Abstract
Background
α-mannan from Candida albicans reportedly induces Th17-mediated pulmonary graft-versus-host disease (GVHD) in mouse models. This study aimed to evaluate the association between candidemia and noninfectious interstitial pneumonia (IP) in allogeneic hematopoietic cell transplantation (HCT) recipients.
Methods
Using a Japanese transplant registry database, we analyzed 9143 pediatric and adult patients with hematological malignancies who underwent their first (n = 7531) or second (n = 1612) allogeneic HCT between 2009 and 2019.
Results
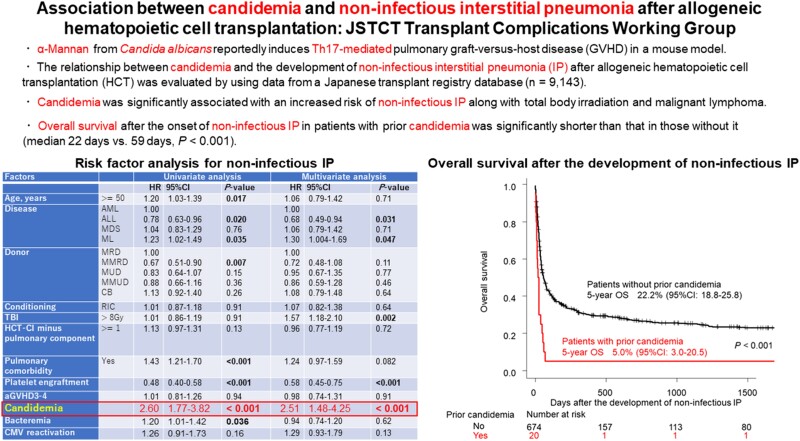
Noninfectious IP was observed in 694 patients at a median (range) of 63 (0–1292) days after HCT. Candidemia occurred in 358 patients at a median (range) of 31 (0–903) days after HCT. Candidemia treated as a time-dependent covariate was significantly associated with an increased incidence of noninfectious IP (hazard ratio [HR], 2.51; 95% CI, 1.48–4.25), along with total body irradiation (>8 Gy; HR, 1.57; 95% CI, 1.18–2.10) and malignant lymphoma (vs acute myeloid leukemia; HR, 1.30; 95% CI, 1.004–1.69). On the other hand, prompt platelet recovery (HR, 0.58; 95% CI, 0.45–0.75) and acute lymphoblastic leukemia (vs acute myeloid leukemia; HR, 0.68; 95% CI, 0.49–0.94) were associated with reduced incidence of noninfectious IP. The median survival after the development of noninfectious IP in patients with prior candidemia was significantly shorter than that in those without it (22 days vs 59 days; P < .001).
Conclusions
Candidemia was associated with an increased incidence of noninfectious IP. The prognosis of noninfectious IP after candidemia was extremely poor.
Keywords: candidemia, hematopoietic cell transplantation–specific comorbidity index, idiopathic pneumonia syndrome, interstitial pneumonia, platelet recovery
Graphical Abstract
Graphical abstract.
Invasive candidiasis is the second most frequent invasive fungal disease after allogeneic hematopoietic cell transplantation (HCT), following only invasive aspergillosis [1, 2]. Candidemia is the most common form of invasive candidiasis [3]. Despite antifungal prophylaxis that covers Candida species, breakthrough candidemia mainly caused by non-albicans Candida species still occurs and is associated with a high mortality rate [4, 5]. In our previous study using a Japanese transplant registry database, the median overall survival (OS) after the development of candidemia was 59 days [6]. One-third of the causes of mortality were noninfectious complications including pulmonary complications such as interstitial pneumonia (IP) and acute respiratory distress syndrome along with graft-versus-host disease (GVHD), thrombotic microangiopathy, and veno-occlusive disease [6].
Infectious complications are closely associated with the development of GVHD through the deterioration of inflammatory cytokines in allogeneic HCT [7]. With regard to the relationship between fungal infection and GVHD, fluconazole prophylaxis against Candida species was associated with a reduced incidence and severity of acute GVHD, particularly involving the gut [8, 9]. In addition, a single-institute retrospective study showed that there was a significant association between invasive fungal disease and chronic GVHD [10].
In mouse models, injection of α-mannan (Mn), a major component of the fungal cell wall, or heat-killed C. albicans exacerbated pulmonary GVHD [11]. Mn-induced donor T-cell polarization toward Th17 and a lung-specific chemokine environment in GVHD led to the accumulation of Th17 cells in the lung. In addition, it has been shown that the detrimental effects of Mn on GVHD depend on donor IL-17A production and host C-type lectin receptor Dectin-2. However, the relationship between candidiasis and pulmonary GVHD after allogeneic HCT has not been clinically evaluated. As the lungs are not the classical target organs of acute GVHD, pulmonary acute GVHD is not clinically defined [12, 13]. In addition, accurate diagnosis and classification of noninfectious pulmonary complications are often difficult [14, 15]. Taking these matters into consideration, this study aimed to evaluate the relationship between candidemia and noninfectious IP after allogeneic HCT by using a Japanese registry database.
