Abstract
Purpose
The purpose of this study was to estimate the clinical efficacy and identify the best beneficiaries of postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) in hepatocellular carcinoma (HCC).
Patients and methods
A total of 749 HCC patients who underwent surgical resection (380 underwent PA-TACE, 369 had resection only) with a high risk of recurrence were reviewed retrospectively. Patients receiving PA-TACE were randomly split into development and validation cohorts. Univariate and multivariate analyses were performed in the development cohort. A novel model for PA-TACE-insensitivity prediction was built based on univariate and multivariate analysis and was multi-dimensionally validated in the validation set and all samples.
Results
After propensity score matching (PSM), in the early-recurrence group, no significant improvement in RFS was achieved with PA-TACE compared to radical hepatic resection alone. PA-TACE insensitive patients were considered as the PA-TACE non-benefit population and were associated with six clinicopathological factors: AFP, node number, tumor capsule, Ki-67 index, MVI, and complications in the development cohort. These factors were incorporated into a nomogram model, which reliably predicted PA-TACE insensitivity, with concordance indices of 0.874 and 0.897 for the development and validation cohort, respectively. In the overall sample, PA-TACE did not significantly improve patients’ RFS and OS in the high-score group, while the low-score group had statistical significance. Recurrence pattern diversity was also found to be a factor leading to PA-TACE insensitivity.
Conclusion
We constructed a new PA-TACE-insensitivity prediction model with potential clinical value. The good predictive performance and availability would allow this model to effectively screen PA-TACE beneficiaries.
KEY MESSAGES
The independent influencing factors of PA-TACE insensitivity in patients who received PA-TACE were analyzed to construct a predictive model and its clinical application performance was verified with multi-dimensional methods.
PA-TACE treatment should be avoided for patients with high scores according to this model, while it should be cautiously recommended for patients with low scores after multiple considerations.
Compared with other related models, this model has obvious advantages in versatility and effectiveness. It can effectively screen the best benefit population of PA-TACE and provide a reliable reference for the selection of precise treatment plans for patients after radical resection of hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma (HCC), post-operative adjuvant transcatheter arterial chemoembolization (PA-TACE), PA-TACE-insensitivity, nomogram, individualized prediction
Introduction
Hepatic carcinoma (HCC) is common cancer with a poor prognosis [1]. Despite recent improvements in the diagnostic and treatment strategies for HCC, curative hepatic resection remains the mainstream therapy, especially for patients with acceptable liver function [2]. However, the recurrence-free survival (RFS) and overall survival (OS) of patients with HCC are still largely unsatisfactory [3–5].
In recent years, Transcatheter arterial chemoembolization (TACE) as adjuvant therapy for curative HCC resection has gained a broad spectrum of attention [6,7]. Although some investigators have argued that postoperative adjuvant TACE (PA-TACE) may reduce the likelihood of relapse in patients with HCC who have undergone hepatectomy [8–10]; however, the opposite opinion was expressed in some studies [11,12]. Even though the controversy persists, some clinicians find that certain patients can benefit from PA-TACE, particularly high-risk individuals with high levels of AFP, microvascular invasion before surgery, and other risk factors [7,13,14], whereas others are insensitive to it. Therefore, screening of PA-TACE benefit groups is of great significance for the precise treatment of HCC patients after hepatectomy.
Ki67 has been used as a biomarker for tumor aggression in a variety of tumors, including lung cancer and soft tissue sarcomas [15–17]. However, the predictive value of Ki67 has not been taken into consideration in HCC.
Studies have shown that for HCC patients with resectable regions, early postoperative recurrence is the leading cause of death, and early postoperative recurrence tends to result in postoperative multiple intrahepatic recurrences, vascular invasion, and distant metastasis [18–20], resulting in extremely poor patient outcomes. PA-TACE has been used for preventing postoperative recurrence as an adjuvant treatment; therefore, we measured its effectiveness by determining whether there is an early recurrence after PA-TACE. On this basis, we divided all patients into two groups according to whether recurrence occurred within 12 months after surgery and compared the tumor-free survival of patients with or without PA-TACE, in each subgroup. We selected the statistically significant group and used the grouping basis as the outcome variable for further analysis. Randomly assigning the patients receiving PA-TACE to a development cohort and a validation cohort, further identified patients who may derive the greatest benefit from PA-TACE through a comprehensive screening of potential predictors, including Ki67 and the construction of a nomogram. We aimed to develop, validate, and assess the performance of a nomogram to screen PA-TACE beneficiaries to achieve precise treatment of HCC patients undergoing hepatectomy.
Materials and methods
Patient cohorts
We reviewed the medical records of 749 consecutive relapse-prone HCC patients who underwent an R0 hepatectomy with or without PA-TACE between January 2012 and December 2017 at our institution. Among them, 369 patients had hepatectomy alone (HR), and 380 patients had PA-TACE treatment after hepatectomy (HR + PA-TACE). This study was in concordance with the Declaration of Helsinki and the Ethics Review Committee of Guangxi Medical University Cancer Hospital, who approved the protocol of this study (LW2022132).
Inclusion criteria included the following: (1) one or more risk factors for postoperative relapse (multiple nodules, tumor size ≥5 cm, PVTT or MVI); (2) hepatic resection with R0, defined as complete macroscopic removal of the tumor, negative resection margins, and no remaining detectable intrahepatic and extrahepatic metastasis lesions; (3) HCC diagnosis based on comparing the pathology with the World Health Organization criteria; and (4) no previous history of anti-cancer therapy.
All patients receiving PA-TACE (n = 380) were randomly assigned to the development cohort (n = 285) and validation cohort (n = 95), with a 3:1 ratio. The factors affecting the efficacy of PA-TACE will be discussed in the group of patients receiving PA-TACE treatment.
Postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE)
TACE was performed four weeks after HR using the Seldinger technique. Under the guidance of hepatic computed tomography (CT) angiography, vascular catheterization was performed via the femoral artery, and a pre-selected hepatic artery was finally reached. Through the catheter, oxaliplatin (25–100 mg), lobaplatin (25–100 mg), pharmorubicin (10–50 mg), or pirarubicin (10–50 mg), as well as lipiodol (2–10 mL) were infused [21]. The specific plan was decided by the treating physician based on the evaluation of the expected efficacy.
Follow-up and end-points
Following therapy, patients were routinely followed up every one to two months by phone calls or outpatient services for the first year and every three months thereafter at our institution Serum AFP level, liver function tests, and abdominal CT or magnetic resonance imaging (MRI) were conducted every 3–6 months. Suspected recurrence was further validated by elevated AFP combined with typical imaging features; hepatic arteriography was recommended. Postoperative complications were defined as events occurring within 30 days after the procedure according to the Clavien-Dindo grading system, which included post-hepatectomy liver failure (PHLF), bleeding in the abdominal cavity, biliary fistula, massive hydrothorax (>500 ml), and intra-abdominal infection, among others. The relapse time and site from surgery until recurrence were also recorded. Patients with early relapse after receiving PA-TACE were considered to be insensitive to preventive treatment. Postoperative relapse within 12 months was defined as ‘early recurrence’, and the type of relapse was classified as pattern I, solitary-intrahepatic recurrence; pattern II, multi-intrahepatic recurrence; pattern III, intrahepatic recurrence with vascular invasion and/or extrahepatic recurrence; and pattern IV, extrahepatic recurrence alone. The final follow-up was conducted on 31 December 2020.
Each patient was followed over 12 months. Recurrence-free survival (RFS) refers to the interval between the date of hepatectomy and the detection of relapse. Overall survival (OS) depends on the time from hepatectomy to the date of the last follow-up visit or death.
Statistical analysis
A 1:1 early recurrence group:not early-recurrence group matching was done using propensity score matching (PSM). The χ2 test or Fisher’s exact test was used to assess the clinicopathological characteristics between the development and validation cohorts. The OS and RFS value were analyzed by log-rank test. Multivariate binary logistic regression was performed using independent factors with p-values <0.05 based on the results of the univariate binary logistic regression analysis. The ‘rms’ package in R was used to develop the final nomogram. An evaluation of validation was conducted using the concordance index (C-index), calibration curves, ROC curve analysis, clinical impact curve analysis, and DCA. To further verify the predictive value of the model, Kaplan-Meier analysis was performed to compare the groups based on the nomogram assessment.
All p values given are two-tailed values, and p < 0.05 indicates statistical significance. The above statistical analyses were performed with SPSS software, version 24 (IBM Corp., New York, NY, USA) and R (version 4.1.1, R Development Core Team), including the ‘MatchIt’, ‘survival’, and ‘rms’ packages.
Results
Clinicopathological characteristics of subgroups before and after matching
Based on recurrence time, the 749 patients were further subdivided into two subgroups, with 452 classified in the early recurrence subgroup (relapsed within 1 year) and 297 in the non-early recurrence subgroup. Clinicopathologic features of the two subgroups are shown in Table 1. Before PSM, there were significant differences in age, AFP level, tumor size, Edmondson grade, node number, tumor capsule, Ki67, MVI, liver cirrhosis, and postoperative complications between the two groups. After propensity matching, 340 patients were included in the analysis: 170 in the early-recurrence group and 170 in the not early-recurrence group. There were no significant differences between the two matched groups (Table 1).
Table 1.
Comparisons of clinical characteristics between the early-recurrence group and the not early-recurrence group before and after matching.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Early recurrence group | Not early-recurrence group | p | Early recurrence group | Not early-recurrence group | p | |
| n | 452 | 297 | 170 | 170 | ||
| Sex | 0.818 | 1 | ||||
