Abstract
Rationale
Transgenic mouse lines expressing Cre-recombinase under the regulation of either dopamine transporter (DAT) or tyrosine hydroxylase (TH) promoters are commonly used to study the dopamine (DA) system. While use of the TH promoter appears to have less liability to changes in native gene expression, transgene insertion in the DAT locus results in reduced DAT expression and function. This confound is sometimes overlooked in genetically targeted behavioral experiments.
Objectives
We sought to evaluate the suitability of DAT-Ires-Cre and TH-Cre transgenic lines for behavioral pharmacology experiments with DA agonists. We hypothesized that DAT-Ires-Cre expression would impact DAT-mediated behaviors, but no impact of TH-Cre expression would be observed.
Methods
DAT-Ires-Cre and TH-Cre mice bred on mixed 129S6/C57BL/6 and pure C57BL/6 backgrounds were evaluated for novelty-induced, baseline, and amphetamine (AMPH)-induced locomotion; and for AMPH and D1 agonist (SKF-38393)-induced preservative behaviors.
Results
DAT-Ires-Cre mice on both mixed 129S6/C57BL/6 and pure C57BL/6 backgrounds displayed increased novelty-induced activity and decreased AMPH-induced locomotion, with mixed results for AMPH-induced stereotypy. TH-Cre mice on both backgrounds showed typical baseline activity and AMPH-induced stereotypy, with a difference in AMPH-induced locomotion observed only on the mixed background. Both transgenic lines displayed unaltered SKF-38393 induced grooming behavior.
Conclusions
Our findings indicate that the DAT-Ires-Cre transgenic line may lead to confounds for experiments that are dependent on DAT expression. The TH-Cre transgenic line studied here may be a more useful option, depending on background strain, because of its lack of baseline and drug-induced phenotypes. These data highlight the importance of appropriate controls in studies employing transgenic mice.
Keywords: Transgenic mice, dopamine transporter, tyrosine hydroxylase, amphetamine, D1 agonist, 129S6/SvEvTac, C57BL/6, inbred strain, novelty-induced locomotion, stereotypic behavior
Introduction
Ventral midbrain dopamine (DA) neurons play important roles in a wide range of brain functions including motor control, emotion regulation, reward processing, reinforcement learning, social interaction, and motivation (Bariselli et al. 2018; da Silva et al. 2018; Friedman et al. 2014; Gunaydin et al. 2014; Jin and Costa 2010; Lammel et al. 2012; Tye et al. 2013). Consistent with this broad set of roles, dysfunction of the DA system is implicated in many different neurologic and neuropsychiatric disorders including Parkinson’s Disease, schizophrenia, attention-deficit hyperactivity disorder, obsessive-compulsive disorder, autism spectrum disorder, and substance use disorders (Cousins et al. 2009; Denys et al. 2013; Denys et al. 2004; Hamilton et al. 2013; Luscher 2016; McCutcheon et al. 2019; Neale et al. 2012; Obeso et al. 2017; Volkow et al. 2007). Consequently, manipulation of gene expression within DA neurons is commonly used as an experimental system to understand the function of specific proteins within these neurons and the downstream impact on DA signaling and behavior. Transgenic Cre-recombinase mouse lines are frequently used to ensure specificity of these manipulations, with the most commonly used transgenic lines employing either the dopamine transporter (DAT) or the tyrosine hydroxylase (TH) promoter to drive Cre expression.
Broadly speaking, a DA neuron is defined as one that synthesizes and releases DA (Berke 2018; Bjorklund and Dunnett 2007; Morales and Margolis 2017; Roeper 2013; Ungless and Grace 2012). DA neurons have therefore traditionally been identified by expression of two genes: 1)Th, which encodes tyrosine hydroxylase (TH), the rate limiting enzyme in DA synthesis; and 2) vesicular monoamine transporter 2 (VMAT2, Slc18a2), which packages DA into vesicles (Bjorklund and Dunnett 2007; Hokfelt et al. 1976; Hokfelt et al. 1977). However, TH is also expressed in cells that produce norepinephrine (NE), and recent evidence suggests that some midbrain DA populations produce mRNA for Th in the absence of detectable amounts of TH protein synthesis (Lammel et al. 2015; Yamaguchi et al. 2015). Thus, genetic tools using the Th promoter may generate both false positive and false negative classification of DA neurons.
Beyond the machinery to synthesize and package DA, most DA neurons express the sodium-dependent dopamine transporter (DAT, Slc6a3), which takes up released DA to terminate its action (Giros et al. 1996; Jones et al. 1998a) and can also efflux DA under certain conditions (Falkenburger et al. 2001; Kahlig et al. 2005; Sulzer et al. 1995). DAT binds to psychotropic medications such as tricyclic antidepressants (Giros and Caron 1993; Vaughan and Foster 2013), as well as drugs of abuse such as cocaine and amphetamine (AMPH). The expression pattern of Slc6a3 appears to be more restricted than that of Th, as shown by a lack of Slc6a3 mRNA expression in Th-expressing locus coeruleus NE cells, suggesting improved specificity. However, Slc6a3 mRNA is not expressed in the medial ventral tegmental area (VTA) DA neurons that project to the medial prefrontal cortex, suggesting issues with sensitivity (Augood et al. 1993; Cardozo Pinto et al. 2019; Ciliax et al. 1995; Lammel et al. 2008; Yip et al. 2018). The Slc6a3 promoter could thus confer better selectivity as a molecular tool for the identification of DA neurons (Cardozo Pinto et al. 2019; Lammel et al. 2015; Stuber et al. 2015), but with the caveat that sensitivity may not be optimal in all neural populations. Notwithstanding their lack of perfect selectivity, both Th/TH and Slc6a3/DAT continue to be used as molecular markers of DA neuron identity (Lammel et al. 2015; Papathanou et al. 2019; Soden et al. 2016; Stuber et al. 2015; Vuong et al. 2015).
The use of the Cre-Lox recombination system (Branda and Dymecki 2004; Tsien et al. 1996) has proven pivotal for understanding the DA system. Several transgenic lines utilize Th and Slc6a3 promotors to direct Cre expression to DA neurons (Backman et al. 2006; Lindeberg et al. 2004; Savitt et al. 2005; Zhuang et al. 2005). In combination with floxed mouse strains and opto- or chemogenetic viral vectors, these transgenic tools have been used to identify DA circuit architecture and test hypotheses regarding involvement of DA pathology in disease (Chaudhury et al. 2013; Cohen et al. 2012; Dabney et al. 2020; Lee et al. 2020; Patriarchi et al. 2018; Pupe and Wallen-Mackenzie 2015; Schiemann et al. 2012; Takeuchi et al. 2016; Tritsch et al. 2012; Tsai et al. 2009; Wang et al. 2011). However, it is essential to demonstrate cell-type specificity of Cre expression and preservation of normal DA system function to ensure valid conclusions from studies using these tools.
Independent of the issues described above related to sensitivity and specificity of TH and DAT for identification of DA cells, all currently available Cre-driver lines that target the DA system have weaknesses to consider when interpreting results. Characterization of Cre expression in TH-Cre driver lines indicates transgene expression in the locus coeruleus, as well as in cells that do not express TH protein in adulthood (Cardozo Pinto et al. 2019; Lammel et al. 2015; Stamatakis et al. 2013; Stuber et al. 2015). In addition, Cre lines driven by Slc6a3, commonly referred to as DAT-Cre (Cardozo Pinto et al. 2019; Lammel et al. 2015), show reduced DAT levels due to interference with one of its alleles (Backman et al. 2006; Zhuang et al. 2005). For example, Cre insertion in the Slc6a3 5’ untranslated region disrupts one copy of the DAT gene in the DAT-Cre knockin mouse line (Zhuang et al. 2005). The DAT-Ires-Cre mouse line was developed to solve this problem, but still results in a 47% reduction of DAT protein levels in the homozygous state and a 17% reduction in heterozygotes (Backman et al. 2006), leading to significantly reduced DA uptake capacity (O’Neill et al. 2017). This is likely to have a cascading impact on DA system function because mice with heterozygous disruption of Slc6a3 (hemizygous DAT knockout mice) show a two-fold increase in extracellular DA tone (Giros et al. 1996; Jones et al. 1998a), as well as a reduction in tissue DA content, TH immunolabeling, and postsynaptic DA receptor transcript and protein expression (Giros et al. 1996; Jones et al. 1998a). Behaviorally, hemizygous DAT knockout mice fail to demonstrate habituation of baseline locomotor activity when tested across multiple days (Spielewoy et al. 2000), show less locomotor response to low dose AMPH (Spielewoy et al. 2001), and display reduced high-dose AMPH-induced stereotypic behavior (Spielewoy et al. 2001).
In the context of concerns about specificity for DA neurons and alteration of DAT expression levels, we evaluated the DAT-Ires-Cre and TH-Cre transgenic lines for their suitability for behavioral pharmacology experiments that target the DA system. We tested AMPH, a DAT substrate that induces DAT-mediated efflux (Freyberg et al. 2016; Kahlig et al. 2005; Sulzer et al. 1995), and SKF-38393, a DA receptor D1 agonist that may be sensitive to changes in baseline DAT activity. We compared baseline and drug-induced behavior in DAT-Ires-Cre, TH-Cre, and littermate control mice. True wildtype littermate controls were included to avoid the common pitfall of solely controlling for expression of the floxed gene and the Cre transgene. We began with mice on the mixed 129S6/C57BL/6 inbred strain background that we planned to use for genetically targeted experiments. We then confirmed findings on a pure C57BL/6 background. Our results highlight the importance of appropriate controls in studies employing transgenic mice to investigate DA neuron function.
Materials and Methods
Animals and Housing.
DAT-Ires-Cre (B6.SJL-Slc6a3tm1.1(cre)Bkmn/J, Stock No: 006660, The Jackson Laboratory) (Backman et al. 2006) and TH-Cre (B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J, Stock No. 008601, The Jackson Laboratory) (Savitt et al. 2005) mice were crossed with 129S6/SvEvTac or C57BL/6J wildtype mice to obtain heterozygous (DAT-Ires-Cre+ or TH-Cre+) and littermate control (DAT-Ires-Cre− or TH-Cre−) mice on a mixed 129S6-B6 or a pure B6 background, respectively. Mice were ear-tagged at weaning. Once genotyped, alternate animals were sequentially assigned to experimental groups. Behavior was run blind to genotypes. Ear-tags were used as animal identifiers throughout all experiments. Adult mice (male and female, 8 weeks old) were used for all experiments. Mice were group housed under a 12 h-light/dark cycle in a temperature-controlled environment with food and water available ad libitum. All experiments were conducted in the light cycle. All animal care and testing were conducted following NIH guidelines and were approved by Institutional Animal Care and Use Committee of New York State Psychiatric Institute.
Behavioral assays
Novelty-induced locomotor activity.
Novelty-induced locomotor activity was tested by placing mice in a novel environment (open field) measuring 40.6 cm long ×40.6 cm wide chambers (SmartFrame Open Field System, Kinder Scientific) fitted with 32 infrared photo-beams (16X & 16Y). Locomotion was detected over a period of 30 mins and analyzed by Motor Monitor software. Testing took place under bright ambient light conditions. After 30 mins, mice were returned to their home cages. Anxiety-like behavior was evaluated by assessing number of visits, distance traveled, and time spent in the center (20 cm x 20 cm) of the open field arena.
Baseline locomotor activity (Saline challenge day).
On the next day, mice were placed in the same activity chambers used on the novelty testing day and allowed to explore for 30 mins. Mice were then removed and intraperitoneally (IP) injected with 0.9% saline (10 ml/kg of body weight). Mice were promptly returned to the chambers, and locomotor activity was monitored for another 60 mins.
