Abstract
Acute pulmonary embolism (PE) is often associated with rapid hemodynamic deterioration or death. Therefore, early therapeutic intervention is important. A 45-year-old man was diagnosed with intermediate-high risk PE, and sequential hybrid therapy consisting of surgical thrombectomy and rivaroxaban intensive therapy was administered. During the course of treatment, echocardiography revealed improvement in pulmonary artery systolic pressure, and thrombus volume analysis by computed tomography revealed a drastic reduction in the size of the thrombus. Sequential hybrid therapy for acute PE not only stabilizes hemodynamics, but may also prevent conversion to chronic thromboembolic pulmonary hypertension by sufficiently reducing the volume of the thrombus.
INTRODUCTION
Recently, with the development of multidetector computed tomography (CT), the accuracy of diagnosis of pulmonary embolism (PE) has dramatically improved. As a result, the number of patients with PE has been increasing every year [1]. Acute PE is often associated with rapid hemodynamic deterioration or mortality; therefore, early therapeutic intervention is clinically pivotal. Surgical thrombectomy has been reported to be effective for acute PE with hemodynamic instability [2], whereas the efficacy of a single oral drug treatment with the Xa inhibitor rivaroxaban for PE with hemodynamic stability has been reported in several randomized controlled trials [3–5]. However, treatment strategy for patients with hemodynamic fluctuations remains unclear. Here, we report a case in which sequential hybrid therapy of surgical thrombectomy and rivaroxaban intensive therapy resulted in a good clinical course and sufficient thrombus reduction in a patient with acute PE with a high thrombus volume, as demonstrated by imaging. This case report has been reported in line with the SCARE criteria [6].
CASE REPORT
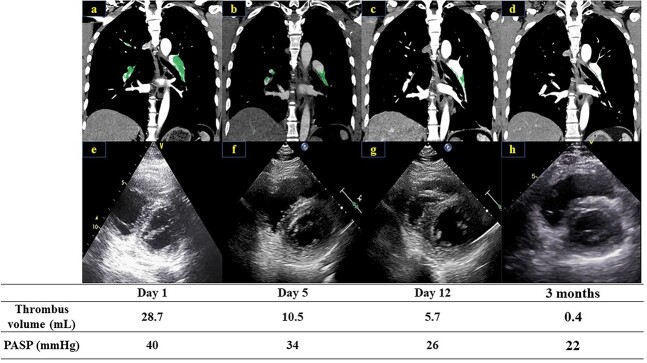
A 45-year-old man was admitted with dyspnea as his chief complaint. He had no past medical history. On admission, his vital signs were as follows: blood pressure, 128/91 mmHg; heart rate, 112 beats/min; respiratory rate, 20 breaths/min; and peripheral oxygen saturation, 89% (in room air). Blood sampling showed elevated D-dimer, N-terminal pro-brain natriuretic peptide (NT-proBNP) and Cardiac Troponin I (Table 1). Contrast-enhanced CT showed a thrombus in the bilateral pulmonary arteries and the right common femoral vein (Fig. 1). Echocardiography revealed enlargement of the right ventricle (D-shape) and a high pulmonary artery systolic pressure (PASP) (Fig. 2). He was considered at intermediate-high risk of 2019 European Society of Cardiology Clinical Practice Guidelines on Acute PE [7]; therefore, anticoagulation with unfractionated heparin was initiated. The following day, the patient remained in a state of hemodynamic instability with persistent tachycardia (HR > 110 bpm). Based on our discussion with the cardiology team, the patient underwent a surgical pulmonary thrombectomy. On Day 3, the patient was extubated to improve oxygenation and rivaroxaban intensive therapy (30 mg/day) was initiated after the confirmation of no renal dysfunction. On Day 5, contrast-enhanced chest CT showed a decrease in the thrombus volume; however, the thrombus remained in the pulmonary lobar artery (Fig. 2). On Day 12, the thrombus in the pulmonary lobar arteries resolved to approximately half of the postoperative level, and the right ventricular load disappeared on echocardiography (Fig. 2). The patient was discharged 14 days after an uncomplicated course. At the 3-month outpatient follow-up, there was no recurrence of PE. D-dimer, NT-proBNP and Cardiac Troponin I were within the normal levels (Table 1), and contrast-enhanced CT showed further thrombus reduction (Fig. 2).
Table 1.
