Abstract
Purpose
The purpose of this prospective longitudinal study was to evaluate the changes in brain surface gyrification in older long-term breast cancer survivors 5 to 15 years after chemotherapy treatment.
Methods
Older breast cancer survivors aged ≥ 65 years treated with chemotherapy (C+) or without chemotherapy (C−) 5–15 years prior and age & sex-matched healthy controls (HC) were recruited (time point 1 (TP1)) and followed up for 2 years (time point 2 (TP2)). Study assessments for both time points included neuropsychological (NP) testing with the NIH Toolbox cognition battery and cortical gyrification analysis based on brain MRI.
Results
The study cohort with data for both TP1 and TP2 consisted of the following: 10 participants for the C+ group, 12 participants for the C− group, and 13 participants for the HC group. The C+ group had increased gyrification in 6 local gyrus regions including the right fusiform, paracentral, precuneus, superior, middle temporal gyri and left pars opercularis gyrus, and it had decreased gyrification in 2 local gyrus regions from TP1 to TP2 (p < 0.05, Bonferroni corrected). The C− and HC groups showed decreased gyrification only (p < 0.05, Bonferroni corrected). In C+ group, changes in right paracentral gyrification and crystalized composite scores were negatively correlated (R = −0.76, p = 0.01).
Conclusions
Altered gyrification could be the neural correlate of cognitive changes in older chemotherapy-treated long-term breast cancer survivors.
Keywords: Gyrification index, Breast cancer, Cancer-related cognitive impairment, Chemotherapy
Introduction
More than 4 million women have a history of breast cancer, and additional newly identified 287,850 cases have been reported as of January 1, 2022 in the United States alone [1]. Besides, more than 2.7 million breast cancer survivors are 65 years and older [1]. Prior studies have shown that chemotherapy-treated breast cancer survivors suffer from cancer-related cognitive impairment (CRCI) [2-4]. CRCI mainly affects memory, attention, and executive functioning in older long-term survivors [5-7].
Neuroimaging studies have shed light on brain structural and functional alterations underlying CRCI in breast cancer survivors [8-10]. Previous studies have found a significant reduction in brain gray matter (GM) and white matter in long-term breast cancer survivors at ten or even twenty years after chemotherapy [11-13]. GM atrophy has been known to have a significant association with cognitive dysfunction amongst breast cancer survivors [14-20]. Our previous study of older breast cancer survivors showed cortical thinning in older long-term breast cancer survivors [9].
Cortical Gyrification is a morphometric feature related to the geometry of the brain surface [21, 22]. Since GM forms an outer layer of the brain surface, the alterations in gyrification result in changes in cortical surface area and cortical GM volume [23]. Gyrification analysis focuses on brain morphometric features that are not identified by GM or cortical thickness [24]. During brain development, gyrification increases and peaks during childhood, promptly decreases during the adolescent stage and then gradually decreases with age [24-27]. Thus, gyrification is expected to decrease with aging [24]. The decreased gyrification is considered an early morphometric biomarker for cognitive changes in patients with Alzheimer’s disease (AD) [28], subjective cognitive impairment [29], autism [30], mild traumatic brain injury [31] and in healthy individuals with normal aging [32]. In addition to the decreased gyrification patterns, prior studies on schizophrenia [24], AD [33], traumatic brain injury [34] and autism [35] have also showed increased gyrification patterns which was associated with cognitive impairment. A previous study of CRCI showed decreased gyrification in patients with breast cancer aged 29 to 68 years shortly after chemotherapy [36]. However, there is limited literature on gyrification in older long-term breast cancer survivors.
Here, we conducted a longitudinal study to assess the brain surface gyrification changes in older breast cancer survivors. We hypothesized that gyrification would be decreased in the older long-term breast cancer survivors with exposure to chemotherapy, which would be correlated with cognitive changes. To test this hypothesis, we assessed brain gyrification on brain MRI and cognitive performance via neuropsychological (NP) testing in older breast cancer survivors who had chemotherapy treatment 5–15 years prior to enrollment and compared this group to the two control groups including the no-chemotherapy group and healthy control group over two years.
Methods
a. Subjects
The study was a neuroimaging sub-study of a multicenter trial of long-term breast cancer survivors (parent trial: Cognition in Older Breast Cancer Survivors: Treatment Exposure, APOE and Smoking History, NCT02122107). Breast cancer survivors treated with chemotherapy (C+) or without chemotherapy (C−) 5-15 years prior and age & sex-matched healthy controls (HC) with no history of cancer were enrolled. All participants were aged ≥ 65 years at the time of initial enrollment. Study assessment included brain MRI and NP attesting with the National Institute of Health (NIH) Toolbox Cognition Battery both at time point 1 (TP1) upon enrollment and at the 2-year interval at time point 2 (TP2). The eligibility criteria for breast cancer survivors were the following: woman aged 65 years and older with a history of stage I-III breast cancer with or without chemotherapy treatment at 5 to 15 years after surviving breast cancer, and no contraindications such as orbital metal or claustrophobia for brain MRI scans. Exclusion criteria included the following: history of stroke, psychiatric disease, metastatic disease, or any other cancer. Age and sex-matched HCs were enrolled with similar criteria except for the history of cancer. The HCs were recruited via local newspaper advertisements, patient referrals, and community health fairs. This study was approved by the Institutional Review Board (IRB) of City of Hope National Medical Center. Written informed consent was obtained from all participants in compliance with institutional guidelines and the Declaration of Helsinki, as well as local, state, and federal regulations from all participating subjects.