Amphetamine-induced locomotor activity.
On the next day, mice were returned to the activity chambers. After an initial exploratory period of 30 mins they were injected with AMPH 3.0 mg/kg (10 ml/kg). Locomotor activity was recorded for 60 mins after AMPH administration. In B6 mice, additional AMPH doses (1.8 and 5.6 mg/kg) were tested after 1 week of drug washout, with each dose preceded by a saline challenge day. After two weeks of drug washout following the final (5.6 mg/kg) dose, mice performed the 8.0 mg/kg AMPH stereotypy assay described below.
Amphetamine-induced stereotypy.
Mice were weighed and placed into novel, clear, empty cages. Following 30 mins acclimatization mice were administered AMPH 8.0 mg/kg IP. Mice were promptly returned to the clear cages, and behavior was recorded with a video camera (Sony Handycam Flash Memory Camcorder, HDRCX405/B) for 2 mins each at 50- and 80-min post AMPH administration. Mouse behavior was analyzed for stationary shuffling and sniffing-like stereotypy as previously described (Zike et al. 2017). Two observers blind to genotype performed analysis by post-hoc scoring of video recordings. The observers recorded time spent performing stationary shuffling- and sniffing-like stereotypy manually with a stopwatch. Following two weeks of washout, mice proceeded to SKF38393 assay described below. B6 mice were additionally tested with 10 mg/kg AMPH dose 2 weeks following the SKF38393-induced grooming assay.
SKF-38393-induced grooming.
Grooming behavior was recorded as previously described (Zike et al. 2017). Mice were weighed and placed into the same clear, empty cages used for the stereotypy assay. Following 30 min acclimatization mice were administered 0.9% saline or SKF-38393 (10 mg/kg, IP) and were placed back in the cages for 60 additional minutes. Behavior was recorded with a video camera for 2 min intervals every 10 min for 60 mins. Following a 1-week washout period, mice received the alternative treatment from week 1. Two trained observers, blind to genotype and drug treatment, manually scored grooming duration during the sample periods.
Drugs.
D-amphetamine (Sigma-Aldrich) was dissolved in 0.9% sterile saline, pH 7.4. SKF-38393 (Tocris Bioscience) was dissolved in 0.25% DMSO/0.9% sterile saline. All drugs were administered IP at a volume of 10 ml/kg.
Statistical analysis.
Data were analyzed using GraphPad Prism (version 8.0.0, GraphPad Software, San Diego, California USA). Two-way repeated measures ANOVA was used to analyze the primary data, except for locomotor data, which were analyzed using nonlinear curve–fit analysis. All data in results and figure legends are reported as mean ± SEM.
Results
129S6-B6 DAT-Ires-Cre mice
129S6-B6 DAT-Ires-Cre+ mice display increased novelty-induced and baseline locomotion compared to WT controls but show preserved habituation
To evaluate whether novelty-induced locomotion is altered in 129S6-B6 DAT-Ires-Cre+ mice, we examined locomotor activity of 129S6-B6 DAT-Ires-Cre+ and littermate 129S6-B6 DAT-Ires-Cre− control mice over a 30 min exploration time in a novel environment (open-field). A curve fit analysis over this period split into six 5-min bins indicated that 129S6-B6 DAT-Ires-Cre+ were hyperlocomotive [F (4, 124) = 7.846, P < 0.0001, Fig. 1b]. However, an absence of significant genotype by time interaction in 2-way repeated measures ANOVA indicated preserved habituation (Fig. 1b). 3-way ANOVA to determine effect sex on locomotor activity revealed a main effect of genotype [F (1, 18) = 7.569, P = 0.0131] and time [F (2.643, 47.58) = 38, P < 0.0001], but no 3-way interaction [F (5, 90) = 1.596, P = 0.1692], sex by genotype interaction [F (1, 18) = 0.03225, P = 0.8595] or a main effect of sex [F (1, 18) = 0.9603, P = 0.3401, n = 6(Cre−, males); 4(Cre+, males); 4(Cre−, females); 7(Cre+, females)]. 129S6-B6 DAT-Ires-Cre+ mice made more visits, spent more time and traveled more distance in the center of the open field arena (Supplementary materials Fig. 3a, c, e), thus exhibiting a low anxiety-like behavior.
Fig. 1. 129S6-B6 DAT-Ires-Cre+ mice display novelty-induced and baseline hyperactivity and show attenuated locomotor and stereotypic response to AMPH but show normal SKF-38393-induced grooming behavior.
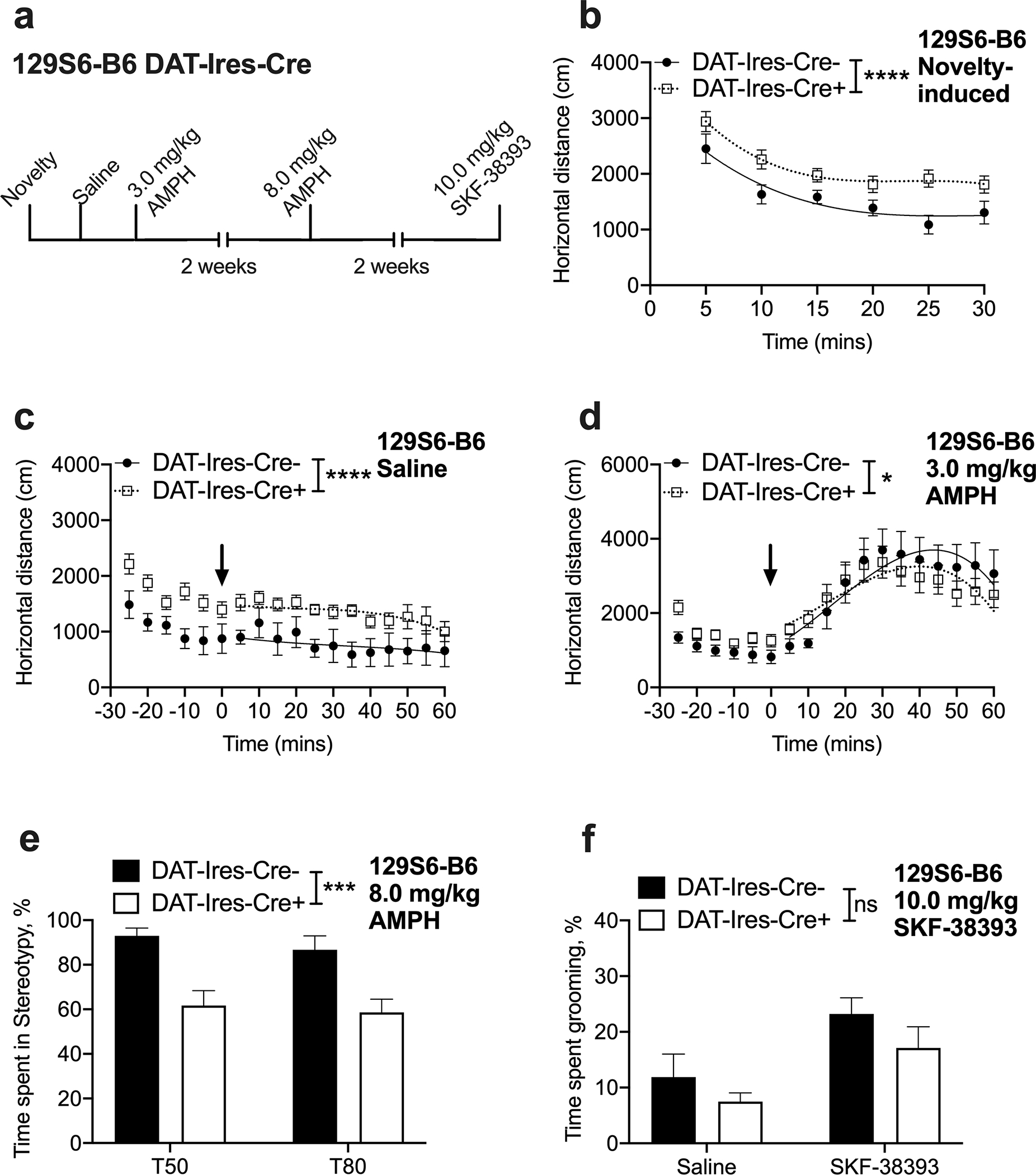
(a) Experimental timeline. (b) 129S6-B6 DAT-Ires-Cre+ mice show increased novelty-induced locomotion [curve–fit analysis, t = 0–30; F (4, 124) = 7.846, ****P < 0.0001, n = 10–12] while displaying habituation to the open field chamber [2-way RM ANOVA; time F (2.4, 48) = 40.38, P < 0.0001, genotype F (1, 20) = 7.205, P = 0.0143, time x genotype interaction F (5, 100) = 1.319, P = 0.2621 n = 10–12]. (c) 129S6-B6 DAT-Ires-Cre+ mice show increased baseline locomotion on saline challenge day [curve–fit analysis, t = 5–60; F (4, 388) = 22.27, ****P < 0.0001, n = 10–12]. (d) 129S6-B6 DAT-Ires-Cre+ mice show attenuated locomotor response to a moderate dose (3.0 mg/kg) of AMPH [curve–fit analysis, t = 5–60; F (4, 388) = 3.271, *P = 0.0118, n = 10–12]. (e) 129S6-B6 DAT-Ires-Cre+ mice display decreased stereotyped behavior following 8.0 mg/kg AMPH injection relative to 129S6-B6 DAT-Ires-Cre− control mice [2-way RM ANOVA; genotype F (1, 20) =16.92, ***P < 0.001, time F (1, 20) =1.238, P = 0.2790, genotype x time interaction F (1, 20) =0.1434, P = 0.7089, n = 10–12]. (f) SKF-38393-induced grooming behavior is not altered in 129S6-B6 DAT-Ires-Cre+ mice [2-way RM ANOVA; drug, F(1, 20) = 9.734, P < 0.01; genotype, F(1, 20) = 2.953, P = 0.1011, drug x genotype interaction F(1, 20) = 0.06414, P = 0.8027, n = 10–12].
To evaluate whether basal locomotion (without the confound of environmental novelty) is altered in 129S6-B6 DAT-Ires-Cre+ mice, we examined locomotor activity of saline injected mice over a 1hr period the day following the novelty testing day. A curve-fit analysis indicated that 129S6-B6 DAT-Ires-Cre+ mice were hyperlocomotive at baseline [F (4,388) = 22.27, P < 0.0001, Fig. 1c]. A repeated measures ANOVA revealed within-session habituation of this locomotor response despite the overall higher basal locomotor activity [2-way RM ANOVA; time F (4.438, 88.76) = 8.114, P < 0.0001, genotype F (1, 20) = 7.624, P = 0.0120, time x genotype interaction F (17, 340) = 0.5638, P = 0.9173, Fig. 1c]. In addition, locomotor responses displayed between-session habituation across the novelty and saline testing days (Supplementary materials Fig. 1a). 3-way ANOVA comparing locomotor activity over this time revealed a main effect of genotype [F (1, 18) = 6.007, P = 0.0247] and time [F (5.065, 91.16) = 7.726, P < 0.0001], but no main effect of sex [F (1, 18) = 1.618, P = 0.2196], sex by genotype interaction [F (1, 18) = 0.05876, P = 0.8112] or a 3-way interaction [F (17, 306) = 0.5361, P = 0.9338].
