Abstract
Endovascular arteriovenous fistulas (eAVFs) are a new and less invasive type of creation for dialysis access. The anastomosis for eAVFs often occurs between the ipsilateral proximal radial artery and vein or proximal ulnar artery and vein. As eAVF creations are in locations that are not traditionally used for surgical AVF creations, the question of how to approach reversal of these fistulas arises. Here we present a case of closure of an ulnar artery to ulnar vein eAVF.
Keywords: Closure of endovascular fistula, endovascular arteriovenous fistulas, endovascular fistula revision, WavelinQ
End-stage renal disease is a prevalent problem that affects nearly 786,000 Americans.1 One of the significant and costly challenges of hemodialysis is access. Interestingly, the radiocephalic fistula was first introduced by Cimino et al in 1966.2 Later the brachiocephalic and brachiobasilic fistulas were employed as well. Most recently, two systems of either percutaneous or endovascular fistulas not requiring traditional surgery were approved by the US Food and Drug Administration in February 2019.3 The fistula creation can be done in an outpatient setting such as an ambulatory surgery center or in office-based labs with regional or local anesthesia. Early studies have been promising regarding patency rates and potentially limited need for reintervention.4 Our case report focuses on the WavelinQTM endovascular arteriovenous fistula (eAVF) by Becton Dickinson, which is an endovascularly created anastomosis between the ipsilateral proximal radial artery and vein or proximal ulnar artery and vein. Percutaneous access is obtained in both the venous and arterial system of the upper extremity, and with the aid of fluoroscopy, two catheters are magnetically guided for precise alignment and joining of adjacent vessels. A burst of radiofrequency energy creates a connection between the artery and vein. The blood then courses from the artery to the deep vein (radial or ulnar) and then to a superficial vein (cephalic or median cubital) via the perforating vein. Thereby, a connection in the deep venous system transmits flow to the superficial veins, which are the veins cannulated with two needles for hemodialysis. Frequently, after the endovascular fistula is created at the index procedure, a coil is placed in one of the brachial veins centrally to the anastomosis to drive more flow into the superficial system.5 One of the questions that arises with these creations is if an EVF needs to be closed, how can it be done? The location of the endovascular fistula in the ulnar location can be a challenging surgical approach not frequently performed and multiple side branches of the vein can make finding the anastomosis challenging. We present a percutaneous closure of an eAVF. To our knowledge, this is the first reported case of the use of a covered stent to endovascularly exclude an eAVF created for hemodialysis access.
CASE DESCRIPTION
An 81-year-old man with end-stage renal disease and a previous eAVF creation presented to the clinic with mild to moderate right forearm swelling secondary to venous hypertension from central deep vein stenosis. On examination, he had palpable radial pulses 2+ bilaterally. A weak thrill over the eAVF and a bruit on Doppler were detected. We reviewed the previous records and attempts to increase flow into the cephalic vein were unsuccessful. We were asked to close the eAVF to alleviate the forearm swelling.
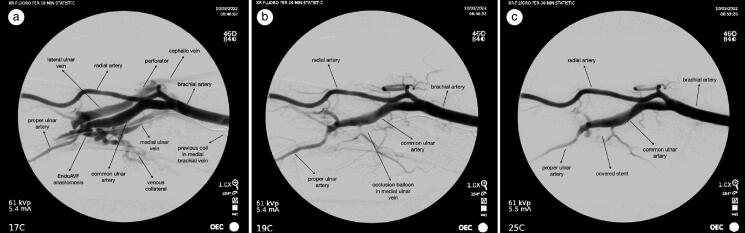
In the operating suite, we accessed the medial ulnar vein with a micropuncture 4 cm proximal to the wrist crease. A 6F sheath was then used for angiography, which showed a patent eAVF with significant collateral filling of deep veins consistent with his forearm swelling (Figure 1a). A second access was obtained in the radial artery just proximal to the wrist crease. Arterial injection to localize the area of the endovascular anastomosis was then performed. To evaluate the feasibility of endovascular occlusion, an angled glide catheter and wire was used to traverse the ulnar vein peripherally and centrally to the anastomosis. A 5 mm × 4 cm balloon was then inflated across the area, and we injected the artery, which showed no further filling of the eAVF (Figure 1b). The balloon was deflated and removed. A covered 5 mm × 5 cm Gore Viabahn stent was placed via the 6F sheath in the medial ulnar vein and deployed under fluoroscopic guidance. Postdeployment injection through the radial artery confirmed occlusion of the endovascular anastomosis (Figure 1c). Injection through the vein confirmed patency of the deep venous system after stent deployment.
Figure 1.
(a) Initial angiogram. (b) Trial balloon occlusion and filling of endoAVF. (c) After stent placement.