Laboratory data at baseline and on follow-up (3 months)
| Investigation | Baseline | Follow-up | Reference range |
|---|---|---|---|
| Hemoglobin (g/L) | 17.0 | 16.3 | 13.7–16.8 |
| Platelet counts (×103/μL) | 243 | 240 | 158–348 |
| Total bilirubin (mg/dL) | 0.27 | 0.41 | 0.40–1.50 |
| Aspartate aminotransferase (IU/L) | 20 | 17 | 13–30 |
| Alanine aminotransferase (IU/L) | 20 | 14 | 10–42 |
| Lactate dehydrogenase (IU/L) | 229 | 159 | 124–222 |
| Serum creatinine (mg/dL) | 1.35 | 1.21 | 0.65–1.07 |
| Blood urea nitrogen (mg/dL) | 22.9 | 16.5 | 8.0–20.0 |
| Creatinine clearance (mL/min) | 79.2 | 88.3 | 82.0–183.0 |
| Serum sodium (mmol/L) | 147 | 144 | 138–145 |
| Serum potassium (mmol/L) | 3.9 | 4.3 | 3.6–4.8 |
| Creatinine kinase (IU/L) | 158 | 78 | 59–248 |
| Creatinine kinase-MB (IU/L) | 7 | 4 | <5 |
| Cardiac Troponin I (ng/mL) | 0.21 | 0.04 | <0.10 |
| NT-proBNP (pg/mL) | 3780 | 16 | <125 |
| D-dimer (μg/mL) | 8.9 | ≤1.0 | ≤1.0 |
Creatinine clearance, (140 − age) × body weight/(72 × serum creatinine).
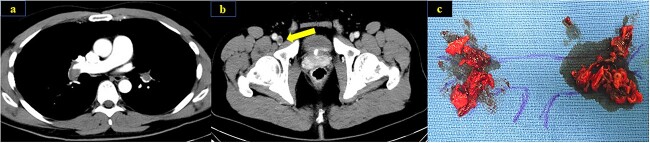
Figure 1.
Contrast-enhanced CT scan at the time of hospitalization showed thrombus in bilateral pulmonary arteries (a) and the right common femoral vein (b). Thrombus in pulmonary arteries were removed by surgical thrombectomy (c).
Figure 2.
Contrast-enhanced chest CT scan on Day 1 (a), Day 5 (b), Day 12 (c) and 3 months (d) trace and measure thrombus volume by using the SYNAPSE VINCENT software (Fujifilm Medical Corporation, Tokyo, Japan). The short-axis image of the echocardiogram is shown on Day 1 (e), Day 5 (f), Day 12 (g) and 3 months (h). The PASP was calculated as 4 × (peak TR velocity)2 + estimated right atrial pressure (RAP). The RAP was estimated from the diameter of the inferior vena cava and its respiratory changes.
DISCUSSION
The patient was diagnosed as PE/DVT because of the following clinical features: dyspnea with tachycardia and decreased oxygen level, an elevated D-dimer, and thrombus in both pulmonary arteries and DVT in the right common femoral vein on contrast-enhanced CT. There was no obvious hemodynamic shock, but the patient was considered at intermediate-high risk based on right heart dysfunction on echocardiography and elevated Cardiac Troponin I. However, the patient did not respond to anticoagulation therapy, and his hemodynamic status was judged to be unstable because of persistent symptoms of respiratory distress and tachycardia. In addition, the patient was young and at low perioperative risk and there was a large amount of thrombus in bilateral pulmonary arteries. Therefore, our team finally decided to perform early thrombectomy rather than catheterization and pharmacological thrombolytic therapy. Indications for surgical thrombectomy should always be considered in cases of high bleeding risk or unresponsiveness to medical therapy [8, 9]. Surgical thrombectomy can rapidly stabilize hemodynamics and improve pulmonary hypertension and right ventricular dysfunction load by removing the thrombus in the central pulmonary artery [10]. Surgical thrombectomy removed the main thrombi in bilateral pulmonary arteries; however, the thrombus remained in the lobar branches. Moreover, pulmonary hypertension persisted, and rivaroxaban intensive therapy was administered to prevent future progression to chronic pulmonary thromboembolism. Experimental studies have reported that rivaroxaban can prevent right heart dysfunction [11]. Rivaroxaban has been reported to be potentially effective as anticoagulation therapy after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension [12]. Nonetheless, there is no literature describing anticoagulation after surgical thrombectomy or quantitative evaluation of thrombus by CT analysis. This case provides clinical insights into not only the potential of rivaroxaban intensive therapy as postsurgical therapy for acute PE, but also clinical importance of serial monitoring of PASP and thrombus reduction by transthoracic echocardiography and CT thrombus volume analysis.