METHODS
Study Patients
We analyzed pediatric and adult patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome (MDS), or malignant lymphoma (ML) including non-Hodgkin lymphoma, Hodgkin lymphoma, and adult T-cell leukemia/lymphoma who underwent their first or second allogeneic HCT from HLA-A, B, and DR serologically 6/6-matched related donors (MRDs), HLA serologically 1–3 antigen mismatched related donors (MMRDs), HLA-A, B, C, DRB1 allele 8/8-matched unrelated donors (MUDs), HLA 1–2 allele mismatched unrelated donors (MMUDs), and cord blood donors (CBs) between 2009 and 2019. In patients who underwent a second HCT, the follow-up of the first HCT was censored at the second HCT. Bone marrow (BM), peripheral blood (PB), and CB were used as stem cell sources. This study included only patients for whom information on IP was available because IP is the most important clinical variable. Clinical data for these patients were obtained from the Transplant Registry Unified Management Program, which is a nationwide data registry managed by the Japanese Society for Transplantation and Cellular Therapy (JSTCT) and the Japanese Data Center for Hematopoietic Cell Transplantation [16–18]. This study was approved by the Data Management Committee of JSTCT and by the Institutional Review Board of Jichi Medical University Saitama Medical Center in accordance with the Declaration of Helsinki.
Definitions
Candidemia was defined as a case in the registry database with “candidemia” or “bloodstream infection” in which the causative organism was reported as Candida species [19]. As idiopathic pneumonia syndrome (IPS) and diffuse alveolar hemorrhage (DAH) were not investigation items in the Japanese registry database, we evaluated noninfectious IP, which was defined as a case in the registry database with “interstitial pneumonia,” in which the causes were not reported as infection such as cytomegalovirus (CMV), other viruses, or pneumocystis pneumonia. Cryptogenic organizing pneumonia (COP), which was an investigation item in the database, was excluded from this study as the onset date of COP was not available. Bronchiolitis obliterans (BOs) was also excluded as BO is closely associated with chronic GVHD, but not with acute GVHD [20].
Hematopoietic cell transplantation–specific comorbidity index (HCT-CI) was calculated as previously reported [21]. Performance status (PS) was evaluated based on the Eastern Cooperative Oncology Group (ECOG) scoring system. PS of 0 or 1 was defined as good, and scores of 2 through 4 were defined as poor. Bacteremia was defined as a case in the registry database with positive blood cultures in which the causative organism was reported as bacteria. CMV reactivation was defined as the beginning of CMV preemptive therapy [22]. With regard to CMV serostatus, CB was considered to test negative for CMV by definition. AML, ALL, and ML in complete remission (CR), MDS refractory anemia (RA), and MDS RA with ringed sideroblasts (RARS) were regarded as standard risk, while others were regarded as high risk. Data regarding antifungal prophylaxis were not available from the database. Conditioning regimens were classified as myeloablative conditioning (MAC) if they included total body irradiation (TBI) >8 Gy, melphalan >140 mg/m2, oral busulfan ≥9 mg/kg, or intravenous busulfan ≥7.2 mg/kg [23]. Other regimens were classified as reduced-intensity conditioning (RIC). In vivo T-cell depletion was defined as the inclusion of antithymocyte globulin (ATG), antilymphocyte globulin, or alemtuzumab in the conditioning regimen for GVHD prophylaxis.
Neutrophil engraftment was defined as the first of 3 consecutive days with a neutrophil count >500/µL. Platelet engraftment was defined as the first of 3 consecutive days with a platelet count >20 000/µL without transfusion in the 7 previous days. Acute and chronic GVHD were diagnosed and graded as previously described [24–26].
Statistical Considerations
Dichotomous and continuous variables were compared using the Fisher exact test and the Mann-Whitney U test, respectively. Kaplan-Meier curves were used to estimate survival probabilities. OS was compared among groups by a log-rank test. The cumulative incidence of noninfectious IP was estimated while treating death as a competing event. In the risk factor analysis, the Cox proportional hazard regression model was used while treating candidemia, bacteremia, neutrophil engraftment, platelet engraftment, CMV reactivation, and acute GVHD as time-dependent covariates. Based on previous studies that reported risk factors for noninfectious IP, the hazard ratio (HR) of candidemia was adjusted for the following variables [27–29]; age (<50 vs ≥50 years), conditioning regimen (MAC vs RIC), TBI (≤8 Gy vs >8 Gy), donor type (MRD vs MMRD, MUD, MMUD, CB), pulmonary comorbidity as defined in the HCT-CI (no vs yes), HCT-CI minus pulmonary component (0 vs ≥1), platelet engraftment, and grade III–IV acute GVHD. In addition, bacteremia and CMV reactivation were also subjected to a multivariate model to evaluate the impact of opportunistic infections other than candidemia. Statistical significance was defined as a 2-tailed P value <.05. All statistical analyses were performed with EZR, version 1.55 (Jichi Medical University Saitama Medical Center, http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [30].