b. MRI acquisition and gyrification analysis
All brain MRI scans were acquired for both TP1 and TP2 in the same in-house 3T VERIO Siemens scanner (Siemens, Erlangen, Germany). Structural three-dimensional (3D) T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images were acquired with the following parameters: TR = 1900 millisecond (ms), TE = 2.94 ms, inversion time = 900 ms, FA = 9°, and voxel size = 0.45 x 0.45 x 1.5. Incidental brain pathology was assessed on the T1-weighted MPRAGE and fluid-attenuated inversion recovery (FLAIR) images by the neuroradiologist in the study (BC). The cortical gyrification analysis was performed using the Computational Anatomy Toolbox (CAT12) [24] from the T1-weighted images. All images were manually re-oriented using the statistical parametric mapping toolbox (version SPM12) (Wellcome Department of Cognitive Neurology, UK). The CAT12 and SPM12 toolboxes for our analysis were based on MATLAB (R2019b). The mean gyrification values were analyzed based on Desikan-Killiany (DK40) cortical atlas [37, 38]. We followed the standard pipeline and settings for preprocessing and gyrification analysis [24]. The main steps were as follows: i) extraction of central surface, ii) estimation of the local absolute mean curvature from each vertex point within the 3 mm of this central surface given point, iii) smoothing and resampling of the gyrification maps using full width at half maximum (FWHM) gaussian filter at 20 mm.
c. NP testing with NIH toolbox for cognition
The NP testing was performed using the NIH Toolbox Cognition Battery [39, 40]. This cognitive testing battery generated seven individual scores for List Sorting Working Memory, Picture Sequence Memory, Pattern Comparison Processing Speed, Oral Reading Recognition, Picture Vocabulary, Flanker Inhibitory Control, and Dimensional Change Card Sorting. Additionally, the crystalized, fluid, and total composite cognition scores, were also generated.
d. Statistical analysis
Clinical and demographic information was assessed using analysis of variance (ANOVA) for continuous variables. Categorical variables were analyzed using Fisher’s exact tests. Threshold of p-value at 0.05 was considered statistically significant for both continuous and categorical variables, and all tests were two-sided.
NP test performance was analyzed using a generalized linear model (GLM) with the correlation of repeated measurements within subjects [9, 41]. Group (C+, C−, HC) and time-point (TP1, TP2) were considered categorical fixed effects in this analysis. Using the GLM, we tested the following: 1) whether there were any differences in NP scores between the 3 groups at TP1 or TP2, 2) whether there were any significant longitudinal differences within group, 3) whether there was a group by time interaction effect. SAS 9.3 (SAS Institute, Cary, NC) was used for data analyses.
Whole brain surface gyrification was compared between groups at TP1 using two-sample t-test. Within group longitudinal change over the 2-year study interval was tested using paired t-tests. In both analyses, effects were corrected for multiple comparisons for the whole brain using Bonferroni correction in the CAT12 software with a significance threshold of p < 0.05. The correlations of the mean gyrification values with NP composite scores were tested using linear regression analysis with a p-value of 0.05 being considered significant. The linear regression analysis and group by time interaction were tested using the statistical package for the social science software (SPSS, v 27, Chicago, IL).
Results
a. Demographic data
At TP1, a total of 60 participants were enrolled with 20 participants for each of the three groups, i.e., C+, C− and HC groups. At TP2, due to attrition from loss to follow-up, new cancer, new memory problems, refusal and death, the cohort consisted of 10 participants for the C+ group, 12 participants for the C− group, and 13 participants for HC group [9]. There were no significant differences among the groups in age (p = 0.75), education (p = 0.80) or race (p = 0.37) (Table 1). More detailed clinical and demographic information for this cohort has been reported [9]. In the C+ group, 80% of survivors had Stage II breast cancer. The C− group consisted of 50% survivors in stage 0, 33% survivors in stage I and 17 % survivors in stage II. In the C+ group, 90% of survivors had treatment with non-trastuzumab regimen and 10% of survivors with trastuzumab regimen (Table 1).
Table 1.
Demographic and clinical information
| Parameters | C+ N=10 |
C− N=12 |
HC N=13 |
p |
|---|---|---|---|---|
| Age years | ||||
| Mean (SD) | 74.70 (5.44) | 76.50 (4.28) | 75.54 (6.63) | 0.752 |
| Median (Range) | 72.5 (68-84) | 75.5 (71-86) | 75.00 (67-88) | |
| Race* (N, %) | ||||
| White or Caucasian | 8 (80) | 11 (92) | 13 (100) | 0.373 |
| Black | 1 (10) | 1 (8) | . | |
| Asian, Native Hawaiian | 1 (10) | . | . | |
| Other | . | . | . | |
| Highest grade (N, %) | ||||
| High school or less | 2 (20) | 4 (33) | 4 (31) | 0.805 |
| College or above | 8 (80) | 8 (67) | 9 (69) | |
| AJCC Stage (N, %) | ||||
| DCIS | 1 (10) | 6 (50) | . | |
| Stage I | 1 (10) | 4 (33) | . | |
| Stage II | 8 (80) | 2 (17) | . | |
| Regimen | ||||
| Non-Trastuzumab Regimen (N, %) | 9 (90) | |||
| Trastuzumab Regimen (N, %) | 1 (10) | |||
Abbreviations: TP1: time point 1, TP2: time point 2, C+: Chemotherapy group, C−: No-chemotherapy group, HC: Healthy control group, BMI: Body mass index, SD: Standard deviation, AJCC: American Joint Committee on Cancer, DCIS: Ductal carcinoma in situ and N = number of subjects. For all the above comparisons, ANOVA or Fisher tests were used (for continuous or categorical data, respectively). Parameters were significant with threshold at p of 0.05.