| Male | 395 (87.4) | 257 (86.5) | 147 (86.5) | 146 (85.9) | ||
| Female | 57 (12.6) | 40 (13.5) | 23 (13.5) | 24 (14.1) | ||
| Age (years) | 46.50 [38.00, 54.00] | 50.00 [44.00, 59.00] | <0.001* | 48.50 [39.00, 57.00] | 48.00 [42.00, 57.00] | 0.494 |
| HBsAg | 0.428 | 1 | ||||
| Negative | 58 (12.8) | 45 (15.2) | 22 (12.9) | 21 (12.4) | ||
| Positive | 394 (87.2) | 252 (84.8) | 148 (87.1) | 149 (87.6) | ||
| HBV DNA (copies/ml) | 0.394 | 0.906 | ||||
| <500 | 142 (31.4) | 103 (34.7) | 51 (30.0) | 53 (31.2) | ||
| ≥500 | 310 (68.6) | 194 (65.3) | 119 (70.0) | 117 (68.8) | ||
| AFP level (ng/ml) | <0.001* | 1 | ||||
| <400 | 165 (36.5) | 182 (61.3) | 84 (49.4) | 85 (50.0) | ||
| ≥400 | 287 (63.5) | 115 (38.7) | 86 (50.6) | 85 (50.0) | ||
| Tumor size (cm)† | 9.00 [6.00, 12.00] | 7.00 [5.00, 9.00] | <0.001* | 8.00 [6.00, 11.00] | 7.00 [5.00, 10.00] | 0.133 |
| Edmondson grade† | <0.001* | 0.825 | ||||
| Poorly differentiated | 222 (49.1) | 90 (30.3) | 70 (41.2) | 67 (39.4) | ||
| Moderately and well-differentiated | 230 (50.9) | 207 (69.7) | 100 (58.8) | 103 (60.6) | ||
| Node number | <0.001* | 1 | ||||
| 1 | 308 (68.1) | 271 (91.2) | 149 (87.6) | 150 (88.2) | ||
| ≥2 | 144 (31.9) | 26 (8.8) | 21 (12.4) | 20 (11.8) | ||
| Tumor capsule | <0.001* | 0.129 | ||||
| Complete | 250 (55.3) | 261 (87.9) | 150 (88.2) | 139 (81.8) | ||
| Incomplete | 202 (44.7) | 36 (12.1) | 20 (11.8) | 31 (18.2) | ||
| Resection margin (cm) | 0.378 | 0.327 | ||||
| <1 | 340 (75.2) | 214 (72.1) | 129 (75.9) | 120 (70.6) | ||
| ≥1 | 112 (24.8) | 83 (27.9) | 41 (24.1) | 50 (29.4) | ||
| Ki67 | 0.40 [0.30, 0.70] | 0.20 [0.10, 0.35] | <0.001* | 0.30 [0.20, 0.40] | 0.30 [0.20, 0.40] | 0.847 |
| MVI | <0.001* | 1 | ||||
| No | 59 (13.1) | 135 (45.5) | 51 (30.0) | 51 (30.0) | ||
| Yes | 393 (86.9) | 162 (54.5) | 119 (70.0) | 119 (70.0) | ||
| PVTT | 0.252 | 0.874 | ||||
| No | 314 (69.5) | 271 (91.2) | 148 (87.1) | 146 (85.9) | ||
| Yes | 138 (30.5) | 26 (8.8) | 22 (12.9) | 24 (14.1) | ||
| Liver cirrhosis | <0.001* | 0.712 | ||||
| None/mild | 338 (74.8) | 210 (70.7) | 127 (74.7) | 123 (72.4) | ||
| Moderate/severe | 114 (25.2) | 87 (29.3) | 43 (25.3) | 47 (27.6) | ||
| Postoperative complications | <0.001* | 0.838 | ||||
| No | 381 (84.3) | 279 (93.9) | 156 (91.8) | 158 (92.9) | ||
| Yes | 71 (15.7) | 18 (6.1) | 14 (8.2) | 12 (7.1) | ||
| PA-TACE | 0.786 | 1 | ||||
| No | 225 (49.8) | 144 (48.5) | 96 (56.5) | 96 (56.5) | ||
| Yes | 227 (50.2) | 153 (51.5) | 74 (43.5) | 74 (43.5) | ||
In cases involving multiple nodes, only the largest was indicated. * indicates p < 0.05. Data are presented as n (%) by group and compared using the Pearson chi-square test or Fisher’s exact test for any cell number < 5. CNLC: China liver cancer; HBV: Hepatitis B virus; HR: Hepatic resection; MVI: Microvascular invasion; PA-TACE: Postoperative adjuvant transarterial chemoembolization; PVTT: Portal vein tumor thrombus.
Survival analysis in all patients and the two matched subgroups
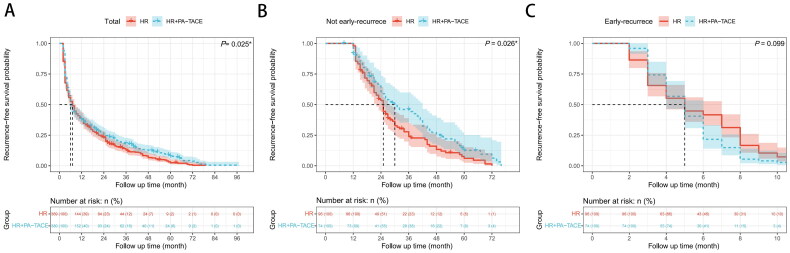
The log-rank test showed that the HR + PA-TACE group exhibited a significant superiority in RFS over the hepatectomy group (p = 0.025) (Figure 1(A)).
Figure 1.
Recurrence-free survival (RFS) of hepatocellular carcinoma patients after hepatic resection (HR) alone or with postoperative adjuvant transarterial chemoembolization (PA-TACE): comparison of RFS (A) among total patients (n = 749), (B) in the matched Early recurrence group (n = 170), (C) in the matched Not early recurrence group (n = 170). The log-rank test was used to determine significant differences between Kaplan–Meier curves.
For patients with early recurrence, there was no significant difference in RFS (p = 0.099) between the HR and HR + PA-TACE groups (Figure 1(B)). However, in the non-early recurrence subgroup, patients who received HR + PA-TACE exhibited a significantly longer RFS than those who received hepatectomy only (p = 0.026) (Figure 1(C)).
Univariate and multivariate analyses to determine the PA-TACE insensitivity factors for patients in the HR + PA-TACE group
From the survival analysis above, we noticed that the efficacy of PA-TACE was limited. We then explored the risk factors that may affect the efficacy of PA-TACE. First, all 380 patients in the HR + PA-TACE group were randomly split into the development (n = 285) and validation (n = 95) cohorts at a ratio of 3:1. The characteristics of the patients were similar between the two cohorts (Table 2).
Table 2.
Comparisons of clinical characteristics between the development cohort and the validation cohort.