RESULTS
Study Patients and Transplantation Outcomes
A total of 9143 patients including 7531 first and 1612 second HCT patients were included in this study (Table 1). The median age (range) was 51 (0–85) years. The median follow-up period for survivors was 1308 days. The 5-year OS was 35.0% (95% CI, 33.9%–36.0%). Neutrophil and platelet engraftment were achieved in 8089 (88.5%) and 5962 (65.2%) patients at a median of 18 and 32 days after HCT, respectively. Grade II–IV and grade III–IV acute GVHD were observed in 3819 (41.8%) and 1739 (19.0%) patients at a median of 29 days and 32 days after HCT, respectively. The 100-day cumulative incidences of grade II–IV and grade III–IV acute GVHD were 41.5% (95% CI, 40.4%–42.5%) and 18.5% (95% CI, 17.7%–19.3%), respectively. Among these patients, 2701 (70.7%) received systemic corticosteroid therapy. Chronic GVHD and extensive chronic GVHD were observed in 2436 and 1518 patients at a median of 127 days after HCT, respectively. The 1-year cumulative incidences of chronic GVHD and extensive chronic GVHD were 25.7% (95% CI, 24.8%–26.6%) and 16.1% (95% CI, 15.3%–16.9%), respectively.
Table 1.
Patient Characteristics (n = 9143)
| Factors | Total (n = 9143) | Candidemia (n = 358) | No candidemia (n = 8785) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Age, y | Median | 51 | 0–85 | 53 | 7–80 | 50 | 0–85 | <.001 |
| <50 | 4370 | (47.8) | 142 | (39.7) | 4228 | (48.1) | .002 | |
| ≥50 | 4773 | (69.5) | 216 | (60.3) | 4557 | (51.9) | ||
| Sex | Male | 5568 | (60.9) | 224 | (62.6) | 5344 | (60.8) | .54 |
| Female | 3575 | (39.1) | 134 | (37.4) | 3441 | (39.2) | ||
| Disease | AML | 4238 | (46.4) | 195 | (54.5) | 4043 | (46.0) | .002 |
| ALL | 1822 | (19.9) | 60 | (16.8) | 1762 | (20.1) | ||
| MDS | 1297 | (14.2) | 32 | (8.9) | 1265 | (14.4) | ||
| ML | 1786 | (19.5) | 71 | (19.8) | 1715 | (19.5) | ||
| Disease risk | Standard | 3983 | (46.4) | 111 | (32.5) | 3872 | (47.0) | <.001 |
| High | 4604 | (53.6) | 231 | (67.5) | 4373 | (53.0) | ||
| NA | 556 | 16 | 540 | |||||
| No. of HCTs | First | 7531 | (82.4) | 274 | (76.5) | 7257 | (82.6) | .005 |
| Second | 1612 | (17.6) | 84 | (23.5) | 1528 | (17.4) | ||
| Donor | MRD | 5280 | (20.1) | 32 | (8.9) | 1322 | (15.0) | <.001 |
| MMRD | 2653 | (10.1) | 42 | (11.7) | 1401 | (15.9) | ||
| MUD | 5943 | (22.7) | 44 | (12.3) | 1586 | (18.1) | ||
| MMUD | 3555 | (13.6) | 32 | (8.9) | 1062 | (12.1) | ||
| CB | 8805 | (33.6) | 208 | (58.1) | 3414 | (38.9) | ||
| Conditioning | MAC | 5365 | (58.7) | 201 | (56.1) | 5164 | (58.8) | .33 |
| RIC | 3778 | (41.3) | 157 | (43.9) | 3621 | (41.2) | ||
| T-cell depletion | No | 7857 | (85.9) | 319 | (89.1) | 7538 | (85.8) | .088 |
| Yes | 1286 | (14.1) | 39 | (10.9) | 1247 | (14.2) | ||
| TBI >8 Gy | No | 6818 | (74.6) | 285 | (79.6) | 6533 | (74.4) | .026 |
| Yes | 2325 | (25.4) | 73 | (20.4) | 2252 | (25.6) | ||
| HCT-CI minus pulmonary component | 0 | 5358 | (59.5) | 137 | (39.4) | 5221 | (60.3) | <.001 |
| ≥1 | 3649 | (40.5) | 211 | (60.6) | 3438 | (39.7) | ||
| NA | 136 | 10 | 126 | |||||
| Pulmonary comorbidity | No | 7141 | (79.0) | 251 | (71.9) | 6890 | (79.3) | .001 |
| Yes | 1897 | (21.0) | 98 | (28.1) | 1799 | (20.7) | ||
| NA | 105 | 9 | 96 | |||||
| PS | 0–1 | 7562 | (82.8) | 252 | (70.4) | 7310 | (83.3) | <.001 |
| ≥2 | 1572 | (17.2) | 106 | (29.6) | 1466 | (16.7) | ||
| NA | 9 | 0 | 9 | |||||
| CMV serostatus, P/D | +/+ | 2744 | (33.0) | 57 | (17.6) | 2687 | (33.7) | <.001 |
| +/− | 4152 | (50.0) | 217 | (67.0) | 3935 | (49.3) | ||
| −/+ | 430 | (5.2) | 14 | (4.3) | 416 | (5.2) | ||
| −/− | 977 | (11.8) | 36 | (11.1) | 941 | (11.8) | ||
| NA | 840 | 34 | 806 | |||||
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CB, cord blood; CMV, cytomegalovirus; D, donor; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; ML, malignant lymphoma; MMRD, HLA-mismatched related donor; MMUD, HLA-mismatched unrelated donor; MRD, HLA-matched related donor; MUD, HLA-matched unrelated donor; NA, not available; P, patient; PS, performance status; RIC, reduced-intensity conditioning; TBI, total body irradiation.