b. Gyrification results
There were no significant gyrification differences at TP1 between C+ versus C−, C+ versus HC, and C− versus HC (p > 0.05, Bonferroni corrected).
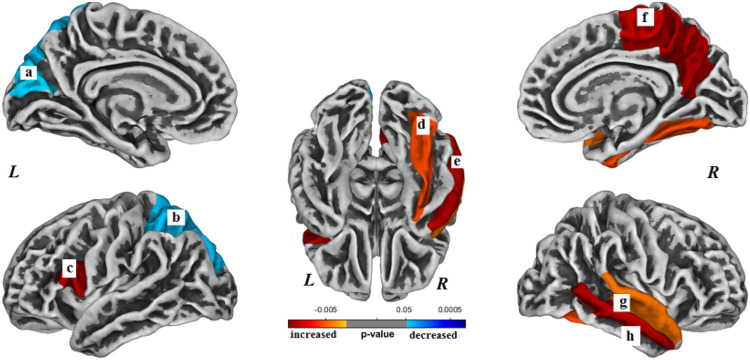
Within the C+ group, gyrification was significantly increased in 6 regions and decreased in 2 regions longitudinally over the 2-year study interval (p < 0.05, Bonferroni corrected) (Table 2). The brain regions with increased surface gyrification in the C+ group included the following (Bonferroni corrected): left pars opercularis gyrus (p < 0.001), right superior temporal gyrus (p < 0.001), right middle temporal gyrus (p < 0.001), right precuneus gyrus (p < 0.001), right paracentral gyrus (p < 0.001) and right fusiform gyrus (p = 0.004) (Fig. 1). The brain regions with decreased surface gyrification in the C+ group included the following (Bonferroni corrected): left superior parietal gyrus (p = 0.030) and left cuneus gyrus (p = 0.030).
Table 2.
Gyrification results
| Changes | Size (vertexes) |
p-value (corrected) |
Overlap of atlas region |
Brain region (DK40) |
|---|---|---|---|---|
| C+ group: | ||||
| TP2>TP1 | 3119 | 0.00034 | 100% | Left Pars opercularis |
| 11925 | 0.00034 | 58% | Right Superior Temporal | |
| 42% | Right Middle Temporal | |||
| 11806 | 0.00025 | 68% | Right Precuneus | |
| 32% | Right Paracentral | |||
| 4661 | 0.00435 | 100% | Right Fusiform | |
| TP2<TP1 | 12086 | 0.03061 | 87% | Left Superior Parietal |
| 13% | Left cuneus | |||
| C− group: | ||||
| TP2<TP1 | 11450 | 0.00001 | 41% | Left Fusiform |
| 37% | Left Lingual | |||
| 22% | Left Isthmus Cingulate | |||
| 8600 | 0.00105 | 100% | Left Paravaginal | |
| 4351 | 0.00616 | 100% | Right Lateral Orbitofrontal | |
| 4198 | 0.0010 | 100% | Right Inferior Temporal | |
| 3494 | 0.00021 | 100% | Right Caudal Middle frontal | |
| HC group: | ||||
| TP2<TP1 | 33739 | 0.00095 | 36% | Left Superior Frontal |
| 28% | Left Postcentral | |||
| 22% | Left Precuneus | |||
| 10% | Left Paracentral | |||
| 4% | Left Caudal Anterior Cingulate | |||
| 1064 | 0.00022 | 100% | Left Transverse Temporal | |
| 15372 | 0.00040 | 77% | Right Superior Frontal | |
| 23% | Right Caudal Middle Frontal | |||
| 8150 | 0.00019 | 100% | Right Supramarginal | |
Abbreviations: TP1: time point 1, TP2: time point 2, C+: Chemotherapy group, C−: No-chemotherapy group, HC: Healthy control group, DK40: Desikan atlas. Cluster size >10. Results were significant with threshold at p of 0.05.
Figure 1.
Brain regions with longitudinal changes in gyrification within the chemotherapy (C+) group. The altered regions are (a) left cuneus, (b) left superior parietal gyrus, (c) left pars opercularis, (d) right fusiform gyrus, (e) right middle temporal gyrus, (f) right precuneus, (g) right superior temporal gyrus, and (h) right middle temporal gyrus. L- left hemisphere, R- right hemisphere. Results were Bonferroni correctedat significant level of 0.05.