| Development cohort | Validation cohort | p | |
|---|---|---|---|
| Characteristic | n = 285 | n = 95 | |
| Sex | 0.05 | ||
| Male | 255 (89.5) | 77 (81.1) | |
| Female | 30 (10.5) | 18 (18.9) | |
| Age (years) | 0.668 | ||
| Median | 47.00 | 47.00 | |
| Range | 23–71 | 24–72 | |
| IQR | 38.00–54.00 | 40.50–54.00 | |
| HBsAg | 0.373 | ||
| Negative | 39 (13.7) | 9 (9.5) | |
| Positive | 246 (86.3) | 86 (90.5) | |
| HBV-DNA (copies/ml) | 0.184 | ||
| <500 | 98 (34.4) | 25 (26.3) | |
| ≥500 | 187 (65.6) | 70 (73.7) | |
| AFP level (ng/ml) | 0.211 | ||
| <400 | 121 (42.5) | 48 (50.5) | |
| ≥400 | 164 (57.5) | 47 (49.5) | |
| Tumor size (cm)† | 0.702 | ||
| Median | 8 | 8 | |
| Range | 2–20 | 3–20 | |
| IQR | 6.00–11.00 | 6.00–11.00 | |
| Edmondson grade† | 0.585 | ||
| Poorly differentiated | 109 (38.2) | 40 (42.1) | |
| Moderately and well-differentiated | 176 (61.8) | 55 (57.9) | |
| Node number | 0.232 | ||
| <3 | 212 (74.4) | 64 (67.4) | |
| ≥3 | 73 (25.6) | 31 (32.6) | |
| Tumor capsule | 0.581 | ||
| Complete | 182 (63.9) | 57 (64.9) | |
| Incomplete | 103 (36.1) | 38 (40.0) | |
| Resection margin (cm) | 0.946 | ||
| <1 | 213 (74.7) | 70 (73.7) | |
| ≥1 | 72 (25.3) | 25 (26.3) | |
| Ki67 | 0.365 | ||
| Median | 0.40 | 0.40 | |
| Range | 0.03–0.95 | 0.05–0.90 | |
| IQR | 0.20–0.60 | 0.15–0.60 | |
| MVI | 0.274 | ||
| No | 55 (19.3) | 24 (25.3) | |
| Yes | 230 (80.7) | 71 (74.7) | |
| PVTT | 1.00 | ||
| No | 215 (75.4) | 72 (75.8) | |
| Yes | 70 (24.6) | 23 (24.2) | |
| Liver cirrhosis | 1.00 | ||
| None/mild | 211 (74.0) | 71 (74.7) | |
| Moderate/severe | 74 (26.0) | 24 (25.3) | |
| Postoperative complications | 0.38 | ||
| No | 253 (88.8) | 94 (92.6) | |
| Yes | 32 (11.2) | 7 (7.4) |
In cases involving multiple nodes, only the largest was indicated. Data are presented as n (%) by group and compared using the Pearson chi-square test or Fisher’s exact test for any cell number < 5. CNLC: China liver cancer; HBV: Hepatitis B virus; HR: Hepatic resection; MVI: Microvascular invasion; PA-TACE: Postoperative adjuvant transarterial chemoembolization; PVTT: Portal vein tumor thrombus.
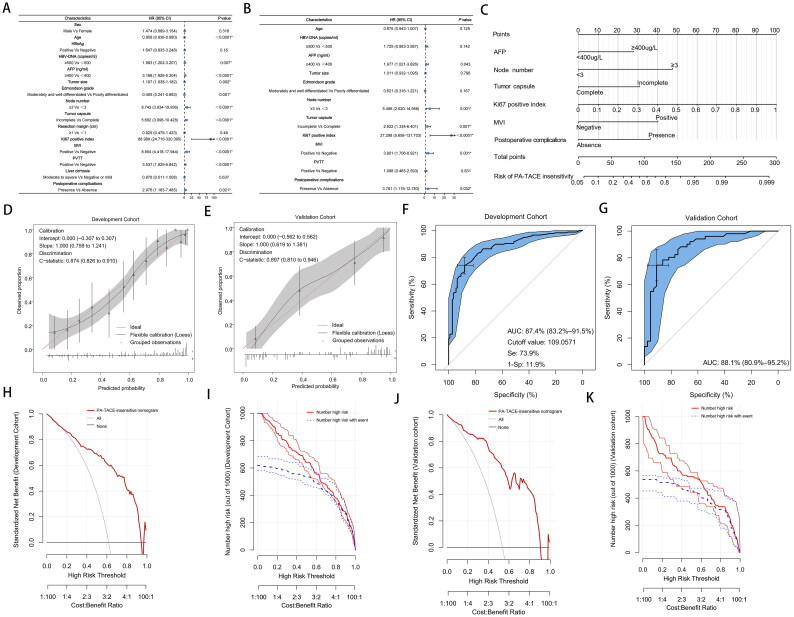
By selecting early recurrence (relapsed within 1 year) as the poor outcome variable for PA-TACE insensitivity, in the development cohort, the univariate analysis showed that PA-TACE insensitivity might be associated with age (p < 0.0001), HBV DNA (p = 0.007), AFP (p < 0.0001), tumor size (p = 0.002), Edmondson grade (p = 0.001), node number (p < 0.0001), tumor capsule (p < 0.0001), Ki67 positive index (p < 0.0001), MVI (p < 0.0001), PVTT (p < 0.0001), and postoperative complications (p = 0.021) (Figure 2(A)). Incorporating the above variables into the multivariate analysis (Figure 2(B)), we revealed that AFP (hazard ration [HR], 1.977; 95% confidence interval [CI], 1.021–3.826; p = 0.043), node number (HR, 5.496; 95% CI, 2.020–14.956; p = 0.001), tumor capsule (HR, 2.922; 95% CI, 1.334–6.401; p = 0.007), Ki67 positive index (HR, 27.298; 95% CI, 5.658–131.703; p < 0.0001), MVI (HR, 3.901; 95% CI, 1.706–8.921; p = 0.001), and postoperative complications (HR, 3.781; 95% CI, 1.119–12.783; p = 0.032) were associated with a greater risk of PA-TACE insensitivity.
Figure 2.
(A) Univariable and (B) multivariable analyses of associations in PA-TACE-insensitive of hepatocellular carcinoma. (C) Postoperative nomogram for predicting the PA-TACE-insensitive of hepatocellular carcinoma. Calibration curve for predicting PA-TACE-insensitive in the (D) development cohort and (E) validation cohort. Receiver operating characteristic curve of the postoperative nomogram in (F) the development cohort and (G) the validation cohort. AUC, Area under the curve. The cutoff value was determined by ROC curve. DCA of the PA-TACE- insensitive nomogram in (H) the development cohort and (I) the validation cohort. The X axis shows the high-risk threshold, and the Y axis represents the standardized net benefit. Clinical impact curves of the PA-TACE-insensitive nomogram in (J) the development cohort and (K) the validation cohort. The number of high-risk patients (blue dotted line) and the number of high-risk patients with events (red solid line) are plotted.