129S6-B6 DAT-Ires-Cre+ mice display blunted locomotor response to a moderate dose of AMPH
Next, we evaluated the locomotor response of 129S6-B6 DAT-Ires-Cre+ mice to a moderate (3.0 mg/kg) dose of the indirect DA agonist AMPH. On the day after the saline testing day (see above), 3.0 mg/kg AMPH IP administration after an acclimatization period of 30 mins resulted in locomotor activation in both groups (Fig. 1d). However, 129S6-B6 DAT-Ires-Cre+ showed an attenuated activation to 3.0 mg/kg AMPH when compared to the 129S6-B6 DAT-Ires-Cre− control group [curve–fit analysis, F (4, 388) = 3.271, P = 0.0118, Fig. 1d]. The blunted AMPH response in 129S6-B6 DAT-Ires-Cre+ is notable because mice continued to display significantly higher overall locomotor activity during the pre-injection acclimatization period on the AMPH test day [curve–fit analysis, t = −25–0; F (4, 124) = 6.595, P < 0.0001, Fig. 1d]. 3-way ANOVA with time as a repeated measure showed a time by genotype interaction [F (17, 306) = 2.198, P = 0.0044], but no sex by genotype interaction [F (1, 18) = 0.4312, P = 0.5197] or 3-way interaction [F (17, 306) = 0.3838, P = 0.9880]. No main effect of sex [F (1, 18) = 2.249, P = 0.1511] was observed.
Reduced AMPH-induced stereotypic behavior but preserved SKF-38393-induced grooming response in 129S6-B6 DAT-Ires-Cre+ mice.
To further investigate diminished sensitivity to AMPH in DAT-Ires-Cre+ mice, we examined AMPH’s ability to induce stereotypic movements by testing a dose (8.0 mg/kg) at which stereotypic behavior dominates over locomotion. Stereotypic behavior was analyzed for stationary shuffling and sniffing-like movements. Video-scoring of stereotypic behavior at 50- and 80-min following AMPH injection in 129S6-B6 DAT-Ires-Cre+ and control mice revealed a significant main effect of genotype [2-way RM ANOVA; genotype F (1, 20) =16.92, P < 0.001, Fig. 1e] suggesting an overall decreased sensitivity to AMPH in the 129S6-B6 DAT-Ires-Cre+ mouse line. 3-way ANOVA to determine the effects of genotype, time and sex on stereotypy ruled out a sex by genotype interaction [F (1, 18) = 0.4358, P = 0.5175] or a main effect of sex [F (1, 18) = 0.1754, P = 0.6803].
To examine stereotyped behavior that is independent of presynaptic DA release we acutely challenged 129S6-B6 DAT-Ires-Cre+ and control mice with the D1 agonist SKF-38393 to induce perseverative grooming. Video-scoring of grooming behavior during six fixed 2-min intervals spread over 1hr post injection revealed a significant main effect of drug [drug, F (1, 20) = 9.734, P < 0.01, Fig. 1f] but no main effect of genotype via a 2-way RM ANOVA [genotype, F (1, 20) = 2.953, P = 0.1011, Fig. 1f], suggesting preserved postsynaptic function in 129S6-B6 DAT-Ires-Cre+ mice. Further, 3-way ANOVA to determine effects of sex, genotype and drug on grooming behavior revealed no sex by genotype interaction [F (1, 18) = 0.04449, P = 0.8353] or a main effect of sex [F (1, 18) = 0.2439, P = 0.6274].
B6 DAT-Ires-Cre mice
B6 DAT-Ires-Cre+ mice display increased novelty-induced hyperlocomotion
To evaluate whether novelty-induced locomotion is altered in B6 DAT-Ires-Cre+ mice, we examined locomotor activity of B6 DAT-Ires-Cre+ and littermate B6 DAT-Ires-Cre− control mice in the same paradigm described above. Similar to the 129S6-B6 DAT-Ires-Cre strain (Fig 1b), a curve fit analysis over the 30 min evaluated period indicated that B6 DAT-Ires-Cre+ are hyperlocomotive in a novel environment [F (4, 106) = 3.820, P = 0.0061, Fig. 2b] and have normal habituation of their locomotor response during the novelty testing session [2-way RM ANOVA; time F (3.397, 57.75) =36, P < 0.0001, genotype F (1, 17) =3.389, P = 0.0832, time x genotype interaction F (5, 85) =0.2600, P = 0.9336, Fig. 2b]. Further, both groups displayed habituation of locomotor response across novelty and the (first) saline testing days (see Supplementary materials Fig. 1c). Further, 3-way ANOVA revealed a main effect of genotype [F (1, 15) = 6.320, P = 0.0238] and time [F (3.282, 49.22) = 27.71, P < 0.0001]. No main effect of sex [F (1, 15) = 3.603, P = 0.0771, n = 3(Cre−, males); 7(Cre+, males); 6(Cre−, females); 3(Cre+, females)], sex by genotype interaction [F (1, 15) = 0.2031, P = 0.6587] or a 3-way interaction [F (5, 75) = 0.5742, P = 0.7195] was found. Evaluation of center activity revealed no genotypic differences over the 30 min session; however, B6 DAT-Ires-Cre+ made fewer visits, spent less time and traveled less distance during the first 5 mins, thus displaying an anxiety-like phenotype during this initial period of exposure (Supplementary materials Fig. 3b, d, f).
Fig. 2. B6 DAT-Ires-Cre+ mice display novelty-induced hyperactivity and attenuated AMPH-induced locomotion but show preserved AMPH- and SKF-38393-induced stereotypic and grooming behaviors.
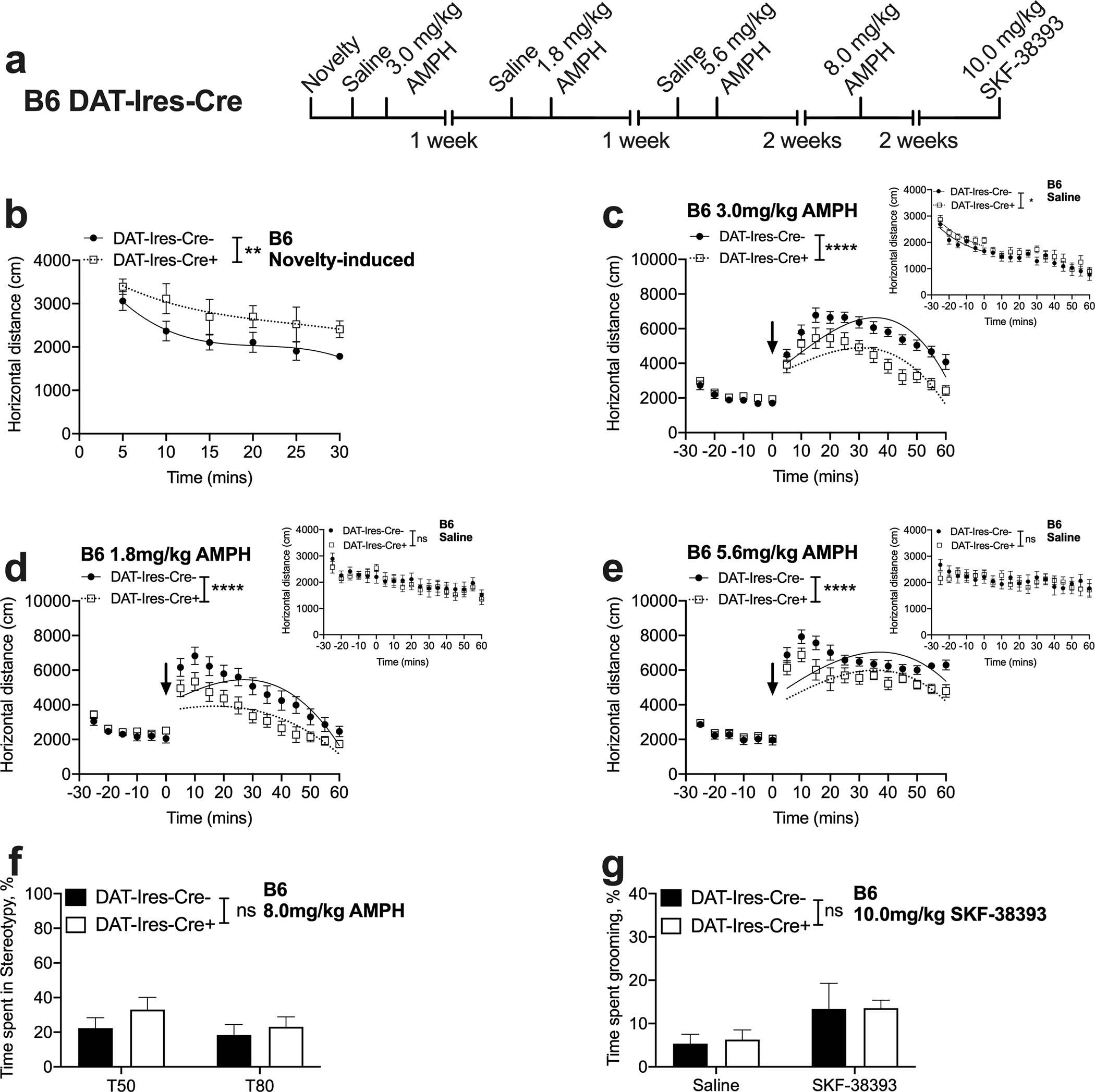
(a) Experimental timeline. (b) B6 DAT-Ires-Cre+ mice show increased novelty-induced locomotion compared to B6 DAT-Ires-Cre− control mice [curve–fit analysis, t = 0–30; F (4, 106) = 3.820, **P = 0.0061, n = 9–10] while displaying habituation to the open field chamber [2-way RM ANOVA; time F (3.397, 57.75) = 36, P < 0.0001, genotype F (1, 17) =3.389, P = 0.0832, time x genotype interaction F (5, 85) = 0.2600, P = 0.9336, n = 9–10]. (c, d, e) B6 DAT-Ires-Cre+ mice show attenuated locomotor responses to low (1.8), moderate (3.0) and high (5.6 mg/kg) doses of AMPH compared to B6 DAT-Ires-Cre− control mice [curve–fit analysis, t = 5–60; low: F (4, 220) = 15.63, ****P < 0.0001; moderate: F (4, 220) = 22.13, ****P < 0.0001; high: F (4, 220) = 13.23, ****P < 0.0001, n = 9–10]. (f) B6 DAT-Ires-Cre+ mice show AMPH-induced stereotyped behavior that is comparable to B6 DAT-Ires-Cre− mice following 8.0 mg/kg AMPH [2-way RM ANOVA; genotype F (1, 7) = 0.9980, P = 0.3511, time F (1, 7) =2.074, P = 0.1930, genotype by time interaction F (1, 7) =0.3762, P = 0.5590, n = 4–5]. (g) SKF-38393 induced grooming behavior is not altered in B6 DAT-Ires-Cre+ mice [2-way RM ANOVA; drug, F(1, 7) = 17.67, P = 0.0040; genotype, F(1, 7) = 0.01774, P = 0.8978, drug x genotype interaction F (1, 7) = 0.04358, P = 0.8406, n = 4–5].
