Abstract
Backgrounds and Aims
Atrial fibrillation (AF) is the most common cardiac arrhythmia among the older patients (≥ 80 years) in clinical practice. The index of neutrophil percentage-to-albumin ratio (NPAR) is a reliable predictor of adverse outcomes in cardiovascular diseases. There is scarce evidence regarding the association between NPAR and mortality among the older patients with AF.
Methods
The research was conducted among 1141 patients with AF between January 2015 and June 2020, hospitalized at Huadong Hospital affiliated with Fudan University. The primary outcome were 28-day all-cause and cardiovascular mortality. Cox regression analysis and Kaplan-Meier survival curves were used to explore the correlation between NPAR and 28-day all-cause or cardiovascular mortality. Receiver operating characteristic (ROC) curve and the area under the curve (AUC) were performed for the predictive values of NPAR on prognosis.
Results
The 28-day death rate from cardiovascular disease and all-causes were 3.3% and 8.7%, respectively. Continuous NPAR levels were positively associated with all-cause (HR 1.13, 95% CI 1.09, 1.16) and cardiovascular (HR 1.16, 95% CI 1.10, 1.23) mortality after adjustment for confounding variables. Relative to patients in the T1 group, those in higher NPAR tertiles also exhibit elevated risks of all-cause (P < 0.001) and cardiovascular mortality (P < 0.001). Furthermore, both all-cause and cardiovascular mortality rates rose with increasing NPAR in all analyzed subgroups.
Conclusion
NPAR values are consistently positively related to 28-day all-cause and cardiovascular mortality rates in patients ≥80 years of age with AF.
Keywords: cardiovascular disease, neutrophil, atrial fibrillation, mortality, older
Introduction
Atrial fibrillation (AF) is an increasingly major contributor to the worldwide medical burden among older adults owing to the progressive aging of the global population, economic development, and rising AF-associated risk factor prevalence rates.1 In population-based studies, AF has been shown to be related to a higher risk of heart failure, stroke, and mortality.2,3 Recent reports have also demonstrated that older AF patients exhibit a higher risk of in-hospital mortality and complications,4,5 underscoring a need for further observational studies aimed at guiding the management of these patients in an effort to improve their clinical outcomes.
Neutrophils are important regulators of immunological and inflammatory diseases. Circulating neutrophil counts are closely related to the incidence of bloodstream infections and to the severity of septicemia.6–8 Albumin is the most abundant protein in human serum wherein it plays an essential role as a transporter for a range of endogenous compounds and exogenous drugs.9 Serum albumin exhibits diverse antithrombotic, antioxidant, and anti-inflammatory proeprties.10 Given the key roles of these two physiological biomarkers, the neutrophil percentage-to-albumin ratio (NPAR) is frequently analyzed to glean relevant information about a variety of clinical conditions. Notably, the NPAR has been shown to be significantly linked to the severity of cardiovascular disease, stroke-related infection, pancreatic cancer, and kidney injury and to the prognosis of affected patients.11–14 However, no studies have examined the NPAR’s association with mortality risk in older individuals with AF.
The purpose of this research was to determine whether there is a correlation between NPAR and 28-day all-cause or cardiovascular death rates in Chinese patients ≥ 80 years old who have been diagnosed with AF.
Methods
Research Subjects
This retrospective study enrolled adults 80 years of age or older that had been diagnosed with AF in the electronic medical records of Huadong Hospital affiliated with Fudan University between January 2015 and June 2020. Since this was a retrospective study, anonymity allowed the Biomedical Research Ethics Committee at Huadong Hospital to accept it, without the necessity for informed consent. Patients were excluded from the study cohort if they lacked neutrophil counts or serum albumin data (n = 24), had been diagnosed with chronic hematological conditions (n = 21), or were lacking 28-day follow-up results (n = 51). In total, 1141 eligible patients were analyzed for this current study (Figure 1).
Figure 1.
Flowchart of patient selection.
Research Methods
Collected data for included patients included sex, age, systolic/diastolic blood pressure (SBP/DBP), drinking habits, smoking habits, new-onset AF, paroxysmal AF, CHA2DS2-VASc scores, HAS-BLED scores,15 comorbidities [including chronic heart failure, COPD, hypertension, diabetes, history of cancer, heart valvular disorders, coronary heart disease, and prior stroke], primary reason for hospital admission [AF, acute coronary syndrome (ACS, including unstable angina, non-STEMI, and STEMI),16 acute heart failure, or other] and the results of examinations performed within 24 h following admission, including white blood cell (WBC), lymphocyte, hemoglobin, platelet counts, C-reactive protein (CRP), blood urea, blood creatine, estimated glomerular filtration rate (eGFR),17 total cholesterol, serum albumin, and brain natriuretic peptide (BNP) levels. A professional sonographer analyzed echocardiographic features including left atrial (LA) diameter, left ventricular (LV) ejection fraction (LVEF), LV interior diameter at end-systolic (LVIDs), left ventricular end-diastolic volume (LVEDV), LV interior diameter at end-diastole (LVIDd), and LV stroke volume (LVSV). Medication use by included patients was determined through inquiries with patients or their relatives on admission, including the use of anticoagulants, antiplatelet drugs, statins, RAS inhibitors, and beta blockers. Chronic heart failure was diagnosed according to the European Society of Cardiology (ESC) guidelines.18
Neutrophil percentage (%) and serum albumin levels (g/dL) were measured using a Beckman Coulter 6800 device. The NPAR was calculated as the ratio between neutrophil percentage and albumin levels.