In summary, sequential hybrid therapy of surgical thrombectomy and rivaroxaban intensive therapy for acute PE could sufficiently and safely reduce the volume of the thrombus and contribute to hemodynamic stabilization.
Contributor Information
Shohei Migita, Division of Cardiology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan.
Nobuhiro Murata, Division of Cardiology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan.
Daisuke Fukamachi, Department of Cardiology, Nihon University Hospital, Tokyo, Japan.
Katsunori Fukumoto, Division of Cardiology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan.
Riku Arai, Division of Cardiology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan.
Hiroe Uchiyama, Department of Radiology, Nihon University School of Medicine, Tokyo, Japan.
Kenichiro Tago, Department of Radiology, Nihon University School of Medicine, Tokyo, Japan.
Masahiro Okada, Department of Radiology, Nihon University School of Medicine, Tokyo, Japan.
Masashi Tanaka, Department of Cardiovascular Surgery, Nihon University School of Medicine, Tokyo, Japan.
Yasuo Okumura, Division of Cardiology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan.
CONFLICT OF INTEREST STATEMENT
Y.O. has received lecture fees, research funding, scholarship funds and donations from Bayer Yakuhin; lecture fees, scholarship funds and donations from Daiichi-Sankyo; research funding from Bristol-Myers Squibb; scholarship funds and donations from Johnson & Johnson; and is associated with endowed departments sponsored by Boston Scientific Japan, Abbott Medical Japan, Medtronic Japan, Nihon Kohden and Japan Lifeline.
FUNDING
None.
ETHICAL APPROVAL
This case was approved by the ethical committee of Nihon University School of Medicine.
CONSENT
Written informed consent was obtained from the patient for publication of this case report.
GUARANTOR
All the authors are nominated guarantors of the manuscript.
DATA AVAILABILITY
The data of this case will not be shared.
REFERENCES
- 1. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006;113:577–82. 10.1161/circulationaha.105.592592. [DOI] [PubMed] [Google Scholar]
- 2. Shiomi D, Kiyama H, Shimizu M, Yamada M, Shimada N, Takahashi Aet al. Surgical embolectomy for high-risk acute pulmonary embolism is standard therapy. Interact Cardiovasc Thorac Surg 2017;25:297–301. 10.1093/icvts/ivx091. [DOI] [PubMed] [Google Scholar]
- 3. Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar Eet al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 4. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus ASet al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 5. Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MHet al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism - the J-EINSTEIN DVT and PE program. Thromb J 2015;13:2. 10.1186/s12959-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int J Surg 2020;84:226–30. 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VPet al. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019;2019:54. 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 8. Neely RC, Byrne JG, Gosev I, Cohn LH, Javed Q, Rawn JDet al. Surgical Embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg 2015;100:1245–51. 10.1016/j.athoracsur.2015.03.111. [DOI] [PubMed] [Google Scholar]
- 9. Fukuda I, Taniguchi S, Fukui K, Minakawa M, Daitoku K, Suzuki Y. Improved outcome of surgical pulmonary embolectomy by aggressive intervention for critically ill patients. Ann Thorac Surg 2011;91:728–32. 10.1016/j.athoracsur.2010.10.086. [DOI] [PubMed] [Google Scholar]
- 10. Masuda M, Okumura M, Doki Y, Endo S, Hirata Y, Kobayashi Jet al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665–97. 10.1007/s11748-016-0695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delbeck M, Nickel KF, Perzborn E, Ellinghaus P, Strassburger J, Kast Ret al. A role for coagulation factor Xa in experimental pulmonary arterial hypertension. Cardiovasc Res 2011;92:159–68. 10.1093/cvr/cvr168. [DOI] [PubMed] [Google Scholar]
- 12. Barati S, Amini H, Ahmadi ZH, Dastan A, Sharif Kashani B, Eskandari Ret al. Evaluating the efficacy and safety of rivaroxaban as a warfarin alternative in chronic thromboembolic pulmonary hypertension patients undergoing pulmonary endarterectomy: a randomized clinical trial. Rev Port Cardiol 2022;42:139–44. 10.1016/j.repc.2021.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this case will not be shared.