To examine baseline locomotion in B6 DAT-Ires-Cre+ mice, we examined locomotor activity of saline injected mice as described above. A curve fit analysis revealed that B6 DAT-Ires-Cre+ mice continued to be hyperlocomotive during the pre-saline injection acclimatization period [curve–fit analysis, F (4, 106) = 3.167, P = 0.0168, Fig. 2c, inset]; however, post-saline injection there was a mitigation of this effect [curve–fit analysis, F (4, 220) = 1.173, P = 0.3235, Fig. 2c, inset]. A repeated measures ANOVA over the post-injection period revealed an absence of main effect of genotype and an absence of time by genotype interaction, suggesting that B6 DAT-Ires-Cre+ mice did not differ from B6 DAT-Ires-Cre− control mice in a familiar environment [2-way RM ANOVA; time F (5.771, 98.10) = 8.785, P < 0.0001, genotype F (1, 17) = 0.6434, P = 0.4336, time x genotype interaction F (11, 187) =0.7518, P = 0.6876, Fig. 2c, inset]. Similarly, a lack of genotypic difference was observed during both pre- and post-saline injection periods on the second [curve–fit analysis, second pre-saline: F (4, 106) = 0.9839, P = 0.4196; second post-saline: F (4, 220) = 0.3801, P = 0.8227, Fig. 2d, inset] and third [curve–fit analysis, third pre-saline: F (4, 106) = 1.062, P = 0.3789; third post-saline: F (4, 220) = 0.1845, P = 0.9463, Fig. 2e, inset] saline exposure days. Further, 3-way ANOVA comparing locomotor activity over this time period on the first saline day revealed a main effect of time [F (5.794, 86.91) = 25.86, P < 0.0001] but no main effect of genotype [F (1, 15) = 3.194, P = 0.0941]. No main effect of sex [F (1, 18) = 1.618, P = 0.2196], sex by genotype interaction [F (1, 18) = 0.05876, P = 0.8112] or a 3-way interaction [F (17, 306) = 0.5361, P = 0.9338] was observed.
B6 DAT-Ires-Cre+ display a blunted locomotor response to AMPH compared to WT controls
Next, we evaluated the locomotor response of B6 DAT-Ires-Cre+ mice to a low (1.8), moderate (3.0) and a high (5.6 mg/kg) IP dose of AMPH. At the low dose, AMPH administration after an acclimatization period of 30 mins resulted in significantly attenuated locomotor activation in B6 DAT-Ires-Cre+ mice relative to B6 DAT-Ires-Cre− control mice [curve-fit analysis, F (4, 220) = 15.63, P < 0.0001, Fig. 2d]. At the moderate dose this effect became even more prominent [curve-fit analysis, F (4, 220) = 22.13, P < 0.0001, Fig. 2c]. The attenuated response persisted at the highest dose tested [curve-fit analysis, F (4, 220) = 13.23, P < 0.0001, Fig. 2e], confirming that B6 DAT-Ires-Cre+ mice have a reduced sensitivity to AMPH’s locomotor stimulating effects. Importantly, and in contrast to the 129S6-B6 strain (Fig 1d), no genotype differences were seen in pre-AMPH injection locomotor activity on AMPH testing days [curve-fit analysis, 1.8 mg/kg: F (4, 106) = 1.513, P = 0.2037, Fig. 2d; 3.0 mg/kg: F (4, 106) = 1.181, P = 0.3236, Fig. 2c; 5.6 mg/kg: F (4, 106) = 0.2838, P = 0.8879, Fig. 2e]. At all the three doses tested, 3-way ANOVAs revealed a time by genotype interaction [1.8 mg/kg: F (17, 255) = 3.526, P < 0.0001; 3.0 mg/kg: F (17, 255) = 8.065, P < 0.0001; 5.6 mg/kg: F (17, 255) = 2.886, P = 0.0002] but no sex by genotype interaction [1.8 mg/kg: F (1, 15) = 0.01772, P = 0.8959; 3.0 mg/kg: F (1, 15) = 0.7466, P = 0.4012; 5.6 mg/kg: F (1, 15) = 0.1485, P = 0.7054].
B6 DAT-Ires-Cre+ mice display normal AMPH-induced stereotypic behavior and SKF-38393 induced grooming
We next examined AMPH’s ability to induce stereotypic behavior by testing 2 doses at which stereotypic behavior dominates locomotor activation (8.0 and 10.0 mg/kg). Video scoring at 50- and 80-min post 8.0 mg/kg AMPH revealed no main effect of genotype [2-way RM ANOVA; genotype F (1, 7) =0.9980, P = 0.3511, time F (1, 7) =2.074, P = 0.1930, time by genotype interaction F (1, 7) = 0.3762, P = 0.5590, Fig. 2f], suggesting preservation of AMPH’s stimulatory effects at higher doses in B6 DAT-Ires-Cre+ mice. Further, 3-way ANOVA to determine the effects of genotype, time and sex ruled out a sex by genotype interaction [F (1, 5) = 3.013, P = 0.1431] or a main effect of sex [F (1, 5) = 0.7375, P = 0.4297]. A similar lack of genotype effect was seen at the 10.0 mg/kg dose (see Supplementary materials Fig. 2a). [Note: B6 mice display an overall lower degree of stereotypic behavior than 129S6-B6 mice at the 8.0 mg/kg IP dose, which likely indicates a strain effect].
Next, we examined SKF-38393 induced grooming in the B6 strain. Video scoring of grooming behavior in B6 DAT-Ires-Cre+ mice revealed a significant main effect of drug but no main effect of genotype [2-way RM ANOVA; drug, F (1, 7) = 17.76, P = 0.0040; genotype, F (1, 7) = 0.01774, P = 0.8978, Fig. 2g] paralleling the lack of genotype difference observed in the 129S6-B6 strain (Fig. 1f). Further, no sex by genotype interaction [F (1, 5) = 1.493, P = 0.2762] or a main effect of sex [F (1, 5) = 0.03511, P = 0.8587] was observed in a 3-way ANOVA.
129S6-B6 TH-Cre mice
Normal novelty-induced and baseline locomotion in 129S6-B6 TH-Cre+ mice
In contrast to 129S6-B6 DAT-Ires-Cre+ mice (Fig. 1b), novelty-induced locomotor response was not altered in 129S6-B6 TH-Cre+ mice [curve-fit analysis, F (4, 166) = 0.7092, P=0.5867, Fig. 3b]. In agreement with the curve-fit analysis, a 3-way ANOVA to determine effects of genotype, time and sex on locomotion revealed no time by genotype interaction [F (5, 125) = 0.09638, P = 0.9926] or main effect of genotype [F (1, 25) = 1.059, P = 0.3132]. Further, no 3-way interaction [F (5, 125) = 1.231, P = 0.2987], sex by genotype interaction [F (1, 25) = 0.7131, P = 0.4064] or a main effect of sex [F (1, 25) = 0.04419, P = 0.8352, n = 11(Cre−, males); 8(Cre+, males); 7(Cre−, females); 3(Cre+, females)] was found. Also, both 129S6-B6 TH-Cre+ and 129S6-B6 TH-Cre− control mice showed habituation of their locomotor response within the novelty testing session (Fig. 3b). No genotypic differences were observed in the number of visits, time spent or distanced traveled in the center of the open field (Supplementary materials Fig. 4a, c, e).
Fig. 3. 129S6-B6 TH-Cre+ exhibit normal novelty-induced activity, baseline locomotion and AMPH- and SKF-38393-induced preservative behaviors but show attenuated AMPH-induced locomotion.
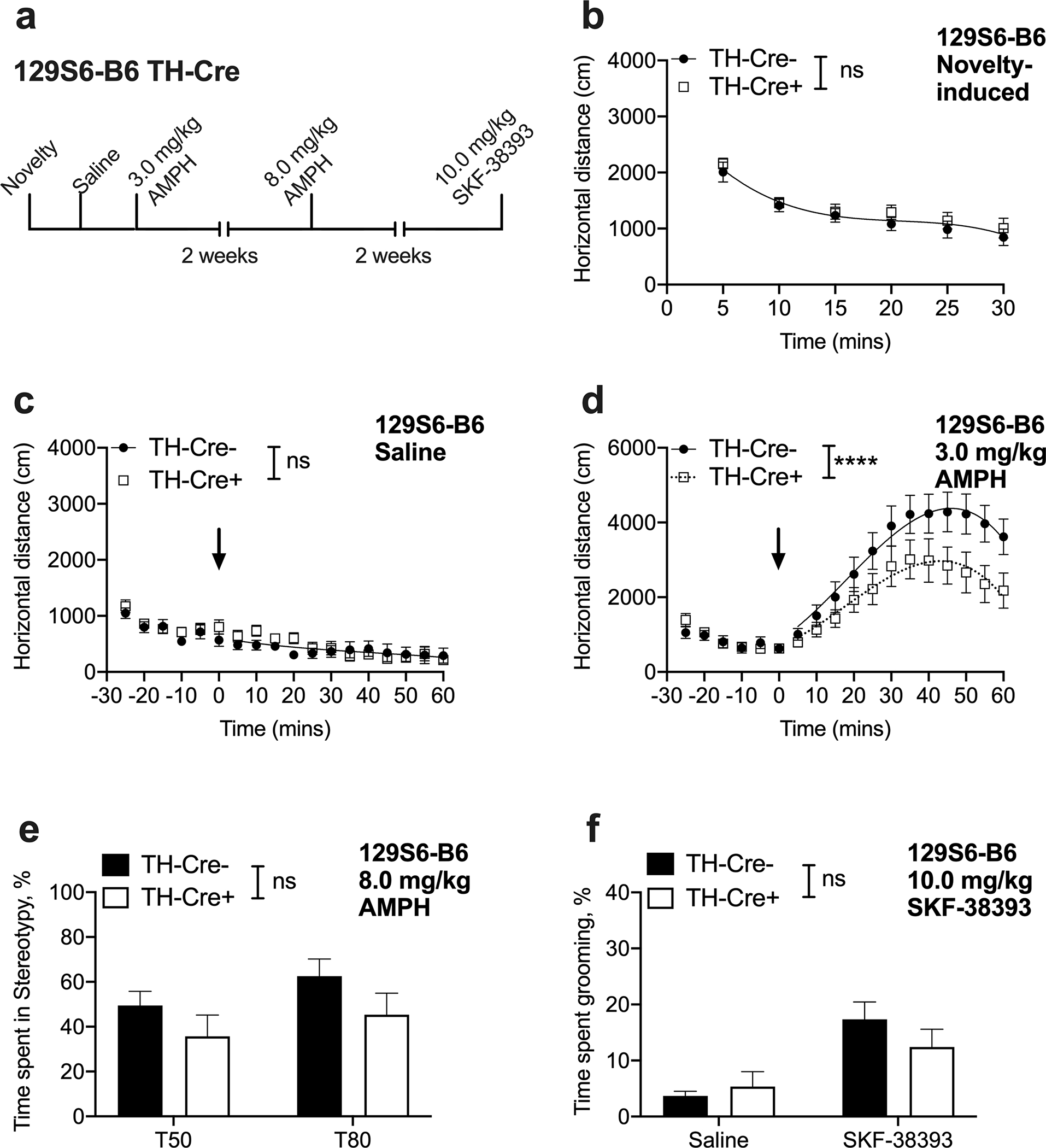
(a) Experimental timeline. (b) Novelty-induced locomotor response is unaltered in 129S6-B6 TH-Cre+ mice [curve–fit analysis, t = 0–30; F (4, 166) = 0.7092, P=0.5867, n = 18–11] and mice display habituation to the open field chamber [2-way RM ANOVA; time F (3.446, 93.05) = 36.10, P < 0.0001, genotype F (1, 27) =0.6412, P = 0.4303, time by genotype interaction F (5, 135) = 0.2016, P = 0.9613 n = 18–11]. (c) Basal locomotor response following saline injection is not altered in 129S6-B6 TH-Cre+ mice [curve–fit analysis, t = 5–60; F (4, 514) = 2.073, P = 0.0831, n = 18–11]. (d) 129S6-B6 TH-Cre+ mice show attenuated locomotor response to a moderate dose (3.0 mg/kg) of AMPH [curve–fit analysis, t = 5–60; F (4, 514) = 12.21, ****P < 0.0001, n = 18–11]. (e) AMPH-induced stereotypic behavior is not altered in 129S6-B6 TH-Cre+ mice at 8.0 mg/kg dose [2-way RM ANOVA; genotype F (1, 27) =1.916, P = 0.1776, time F (1, 27) =10.54, P = 0.0031, genotype x time interaction F (1,27) =0.2391, P = 0.6288, n = 18–11]. (f) SKF-38393-induced grooming behavior is not altered in 129S6-B6 TH-Cre+ mice [2-way RM ANOVA; drug, F (1, 27) = 20.57, P < 0.0001; genotype, F (1, 27) = 0.324, P = 0.5738, drug x genotype interaction F (1, 27) = 2.097, P = 0.1591, n = 18–11].