Candidemia and Noninfectious Interstitial Pneumonia
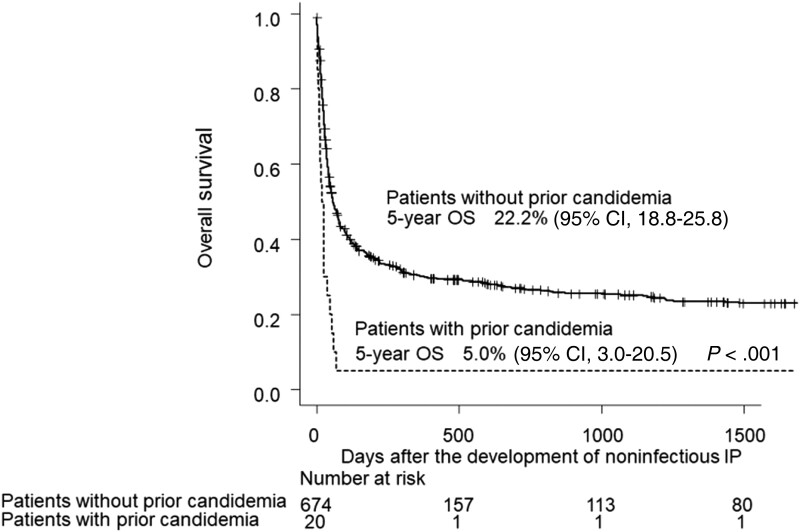
Candidemia and noninfectious IP were observed in 358 and 694 patients at a median (range) of 31 (0–903) days and 63 (0–1292) days after HCT, respectively. The 1-year cumulative incidences of candidemia and noninfectious IP were 3.9% (95% CI, 3.5%–4.3%) and 7.4% (95% CI, 6.9%–8.0%). Among the patients with candidemia, 20 (5.6%) developed noninfectious IP after candidemia at a median (range) of 53.5 (11–333) days after HCT (Supplementary Table 1). The median interval between candidemia and noninfectious IP (range) was 20.5 (1–299) days. The etiologies of noninfectious IP in these 20 patients were reported as follows: IPS (n = 2), pulmonary GVHD (n = 3), idiopathic (n = 11), and unknown (n = 4). Compared with patients who developed noninfectious IP without prior candidemia, a greater proportion of high-risk disease status (78.9% vs 54.2%; P = .036), higher HCT-CI minus pulmonary component (65.0% vs 38.5%; P = .02), higher proportion of patient-positive/donor-negative CMV serostatus (90.0% vs 52.3%; P = .014), and lower achievement rate of platelet engraftment (15.0% vs 63.9%; P < .001) were observed in those who developed noninfectious IP with prior candidemia (Supplementary Table 1). The median OS after the development of noninfectious IP was 55 days (95% CI, 46–69). The median OS in patients who developed noninfectious IP after candidemia was significantly shorter than that in those without prior candidemia (median, 22 days; 95% CI, 10–38; vs 59 days; 95% CI, 49–74; P < .001) (Figure 1). The 5-year OS was 5.0% (95% CI, 3.0%–20.5%) and 22.2% (95% CI, 18.8%–25.8%), respectively. The influence of prior candidemia on OS after the development of noninfectious IP was still significant after being adjusted by disease risk (data not shown). The causes of mortality in 19 patients who developed noninfectious IP after candidemia were noninfectious IP (n = 12 [63.2%]), disease relapse (n = 2 [10.5%]), infection (n = 2 [10.5%]), and other (n = 3 [15.8%]). The causes of mortality in 529 patients who developed noninfectious IP without prior candidemia were noninfectious IP (n = 282 [54.9%]), respiratory failure (n = 29 [5.5%]), disease relapse (n = 75 [14.2%]), infection (n = 55 [10.4%]), GVHD (n = 24 [4.5%]), veno-occlusive disease (n = 8 [1.5%]), thrombotic microangiopathy (n = 6 [1.1%]), and other (n = 50 [9.5%]).
Figure 1.
Overall survival after the development of noninfectious interstitial pneumonia. The median overall survival in patients who developed noninfectious interstitial pneumonia after candidemia was significantly shorter than that in those without prior candidemia (22 days; 95% CI, 10–38; vs 59 days; 95% CI, 49–74; P < .001). The 5-year OS was 5.0% (95% CI, 3.0–20.5) and 22.2% (95% CI, 18.8–25.8), respectively. Abbreviations: IP, interstitial pneumonia; OS, overall survival.
The most frequently identified Candida species in those who suffered from candidemia was C. parapsilosis (n = 113 [31.6%]), followed by C. albicans (n = 47 [13.1%]) and C. glabrata (n = 42 [11.7%]) (Table 2). However, C. albicans (n = 9 [45.0%]) was the leading causative pathogen in patients who developed noninfectious IP after candidemia, while C. parapsilosis was seen only in 1 patient (5%). There was no significant difference in patient background between patients with candidemia caused by C. albicans and patients in whom candidemia was caused by non-albicans Candida (Supplementary Table 2).
Table 2.