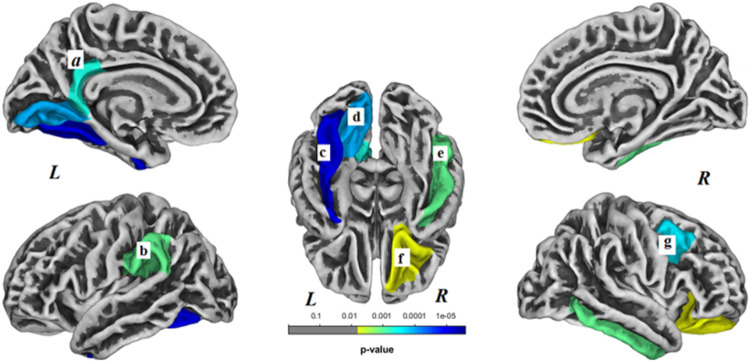
Within the C− group, brain surface gyrification was significantly decreased in 7 regions (p < 0.05, Bonferroni corrected) and no regions showed increased gyrification. Decreased surface gyrification within the C− group was noted in left fusiform gyrus (p < 0.001), left lingual gyrus (p < 0.001), left isthmus cingulate gyrus (p < 0.001), left supramarginal gyrus (p = 0.001), right lateral orbitofrontal gyrus (p = 0.006), right inferior temporal gyrus (p = 0.001) and right caudal middle frontal gyrus (p < 0.001) (Fig. 2).
Figure 2.
Brain regions with decreased gyrification within the non-chemotherapy control (C−) group. These regions included the following: (a) left isthmus cingulate gyrus, (b) left supramarginal gyrus, (c) left fusiform gyrus, (d) left lingual gyrus, (e) right inferior temporal gyrus, (f) right lateral orbitofrontal gyrus, (g) right caudal middle frontal gyrus. L- left hemisphere, R- right hemisphere. Results were Bonferroni corrected at significant level of 0.05.
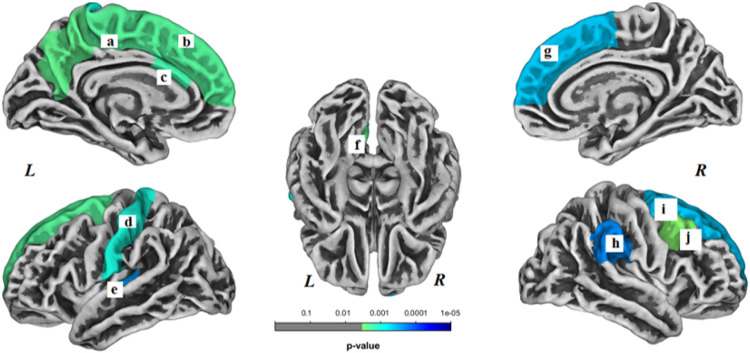
In the HC group, brain surface gyrification was significantly decreased in 9 regions (p < 0.05, Bonferroni corrected) and no regions showed increased gyrification longitudinally. Decreased brain surface gyrification was noted in the following regions: left superior frontal gyrus (p < 0.001), left postcentral gyrus (p < 0.001), left precuneus gyrus (p < 0.001), left paracentral gyrus (p < 0.001), left caudal anterior cingulate gyrus (p < 0.001), left transverse temporal gyrus (p < 0.001), right superior frontal gyrus (p < 0.001), right caudal middle frontal gyrus (p < 0.001) and right supramarginal gyrus (p < 0.001) (Fig. 3).
Figure 3.
Brain regions with decreased gyrification within the healthy control (HC) group. These regions included the following: (a) left paracentral gyrus, (b) left superior frontal gyrus, (c) left caudal anterior cingulate gyrus, (d) left postcentral gyrus, (e) left transverse temporal gyrus, (f) left precuneus, (g) right superior frontal gyrus, (h) right supramarginal gyrus, (i) right superior frontal gyrus, and (j) right caudal middle frontal gyrus. L- left hemisphere, R- right hemisphere. Results were Bonferroni correctedat significant level of 0.05.
There was no significant gyrification difference noted in group-by-time interaction analysis (p > 0.05, Bonferroni corrected).
c. NP testing scores
The detailed results of the NIH Toolbox cognition battery testing scores have been reported in our prior study of cortical thickness in the same cohort [9]. Briefly, the C+ group showed significantly decreased total composite score (p = 0.01), fluid composite score (p = 0.03) and picture vocabulary score (p = 0.04) across the 2-year interval. No significant changes in NP scores were noted in C− and HC group at a threshold of p values at 0.05.
d. Correlation between gyrification and NP scores
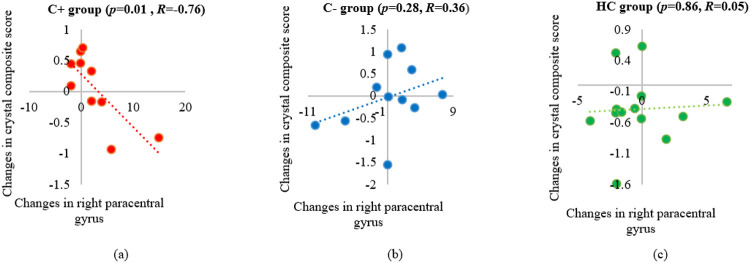
The correlation analysis was performed between the significant gyrification alterations within each group over time and the 3 NP composite scores. A significant negative correlation was noted between longitudinal changes in the crystallized composite scores and right paracentral gyrification values in the C+ group (p = 0.01, R = −0.76). No significant correlations were noted in the C− or the HC group (Fig. 4).
