen consumption rate than individual treatments and controls (Fig. 4A), thereby indicating the disruption of mitochondrial function.
Fig. 4.
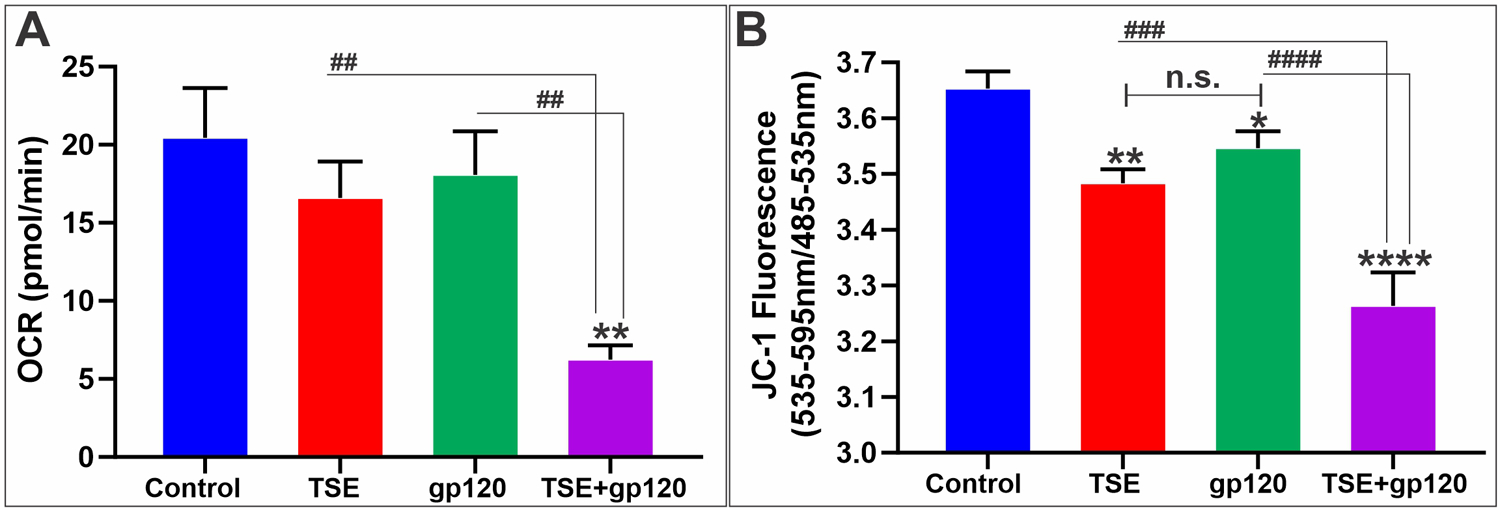
A: Co-treatment of TSE-gp120 diminished basal oxygen consumption rate (OCR) in co-treated group when compared with individual treatments and control. B: Reduced mitochondrial membrane potential in TSE-gp120 combination treatment. n = 3 biological replicates/group. *p < 0.05, **p < 0.01, ****p < 0.0001 vs. control; ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. TS + gp120 co-treatment. n.s. = non statistical significance.
A similar trend was observed when we tested for mitochondrial membrane potential using JC-1 dye that can selectively enter into mitochondria and form aggregates (red = healthy) or remain mono-meric (green = unhealthy) based on the mitochondrial membrane potential. The TSE-gp120 co-treatment group exhibited a significantly reduced red/green ratio, meaning more cells with damaged mitochondria than others (Fig. 4B).
3.6. TSE and HIV-1 gp120 disrupt the tight junction proteins and impair BBB integrity
Zonula occludens 1 (ZO1) is a scaffolding protein that links TJ transmembrane proteins such as claudins and occludin to the actin cytoskeleton. Occludin plays a role in the formation and regulation of the TJs. Both are essential to the maintenance of barrier integrity (Daneman and Prat, 2015; Jiao et al., 2011). Our results showed that exposure to TSE-gp120 negatively impacted the barrier and decreased the expression of ZO1 and Occludin, as shown by western blot and immunofluorescence analyses (Fig. 5 A–D). As expected, the downregulation of TJs expression negatively impacted the integrity of the endothelial barrier. As shown in Fig. 5E we observed a significant reduction in TEER values in the TSE-gp120 treated compared to the control group and individual treatments and a corresponding significant increase in fluorescein permeability (Fig. 5F).
Fig. 5. Effects of TSE-Gp120 exposure on tight junction proteins and BBB integrity.