Again, in contrast to 129S6-B6 DAT-Ires-Cre+ mice (Fig. 1c), baseline locomotor response of saline injected 129S6-B6 TH-Cre+ mice in a familiar environment did not differ from 129S6-B6 TH-Cre− controls on the saline challenge day [curve fit-analysis, F (4, 514) = 2.073, P=0.0831, Fig. 3c], with both the 129S6-B6 TH-Cre+ and 129S6-B6 TH-Cre− groups showing habituation of their locomotor response within the saline testing session [2-way RM ANOVA; time F (5.944, 160.5) =16.28, P < 0.0001, genotype F (1, 27) =0.2843, P = 0.5983, time x genotype interaction F (17, 549) =1.255, P = 0.2179, Fig. 3c] and across the novelty and saline testing days (see Supplementary materials Fig. 1b). No main effect of sex [F (1, 25) = 2.879, P = 0.1021], genotype [F (1, 25) = 0.5968, P = 0.4470] or sex by genotype interaction [F (1, 25) = 0.05033, P = 0.8243] was found on locomotion on saline day using a 3-way ANOVA.
Blunted locomotor response to a moderate dose of AMPH in 129S6-B6 TH-Cre+ mice
Evaluation of the locomotor response of 129S6-B6 TH-Cre+ mice to a 3.0 mg/kg dose of AMPH surprisingly revealed an attenuated response in 129S6-B6 TH-Cre+ mice when compared to the 129S6-B6 TH-Cre− control group [curve–fit analysis, F (4, 514) = 12.21, P < 0.0001, Fig. 3d]. 3-way ANOVA with time as a repeated measure showed a time by genotype interaction [F (17, 425) = 3.621, P < 0.0001], but no sex by genotype interaction [F (1, 25) = 0.5433, P = 0.4679] or 3-way interaction [F (17, 425) = 1.127, P = 0.3246]. Further, no main effect of sex [F (1, 25) = 0.07463, P = 0.7870] was observed, suggesting that both males and females displayed attenuated AMPH response. Importantly, locomotor activity of 129S6-B6 TH-Cre+ mice during pre-AMPH injection acclimatization period did not differ from control mice [curve–fit analysis, t = −25–0; F (4, 166) = 0.7740, P = 0.5436, Fig. 3d].
Normal AMPH-induced stereotypic behavior and SKF-38393 induced grooming in 129S6-B6 TH-Cre+ mice.
We next found that stereotypic behavior in 129S6-B6 TH-Cre+ mice was not different from 129S6-B6 TH-Cre− control mice [2-way RM ANOVA; genotype F (1, 27) =1.916, P = 0.1776, Fig. 3e], suggesting a reduced sensitivity to AMPH only at lower doses in 129S6-B6 TH-Cre+ mice. Further, 3-way ANOVA to determine the effects of genotype, time and sex ruled out a 3 -way interaction [F (1, 25) = 0.9931, P = 0.3285], time by genotype interaction [F (1, 25) = 0.7401, P = 0.3978] and sex by genotype interaction [F (1, 25) = 0.1115, P = 0.7412].
Similarly, SKF-38393 injection to induce perseverative grooming behavior revealed a main effect of drug and an absence of main effect of genotype in 129S6-B6 TH-Cre mouse line [2-way RM ANOVA; drug, F(1, 27) = 20.57, P = 0.0001; genotype, F(1, 27) = 0.3241, P = 0.5738, Fig. 3f], suggesting preserved postsynaptic receptor function in the mixed strain. Further, a 3-way ANOVA revealed no drug by sex by genotype interaction [F (1, 26) = 2.160, P = 0.1536] or a sex by genotype interaction [F (1, 26) = 0.2550, P = 0.6179].
B6 TH-Cre mice
Normal novelty-induced and baseline locomotion in B6 TH-Cre+ mice
Similar to the 129S6-B6 TH-Cre strain (Fig. 3b), novelty-induced locomotor response was not altered in B6 TH-Cre+ mice [curve-fit analysis, F (4, 112) = 1.939, P = 0.1089, Fig. 4b], with both the B6 TH-Cre+ and B6 TH-Cre− control mice displaying habituation of their locomotor response both during the novelty testing session [2-way RM ANOVA; time F (3.593, 64.67) = 49.37, P < 0.0001, genotype F (1, 18) = 1.557, P = 0.2281, time x genotype interaction F (5, 90) = 0.8312, P = 0.5309, Fig. 4b] and across novelty and the first saline testing days (see Supplementary materials Fig. 1d). Further, a 3-way ANOVA with time as a repeated factor revealed no main effect of genotype [F (1, 16) = 0.7351, P = 0.4039], sex [F (1, 16) = 1.221, P = 0.2855, n = 6(Cre−, males); 7(Cre+, males); 3(Cre−, females); 4(Cre+, females)], sex by genotype interaction [F (1, 16) = 0.3901, P = 0.5411] or a 3-way interaction [F (5, 80) = 0.2354, P = 0.9458]. Evaluation of center activity revealed no genotypic differences over the 30 min session (Supplementary materials Fig. 4b, d, f).
Fig. 4. B6 TH-Cre+ mice display normal novelty-induced activity, AMPH-induced locomotion and AMPH and SKF-38393-induced stereotypic and grooming behaviors.
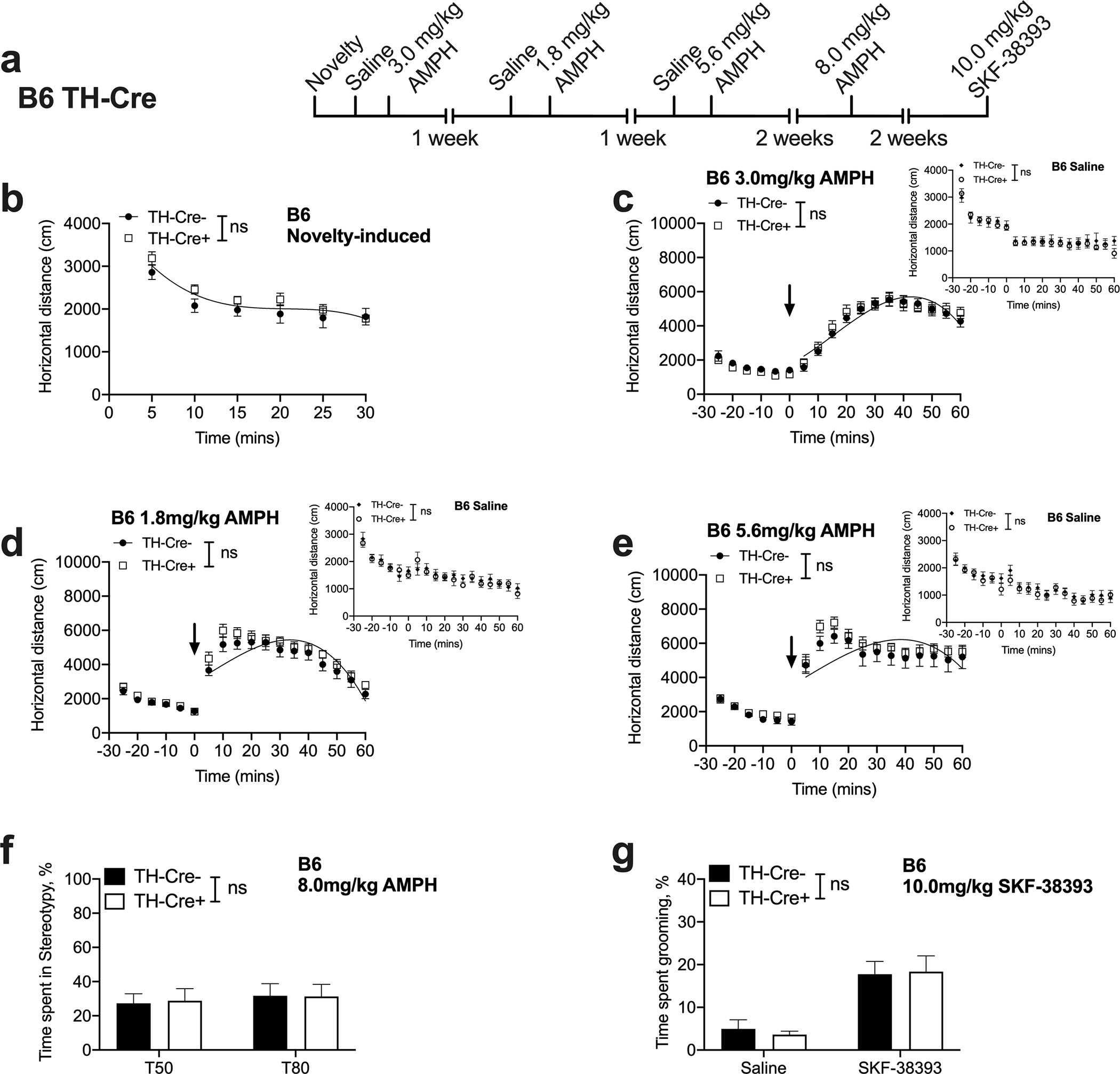
(a) Experimental timeline. (b) B6 TH-Cre+ mice show comparable novelty-induced locomotion to B6 TH-Cre− control mice [curve–fit analysis, t = 0–30; F (4, 112) = 1.939, P = 0.1089, n = 9–11] and display habituation to the open field chamber [B6 TH-Cre, 2-way RM ANOVA; time F (3.593, 64.67) = 49.37, P < 0.0001, genotype F (1, 18) = 1.557, P = 0.2281, time x genotype interaction F (5, 90) = 0.8312, P = 0.5309 n = 9–11]. (c, d, e) Locomotor responses of B6 TH-Cre+ mice to low (1.8), moderate (3.0) and high (5.6 mg/kg) AMPH doses do not differ from B6 TH-Cre− control mice’ responses. [curve–fit analysis, t = 5–60; low: F (4, 232) = 1.950, P = 0.1030; moderate: F (4, 232) = 0.8037, P = 0.5239; high: F (4, 232) = 0.1855, P = 0.9458, n = 9–11]. (f) AMPH-induced stereotyped behavior is not altered in B6 TH-Cre+ mice [2-way RM ANOVA; genotype F (1, 18) = 0.0038, P = 0.9515, time F (1, 18) = 0.7070, P = 0.4115, genotype by time interaction F (1, 18) = 0.05190, P = 0.8224, n = 9–11]. (g) SKF-38393 induced grooming behavior is not altered in B6 TH-Cre+ mice [2-way RM ANOVA; drug, F (1, 18) = 24.74, P < 0.0001; genotype, F (1, 18) = 0.01953, P = 0.8904, drug x genotype interaction F (1, 18) = 0.1207, P = 0.7323, n = 9–11].