Figure 4.
Correlation of longitudinal changes between the right paracentral gyrification values and the crystallized composite scores. (a) chemotherapy (C+) group, (b) no-chemotherapy (C−) group, and (c) healthy control (HC) group. R: the Pearson’s correlation coefficient with significance set at p ≤ 0.05.
Discussion
We identified altered gyrification in the older long-term survivors of breast cancer with exposure to chemotherapy. We found the mostly increased gyrification in the chemotherapy-treated group while only decreased gyrification in the control groups over the 2-year study interval. In addition, we also found a significant correlation between the increased gyrification and the changes in cognitive testing scores. To the best of our knowledge, this was the first prospective longitudinal study of the effect of chemotherapy on gyrification in older long-term survivors of breast cancer.
Our C+ group showed increased gyrification in the right superior temporal gyrus. In contrast, a previous study of breast cancer patients with neoadjuvant chemotherapy showed decreased gyrification in the same region [36]. The divergent results might be due to differences in study designs. The prior study focused on the acute effects of chemotherapy within 2 months after treatment and assessed the pre- and post-chemotherapy differences in patients of 29 to 68 years of age [36]. Therefore, this prior study assessed acute changes stimulated by neurotoxic effects of chemotherapy while our study assessed the chronic chemotherapy-related neurotoxicity in older breast cancer survivors. Literature supports this pattern of gyrification alteration with decreased gyrification in acute phase as noted in mild traumatic brain injury within 3 months of brain injury [31] and increased gyrification in a cohort with childhood traumatic brain injury after 6 to 15 years of post-injury [34]. Brain changes associated with chemotherapy tend to be subtle and are similar to mild traumatic brain injury. One speculation for the increased gyrification relies on the phenomena of neurogenesis [33], in which the brain might expand by increasing gyrification to accommodate newly generated neurons. In addition, the right superior temporal gyrus plays a role in social cognitive function such as auditory and language processing [42]. The oral reading recognition score from the NP testing in our study assessed language and auditory skills [43] and was decreased within the C+ group, thus implying the brain structure including the superior temporal gyrus underlying these functions, may be altered. Therefore, we speculate that the increased right superior temporal gyrification might be a compensatory measure to accommodate the newly generated neurons to counter neurotoxicity of chemotherapy [33].
We found increased gyrification in the right medial temporal gyrus in the C+ group, which is in general agreement with a prior study in patients with early stages of dementia [33]. Patients with mild cognitive impairment (MCI) and Alzheimer’s Dementia (AD) [33] had increased gyrification and atrophy in entorhinal cortex, which is a part of the medial temporal gyrus. The medial temporal lobe is associated with episodic memory [44]. We also found a decreased picture vocabulary testing score in the C+ group, indicating diminished episodic memory. Our findings support the notion that gyrification alteration in the medial temporal lobe may be useful as an imaging biomarker for CRCI and AD in older cancer survivors. The right fusiform gyrus, close to the medial temporal gyrus, also showed increased gyrification in our C+ group. The fusiform gyrus plays an important role in semantic memory such as face recognition [45], visual perception [46] and face stimuli [46]. Our own prior study noted GM reduction in the right fusiform cortex in the chemotherapy-treated group [47]. Overall, our findings implicate the temporal lobe structures as being vulnerable to chemotherapy neurotoxicity.
We found increased gyrification in the paracentral gyrus within the C+ group over time and our findings were consistent with a prior study showing decreased sulcus depth in the paracentral gyrus during the early post-chemotherapy phase in breast cancer patients [36]. The paracentral gyrus is the medial continuation of the precentral and postcentral regions, which controls motor and sensory innervations of the contralateral lower extremity [48]. Our findings implicate the paracentral gyrification as a potential neural correlate for CRCI in older long-term cancer survivors who had chemotherapy treatment many years ago. The increased gyrification in the paracentral gyrus region had a significant negative association with the crystallized composite scores in the C+ group. The crystalized intelligence consisted of picture vocabulary and oral reading recognition based on past learning experiences [49]. Nevertheless, the crystalized cognition score was only marginally significant overtime in our C+ group and this score has been known to be resilient to change [50]. More studies in larger samples are needed to confirm the association of crystalized composite score and paracentral gyrification changes in the older survivors treated with chemotherapy.
We found decreased gyrification in the left superior parietal lobe in the older long-term breast cancer survivors with history of chemotherapy treatment. A prior study showed similar findings in a cohort of breast cancer patients shortly after chemotherapy [36]. The parietal lobe is important for cognitive function, and atrophy of the superior partial lobe is associated with impairment of working memory, attention and visuomotor functions [51, 52]. Taken together, the diminished left superior parietal gyrification may have occurred shortly after chemotherapy and persisted into long-term survivorship. Nevertheless, a longitudinal study including a pre-chemotherapy baseline and long-term follow-up is needed to assess the trajectory of gyrification alterations.