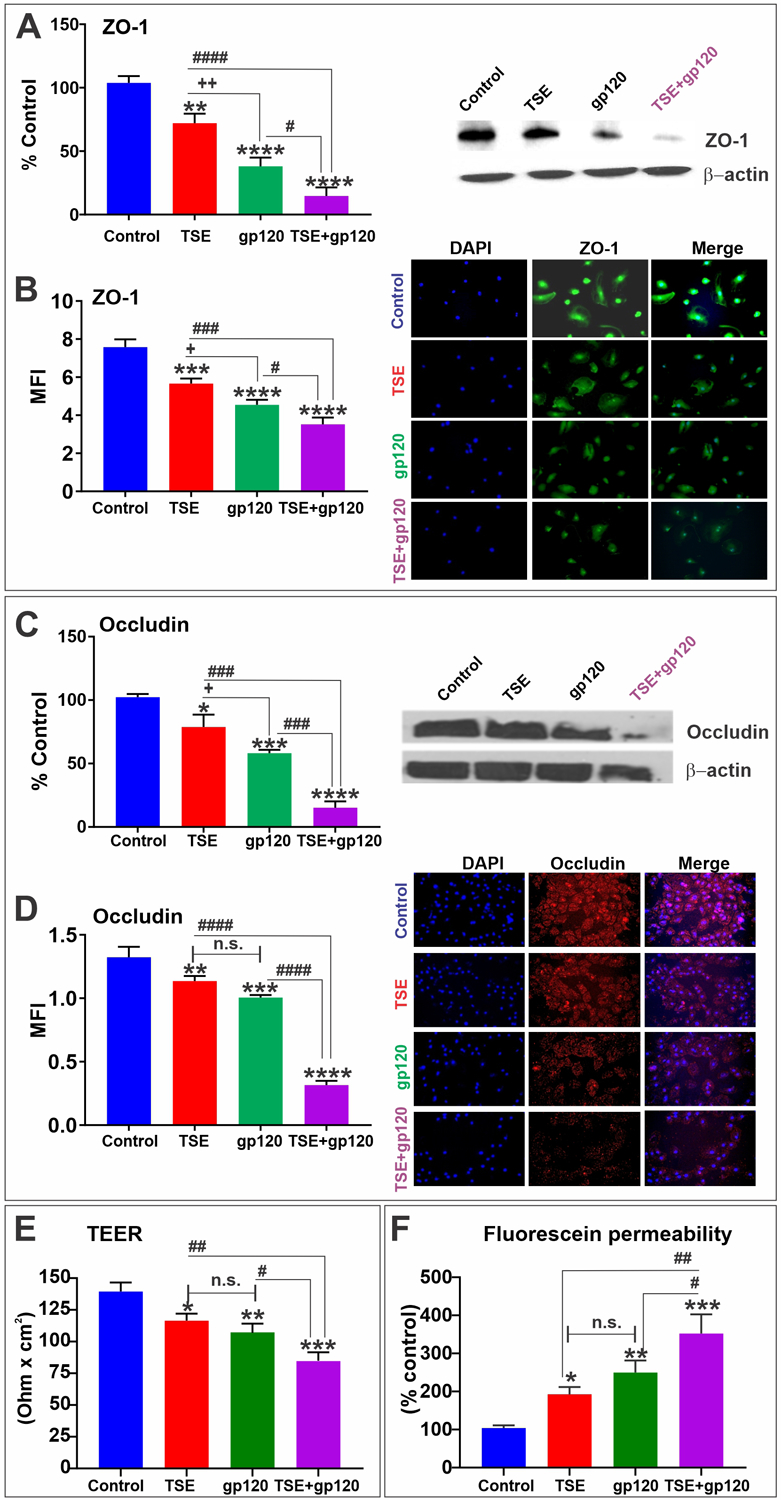
Western blotting analysis showing the downregulation of ZO-1 (A) along with the corresponding Immunofluorescence images showing protein expression and distribution (B). Western blotting analysis showing the downregulation of Occludin (C) along with the corresponding Immunofluorescence images showing protein expression and distribution (D). TEER (E) and fluorescein permeability (F) demonstrating a significant decrease of BBB integrity. *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control; +p < 0.05, ++p < 0.01 vs. gp120; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. TS + gp120 co-treatment. n.s. = non statistical significance.
4. Discussion
Oxidative stress, defined as the loss of physiological redox equilibrium, can have several exogenous and endogenous causative factors (Birben et al., 2012). Reactive Oxygen Species or pro-oxidants are normally involved in the maintenance and regulation of cells; however, excess ROS levels can damage DNA, proteins, and lipids leading to a loss of cellular redox homeostasis (Gilgun-Sherki et al., 2001). Tobacco smoke contains more than 7000 toxic chemicals (Bhalerao and Cucullo, 2019). The oxidative potential of tobacco smoke is well studied. Our group has previously shown the harmful effects of oxidative damage caused by tobacco smoke on the cerebrovascular system with severe implications for stroke, diabetes, and post-traumatic brain injury (Prasad et al., 2015; Kaisar et al., 2017; Sivandzade et al., 2021, 2020).