Identified Candida Species
| Total (n = 358), No. (%) |
Cases Followed by Noninfectious IP (n = 20), No. (%) |
|
|---|---|---|
| C. albicans | 47 (13.1) | 9 (45.0) |
| C. glabrata | 42 (11.7) | 4 (20.0) |
| C. krusei | 25 (7.0) | 0 |
| C. parapsilosis | 113 (31.6) | 1 (5.0) |
| C. tropicalis | 3 (0.8) | 1 (5.0) |
| C. guilliermondii | 28 (7.8) | 1 (5.0) |
| C. lusitaniae | 7 (2.0) | 0 |
| C. famata | 9 (2.5) | 2 (10.0) |
| Other Candida species | 6 (1.7) | 0 |
| Mixed Candida infection | 4 (1.1) | 1 (5.0) |
| Not identified | 74 (20.7) | 1 (5.0) |
Abbreviation: IP, interstitial pneumonia.
Risk Factor Analysis for Noninfectious Interstitial Pneumonia
We analyzed risk factors for the development of noninfectious IP after HCT (Table 3). Candidemia was significantly associated with an increased risk of noninfectious IP (HR, 2.51; 95% CI, 1.48–4.25), along with ML (HR, 1.30; 95% CI, 1.004–1.69) and TBI (>8 Gy; HR, 1.57; 95% CI, 1.18–2.10). Although pulmonary comorbidity and older age affected the risk of noninfectious IP in a univariate analysis, they were not significant in a multivariate analysis. On the other hand, ALL (HR, 0.68; 95% CI, 0.49–0.94) and platelet engraftment (HR, 0.58; 95% CI, 0.45–0.75) were associated with a reduced incidence of noninfectious IP. Although bacteremia was also associated with an increased incidence of noninfectious IP in a univariate analysis (HR, 1.20; 95% CI, 1.01–1.42; P = .036), there was no significant association in a multivariate analysis (HR, 0.94; 95% CI, 0.74–1.20; P = .62). There was no significant association between CMV reactivation as a time-dependent covariate and noninfectious IP either (HR, 1.29; 95% CI, 0.93–1.79; P = .13).
Table 3.
Risk Factor Analysis for Noninfectious Interstitial Pneumonia
| Factors | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Age, y | ≥50 | 1.20 | 1.03–1.39 | .017 | 1.06 | 0.79–1.42 | .71 |
| Disease | AML | 1.00 | .003a | 1.00 | .004a | ||
| ALL | 0.78 | 0.63–0.96 | .020 | .68 | 0.49–0.94 | .031 | |
| MDS | 1.04 | 0.83–1.29 | .76 | 1.06 | 0.79–1.42 | .71 | |
| ML | 1.23 | 1.02–1.49 | .035 | 1.30 | 1.004–1.69 | .047 | |
| Donor | MRD | 1.00 | <.001a | 1.00 | .19a | ||
| MMRD | 0.67 | 0.51–0.90 | .007 | .72 | 0.48–1.08 | .11 | |
| MUD | 0.83 | 0.64–1.07 | .15 | .95 | 0.67–1.35 | .77 | |
| MMUD | 0.88 | 0.66–1.16 | .36 | .86 | 0.59–1.28 | .46 | |
| CB | 1.13 | 0.92–1.40 | .26 | 1.08 | 0.79–1.48 | .64 | |
| Conditioning | RIC | 1.01 | 0.87–1.18 | .91 | 1.07 | 0.82–1.38 | .64 |
| TBI | >8 Gy | 1.01 | 0.86–1.19 | .91 | 1.57 | 1.18–2.10 | .002 |
| HCT-CI minus pulmonary component | ≥1 | 1.13 | 0.97–1.31 | .13 | .96 | 0.77–1.19 | .72 |
| Pulmonary comorbidity | Yes | 1.43 | 1.21–1.70 | <.001 | 1.24 | 0.97–1.59 | .082 |
| Platelet engraftmentb | … | 0.48 | 0.40–0.58 | <.001 | .58 | 0.45–0.75 | <.001 |
| aGVHD3–4b | … | 1.01 | 0.81–1.26 | .94 | .98 | 0.74–1.31 | .91 |
| Candidemiab | … | 2.60 | 1.77–3.82 | <.001 | 2.51 | 1.48–4.25 | <.001 |
| Bacteremiab | … | 1.20 | 1.01–1.42 | .036 | .94 | 0.74–1.20 | .62 |
| CMV reactivationb | … | 1.26 | 0.91–1.73 | .16 | 1.29 | 0.93–1.79 | .13 |
Abbreviations: aGVHD3–4, grade III-IV acute graft-vs-host disease; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CB, cord blood; CMV, cytomegalovirus; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HR, hazard ratio; MDS, myelodysplastic syndrome; ML, malignant lymphoma; MMRD, HLA-mismatched related donor; MMUD, HLA-mismatched unrelated donor; MRD, HLA-matched related donor; MUD, HLA-matched unrelated donor; RIC, reduced-intensity conditioning; TBI, total body irradiation.
Overall comparison.
Time-dependent covariate.
Additional Analysis Focusing on Noninfectious IP Within 1 Year After HCT and Treating Disease Relapse as a Censoring Event
As most noninfectious IP occurred within 1 year after HCT, we additionally performed an analysis excluding those that occurred 1 year or more after HCT. In addition, follow-up of the patients who experienced disease relapse was censored at the time of relapse, as leukemic infiltration and chemotherapy-related toxicities can be the cause of noninfectious IP. The results of this additional analysis are shown in Supplementary Table 3.