Similar to the 129S6-B6 TH-Cre strain (Fig. 3c), basal locomotor response of saline injected B6 TH-Cre+ mice did not differ from B6 TH-Cre− control mice on either the first, second or third saline exposure days [curve–fit analysis, first pre-saline: F (4, 112) = 0.4198, P = 0.7940; first post-saline: F (4, 232) = 0.1278, P = 0.2795, Fig. 4c, inset; second pre-saline: F (4, 112) = 0.3361, P = 0.8531, second post-saline: F (4, 232) = 0.8178, P = 0.5149, Fig. 4d, inset; third pre-saline: F (4, 112) = 0.7267, P = 0.5755, third post-saline: F (4, 232) = 0.8445, P = 0.4982, Fig. 4e, inset]. Additionally, a 3-way ANOVA to assess effects of genotype, time and sex revealed no sex by genotype interaction [F (1, 16) = 0.0047, P = 0.9459], main effect of sex [F (1, 16) = 0.8022, P = 0.3837] or a time by sex by genotype interaction [F (17, 272) = 0.9753, P = 0.4864] on the first saline day.
Normal locomotor response to AMPH in B6 TH-Cre+ mice
Next, we evaluated the locomotor response of B6 TH-Cre+ mice to 1.8, 3.0 and 5.6 mg/kg IP doses of AMPH. In contrast to B6 DAT-Ires-Cre+ mice (Figs. 2c, d, e), B6 TH-Cre+ mice did not differ from B6 TH-Cre− control mice in their locomotor activity at the three AMPH doses tested [curve–fit analysis, t = 5–60; 1.8 mg/kg: F (4, 232) = 1.950, P = 0.1030; 3.0 mg/kg: F (4, 232) = 0.8037, P = 0.5239; 5.6 mg/kg: F (4, 232) = 0.1855, P = 0.9458, Figs. 4c, d, e]. Differences in pre-AMPH injection activity levels were ruled out on all days, suggesting that AMPH sensitivity is preserved in B6 TH-Cre− mice. These data also suggest that the diminished locomotor activation seen in 129S6-B6 TH-Cre+ mice (Fig. 3d) is likely due to a mixed background strain effect. In agreement with the curve fit analysis, 3-way ANOVAs at all three doses revealed no time by genotype interaction [1.8 mg/kg: F (17, 289) = 0.38834, P = 0.9871; 3.0 mg/kg: F (17, 289) = 0.6929, P = 0.8095; 5.6 mg/kg: F (17, 289) = 0.2541, P = 0.9990] or sex by genotype interaction [1.8 mg/kg: F (1, 17) = 0.09503, P = 0.7616; 3.0 mg/kg: F (1, 17) = 0.03386, P = 0.8562; 5.6 mg/kg: F (1, 17) = 0.1563, P = 0.6975] or a main effect of sex [1.8 mg/kg: F (1, 17) = 0.9652, P = 0.3397; 3.0 mg/kg: F (1, 17) = 1.311, P = 0.2681; 5.6 mg/kg: F (1, 17) = 1.470, P = 0.2419].
B6 TH-Cre+ display normal AMPH- and SKF-38393 induced repetitive behavior
Similar to 129S6-B6 TH-Cre strain (Fig 3e), stereotypic behavior in B6 TH-Cre+ mice did not differ from B6 TH-Cre− mice at 8.0 mg/kg [2-way RM ANOVA; genotype F (1, 18) = 0.0038, P = 0.9515, time F (1, 18) = 0.7070, P = 0.4115, time by genotype interaction F (1, 18) = 0.0519, P = 0.8224, Fig. 4f], or 10.0 mg/kg (see Supplementary materials Fig. 2b). Further, 3-way ANOVA to determine the effects of genotype, time and sex ruled out a sex by genotype interaction [F (1, 18) = 0.7159, P = 0.4086] or a main effect of sex [F (1, 18) = 1.586, P = 0.2240]. Taken together, these data suggest that B6 TH-Cre+ mice resemble B6 TH-Cre− mice in their AMPH sensitivity.
Finally, we examined the SKF-38393 induced grooming response in the B6 TH-Cre strain. Similar to 129S6-B6 TH-Cre mice (Fig 3f), a lack of genotypic effect was seen, suggesting that TH-Cre+ mice do not differ from TH-Cre− mice in SKF-38393 induced grooming response [2-way RM ANOVA; drug, F (1, 18) = 24.74, P < 0.0001; genotype, F (1, 18) = 0.01953, P = 0.8904, Fig. 4g]. Further, no drug by genotype interaction [F (1, 21) = 0.07524, P = 0.7865], sex by genotype interaction [F (1, 21) = 0.03033, P = 0.8634] or a main effect of sex [F (1, 21) = 1.517, P = 0.2317] was observed in a 3-way ANOVA.
Discussion
Transgenic mouse lines expressing Cre-recombinase under the regulation of the DAT or TH promotor are frequently used to study the DA system. Our findings point to behavioral phenotypes in DAT-Ires-Cre mice, including increased locomotor activity in a novel environment and decreased responsiveness to the psychostimulant AMPH, a DAT substrate. In contrast, TH-Cre mice bred on a pure C57BL/6 background display unchanged baseline and AMPH-induced behaviors when compared with wildtype controls. Additionally, comparison of the mixed 129S6/C57BL/6 inbred and pure C57BL/6 strains of DAT-Ires-Cre and TH-Cre mice revealed that genetic background affects the transgenic lines’ basal locomotor phenotypes and responses to AMPH (Table 1).
Table 1.
Summary of behavioral phenotypes in DAT-Ires-Cre and TH-Cre mice bred on mixed 129S6/C57BL/6 and pure C57BL/6 inbred strain backgrounds
| Mouse line | DAT-Ires-Cre | TH-Cre | ||
|---|---|---|---|---|
| Background strain | 129S6/C57BL/6 | C57BL/6 | 129S6/C57BL/6 | C57BL/6 |
| Novelty-induced locomotion | ↑ | ↑ | ↔ | ↔ |
| Baseline locomotion | ↑ | ↔ | ↔ | ↔ |
| AMPH- induced locomotion | ↓ | ↓ | ↓ | ↔ |
| AMPH-induced stereotypy | ↓ | ↔ | ↔ | ↔ |
| SKF-38393 induced grooming | ↔ | ↔ | ↔ | ↔ |
revious studies have reported significant phenotypic variation in transgenic and knock-in mouse lines (Chen et al. 2018; Crittenden et al. 2014; Galichet et al. 2010; Kim et al. 2013; Kolisnyk et al. 2013; Kramer et al. 2011; Steinmetz et al. 2017). One of the first knock-in lines targeting the monoaminergic system, the DAT-Cre knock-in mouse line (Zhuang et al. 2005), had one copy of the DAT gene disrupted, which made it comparable to hemizygous DAT knockout mice known to exhibit profound alterations in DAT function (Giros et al. 1996; Jones et al. 1998a; Spielewoy et al. 2001; Spielewoy et al. 2000). The DAT-Ires-Cre mouse line was developed to avoid this disruption of the DAT gene; however, DAT protein levels were found to be reduced by 47% in homozygous mice and by 17% in heterozygotes (Backman et al. 2006). Strikingly, the modest reduction of DAT protein levels in heterozygotes was associated with significantly reduced DA uptake capacity (O’Neill et al. 2017).
Physiologically, a decrease in DAT function should increase DA tone, and subsequently alter DA-mediated behaviors. Congruent with this, mice with deficient DAT function display increased DA tone and a basal hyperlocomotor phenotype. Specifically, while DAT knockout and knockdown mice (expressing 0% and 10% of wildtype DAT levels, respectively) display spontaneous hyperlocomotion and loss of locomotor habituation (Giros et al. 1996; Tilley et al. 2007; Zhuang et al. 2001), hemizygous DAT knockout and DAT-low expressor mice (both expressing 30% of wildtype DAT levels) still show basal hyperlocomotion (Rao et al. 2013) and loss of locomotor habituation (Rao et al. 2013; Spielewoy et al. 2000). Further, cocaine-insensitive DAT knock-in mice that have a 50% reduction of DAT activity display spontaneous hyperactivity (Chen et al. 2006). Our results reveal that DAT-Ires-Cre mice, with a previously described reduction in DAT expression and function (Backman et al. 2006; O’Neill et al. 2017), are hyperlocomotive in a novel environment but show preserved habituation of locomotor activity, indicating that the Ires-Cre transgene impacts DAT-mediated behaviors. It is conceivable that increases in DA tone lead to increases in novelty-induced behavior in the presence of preserved habituation of locomotor activity, as has been recently shown in mice following chemogenetic activation of DA neurons (Runegaard et al. 2018). Moreover, it is also possible that an interplay of DA and adaptation of other neurotransmitter systems mediates our observed novelty-driven hyperactivity (Gainetdinov et al. 1999), thus requiring further examination of the underlying mechanisms.
Our results also show that DAT-Ires-Cre mice display attenuated locomotor activation to AMPH. DAT is a well-known target for AMPH, mediating DA release by reverse DAT-mediated transport (Freyberg et al. 2016; Kahlig et al. 2005; Sulzer et al. 1995). Accordingly, AMPH is unable to affect extracellular DA levels in DAT knockout mice (Jones et al. 1998b) and induces a paradoxical hypolocomotor effect (Giros et al. 1996; Spielewoy et al. 2001; Zhuang et al. 2001) and reduced stereotypic behavior (Spielewoy et al. 2001) in mice with deficient DAT function. A reduction of 40% in DAT activity that follows small interfering RNA (siRNA) injections in wildtype mice translates into roughly 40% reduced level of locomotor activity post-AMPH (Salahpour et al. 2007). Here, in DAT-Ires-Cre mice, we similarly see a blunting of post-AMPH activity that parallels the degree of reduction in DAT expression and function (Backman et al. 2006; O’Neill et al. 2017). The blunted response to AMPH may most easily be explained by a less than optimal DAT-mediated DA efflux, but other mechanisms are also possible, including adaptations in serotonergic-DA interactions (Gainetdinov et al. 1999), possible toxic effects of Cre (Forni et al. 2006; Kim et al. 2013; Schmidt et al. 2000; Schmidt-Supprian and Rajewsky 2007), or changes in pre- or post-synaptic receptor sensitivity to DA. However, we ruled out changes in D1 function in our SKF-38393 induced grooming assay.
One of the events downstream of DAT-mediated AMPH uptake is internalization of excitatory amino acid transporter 3 (EAAT3, encoded by Slc1a1) from the neuronal surface, reducing glutamate clearance and increasing excitatory transmission (Li et al. 2017; Underhill et al. 2019; Underhill et al. 2014). We previously found that loss of Slc1a1/EAAT3 in mice leads to diminished AMPH-induced DA release, locomotor activity and stereotypic behavior (Zike et al. 2017). We anticipated that DAT-Ires-Cre animals would be a suitable tool for rescue of Slc1a1/EAAT3 expression in Slc1a1-STOP mice to complement our previous data with viral rescue (Zike et al. 2017). However, our findings indicate that the presence of an intrinsically diminished AMPH response in DAT-Ires-Cre mice would significantly confound DAT-Ires-Cre-mediated EAAT3 rescue results. Moreover, our findings call for caution in interpreting data from experiments that are dependent on DAT expression and employ DAT-Ires-Cre or DAT-Cre manipulations (Bariselli et al. 2018; Bello et al. 2011; Beutler et al. 2011; Diaz-Ruiz et al. 2012; Kosillo et al. 2019; Luo et al. 2010; McCall et al. 2019; Zweifel et al. 2008).