Nomogram model for PA-TACE non-benefit patient prediction
Given that PA-TACE insensitive patients may form PA-TACE non-benefit populations, a nomogram model was built based on six risk factors identified in univariate and multivariate analyses to predict PA-TACE non-benefit patients in the development cohort, and the predictive accuracy was shown by the calibration curves (Figures 2(C–E)). The discrimination and prognostic accuracy of the prognostic model were calculated using the C-index in the development cohort and in the validation cohort at 0.874 (95% CI, 0.826–0.910) and 0.897 (95% CI, 0.810–0.946), respectively (Figures 2(D,E)). Based on the nomogram model, the score of each patient was calculated, and ROC curve analysis showed area under the curve (AUC) values of 0.874 (95% CI, 0.832–0.915) in the development cohort (Figures 2(F,G)). The optimal cut-off score for predicting PA-TACE non-benefit patients was 109.057, and the specificity and sensitivity were 88.1 and 73.9%, respectively (Figure 2(F)). Similar results were obtained in the validation cohort, with AUC values of 0.881 (95% CI, 0.809–0.952) (Figure 2(G)). DCA indicated that this nomogram can serve as a valuable forecasting tool for PA-TACE non-benefit patient prediction (Figures 2(H,I)). Clinical impact curves also showed a remarkable predicted efficacy of the nomogram (Figures 2(J,K)). The novel nomogram for predicting the insensitivity of PA-TACE was reliably validated and had good clinical utility.
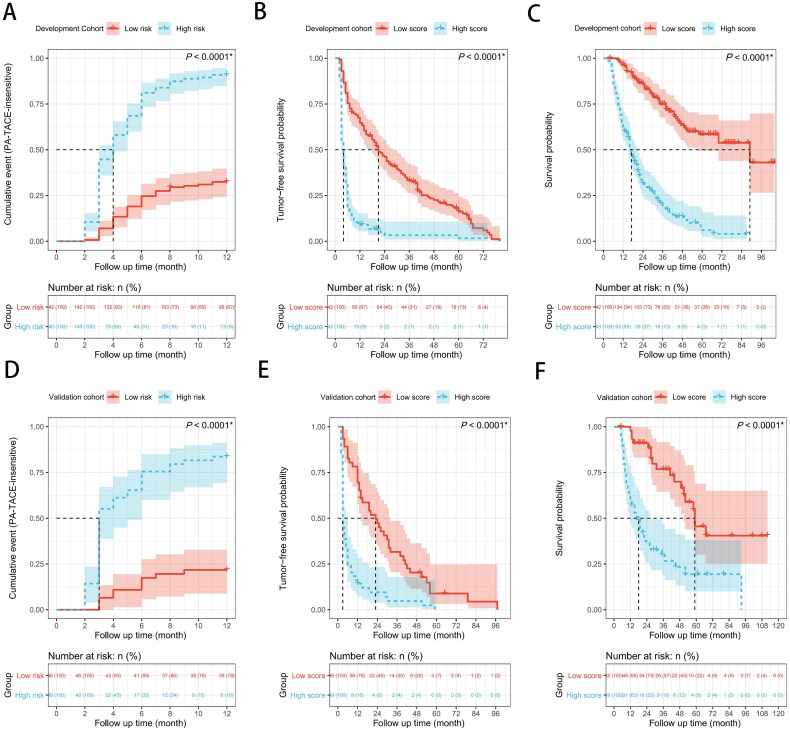
In addition, with a threshold score of 109.057, patients were partitioned into a high-score group (≥109.057) and a low-score group (<109.057). The high-score group had significantly lower TFS and OS than the low-score group, according to the log-rank test (all p < 0.0001, Figures 3(A–F)).
Figure 3.
(A) Possibility of PA-TACE-insensitive, (B) recurrence-free survival (RFS), and (C) overall survival (OS) between the low score group and the high score group in the development cohort. (D) Possibility of PA-TACE-insensitive, (E) recurrence-free survival (RFS), and (F) overall survival (OS) between the low score group and the high score group in the validation cohort. The log-rank test was used to determine significant differences between Kaplan–Meier curves.
Survival analysis with respect to the score differences between HR and HR + PA-TACE groups
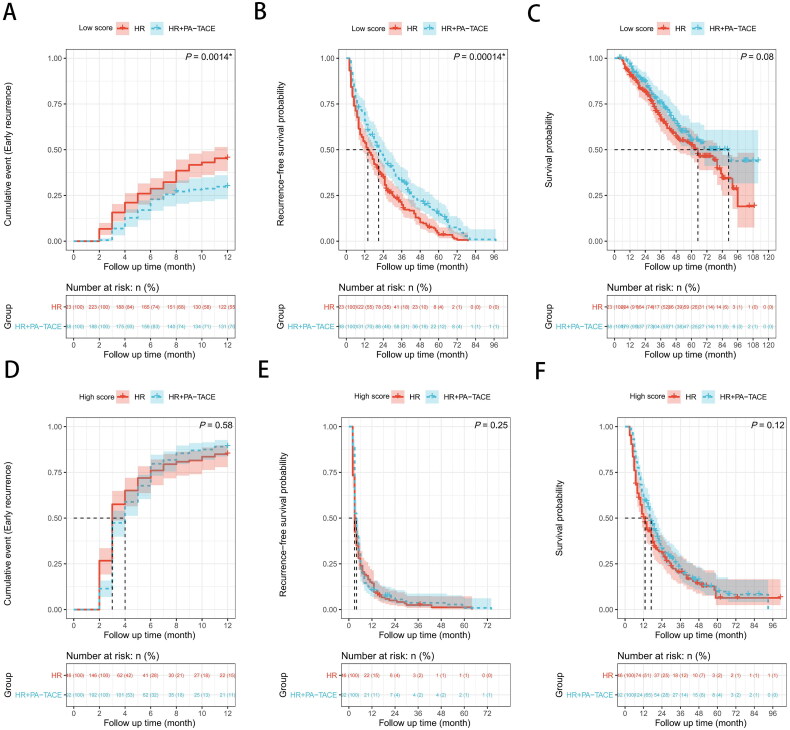
Among the patients with low scores, the HR + PA-TACE group showed a significantly lower PA-TACE-insensitive probability than the HR only group, based on the results of the log-rank test (p = 0.0014, Figure 4(A)). Additionally, the HR + PA-TACE group exhibited a significant superiority in RFS (p = 0.00014, Figure 4(B)), but there was no significant difference in OS (p = 0.08, Figure 4(C)). However, among the patients with high scores, the log-rank test revealed that a non-significant difference was found between the two groups in terms of PA-TACE-insensitive probability, RFS, and OS (PA-TACE-insensitive probability, p = 0.58; RFS, p = 0.25; OS, p = 0.12; Figures 4(D–F)).