The control groups in our study showed only decreased gyrification over time with no increase noted, which was consistent with prior studies of normal aging. For instance, a prior study has shown decreased gyrification in the older population as compared to the younger population [32]. The decreased gyrification in the left lingual and right lateral orbitofrontal gyrus in our C− group and in the left postcentral and precuneus in the HC group were in line with previous longitudinal study of healthy aging [33]. The underlying neural mechanism for decreased gyrification in the aging studies is not well known [24]. We speculate that it could be partly due to age-related brain volume loss, leading to less folding of gyrus thus decreased gyrification during the aging process [24].
There were limitations to this study. First, our study cohort was small and there was severe attrition during the 2-year study interval. We will implement measurements and lessons from this study to decrease attrition in our future studies. Second, our study cohort included mostly non-Hispanic white women, decreasing the generalizability of our gyrification results to other racial and ethnic groups. Third, though gyrification is a significant surface parameter to assess brain alterations, other surface morphology parameters such as sulcal depth may help confirm brain changes. Further analysis of brain surface parameters is ongoing. Lastly, we only identified longitudinal changes over a 2-year interval but not at TP 1 during the initial enrollment. We believe a larger sample size may have detected subtle differences among the groups at TP 1. Despite the limitations, this study has merits. This was the first longitudinal study to assess the effect of chemotherapy on gyrification in older long-term survivors of breast cancer. We contributed novel brain structural and functional information to advance CRCI research in older cancer survivors.
Conclusions
We identified altered brain surface gyrification and its association with cognitive function in long-term breast cancer survivors who had chemotherapy many years ago. This study implicated gyrification as a possible underlying neural correlate of CRCI in older long-term survivors of cancer.
Funding:
This study was partially funded by National Institutes of Health/National Institute on Aging grants R01 CA172119 (TA), U54 CA137788 (TA), P30 CA008748 (TA), and K24 AG055693-01 (WD). BTC received funding support from the City of Hope Center for Cancer and Aging.
Footnotes
Competing Interests:
The authors had no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures involving human participants were performed in accordance with the ethical standards of the Institutional Review Board of City of Hope and with the 1964 Helsinki Declaration and its later amendments, as well as with all local, state, and federal regulations. Informed consent was obtained from all participants in the study. The parent study for this neuroimaging sub study has been registered on ClinicalTrials.gov (NCT02122107).
Consent to participate:
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Ebenezer Daniel, City of Hope National Medical Center: City of Hope.
Frank Deng, City of Hope National Medical Center: City of Hope.
Sunita K. Patel, City of Hope National Medical Center
Mina S. Sedrak, City of Hope National Medical Center: City of Hope
Heeyoung Kim, City of Hope National Medical Center: City of Hope.
Marianne Razavi, City of Hope National Medical Center.
Can-Lan Sun, City of Hope National Medical Center.
James C. Root, Memorial Sloan Kettering Cancer Center
Tim A. Ahles, Memorial Sloan Kettering Cancer Center
William Dale, Memorial Sloan Kettering Cancer Center.
Bihong T. Chen, City of Hope National Medical Center
Data Availability:
The datasets generated during the current study are not publicly available due to lack of relevant public database to deposit the data but are available from the corresponding author on reasonable request.
References
- 1.Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL (2022) Cancer treatment and survivorship statistics, 2022. CA: A Cancer Journal for Clinicians 72:409–436. doi: 10.3322/caac.21731 [DOI] [PubMed] [Google Scholar]
- 2.Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M (2010) Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med 52:219–227. doi: 10.1097/JOM.0b013e3181d0bef7 [DOI] [PubMed] [Google Scholar]
- 3.Lyon DE, Cohen R, Chen H, Kelly DL, Starkweather A, Ahn HC, Jackson-Cook CK (2016) The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: a 2-year longitudinal study. J Cancer Res Clin Oncol 142:1461–1474. doi: 10.1007/s00432-016-2163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Jacobsen PB, Ahles T (2014) Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol 32:2617–2626. doi: 10.1200/JCO.2014.55.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Országhová Z, Mego M, Chovanec M (2021) Long-Term Cognitive Dysfunction in Cancer Survivors. Frontiers in molecular biosciences 8:770413. doi: 10.3389/fmolb.2021.770413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendergrass JC, Targum SD, Harrison JE (2018) Cognitive Impairment Associated with Cancer: A Brief Review. Innov Clin Neurosci 15:36–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Joly F, Giffard B, Rigal O, De Ruiter MB, Small BJ, Dubois M, LeFel J, Schagen SB, Ahles TA, Wefel JS, Vardy JL, Pancré V, Lange M, Castel H (2015) Impact of Cancer and Its Treatments on Cognitive Function: Advances in Research From the Paris International Cognition and Cancer Task Force Symposium and Update Since 2012. Journal of Pain and Symptom Management 50:830–841. doi: 10.1016/j.jpainsymman.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 8.Sousa H, Almeida S, Bessa J, Pereira MG (2020) The Developmental Trajectory of Cancer-Related Cognitive Impairment in Breast Cancer Patients: A Systematic Review of Longitudinal Neuroimaging Studies. Neuropsychol Rev 30:287–309. doi: 10.1007/s11065-020-09441-9 [DOI] [PubMed] [Google Scholar]