While the locomotor phenotypes in both the mixed 129S6/C57BL/6 inbred and pure C57BL/6 strains of DAT-Ires-Cre+ mice were found to be largely comparable, a few differences were observed. Specifically, we found that inbred 129S6-B6 DAT-Ires-Cre+ mice displayed low anxiety-like behavior in a novel environment and displayed sustained basal hyperactivity in familiar environments. In contrast, B6 DAT-Ires-Cre+ mice displayed an initial anxiogenic phenotype in a novel phenotype; further, the locomotor activity became indistinguishable from wildtype siblings in a familiar environment. A low anxiety-like phenotype has previously been reported in DAT knockout mice and mice with reduced DAT expression (Carpenter et al. 2012; Pogorelov et al. 2005; Tian et al. 2010); whereas DAT knockout mice were also shown to exhibit an initial anxiety-like phenotype during the first five minutes in an elevated zero maze (Pogorelov et al. 2005). In parallel to the exaggerated basal hyperactivity, a significantly reduced AMPH-induced stereotypic behavior was found in 129S6-B6 DAT-Ires-Cre+ mice, an effect that was absent in the B6 mice. Notably, the degree of stereotypic behavior also differed across the two strains, with the mixed 129-B6 strain showing increased stereotypic movements at 8.0 mg/kg, consistent with the generally reduced levels of locomotion exhibited by this strain (O’Neill and Gu 2013). No genotypic differences were seen in D1-agonist (SKF-38393) induced grooming response in either of the strains. A lack of difference in grooming behavior in 129-B6 DAT-Ires-Cre+ mice is notable as the increased basal locomotion would predict chronically elevated DA levels with consequent downregulation of D1 receptor function. However, hyperdopaminergia is known to induce variable and contrasting effects on D1 receptor mRNA levels depending on the degree and duration of increased DA tone (Choi and Ronnekleiv 1996; Leslie et al. 1994). Our findings are consistent with the lack of changes in plasma membrane D1 receptor expression previously reported in hemizygous DAT knockout mice that have two-fold increase in DA levels (Dumartin et al. 2000), as well as the lack of changes in D1 receptor mRNA levels previously reported in DAT-Ires-Cre mice (Backman et al. 2006). Strain differences are well-known to affect development of behavioral profiles, which are accentuated in genetically manipulated mice (Abramov et al. 2008; Crabbe et al. 1999; Crusio 2004; Gerlai 1996; O’Neill and Gu 2013; Rodgers et al. 2002; Voikar et al. 2004). Taken together, these data highlight the importance of including true wildtype sibling controls in experimental design.
Our current work also characterized a prominent TH-Cre transgenic mouse line (Savitt et al. 2005), which was recently reported to exhibit normal levels of DAT and other DA-related markers (Runegaard et al. 2017). Our results in the pure B6 strain are consistent with the previously reported lack of basal locomotor phenotype in these mice. Further, we now show that TH-Cre mice exhibit normal AMPH-induced locomotor activity and stereotypic behavior, as well as normal SKF-38393-induced grooming behavior. However, we did find a blunted response to a moderate dose of AMPH in TH-Cre+ mice in the mixed 129S6-B6 inbred background strain that is likely to be a strain effect. Though this study found a lack of baseline and drug-induced phenotypes, TH-Cre mice have previously been shown to display substantial ectopic expression in TH-immunonegative neurons (Lammel et al. 2015; Lindeberg et al. 2004; Savitt et al. 2005; Vuong et al. 2015; Yamaguchi et al. 2015), which could counter their utility as an appropriate genetic tool to achieve DA selectivity in optogenetics, chemogenetics or circuit tracing studies (Lammel et al. 2015; Stuber et al. 2015). In contrast, the DAT-Ires-Cre driver does not appear to drive recombination in TH-immunonegative neurons in the midbrain (Backman et al. 2006; Zhuang et al. 2005); however recent evidence has suggested ectopic DAT-Ires-Cre driven reporter expression in some limbic regions (Nouri and Awatramani 2017; Papathanou et al. 2019; Soden et al. 2016). Further, low DAT expression levels in DA neurons that project to the medial prefrontal cortex may result in less efficient recombination in this population (Lammel et al. 2008). Notwithstanding the reduced DAT function described above, the DAT-Ires-Cre transgenic line still appears be an important genetic tool for cell identification and in circuit tracing studies, particularly when selectivity could be further enhanced by employing projection-specific retrograde or intersectional approaches (Beier et al. 2015; Lerner et al. 2015).
In summary, our behavioral characterization of two different strains of two of the mostly widely used DA Cre-driver lines uncovered baseline and psychostimulant-induced behavioral phenotypes (Table 1). In particular, our findings indicate that DAT-Ires-Cre may not be the ideal transgenic line for experiments that are dependent on DAT expression. With substantial non-DA neuron expression (Cardozo Pinto et al. 2019; Lammel et al. 2015), TH-Cre mice may also not be ideal, but they may be preferable for DAT-dependent behaviors because they lack baseline and drug-induced phenotypes. Investigators may also want to consider alternate approaches, such as the use of viruses (Gompf et al. 2015; Stauffer et al. 2016) or the recently generated improved bacterial artificial chromosome (BAC)-transgenic mice (Kaiser et al. 2016; Krol et al. 2019; Ting and Feng 2014) that lack Cre sequence insertions into the endogenous gene’s UTR while avoiding the confounds of overexpression of extra genes seen in traditional BAC transgenic lines (Chen et al. 2018; Crittenden et al. 2014; Kramer et al. 2011). Finally, the most important consideration in experimental design should always be to include the appropriate behavioral controls.
Supplementary Material
Acknowledgements
This work was funded by NIH Grant MH114296 (to SA and JV).
Footnotes
Conflict of interest
JV has served on advisory boards for Roche, Novartis, and SynapDx. He has received research funding from Roche, Novartis, Forest, Janssen, SynapDx, and Seaside Therapeutics. He has received stipends for editorial work from Wiley and Springer. The other authors declare no biomedical conflicts of interest.
References
- Abramov U, Puussaar T, Raud S, Kurrikoff K, Vasar E (2008) Behavioural differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment Neurosci Lett 443:223–227 doi: 10.1016/j.neulet.2008.07.075 [DOI] [PubMed] [Google Scholar]
- Augood SJ, Westmore K, McKenna PJ, Emson PC (1993) Co-expression of dopamine transporter mRNA and tyrosine hydroxylase mRNA in ventral mesencephalic neurones Brain Res Mol Brain Res 20:328–334 doi: 10.1016/0169-328x(93)90059-x [DOI] [PubMed] [Google Scholar]
- Backman CM et al. (2006) Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus Genesis 44:383–390 doi: 10.1002/dvg.20228 [DOI] [PubMed] [Google Scholar]
- Bariselli S, Hornberg H, Prevost-Solie C, Musardo S, Hatstatt-Burkle L, Scheiffele P, Bellone C (2018) Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction Nat Commun 9:3173 doi: 10.1038/s41467-018-05382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT et al. (2015) Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping Cell 162:622–634 doi: 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP et al. (2011) Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors Nat Neurosci 14:1033–1038 doi: 10.1038/nn.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018) What does dopamine mean? Nat Neurosci 21:787–793 doi: 10.1038/s41593-018-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD (2011) Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization Proc Natl Acad Sci U S A 108:4206–4211 doi: 10.1073/pnas.1101424108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update Trends Neurosci 30:194–202 doi: 10.1016/j.tins.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM (2004) Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice Dev Cell 6:7–28 doi: 10.1016/s1534-5807(03)00399-x [DOI] [PubMed] [Google Scholar]
- Cardozo Pinto DF et al. (2019) Characterization of transgenic mouse models targeting neuromodulatory systems reveals organizational principles of the dorsal raphe Nat Commun 10:4633 doi: 10.1038/s41467-019-12392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Saborido TP, Stanwood GD (2012) Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice Dev Neurosci 34:250–257 doi: 10.1159/000336824 [DOI] [PubMed] [Google Scholar]
- Chaudhury D et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons Nature 493:532–536 doi: 10.1038/nature11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, Fowler CD (2018) Altered Baseline and Nicotine-Mediated Behavioral and Cholinergic Profiles in ChAT-Cre Mouse Lines J Neurosci 38:2177–2188 doi: 10.1523/JNEUROSCI.1433-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R et al. (2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter Proc Natl Acad Sci U S A 103:9333–9338 doi: 10.1073/pnas.0600905103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Ronnekleiv OK (1996) Effects of in utero cocaine exposure on the expression of mRNAS encoding the dopamine transporter and the D1, D2 and D5 dopamine receptor subtypes in fetal rhesus monkey Brain Res Dev Brain Res 96:249–260 doi: 10.1016/0165-3806(96)00123-x [DOI] [PubMed] [Google Scholar]
- Ciliax BJ et al. (1995) The dopamine transporter: immunochemical characterization and localization in brain J Neurosci 15:1714–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area Nature 482:85–88 doi: 10.1038/nature10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins DA, Butts K, Young AH (2009) The role of dopamine in bipolar disorder Bipolar Disord 11:787–806 doi: 10.1111/j.1399-5618.2009.00760.x [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment Science 284:1670–1672 doi: 10.1126/science.284.5420.1670 [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Lacey CJ, Lee T, Bowden HA, Graybiel AM (2014) Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice Front Neural Circuits 8:57 doi: 10.3389/fncir.2014.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE (2004) Flanking gene and genetic background problems in genetically manipulated mice Biol Psychiatry 56:381–385 doi: 10.1016/j.biopsych.2003.12.026 [DOI] [PubMed] [Google Scholar]
- da Silva JA, Tecuapetla F, Paixao V, Costa RM (2018) Dopamine neuron activity before action initiation gates and invigorates future movements Nature 554:244–248 doi: 10.1038/nature25457 [DOI] [PubMed] [Google Scholar]
- Dabney W, Kurth-Nelson Z, Uchida N, Starkweather CK, Hassabis D, Munos R, Botvinick M (2020) A distributional code for value in dopamine-based reinforcement learning Nature 577:671–675 doi: 10.1038/s41586-019-1924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D et al. (2013) Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder Eur Neuropsychopharmacol 23:1423–1431 doi: 10.1016/j.euroneuro.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Denys D, Zohar J, Westenberg HG (2004) The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence J Clin Psychiatry 65 Suppl 14:11–17 [PubMed] [Google Scholar]
- Diaz-Ruiz O et al. (2012) Attenuated response to methamphetamine sensitization and deficits in motor learning and memory after selective deletion of beta-catenin in dopamine neurons Learn Mem 19:341–350 doi: 10.1101/lm.026716.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumartin B, Jaber M, Gonon F, Caron MG, Giros B, Bloch B (2000) Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice Proc Natl Acad Sci U S A 97:1879–1884 doi: 10.1073/pnas.97.4.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM (2001) Dendrodendritic inhibition through reversal of dopamine transport Science 293:2465–2470 doi: 10.1126/science.1060645 [DOI] [PubMed] [Google Scholar]
- Forni PE et al. (2006) High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly J Neurosci 26:9593–9602 doi: 10.1523/JNEUROSCI.2815-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z et al. (2016) Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain Nat Commun 7:10652 doi: 10.1038/ncomms10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK et al. (2014) Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience Science 344:313–319 doi: 10.1126/science.1249240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity Science 283:397–401 doi: 10.1126/science.283.5400.397 [DOI] [PubMed] [Google Scholar]
- Galichet C, Lovell-Badge R, Rizzoti K (2010) Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland PLoS One 5:e11443 doi: 10.1371/journal.pone.0011443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R (1996) Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci 19:177–181 doi: 10.1016/s0166-2236(96)20020-7 [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG (1993) Molecular characterization of the dopamine transporter Trends Pharmacol Sci 14:43–49 doi: 10.1016/0165-6147(93)90029-j [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter Nature 379:606–612 doi: 10.1038/379606a0 [DOI] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE (2015) Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals Front Behav Neurosci 9:152 doi: 10.3389/fnbeh.2015.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA et al. (2014) Natural neural projection dynamics underlying social behavior Cell 157:1535–1551 doi: 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ et al. (2013) De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder Mol Psychiatry 18:1315–1323 doi: 10.1038/mp.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1976) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mes- and diencephalon Med Biol 54:427–453 [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1977) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon Med Biol 55:21–40 [PubMed] [Google Scholar]