Figure 4.
Possibility of PA-TACE-insensitive, recurrence-free survival (RFS) and overall survival (OS) of hepatocellular carcinoma patients after hepatic resection (HR) alone or with postoperative adjuvant transarterial chemoembolization (PA-TACE) among total 749 patients: comparison of possibility of PA-TACE-insensitive, RFS, or OS (A–C) in the low score group (n = 338), (D–F) in the high score group (n = 411). The log-rank test was used to determine significant differences between Kaplan–Meier curves.
Characteristic association and prognosis of relapsed patients
The overall tumor recurrence rate was 94.53% (708/749) at the end of follow-up, including 354 patients in the HR group and 354 in the HR + PA-TACE group. The four most common recurrence patterns are summarized as follows: pattern I (36.44%), pattern II (26.13%), pattern III (25.99%), and pattern IV (11.44%).
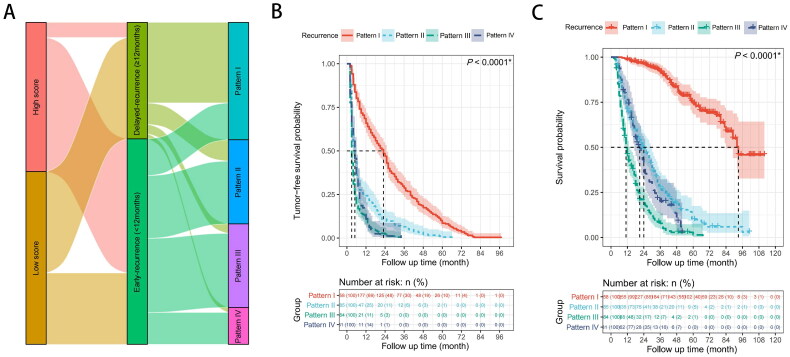
The Sankey diagram shows that most of the patients with high scores (89.94%) displayed early recurrence. However, early recurrence only occurred in less than half of patients with low scores. In addition, Compared with patients without early recurrence, patients with early postoperative recurrence showed much higher rates of pattern IV recurrence (15.49 vs. 4.30%), pattern III recurrence (36.06 vs. 8.20%), and pattern II recurrence (30.53 vs. 18.36%). Pattern I was the most frequent type of recurrence among patients with delayed recurrence (69.14%) (Figure 5(A)).
Figure 5.
(A) Characteristic association and outcome flow of relapsed patients. (B,C) Recurrence-free survival (RFS) or overall survival (OS) of relapsed patients (n = 708): comparison of RFS (B) or OS (C) in four groups. The log-rank test was used to determine significant differences between Kaplan–Meier curves. Pattern I: solitary-intrahepatic recurrence; Pattern II: multi-intrahepatic recurrence; Pattern III: intrahepatic recurrence with vascular invasion and/or extrahepatic recurrence; Pattern IV: extrahepatic recurrence alone.
Of the 708 patients who relapsed after liver resection, the log-rank test revealed that the RFS and OS of relapsed patients with patterns II, III, and VI were much shorter than those of pattern I (both p < 0.0001, Figures 5(B,C)).
Discussion
Whether PA-TACE is treated as an adjunct treatment option to reduce the risk of recurrence in patients with a high rate of relapse remains controversial. Because the criterion of PA-TACE recommended patients only use general ‘high-risk factors’, it does not meet the requirements of precision medicine. Our results suggest that the efficacy of PA-TACE is limited. We noticed that the tumor-free survival curve showed statistical differences between the HR + PA-TACE and HR groups, while the median tumor free-survival (MTFS) was similar, especially in patients with early recurrence. This indicates that some underlying factors may also exist in HCC patients, which may result in PA-TACE-insensitivity in patients. Early recurrence predicting poor prognosis in HCC patients is generally agreed upon. Therefore, in the present study, we consider that patients who received PA-TACE still experienced early recurrence as often as PA-TACE-insensitive patients, and it is clinically significant to identify the independent and related risk factors. Using univariate and multivariate analyses for HR + PA-TACE patients in this study, we identified six clinicopathologic factors associated with PA-TACE-insensitivity in patients, with four of these being recognized tumor-associated factors that reflect primary tumor malignant-behavior characteristics (incomplete tumor capsule, multi-tumor nodes, MVI, and high Ki67 index). This suggests that the high malignant potential of primary tumors may play an essential role in PA-TACE-insensitivity in patients. Interestingly, three of the four malignant behavior factors (incomplete tumor capsule, multi-tumor nodes, and MVI) were the general ‘high-risk factors’ that were used as a basis for PA-TACE recommendations before, which to a certain extent, reflected the limitation of traditional standards for PA-TACE beneficiaries.