DISCUSSION
As eAVFs are fistulas, they present with many of the same challenges as surgical fistulas with a few caveats regarding access. The most common complication at the time of creation is a hematoma at the arterial access site, in which the complication rate for WavelinQ was 8.59% in a meta-analysis.6 Other complications for percutaneous AVFs include pseudoaneurysms at the creation or access site, early thrombosis, pulmonary embolism from coil migration, ischemic monomelic neuropathy, and risk of median nerve injury with brachial artery access.7–9 In this case report, the complication was multifaceted, in which a combination of proximal stenosis and venous hypertension from previous coil embolization caused congestion in the deep system, as there were not adequate connections to the superficial system (i.e., cephalic vein) through perforators.
Surgical AVFs are classically created in the easier to approach radial or brachial artery, but the common ulnar artery is difficult to approach surgically.10 eAVFs are frequently commonly created between the common ulnar artery (segment between the brachial bifurcation and prior to the takeoff of the interosseus and proper ulnar artery) and medial ulnar vein5 secondary to the larger size of both the common ulnar arteries and veins in this area. This endovascular advantage in access to the ulnar artery during eAVF creation translates similarly for the patient in this case report when considering approaches to occlusion of eAVFs.
In this case report, the patient had a previous eAVF with coil embolization in the brachial vein, resulting in difficulty gaining access in an antegrade manner. It was optimal to gain access percutaneously from the wrist and forearm to avoid working against the coil and vessel valves, and the veins in this location were dilated secondary to the venous hypertension, allowing easier access. Additionally, arterial access from the radial or ulnar artery was gained simultaneously, which is important—especially when working on eAVFs that have been in place for a few weeks to months, as a large number of collateral dilations can make locating the anastomosis quite challenging. This simultaneous arterial and venous access allows accurate delineation of the location of the anastomosis, as unlike with surgical AVFs, it is more difficult to identify the location of the anastomosis of eAVFs because it is a side-to-side anastomosis with multiple collateral filling. Another important consideration when approaching covered stent occlusion of the fistula is that the placement of the stent should be on the venous side to prevent future arterial complications. We also found it quite helpful to inflate a balloon prior to stent placement to ensure the access would be occluded if a covered stent was placed in that location.
When endovascular fistulas were approved by the Food and Drug Administration in February 2019, attention was centered around creation. More recently, the focus has shifted to understand the procedural approach to malfunctioning fistulas, including the occlusion of eAVFs if needed. The exact role that eAVFs will play in dialysis access creation is still being elucidated, yet the advent of percutaneous creation of dialysis access has broadened the scope of approach to not only fistula creation, but also fistula revision. Our unique approach allowed for the endovascular occlusion of a fistula created endovascularly.
Disclosure statement/Funding
Stephen E. Hohmann is a consultant and speaker for Becton Dickinson, who produces the WavelinQ. Erin Cha has no potential conflict to report. The authors report no funding. The patient gave permission for publication of this report.
References
- 1.US Renal Data System . 2022. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2022. https://adr.usrds.org/2020/end-stage-renal-disease. [Google Scholar]
- 2.Bressica MJ, Cimino JE, Appell K, Hurwich BJ, Scribner BH.. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. J Am Soc Nephrol. 1999;10(1):193–199. [PubMed] [Google Scholar]
- 3.WavelinQ 4F EndoAVF System 510(k) Premarket Notification . Page last updated: 01/02/2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K182796.
- 4.Lok CE, Rajan DK, Clement J, et al. ; NEAT Investigators . Endovascular proximal forearm arteriovenous fistula for hemodialysis access: results of the prospective, multicenter Novel Endovascular Access Trial (NEAT). Am J Kidney Dis. 2017;70(4):486–497. doi: 10.1053/j.ajkd.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Rajan DK, Ebner A, Desai S, Rios J, Cohn W.. Percutaneous creation of an arteriovenous fistula for hemodialysis access. J Vasc Interv Radiol. 2015;26(4):484–490. doi: 10.1016/j.jvir.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Yan Wee IJ, Yap HY, Tang TY, Chong TT.. A systematic review, meta-analysis and meta-regression of the efficacy and safety of endovascular arteriovenous fistula creation. J Vasc Surg. 2020;71(1):309–317.e5. doi: 10.1016/j.jvs.2019.07.057. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AM, Grocott M, Schwartz MS, Modarres H, Scott M, Schon F.. Median nerve injury: an underrecognized complication of brachial artery cardiac catheterisation? J Neurol Neurosurg Psychiatry. 1997;63(4):542–546. doi: 10.1136/jnnp.63.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda K, Osamura N.. Median nerve palsy: a complication of brachial artery cardiac catheterization. Hand Surg. 2011;16(3):343–345. doi: 10.1142/S0218810411005667. [DOI] [PubMed] [Google Scholar]
- 9.Chorney MA, Marino AG, Perez Lozada JCL.. Ischemic monomelic neuropathy after percutaneous fistula creation. J Vasc Interv Radiol. 2021;32(4):624–626. doi: 10.1016/j.jvir.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Palmes D, Kebschull L, Schaefer RM, Pelster F, Konner K.. Perforating vein fistula is superior to forearm fistula in elderly haemodialysis patients with diabetes and arterial hypertension. Nephrol Dial Transplant. 2011;26(10):3309–3314. doi: 10.1093/ndt/gfr004. [DOI] [PubMed] [Google Scholar]