- 9.Daniel E, Deng F, Patel SK, Sedrak MS, Kim H, Razavi M, Sun C-L, Root JC, Ahles TA, Dale W, Chen BT (2022) Cortical thinning in chemotherapy-treated older long-term breast cancer survivors. Brain Imaging and Behavior. doi: 10.1007/s11682-022-00743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wefel JS, Kesler SR, Noll KR, Schagen SB (2015) Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin 65:123–138. doi: 10.3322/caac.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB (2012) Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp 33:2971–2983. doi: 10.1002/hbm.21422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB (2015) Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav 9:275–284. doi: 10.1007/s11682-014-9305-0 [DOI] [PubMed] [Google Scholar]
- 13.Koppelmans V, de Groot M, de Ruiter MB, Boogerd W, Seynaeve C, Vernooij MW, Niessen WJ, Schagen SB, Breteler MM (2014) Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp 35:889–899. doi: 10.1002/hbm.22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrier J, Viard A, Levy C, Morel N, Allouache D, Noal S, Joly F, Eustache F, Giffard B (2020) Longitudinal investigation of cognitive deficits in breast cancer patients and their gray matter correlates: impact of education level. Brain Imaging Behav 14:226–241. doi: 10.1007/s11682-018-9991-0 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Chen H, Lv Y, Chao HH, Gong L, Li C-SR, Cheng H (2018) Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Scientific Reports 8:13801. doi: 10.1038/s41598-018-32257-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesler SR, Rao V, Ray WJ, Rao A (2017) Probability of Alzheimer's disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 9:67–75. doi: 10.1016/j.dadm.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini SMH, Koovakkattu D, Kesler SR (2012) Altered small-world properties of gray matter networks in breast cancer. BMC Neurology 12:28. doi: 10.1186/1471-2377-12-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, MacKenzie J, Scherling C (2014) A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. SpringerPlus 3:444. doi: 10.1186/2193-1801-3-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen BT, Sethi SK, Jin T, Patel SK, Ye N, Sun C-L, Rockne RC, Haacke EM, Root JC, Saykin AJ, Ahles TA, Holodny AI, Prakash N, Mortimer J, Waisman J, Yuan Y, Somlo G, Li D, Yang R, Tan H, Katheria V, Morrison R, Hurria A (2018) Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Research 20:38. doi: 10.1186/s13058-018-0965-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123:819–828. doi: 10.1007/s10549-010-1088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao B, Mwangi B, Passos IC, Wu M-J, Keser Z, Zunta-Soares GB, Xu D, Hasan KM, Soares JC (2017) Lifespan Gyrification Trajectories of Human Brain in Healthy Individuals and Patients with Major Psychiatric Disorders. Scientific Reports 7:511. doi: 10.1038/s41598-017-00582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luders E, Kurth F, Mayer E, Toga A, Narr K, Gaser C (2012) The Unique Brain Anatomy of Meditation Practitioners: Alterations in Cortical Gyrification. Frontiers in human neuroscience 6. doi: 10.3389/fnhum.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White T, Su S, Schmidt M, Kao CY, Sapiro G (2010) The development of gyrification in childhood and adolescence. Brain Cogn 72:36–45. doi: 10.1016/j.bandc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spalthoff R, Gaser C, Nenadić I (2018) Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophrenia Research 202:195–202. doi: 10.1016/j.schres.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Gregory MD, Kippenhan JS, Dickinson D, Carrasco J, Mattay VS, Weinberger DR, Berman KF (2016) Regional Variations in Brain Gyrification Are Associated with General Cognitive Ability in Humans. Current Biology 26:1301–1305. doi: 10.1016/j.cub.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan CR (2021) Age-related decrements in cortical gyrification: Evidence from an accelerated longitudinal dataset. Eur J Neurosci 53:1661–1671. doi: 10.1111/ejn.15039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS (2011) Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 68:871–880. doi: 10.1001/archgenpsychiatry.2011.88 [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Lipnicki DM, Zhu W, Tao D, Zhang C, Cui Y, Jin JS, Sachdev PS, Wen W (2012) Cortical gyrification and sulcal spans in early stage Alzheimer's disease. PloS one 7:e31083. doi: 10.1371/journal.pone.0031083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn H, Choi M, Lee S, Kim D, Suh S, Han CE, Jeong HG (2021) Decreased Cortical Thickness and Local Gyrification in Individuals with Subjective Cognitive Impairment. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology 19:640–652. doi: 10.9758/cpn.2021.19.4.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaer M, Ottet M-C, Scariati E, Dukes D, Franchini M, Eliez S, Glaser B (2013) Decreased frontal gyrification correlates with altered connectivity in children with autism. Frontiers in Human Neuroscience 7. doi: 10.3389/fnhum.2013.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharehgazlou A, Jetly R, Rhind SG, Reichelt AC, Da Costa L, Dunkley BT (2022) Cortical Gyrification Morphology in Adult Males with Mild Traumatic Brain Injury. Neurotrauma reports 3:299–307. doi: 10.1089/neur.2021.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamballais S, Vinke EJ, Vernooij MW, Ikram MA, Muetzel RL (2020) Cortical gyrification in relation to age and cognition in older adults. NeuroImage 212:116637. doi: 10.1016/j.neuroimage.2020.116637 [DOI] [PubMed] [Google Scholar]