Ki67, an important marker of cellular proliferation that influences the cell cycle, has been rarely mentioned when assessing whether a patient would be a beneficiary of PA-TACE treatment. Studies suggest that the rapid recurrence after radical resection of HCC may be due to the presence of occult micro-metastasis of the high proliferative initial tumor before [22–25] There is a reasonable likelihood that HCCs with high expression of Ki67 exhibit strong proliferation potential, as with disseminated tumor cells (DTCs) in blood. If the primary tumor cells of these patients have high Ki67 expression levels, the tumor cells scattered in the blood may also have homogeneity to the primary tumor cells. When these highly proliferative DTCs colonize the residual liver or reach distant fields, such as the lungs, brain, or bones, they will retain the homogeneity of the primary tumor and thus, lead to early recurrence. This has been well reflected in the present study. As a representative variable of tumor proliferation and stemness characteristics, Ki67 occupies a significant weight in this model. Our results showed that high-risk patients who were not sensitive to PA-TACE tended to relapse early after surgery and had extrahepatic metastasis as one of the main modes of recurrence. In addition, most of the proliferative tumors are often characterized by immune deficiency and lower aerobic metabolism [26], which may offset the hypoxia suppression effect caused by PA-TACE. Together, the role of Ki67 in promoting this flexibility may explain why patients with a high Ki67-index are insensitive to PA-TACE. It is worth mentioning that several studies have shown that tumors with lower Ki67 expression are more sensitive to chemotherapeutics but are more easily recognized by the immune system. Therefore, this suggests that they may have a favorable response to immunotherapy [27,28].
Another interesting factor is postoperative complications, which may influence the patient’s immune status and contribute to tumor recurrence and prognosis. This may indicate that PA-TACE is unsuitable for patients with severe postoperative complications. However, postoperative complications were the only modifiable risk factor among the six factors identified. Thus, reducing the incidence of postoperative complications by improving the perioperative management of patients may improve the outcomes of HCC.
Additionally, it was noted that PVTT is not an independent risk factor for PA-TACE insensitivity. For HCC patients with PVTT, PA-TACE was perceived as a beneficial option [29,30]. According to our findings, this is indeed the case, and patients with PVTT have more hope in preventing early postoperative recurrence and improving short-term or long-term survival by PA-TACE.
Several studies have developed prognostic models related to PA-TACE or early relapse in patients with HCC. In 2016, Hu et al. published a nomogram to predict the prognosis of PA-TACE in patients with HBV-related HCC. The study set comprised 235 samples, and the nomogram demonstrated good accuracy with a C index of 0.75 (95% CI, 0.67–0.83), but the population was restricted to HBV–related HCC, which may limit the generalizability of the system [31]. In 2020, Liu et al. developed a PA-TACE-associated scoring system by retrospective analysis of 293 HCC patients with PVTT with a prognosis of 30.7% in patients who received PA-TACE once a month after hepatectomy. The focus of the study was the survival of HCC patients with PVTT, rather than the screening of PA-TACE beneficiaries. The two recent studies have limitations in versatility, and focus on RFS or OS, but lack the exploration of recurrence types [29]. Several other studies have shown similar limitations [32,33].
However, there is still no effective predictive model for PA-TACE insensitivity. Given that PA-TACE insensitivity of patients may be multifactorial, an ideal predictive model would be generated based on the risk variables selected from these six clinicopathologic factors. In the present study, based on the six variables mentioned above, we established a nomogram model to predict PA-TACE insensitivity. Multi-dimensional verification of the model exhibited excellent performance. The model demonstrated stronger diagnostic capabilities, as shown by calibration plots and ROC analysis (in the development cohort and validation cohort: C index, 0.874 and 0.897 (95% CI, 0.826–0.910 and 0.810–0.946); AUC: 0.874 and 0.881 (95% CI, 0.832–0.915 and 0.809–0.952). Clinical impact curve and DCA analysis also indicated that the nomogram had satisfactory predictive power, and the availability of the variables would make this model easy to use in real-world clinical applications. According to this model, patients with high scores should not be recommended for PA-TACE treatment, while patients with low scores should be cautiously recommended after multiple considerations.
Survival analysis further confirmed the reliability of the model in clinical applications. With a total of 749 patients, according to the cutoff value determined by the ROC curve, patients with low-scores have a significantly lower PA-TACE-insensitivity rate and better OS than did those with high-scores. Although PA-TACE has not shown a statistically significant improvement in OS in the low-scoring group, this was probably due to our limited follow-up time. These findings indicate that patients with a low score rather than that with a high score based on our model may serve as potential target therapeutic candidate populations for PA-TACE. From this, we can recognize the important theoretical and practical value of identifying and expanding follow-up indicators in HCC patients undergoing resection, as these are of great significance in guiding the design of post-operative protocol.
Compared with previous studies, this study has an innovative design. Patient survival time is the result of the combined effects of many factors, including, but not limited to, tumors, such as the patient’s other underlying diseases, and even emotional life. In comparison, the recurrence time is determined more by the heterogeneity of the tumor itself. We first grouped all patients into the early-recurrence group and the non-early-recurrence group according to whether there was a relapse within one year after surgery and compared the recurrence-free survival of each group of patients with or without PA-TACE treatment, to identify the sensitivity of each group to PA-TACE. Then, for the group where PA-TACE did not improve survival, patients at risk of such relapses are insensitive to PA-TACE, so as to reversely deduce high-risk factors that are not sensitive to PA-TACE. According to our survival analysis of recurrence groups, patients with early recurrence clearly indicate a poor prognosis. Therefore, evaluating the prognosis of PA-TACE patients with early recurrence as the endpoint can eliminate bias caused by non-tumor-related factors in predicting OS, and it is more consistent with the original intention of PA-TACE, to prevent early relapse. This research path of finely dividing patient outcomes and inferring inversely is more in line with the clinical situation.
The primary limitation of the current study is its retrospective design. The lack of external verification is also a shortcoming of this research. In addition, single-center studies may also have some inevitable selection bias, and the samples included in this study are all from China, which has a high incidence of HBV-related HCC. Thus, multi-center and large-scale randomized controlled trials are necessary to verify the clinical application value of our nomogram.