Similar to the main analysis, candidemia was significantly associated with an increased risk of noninfectious IP (HR, 2.11; 95% CI, 1.44–3.88), along with ML (HR, 1.43; 95% CI, 1.10–1.88), while bacteremia and CMV reactivation were not. ALL (HR, 0.67; 95% CI, 0.48–0.93) and platelet engraftment (HR, 0.57; 95% CI, 0.44–0.75) were associated with a reduced incidence of noninfectious IP.
DISCUSSION
In this study, we revealed that candidemia was significantly associated with the development of noninfectious IP after allogeneic HCT. To the best of our knowledge, this is the first study to clarify the clinical association between candidemia and noninfectious pulmonary complications. In contrast to the results in the overall cohort, C. albicans was the leading causative pathogen in patients who developed noninfectious IP after candidemia. There was no significant difference in patient characteristics between candidemia patients with and without C. albicans. Although the reason for the difference in Candida species is not clear, a structural difference in Candida Mn depending on the Candida species might play a role in causing pulmonary GVHD [31, 32].
While the release of cytokines secondary to systemic infection might cause acute respiratory distress syndrome [33], bacteremia was not identified as a risk factor of noninfectious IP in this study. In a previous study in experimental mouse models of IPS, lipopolysaccharide caused lung injury and played a role in the development of IPS [34]. As lipopolysaccharide is a central component of the outer membrane in gram-negative bacteria [35], the impact of gram-positive and gram-negative bacteria on the development of IPS could differ. However, this issue is outside the scope of this study and should be evaluated separately.
The OS after the development of noninfectious IP was poor, particularly in patients with prior candidemia. Although the details of the treatment for noninfectious IP were not available from the registry database, the response to treatment for noninfectious IP might be poor in patients with prior candidemia. It has been reported that corticosteroid paradoxically increases the pathogenic Th17-mediated immune response and may lead to corticosteroid-resistant GVHD [36], which might explain the extremely poor outcomes in patients with noninfectious IP secondary to candidemia.
In previous studies, older age, MDS or ML, pulmonary comorbidity, higher HCT-CI, myeloablative regimen, TBI, HCT from an alternative donor, and severe acute GVHD have been reported as risk factors for noninfectious pulmonary complications [27–29]. Platelet recovery helped protect against IPS and DAH [28]. Therefore, the HR of candidemia was adjusted for these established risk factors in this study, which confirmed the significant association between candidemia and the development of noninfectious IP in a multivariate analysis. Aside from candidemia, the significant associations of ML, TBI, and platelet recovery were confirmed in this study. The common adverse effect of TBI is pulmonary toxicity, and TBI has been reported as a risk factor of IPS [27, 37]. Although this finding was not statistically significant, pulmonary comorbidity also tended to be associated with an increased incidence of noninfectious IP. On the other hand, acute GVHD did not have a significant impact in this study. A previous study using a Japanese registry database also reported that acute GVHD did not influence the development of noninfectious pulmonary complications [29]. Immunosuppressive therapy for GVHD might inhibit the development of noninfectious IP. With regard to the underlying disease, ALL was associated with a reduced risk of noninfectious IP in this study, which has not been reported in previous studies [27–29]. Although the type and intensity of pretransplant treatment might determine the severity of pretransplant pulmonary toxicity and influence the incidence of noninfectious IP, the definitive reason why ALL was identified as a predictive factor was not clear.
This study has some important limitations. First, the number of patients who developed noninfectious IP after candidemia was small. Therefore, while the relationship between candidemia and noninfectious IP was significant, the clinical impact of this study might be limited. Second, some important clinical variables such as antifungal prophylaxis and treatment of candidemia were not available from the registry database. In addition, candidemia and noninfectious IP shared similar predisposing factors. In these respects, it might be difficult to conclude a causal relationship between candidemia and noninfectious IP. Third, the type of noninfectious IP, particularly IPS and DAH, was not available from the database. In addition, COP was excluded from this study. Therefore, the clinical phenotype of pulmonary GVHD caused by Candida could not be investigated in this study. Fourth, although a previous study that found Th17-mediated pulmonary GVHD in a mouse model inspired this clinical study, the mechanisms of the interaction between candidemia and noninfectious IP could not be investigated in this study using this database. Fifth, as information on the treatment for noninfectious IP was not available from the database, the treatment strategy for noninfectious IP after candidemia could not be assessed in this study. Finally, it was difficult to strictly exclude pulmonary infections from IP [38]. Although it was assumed that the possible diagnostic workup was performed in patients with pulmonary complications after allogeneic HCT, the detailed data on the diagnostic workup such as sputum test, bronchoscopy, and lung biopsy in each case were not available from the registry database. Therefore, the diagnosis of noninfectious IP might have been loosely established based on clinical suspicion. Although the rate of bronchoscopy was not available from the database, it is often difficult to perform in patients with myelosuppression or severe condition.
In conclusion, candidemia was associated with an increased risk of noninfectious IP after allogeneic HCT. According to an in vitro study showing that α-mannan induced Th17-mediated pulmonary GVHD, it is assumed that an allogeneic adverse response specific to the lung would at least partly play a role in the mechanisms of noninfectious IP after the development of candidemia. Considering the extremely poor prognosis and possible mechanism of Th17-mediated pulmonary GVHD in noninfectious IP after candidemia, a novel treatment approach such as the use of IL-17 inhibitors might be worth evaluating in this condition [39]. Further studies are necessary to clinically elucidate the mechanisms of the relationship between candidemia and noninfectious IP and to assess the appropriate treatment approach in this condition.