HIV/AIDS is a disease that is characterized by severe loss of the body’s inherent immunity leading to increased susceptibility to opportunistic infections. Evidence suggests that HIV-infected individuals are exposed to chronic oxidative stress (Pace and Leaf, 1995). Although several components of the virus are implicated in oxidative stress, HIV envelope glycoprotein gp120 is known to cause oxidative stress and is involved in disrupting the BBB (Price et al., 2005).
In the present study, we treated HBMECs with TSE and gp120 to study the combined effects on BBB. Our results also show a significant increase in the ROS levels in response to co-treatment. NRF2 is an important transcription factor that regulates the cellular antioxidant stress response to oxidative stress (Sivandzade et al., 2019). As seen in our results decrease in NRF2 levels indicates that the expression of many antioxidants and neuroprotective genes may be severely affected in the context of smoking and HIV infection, thereby giving rise to subsequent pathophysiological consequences.
Both NRF2 and NF-kB are interlinked in many ways in neurodegenerative disorders. NF-kB is a nuclear transcription factor in numerous cell types and regulates the expression of many genes mediating an inflammatory response (Sivandzade et al., 2019). Our results show elevated NF-kB mRNA and protein levels in response to co-treatment. Activation of NF-kB causes transcription of related inflammatory factors, leading to vascular endothelial cell injury (Asare et al., 2013).
Next, we wanted to evaluate the effect of our treatments on mitochondrial function. Mitochondria play a key role in generating energy for cells to conduct various activities. Inadequate NRF2 function and the pro-inflammatory role of NF-kB are both implicated in promoting mitochondrial dysfunction (Nisr et al., 2019; Holmstrom et al., 2016). Our results show that the diminished basal oxygen consumption rate and mitochondrial membrane potential indicate that mitochondrial functioning is severely compromised in the TSE-gp120 treated group. Thus, as seen above, impairment of mitochondrial function could have harmful consequences for the cells involved in maintaining the integrity of BBB and opening a pathway for the onset of neurological diseases. As demonstrated by our results, TSE-gp120 induced disruption in tight junction proteins, ZO-1, and occludin. The same was accompanied by a loss of BBB integrity, as demonstrated by the decrease in TEER values of the endothelial monolayers and the corresponding increase in paracellular permeability. Overall, a significant reduction in HBMEC cell viability was observed in the TSE-gp120 treated group suggesting an amplification of harmful effects when tobacco smoke and HIV gp120 act on the BBB, causing loss of characteristics and function.
5. Conclusions
Our results suggest that oxidative stress, inflammation, and mitochondrial dysfunction seem to be the major underlying mechanisms through which tobacco smoke and HIV-1 envelope protein gp120 may synergistically impact the integrity and viability of the BBB. The resulting effect could potentially increase the burden of NeuroAIDS related morbidity and mortality in HIV infected individuals who smoke (Bhalerao and Cucullo, 2020). In the context of HIV/AIDS patients leading otherwise normal lives due to combination antiretroviral therapy (cART), this study could raise awareness of the negative impact of smoking on the onset and/or progression of HIV/AIDS-associated neurological disorders.
Funding
This work was supported by the National Institutes of Health/National Institute on Drug Abuse 2R01-DA029121 and 1R01-DA049737 and the National Institute of Neurological Disorders and Stroke 1R01NS117906 to Dr. Luca Cucullo.
Footnotes
CRediT authorship contribution statement
AB conceived the study and prepared the drafting of the manuscript. LC oversaw the study, assisted with drafting the manuscript and preparing the figures and tables, made revisions, and provided the necessary funding. All authors reviewed the manuscript.
Competing interests
The authors declare that they have no competing interests.
Data Availability
Data will be made available on request.
Availability of data and materials
All data generated during this study are included in this published article. The original datasets analyzed in this study are available from the corresponding author upon reasonable request.