Conclusion
In conclusion, in this study, the curative effect of PA-TACE for patients with HCC after curative hepatectomy was verified, and a validated novel applicable nomogram model was constructed to identify target populations that derive the greatest benefit from PA-TACE, including patients with AFP, tumor capsule, Ki67 positive index, MVI, and postoperative complications. This scoring system would be clinically convenient to facilitate the individualized prediction of the necessity of PA-TACE in HCC.
Ethical approval
The protocol for this study was approved by the Ethics Review Committee of Guangxi Medical University Cancer Hospital (LW20222132).
Funding Statement
This work was supported by the National Natural Science Foundation of China (81972306 and 82273405).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Lu-Nan Qi: conceptualization, funding acquisition, and writing—review and editing; Yu-Chong Peng: conceptualization, data curation, project administration, and resources; Jing-Xuan Xu: formal analysis, methodology, and writing—original draft; Shui-Lin Qin: methodology and investigation; Hao-wen Wei: validation and visualization; Yuan-Yuan Chen: resources, validation, and supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Bruix J, Gores GJ, Mazzaferro V.. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J.. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. [DOI] [PubMed] [Google Scholar]
- 3.Imamura H, Matsuyama Y, Tanaka E, et al. . Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. [DOI] [PubMed] [Google Scholar]
- 4.Poon RTP. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54(3):757–759. [DOI] [PubMed] [Google Scholar]
- 5.Du Z-G, Wei Y-G, Chen K-F, et al. . Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution. Hepatobiliary Pancreat Dis Int. 2014;13(2):153–161. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T, Imazeki F, Wu S, et al. . The assessment of serum hepatitis C virus RNA 12 weeks after the end of treatment using TaqMan polymerase chain reaction is less relevant than after 24 weeks for predicting sustained virological response. Hepatology. 2011;54(4):1482; author reply 1482–1483. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Zhang X, Cui R, et al. . Prognostic predictors for patients with hepatocellular carcinoma receiving adjuvant transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2019;31(7):836–844. [DOI] [PubMed] [Google Scholar]
- 8.Tao X, Lai ECH, Min AR, et al. . Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: a non-randomized comparative study. Hepato-gastroenterology. 2012;59(116):1198. [DOI] [PubMed] [Google Scholar]
- 9.Jing JS, Kang W, Zhang CZ, et al. . Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2015;23(4):1344–1351. [DOI] [PubMed] [Google Scholar]
- 10.Tong Y, Li Z, Liang Y, et al. . Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? a propensity score analysis. Oncotarget. 2017;8(16):26671–26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz JD, Schwartz M, Mandeli J, et al. . Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3(10):593–603. [DOI] [PubMed] [Google Scholar]
- 12.Jiang JH, Guo Z, Lu HF, et al. . Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol. 2015;21(15):4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng-Gang R, Zhi-Ying L, Jing-Lin X, et al. . Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;19:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Zheng W, Zhenggang R, et al. . Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Kaufman PD.. Ki-67: more than a proliferation marker. Chromosoma. 2018;127(2):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorbye SW, Kilvaer TK, Andrej V, et al. . Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLOS One. 2012;7(10):e47068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciancio N, Galasso MG, Campisi R, et al. . Prognostic value of p53 and Ki67 expression in fiberoptic bronchial biopsies of patients with non-small cell lung cancer. Multidiscip Respir Med. 2012;7(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai T-J, Chau G-Y, Lui W-Y, et al. . Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127(6):603–608. [DOI] [PubMed] [Google Scholar]
- 19.Cho YB, Lee KU, Lee HW, et al. . Outcomes of hepatic resection for a single large hepatocellular carcinoma. World J Surg. 2007;31(4):795–801. [DOI] [PubMed] [Google Scholar]
- 20.Zhu AX, Duda DG, Sahani DV, et al. . HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ya-Peng Q, Jian-Hong Z, Zhi-Yin L, et al. . Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217(4):739–744. [DOI] [PubMed] [Google Scholar]
- 22.Xing H, Zhang WG, Cescon M, et al. . Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB. 2020;22(5):677–689. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Li J, Yan J, et al. . Early recurrence after curative resection in oligonodular hepatocellular carcinoma. Hepato-gastroenterology. 2013;60(121):28–31. [DOI] [PubMed] [Google Scholar]
- 24.Lee HY, Rhim H, Lee MW, et al. . Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23(1):190–197. [DOI] [PubMed] [Google Scholar]
- 25.Shimoda M, Tago K, Shiraki T, et al. . Risk factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg. 2016;40(10):2466–2471. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Zhu H, Dong L, et al. . Integrated proteogenomic characterization of HBV-Related hepatocellular carcinoma. Cell. 2019;179(5):1240. [DOI] [PubMed] [Google Scholar]
- 27.Mrouj K, Andrés-Sánchez N, Dubra G, et al. . Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc Natl Acad Sci USA. 2021;118(10):e2026507118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cidado J, Hong YW, Rosen DM, et al. . Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget. 2016;7(5):6281–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Guo X, Dong W, et al. . Postoperative adjuvant TACE-associated nomogram for predicting the prognosis of resectable hepatocellular carcinoma with portal vein tumor thrombus after liver resection. Int J Biol Sci. 2020;16(16):3210–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Guo L, Li H, et al. . Postoperative adjuvant trans-arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol. 2018;25(7):2098–2104. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Han XK, Long XR, et al. . Prognostic nomogram for post-surgical treatment with adjuvant TACE in hepatitis B virus-related hepatocellular carcinoma. Oncotarget. 2016;7(36):58302–58314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu S, Gan W, Qiao L, et al. . A new prognostic algorithm predicting HCC recurrence in patients with Barcelona clinic liver cancer stage B who received PA-TACE. Front. Oncol. 2021;11:4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao S, Shan Y, Yu X, et al. . A new prognostic model predicting hepatocellular carcinoma early recurrence in patients with microvascular invasion who received postoperative adjuvant transcatheter arterial chemoembolization. Eur J Surg Oncol. 2023;49(1):129–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.