Supplementary Material
Acknowledgments
The authors are grateful for the work of all of the physicians and data managers at the centers that contributed valuable data on transplantation to the JSTCT. We would also like to thank all of the members of the Transplant Registry Unified Management committees at JSTCT for their dedicated data management.
Author contributions. S.-I.K. designed the study, analyzed the data, and wrote the manuscript. Y.Aa., S.S., K.O., and K.H. advised on methods and revised the manuscript. N.U., N.D., K.I., H.Nakam., M.T., S.T., T.K., K.-I.M., T.A., S.O., and M.S. collected data and revised the manuscript. M.O., T.F., and Y.K. collected data, revised the manuscript, and were responsible for data management at JSTCT. Y.At. managed the unified registry database and revised the manuscript. H.Nakas. designed the study, advised on the methods, wrote the manuscript, and was responsible for the project of the JSTCT Transplant Complications Working Group.
Patient consent. All patients provided written informed consent for data reporting. This study was approved by the Data Management Committee of JSTCT and by the Institutional Review Board of Jichi Medical University Saitama Medical Center.
Financial support. S.-I.K. received a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP20K17406. H.N. was a recipient of a grant for the Japan Program for Infectious Diseases Research and Infrastructure from the Japan Agency of Medical Research and Development (AMED; JP21wm0325046) and Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP21K07070.
Contributor Information
Shun-ichi Kimura, Division of Hematology, Jichi Medical University Saitama Medical Center, Saitama, Japan; Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan.
Yu Akahoshi, Division of Hematology, Jichi Medical University Saitama Medical Center, Saitama, Japan; Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan; Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Souichi Shiratori, Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan; Department of Hematology, Hokkaido University Hospital, Hokkaido, Japan.
Keiji Okinaka, Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan; Department of General Medicine and Infectious Diseases, National Cancer Center Hospital East, Chiba, Japan; Department of Hematopoietic Stem Cell Transplantation, National Cancer Center Hospital, Tokyo, Japan.
Kaito Harada, Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan; Department of Hematology and Oncology, Tokai University School of Medicine, Kanagawa, Japan.
Naoyuki Uchida, Department of Hematology, Federation of National Public Service Personnel Mutual Aid Associations Toranomon Hospital, Tokyo, Japan.
Noriko Doki, Hematology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Tokyo, Japan.
Kazuhiro Ikegame, Department of Hematology, Hyogo College of Medicine Hospital, Hyogo, Japan.
Hirohisa Nakamae, Department of Hematology, Osaka Metropolitan University Hospital, Osaka, Japan.
Masatsugu Tanaka, Department of Hematology, Kanagawa Cancer Center, Kanagawa, Japan.
Satoru Takada, Leukemia Research Center, Saiseikai Maebashi Hospital, Gunma, Japan.
Toshiro Kawakita, Department of Hematology, National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan.
Ken-ichi Matsuoka, Department of Hematology and Oncology, Okayama University Hospital, Okayama, Japan.
Takahide Ara, Department of Hematology, Hokkaido University Hospital, Hokkaido, Japan.
Shuichi Ota, Department of Hematology, Sapporo Hokuyu Hospital, Hokkaido, Japan.
Masashi Sawa, Department of Hematology and Oncology, Anjo Kosei Hospital, Aichi, Japan.
Makoto Onizuka, Department of Hematology and Oncology, Tokai University School of Medicine, Kanagawa, Japan.
Takahiro Fukuda, Department of Hematopoietic Stem Cell Transplantation, National Cancer Center Hospital, Tokyo, Japan.
Yoshiko Atsuta, Japanese Data Center for Hematopoietic Cell Transplantation, Aichi, Japan; Department of Registry Science for Transplant and Cellular Therapy, Aichi Medical University School of Medicine, Aichi, Japan.
Yoshinobu Kanda, Division of Hematology, Jichi Medical University Saitama Medical Center, Saitama, Japan; Division of Hematology, Jichi Medical University, Tochigi, Japan.