References
- Abbott NJ, et al. , 2010. Structure and function of the blood–brain barrier. Neurobiol. Dis 37 (1), 13–25. [DOI] [PubMed] [Google Scholar]
- Asare Y, et al. , 2013. Endothelial CSN5 impairs NF-kappaB activation and monocyte adhesion to endothelial cells and is highly expressed in human atherosclerotic lesions. Thromb. Haemost 110 (1), 141–152. [DOI] [PubMed] [Google Scholar]
- Banks WA, Akerstrom V, Kastin AJ, 1998. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J. Cell Sci 111 (4), 533–540. [DOI] [PubMed] [Google Scholar]
- Bhalerao A, Cucullo L, 2019. Impact of tobacco smoke in HIV progression: a major risk factor for the development of NeuroAIDS and associated CNS disorders. J. Public Health 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao A, Cucullo L, 2020. Impact of tobacco smoke in HIV progression: a major risk factor for the development of NeuroAIDS and associated of CNS disorders. Z. Gesund Wiss 28 (3), 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E, et al. , 2012. Oxidative stress and antioxidant defense. World Allergy Organ. J 5 (1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood GAC, 2016. Human immunodeficiency virus (HIV). Transfus. Med. Hemother 43 (3), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissé L, Gill MJ, Power C, 2008. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol. Clin 26 (3), 799–819. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, et al. , 2018. Participant characteristics and clinical trial decision-making factors in AIDS malignancy consortium treatment trials for HIV-infected persons with cancer (AMC# S006). HIV Clin. Trials 19 (6), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S, et al. , 2013. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. J. Neuroimmune Pharm 8 (5), 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins NW, Rizza SA, Badley AD, 2010. How much gp120 is there? J. Infect. Dis 201 (8) (1273–1273). [DOI] [PubMed] [Google Scholar]
- Daneman R, Prat A, 2015. The blood–brain barrier. Cold Spring Harb. Perspect. Biol 7 (1), a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV, 2013. The end of AIDS: HIV infection as a chronic disease. Lancet 382 (9903), 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D, 2001. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40 (8), 959–975. [DOI] [PubMed] [Google Scholar]
- Gorry PR, et al. , 2003. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr. HIV Res 1 (4), 463–473. [DOI] [PubMed] [Google Scholar]
- Helleberg M, et al. , 2015. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 29 (2), 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom KM, Kostov RV, Dinkova-Kostova AT, 2016. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol 1, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, et al. , 2011. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood–brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci 44 (2), 130–139. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, et al. , 2017. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure? Redox Biol. 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, et al. , 2007. HIV-1 gp120 compromises blood–brain barrier integrity and enhance monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab 27 (1), 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, 2011. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top. Antivir. Med 19 (4), 137. [PMC free article] [PubMed] [Google Scholar]
- Liu T, et al. , 2017. NF-κB signaling in inflammation. Signal Transduct. Target. Ther 2 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, et al. , 2008. NeuroAIDS. Mol. Diagn. Ther 12 (1), 25–43. [DOI] [PubMed] [Google Scholar]
- Mollace V, et al. , 2001. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 24 (7), 411–416. [DOI] [PubMed] [Google Scholar]
- Naik P, et al. , 2014. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 15 (1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P, et al. , 2015. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC Neurosci. 16 (1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, et al. , 2000. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc 47 (2), 186–194. [PubMed] [Google Scholar]
- Nisr RB, et al. , 2019. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell. Mol. Life Sci 76 (24), 4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace GW, Leaf CD, 1995. The role of oxidative stress in HIV disease. Free Radic. Biol. Med 19 (4), 523–528. [DOI] [PubMed] [Google Scholar]
- Prasad S, et al. , 2015. Impact of cigarette smoke extract and hyperglycemic conditions on blood–brain barrier endothelial cells. Fluids Barriers CNS 12 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, et al. , 2017. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 12, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TO, et al. , 2005. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 1045 (1–2), 57–63. [DOI] [PubMed] [Google Scholar]
- Reynolds NR, 2009. Cigarette smoking and HIV: more evidence for action. AIDS Educ. Prev 21 (3_suppl.), S106–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, et al. , 2004. Role of microglia in central nervous system infections. Clin. Microbiol. Rev 17 (4), 942–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Bairy I, Shivananda P, 2003. Spectrum of opportunistic infections in AIDS cases. Indian J. Med. Sci 57 (1), 16–21. [PubMed] [Google Scholar]
- Sivandzade F, et al. , 2019. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 21, 101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, et al. , 2020. The cerebrovascular and neurological impact of chronic smoking on post-traumatic brain injury outcome and recovery: an in vivo study. J. Neuroinflamm 17 (1), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Alqahtani F, Cucullo L, 2021. Impact of chronic smoking on traumatic brain microvascular injury: an in vitro study. J. Cell Mol. Med 25 (15), 7122–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW, 2012. HIV: cell binding and entry. Cold Spring Harb. Perspect. Med 2 (8), a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Gust SW, Strathdee SA, 2006. Drug abuse and HIV/AIDS: international research lessons and imperatives. Drug Alcohol Depend. 82, S1–S5. [DOI] [PubMed] [Google Scholar]
- Wyatt R, et al. , 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393 (6686), 705–711. [DOI] [PubMed] [Google Scholar]
- Yadav A, Collman RG, 2009. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J. Neuroimmune Pharm 4 (4), 430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayyad Z, Spudich S, 2015. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr. HIV/Aids Rep 12 (1), 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
All data generated during this study are included in this published article. The original datasets analyzed in this study are available from the corresponding author upon reasonable request.