- 33.Núñez C, Callén A, Lombardini F, Compta Y, Stephan-Otto C (2020) Different Cortical Gyrification Patterns in Alzheimer's Disease and Impact on Memory Performance. Annals of neurology 88:67–80. doi: 10.1002/ana.25741 [DOI] [PubMed] [Google Scholar]
- 34.Wilde EA, Merkley TL, Lindsey HM, Bigler ED, Hunter JV, Ewing-Cobbs L, Aitken ME, MacLeod MC, Hanten G, Chu ZD, Abildskov TJ, Noble-Haeusslein LJ, Levin HS (2021) Developmental Alterations in Cortical Organization and Socialization in Adolescents Who Sustained a Traumatic Brain Injury in Early Childhood. Journal of neurotrauma 38:133–143. doi: 10.1089/neu.2019.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang DYJ, Beam D, Pelphrey KA, Abdullahi S, Jou RJ (2016) Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Molecular Autism 7:11. doi: 10.1186/s13229-016-0076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Tan Y, Yu H, Liu J, Lan X, Deng Y, Yu F, Wang C, Chen J, Zeng X, Liu D, Zhang J (2022) Early alterations in cortical morphology after neoadjuvant chemotherapy in breast cancer patients: A longitudinal magnetic resonance imaging study. Hum Brain Mapp 43:4513–4528. doi: 10.1002/hbm.25969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary S, Kumaran SS, Goyal V, Kaloiya GS, Kalaivani M, Jagannathan NR, Sagar R, Mehta N, Srivastava AK (2020) Cortical thickness and gyrification index measuring cognition in Parkinson’s disease. International Journal of Neuroscience:1–10. doi: 10.1080/00207454.2020.1766459 [DOI] [PubMed] [Google Scholar]
- 38.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 39.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC (2013) Cognition assessment using the NIH Toolbox. Neurology 80:S54–64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ (2013) NIH toolbox for assessment of neurological and behavioral function. Neurology 80:S2–6. doi: 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird NM, Ware JH (1982) Random-effects models for longitudinal data. Biometrics 38:963–974. [PubMed] [Google Scholar]
- 42.Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE (2007) Superior temporal gyrus, language function, and autism. Developmental neuropsychology 31:217–238. doi: 10.1080/87565640701190841 [DOI] [PubMed] [Google Scholar]
- 43.Gershon RC, Cook KF, Mungas D, Manly JJ, Slotkin J, Beaumont JL, Weintraub S (2014) Language measures of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc 20:642–651. doi: 10.1017/s1355617714000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickerson BC, Eichenbaum H (2010) The Episodic Memory System: Neurocircuitry and Disorders. Neuropsychopharmacology 35:86–104. doi: 10.1038/npp.2009.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai S, Chong T, Zhang Y, Li J, von Deneen KM, Ren J, Dong M, Huang L, ftAsDNI (2015) Altered Functional Connectivity of Fusiform Gyrus in Subjects with Amnestic Mild Cognitive Impairment: A Resting-State fMRI Study. Frontiers in Human Neuroscience 9. doi: 10.3389/fnhum.2015.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Möller HJ, Hampel H (2006) Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain : a journal of neurology 129:1113–1124. doi: 10.1093/brain/awl051 [DOI] [PubMed] [Google Scholar]
- 47.Chen BT, Jin T, Patel SK, Ye N, Sun CL, Ma H, Rockne RC, Root JC, Saykin AJ, Ahles TA, Holodny AI, Prakash N, Mortimer J, Waisman J, Yuan Y, Li D, Somlo G, Vazquez J, Levi A, Tan H, Yang R, Katheria V, Hurria A (2018) Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 172:363–370. doi: 10.1007/s10549-018-4911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patra A, Kaur H, Chaudhary P, Asghar A, Singal A (2021) Morphology and Morphometry of Human Paracentral Lobule: An Anatomical Study with its Application in Neurosurgery. Asian journal of neurosurgery 16:349–354. doi: 10.4103/ajns.AJNS_505_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doucet GE, Hamlin N, Kruse JA, Taylor BK, Poirel N (2022) Link between fluid/crystallized intelligence and global/local visual abilities across adulthood. Conscious Cogn 106:103429. doi: 10.1016/j.concog.2022.103429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajpai S, Upadhayay AD, Banerjee J, Chakrawarthy A, Chatterjee P, Lee J, Dey AB (2022) Discrepancy in Fluid and Crystallized Intelligence: An Early Cognitive Marker of Dementia from the LASI-DAD Cohort. Dementia and geriatric cognitive disorders extra 12:51–59. doi: 10.1159/000520879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alahmadi AAS (2021) Investigating the sub-regions of the superior parietal cortex using functional magnetic resonance imaging connectivity. Insights into Imaging 12:47. doi: 10.1186/s13244-021-00993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koenigs M, Barbey AK, Postle BR, Grafman J (2009) Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29:14980–14986. doi: 10.1523/jneurosci.3706-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available due to lack of relevant public database to deposit the data but are available from the corresponding author on reasonable request.