Hideki Nakasone, Division of Hematology, Jichi Medical University Saitama Medical Center, Saitama, Japan; Transplant Complications Working Group of the Japan Society for Transplantation and Cellular Therapy (JSTCT), Aichi, Japan.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Neofytos D, Horn D, Anaissie E, et al. . Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis 2009; 48:265–73. [DOI] [PubMed] [Google Scholar]
- 2. Kontoyiannis DP, Marr KA, Park BJ, et al. . Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 3. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med 2015; 373:1445–56. [DOI] [PubMed] [Google Scholar]
- 4. Kimura M, Araoka H, Yamamoto H, et al. . Clinical and microbiological characteristics of breakthrough candidemia in allogeneic hematopoietic stem cell transplant recipients in a Japanese hospital. Antimicrob Agents Chemother 2017; 61:e01791-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen XC, Xu J, Wu DP. Clinical characteristics and outcomes of breakthrough candidemia in 71 hematologic malignancy patients and/or allogeneic hematopoietic stem cell transplant recipients: a single-center retrospective study from China, 2011-2018. Clin Infect Dis 2020; 71(Suppl 4):S394–9. [DOI] [PubMed] [Google Scholar]
- 6. Kimura SI, Kameda K, Harada K, et al. . Risk and predictive factors for candidemia after allogeneic hematopoietic cell transplantation: JSTCT Transplant Complications Working Group. Transplant Cell Ther 2022; 28:209.e1–9. [DOI] [PubMed] [Google Scholar]
- 7. Kameda K, Kimura SI, Misaki Y, et al. . Associations between febrile neutropenia-related parameters and the risk of acute GVHD or non-relapse mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2019; 54:707–16. [DOI] [PubMed] [Google Scholar]
- 8. Marr KA, Seidel K, Slavin MA, et al. . Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96:2055–61. [PubMed] [Google Scholar]
- 9. van der Velden WJ, Netea MG, de Haan AF, Huls GA, Donnelly JP, Blijlevens NM. Role of the mycobiome in human acute graft-versus-host disease. Biol Blood Marrow Transplant 2013; 19:329–32. [DOI] [PubMed] [Google Scholar]
- 10. Jin H, Fan Z, Huang F, et al. . Invasive fungal disease is associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplant: a single center, retrospective study. Infection 2019; 47:275–84. [DOI] [PubMed] [Google Scholar]
- 11. Uryu H, Hashimoto D, Kato K, et al. . Alpha-mannan induces Th17-mediated pulmonary graft-versus-host disease in mice. Blood 2015; 125:3014–23. [DOI] [PubMed] [Google Scholar]
- 12. Cooke KR, Yanik G. Acute lung injury after allogeneic stem cell transplantation: is the lung a target of acute graft-versus-host disease? Bone Marrow Transplant 2004; 34:753–65. [DOI] [PubMed] [Google Scholar]
- 13. Zeiser R, Teshima T. Nonclassical manifestations of acute GVHD. Blood 2021; 138:2165–72. [DOI] [PubMed] [Google Scholar]
- 14. Sharma S, Nadrous HF, Peters SG, et al. . Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest 2005; 128:1385–92. [DOI] [PubMed] [Google Scholar]
- 15. Huaringa AJ, Leyva FJ, Signes-Costa J, et al. . Bronchoalveolar lavage in the diagnosis of pulmonary complications of bone marrow transplant patients. Bone Marrow Transplant 2000; 25:975–9. [DOI] [PubMed] [Google Scholar]
- 16. Atsuta Y, Suzuki R, Yoshimi A, et al. . Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP system. Int J Hematol 2007; 86:269–74. [DOI] [PubMed] [Google Scholar]
- 17. Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol 2016; 103:3–10. [DOI] [PubMed] [Google Scholar]
- 18. Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol 2016; 103:11–9. [DOI] [PubMed] [Google Scholar]
- 19. Donnelly JP, Chen SC, Kauffman CA, et al. . Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jagasia MH, Greinix HT, Arora M, et al. . National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21:389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takenaka K, Nishida T, Asano-Mori Y, et al. . Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-Related Complication Working Group. Biol Blood Marrow Transplant 2015; 21:2008–16. [DOI] [PubMed] [Google Scholar]
- 23. Giralt S, Ballen K, Rizzo D, et al. . Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 2009; 15:367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Przepiorka D, Weisdorf D, Martin P, et al. . 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15:825–8. [PubMed] [Google Scholar]
- 25. Shulman HM, Sale GE, Lerner KG, et al. . Chronic cutaneous graft-versus-host disease in man. Am J Pathol 1978; 91:545–70. [PMC free article] [PubMed] [Google Scholar]
- 26. Sullivan KM, Shulman HM, Storb R, et al. . Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981; 57:267–76. [PubMed] [Google Scholar]
- 27. Fukuda T, Hackman RC, Guthrie KA, et al. . Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003; 102:2777–85. [DOI] [PubMed] [Google Scholar]
- 28. Patel SS, Ahn KW, Khanal M, et al. . Noninfectious pulmonary toxicity after allogeneic hematopoietic cell transplantation. Transplant Cell Ther 2022; 28:310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onizuka M, Fujii N, Nakasone H, et al. . Risk factors and prognosis of non-infectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Int J Hematol 2022; 115:534–44. [DOI] [PubMed] [Google Scholar]
- 30. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kogan G, Pavliak V, Masler L. Structural studies of mannans from the cell walls of the pathogenic yeasts Candida albicans serotypes A and B and Candida parapsilosis. Carbohydr Res 1988; 172:243–53. [DOI] [PubMed] [Google Scholar]
- 32. Shibata N, Ikuta K, Imai T, et al. . Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem 1995; 270:1113–22. [DOI] [PubMed] [Google Scholar]
- 33. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377:1904–5. [DOI] [PubMed] [Google Scholar]
- 34. Cooke KR, Kobzik L, Martin TR, et al. . An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: i. The roles of minor H antigens and endotoxin. Blood 1996; 88:3230–9. [PubMed] [Google Scholar]
- 35. Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 2014; 83:99–128. [DOI] [PubMed] [Google Scholar]
- 36. Toubai T, Magenau J. Immunopathology and biology-based treatment of steroid-refractory graft-versus-host disease. Blood 2020; 136:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vogel J, Hui S, Hua CH, et al. . Pulmonary toxicity after total body irradiation - critical review of the literature and recommendations for toxicity reporting. Front Oncol 2021; 11:708906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seo S, Renaud C, Kuypers JM, et al. . Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reich K, Warren RB, Lebwohl M, et al. . Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med 2021; 385:142–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.