- Jin X, Costa RM (2010) Start/stop signals emerge in nigrostriatal circuits during sequence learning Nature 466:457–462 doi: 10.1038/nature09263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998a) Profound neuronal plasticity in response to inactivation of the dopamine transporter Proc Natl Acad Sci U S A 95:4029–4034 doi: 10.1073/pnas.95.7.4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998b) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter J Neurosci 18:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel Proc Natl Acad Sci U S A 102:3495–3500 doi: 10.1073/pnas.0407737102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser T, Ting JT, Monteiro P, Feng G (2016) Transgenic labeling of parvalbumin-expressing neurons with tdTomato Neuroscience 321:236–245 doi: 10.1016/j.neuroscience.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Richards K, Heng J, Petrou S, Reid CA (2013) Two lines of transgenic mice expressing cre-recombinase exhibit increased seizure susceptibility Epilepsy Res 104:11–16 doi: 10.1016/j.eplepsyres.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Kolisnyk B et al. (2013) ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains J Neurosci 33:10427–10438 doi: 10.1523/JNEUROSCI.0395-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P et al. (2019) Tsc1-mTORC1 signaling controls striatal dopamine release and cognitive flexibility Nat Commun 10:5426 doi: 10.1038/s41467-019-13396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PF et al. (2011) Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice J Neurosci 31:126–132 doi: 10.1523/JNEUROSCI.4287-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A, Lopez-Huerta VG, Corey TEC, Deisseroth K, Ting JT, Feng G (2019) Two eARCHT3.0 Lines for Optogenetic Silencing of Dopaminergic and Serotonergic Neurons Front Neural Circuits 13:4 doi: 10.3389/fncir.2019.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system Neuron 57:760–773 doi: 10.1016/j.neuron.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Lammel S et al. (2012) Input-specific control of reward and aversion in the ventral tegmental area Nature 491:212–217 doi: 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC (2015) Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons Neuron 85:429–438 doi: 10.1016/j.neuron.2014.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K et al. (2020) Temporally restricted dopaminergic control of reward-conditioned movements Nat Neurosci 23:209–216 doi: 10.1038/s41593-019-0567-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN et al. (2015) Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits Cell 162:635–647 doi: 10.1016/j.cell.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Jung AB, Liebermann J, Bennett JP Jr. (1994) Effects of prenatal cocaine exposure upon postnatal development of neostriatal dopaminergic function Synapse 17:210–215 doi: 10.1002/syn.890170311 [DOI] [PubMed] [Google Scholar]
- Li MH, Underhill SM, Reed C, Phillips TJ, Amara SG, Ingram SL (2017) Amphetamine and Methamphetamine Increase NMDAR-GluN2B Synaptic Currents in Midbrain Dopamine Neurons Neuropsychopharmacology 42:1539–1547 doi: 10.1038/npp.2016.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T (2004) Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus Genesis 40:67–73 doi: 10.1002/gene.20065 [DOI] [PubMed] [Google Scholar]
- Luo Y et al. (2010) NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization PLoS One 5:e12141 doi: 10.1371/journal.pone.0012141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C (2016) The Emergence of a Circuit Model for Addiction Annu Rev Neurosci 39:257–276 doi: 10.1146/annurev-neuro-070815-013920 [DOI] [PubMed] [Google Scholar]
- McCall NM, Marron Fernandez de Velasco E, Wickman K (2019) GIRK Channel Activity in Dopamine Neurons of the Ventral Tegmental Area Bidirectionally Regulates Behavioral Sensitivity to Cocaine J Neurosci 39:3600–3610 doi: 10.1523/JNEUROSCI.3101-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon RA, Abi-Dargham A, Howes OD (2019) Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms Trends Neurosci 42:205–220 doi: 10.1016/j.tins.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour Nat Rev Neurosci 18:73–85 doi: 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- Neale BM et al. (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders Nature 485:242–245 doi: 10.1038/nature11011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri N, Awatramani R (2017) A novel floor plate boundary defined by adjacent En1 and Dbx1 microdomains distinguishes midbrain dopamine and hypothalamic neurons Development 144:916–927 doi: 10.1242/dev.144949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Gu HH (2013) Amphetamine-induced locomotion in a hyperdopaminergic ADHD mouse model depends on genetic background Pharmacol Biochem Behav 103:455–459 doi: 10.1016/j.pbb.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Patel JC, Rice ME (2017) Characterization of Optically and Electrically Evoked Dopamine Release in Striatal Slices from Digenic Knock-in Mice with DAT-Driven Expression of Channelrhodopsin ACS Chem Neurosci 8:310–319 doi: 10.1021/acschemneuro.6b00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA et al. (2017) Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy Mov Disord 32:1264–1310 doi: 10.1002/mds.27115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanou M, Dumas S, Pettersson H, Olson L, Wallen-Mackenzie A (2019) Off-Target Effects in Transgenic Mice: Characterization of Dopamine Transporter (DAT)-Cre Transgenic Mouse Lines Exposes Multiple Non-Dopaminergic Neuronal Clusters Available for Selective Targeting within Limbic Neurocircuitry eNeuro 6 doi: 10.1523/ENEURO.0198-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T et al. (2018) Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors Science 360 doi: 10.1126/science.aat4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC (2005) Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene Neuropsychopharmacology 30:1818–1831 doi: 10.1038/sj.npp.1300724 [DOI] [PubMed] [Google Scholar]
- Pupe S, Wallen-Mackenzie A (2015) Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA Trends Neurosci 38:375–386 doi: 10.1016/j.tins.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Rao A, Sorkin A, Zahniser NR (2013) Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness Synapse 67:668–677 doi: 10.1002/syn.21671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A (2002) Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour Physiol Behav 77:301–310 doi: 10.1016/s0031-9384(02)00856-9 [DOI] [PubMed] [Google Scholar]
- Roeper J (2013) Dissecting the diversity of midbrain dopamine neurons Trends Neurosci 36:336–342 doi: 10.1016/j.tins.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Runegaard AH, Jensen KL, Fitzpatrick CM, Dencker D, Weikop P, Gether U, Rickhag M (2017) Preserved dopaminergic homeostasis and dopamine-related behaviour in hemizygous TH-Cre mice Eur J Neurosci 45:121–128 doi: 10.1111/ejn.13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runegaard AH et al. (2018) Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons eNeuro 5 doi: 10.1523/ENEURO.0345-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG (2007) Local knockdown of genes in the brain using small interfering RNA: a phenotypic comparison with knockout animals Biol Psychiatry 61:65–69 doi: 10.1016/j.biopsych.2006.03.020 [DOI] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM (2005) Bcl-x is required for proper development of the mouse substantia nigra J Neurosci 25:6721–6728 doi: 10.1523/JNEUROSCI.0760-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann J et al. (2012) K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration Nat Neurosci 15:1272–1280 doi: 10.1038/nn.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR (2000) Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids Proc Natl Acad Sci U S A 97:13702–13707 doi: 10.1073/pnas.240471297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K (2007) Vagaries of conditional gene targeting Nat Immunol 8:665–668 doi: 10.1038/ni0707-665 [DOI] [PubMed] [Google Scholar]
- Soden ME, Miller SM, Burgeno LM, Phillips PEM, Hnasko TS, Zweifel LS (2016) Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior Cell Rep 16:304–313 doi: 10.1016/j.celrep.2016.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B (2001) Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter Psychopharmacology (Berl) 159:2–9 doi: 10.1007/s002130100901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B (2000) Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice Behav Pharmacol 11:279–290 doi: 10.1097/00008877-200006000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM et al. (2013) A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward Neuron 80:1039–1053 doi: 10.1016/j.neuron.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W (2016) Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques Cell 166:1564–1571 e1566 doi: 10.1016/j.cell.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA et al. (2017) Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines eNeuro 4 doi: 10.1523/ENEURO.0207-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA (2015) Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry Neuron 85:439–445 doi: 10.1016/j.neuron.2014.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport J Neurosci 15:4102–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T et al. (2016) Locus coeruleus and dopaminergic consolidation of everyday memory Nature 537:357–362 doi: 10.1038/nature19325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG (2010) Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice Synapse 64:432–439 doi: 10.1002/syn.20746 [DOI] [PubMed] [Google Scholar]
- Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH (2007) Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression BMC Neurosci 8:42 doi: 10.1186/1471-2202-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Feng G (2014) Recombineering strategies for developing next generation BAC transgenic tools for optogenetics and beyond Front Behav Neurosci 8:111 doi: 10.3389/fnbeh.2014.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA Nature 490:262–266 doi: 10.1038/nature11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning Science 324:1080–1084 doi: 10.1126/science.1168878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ et al. (1996) Subregion- and cell type-restricted gene knockout in mouse brain Cell 87:1317–1326 doi: 10.1016/s0092-8674(00)81826-7 [DOI] [PubMed] [Google Scholar]
- Tye KM et al. (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour Nature 493:537–541 doi: 10.1038/nature11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill SM, Ingram SL, Ahmari SE, Veenstra-VanderWeele J, Amara SG (2019) Neuronal excitatory amino acid transporter EAAT3: Emerging functions in health and disease Neurochem Int 123:69–76 doi: 10.1016/j.neuint.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (2014) Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons Neuron 83:404–416 doi: 10.1016/j.neuron.2014.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons Trends Neurosci 35:422–430 doi: 10.1016/j.tins.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Foster JD (2013) Mechanisms of dopamine transporter regulation in normal and disease states Trends Pharmacol Sci 34:489–496 doi: 10.1016/j.tips.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Vasar E, Rauvala H (2004) Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens Genes Brain Behav 3:27–38 doi: 10.1046/j.1601-183x.2003.0044.x [DOI] [PubMed] [Google Scholar]
- Volkow ND et al. (2007) Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder Arch Gen Psychiatry 64:932–940 doi: 10.1001/archpsyc.64.8.932 [DOI] [PubMed] [Google Scholar]
- Vuong HE, Perez de Sevilla Muller L, Hardi CN, McMahon DG, Brecha NC (2015) Heterogeneous transgene expression in the retinas of the TH-RFP, TH-Cre, TH-BAC-Cre and DAT-Cre mouse lines Neuroscience 307:319–337 doi: 10.1016/j.neuroscience.2015.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ (2011) NMDA receptors in dopaminergic neurons are crucial for habit learning Neuron 72:1055–1066 doi: 10.1016/j.neuron.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area Eur J Neurosci 41:760–772 doi: 10.1111/ejn.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SH, York J, Hyland B, Bunn SJ, Grattan DR (2018) Incomplete concordance of dopamine transporter Cre (DAT(IREScre))-mediated recombination and tyrosine hydroxylase immunoreactivity in the mouse forebrain J Chem Neuroanat 90:40–48 doi: 10.1016/j.jchemneu.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R (2005) Targeted gene expression in dopamine and serotonin neurons of the mouse brain J Neurosci Methods 143:27–32 doi: 10.1016/j.jneumeth.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R (2001) Hyperactivity and impaired response habituation in hyperdopaminergic mice Proc Natl Acad Sci U S A 98:1982–1987 doi: 10.1073/pnas.98.4.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zike ID et al. (2017) OCD candidate gene SLC1A1/EAAT3 impacts basal ganglia-mediated activity and stereotypic behavior Proc Natl Acad Sci U S A 114:5719–5724 doi: 10.1073/pnas.1701736114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD (2008) Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors Neuron 59:486–496 doi: 10.1016/j.neuron.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