Statistical Analysis
One-way analysis of variance (ANOVA) and the Kruskal–Wallis test were used to compare normally and non-normally distributed continuous data, where means ± SD or medians and interquartile ranges were calculated. Chi-square tests are used to compare numerical or percentage-based categorical data.
Cox proportional hazards models were applied to investigate the correlations between NPAR levels and 28-day all-cause or cardiovascular death outcomes. Multivariate analysis findings are reported as hazard ratios (HRs) and 95% confidence intervals (CIs), with results being assessed using two multivariate models. The first of these models was minimally adjusted, only controlling for age, sex, and SBP. The second was the fully adjusted model, which controlled for age, sex, SBP, primary reason for hospital admission, eGFR, AF type, HAS-BLED scores, history of cancer, CRP levels, LVEF, hemoglobin levels, urea levels, BNP levels, and total cholesterol levels. The confounding variables included in the fully adjusted model were those that were related to mortality or exhibited > 10% changes in effect estimates.19 Comparing outcomes across NPAR tertiles was done using Kaplan-Meier curves and Log rank testing. Receiver operating characteristic (ROC) curve area under the curve (AUC) values were used to compare the predictive value of NPAR, neutrophil percentage, and albumin levels for all-cause and cardiovascular mortality.
The association between NPAR and 28-day mortality outcomes was further investigated using subgroup analysis. R version 3.4.3 (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com) were used for all statistical analyses. The cutoff for significance was set at P < 0.05.
Results
Patient Characteristics
In total, this study enrolled 1141 older AF patients, with Table 1 compiling the demographic characteristics of these patients when separated into NPAR tertiles (T1: ≤ 16.4; T2: > 16.4, ≤ 20.3; T3: > 20.3). Overall, 528 (46.3%) of these patients were male and 344 (30.1%) had been diagnosed with paroxysmal AF. The T1, T2, and T3 groups contained 380, 380, and 381 patients, respectively. Patients in the T3 group exhibited a higher chance of being older, reporting new-onset AF, suffering from COPD or chronic heart failure, and exhibiting higher WBC, platelet, neutrophil percentage, CRP, BNP, blood creatine, and blood urea levels as well as lower SBP, total cholesterol, eGFR, hemoglobin, LVEF, albumin, and LA diameters relative to other patients. Of the overall patient cohort, 416 (36.5%) and 464 (40.7%) patients had been hospitalized for AF and other causes, respectively, with AF being the most common single cause of hospitalization. No differences were observed among groups with respect to sex, DBP, smoking, drinking, HAS-BLED scores, CHA2DS2-VASc scores, history of diabetes, coronary heart disease, history of heart valvular disease, cancer, and previous stroke, LVIDd, LVIDs, LVEDV measurements, or medication use.
Table 1.
Baseline Characteristics of Patients
| Variables | Total | Neutrophil Percentage-to-Albumin Ratio | P-value | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| (≤16.4) | (16.4–20.3) | (>20.3) | |||
| N | 1141 | 380 | 380 | 381 | |
| Age, y | 84.7 ± 3.9 | 83.9 ± 3.6 | 84.6 ± 3.7 | 85.6 ± 4.3 | <0.001 |
| Male, n (%) | 528 (46.3) | 164 (43.2) | 182 (47.9) | 182 (47.8) | 0.328 |
| SBP, mmHg | 133.7 ± 18.7 | 136.1 ± 19.1 | 133.4 ± 17.7 | 131.6 ± 19.0 | 0.004 |
| DBP, mmHg | 76.3 ± 10.8 | 76.8 ± 10.7 | 76.5 ± 10.5 | 75.5 ± 11.2 | 0.216 |
| Smoking habits, n (%) | 135 (12.3) | 46 (12.4) | 45 (12.3) | 44 (12.2) | 0.995 |
| Drinking habits, n (%) | 60 (5.5) | 21 (5.7) | 23 (6.3) | 16 (4.4) | 0.535 |
| Paroxysmal AF, n (%) | 344 (30.1) | 133 (35.0) | 112 (29.5) | 99 (26.0) | 0.024 |
| New-onset AF, n (%) | 97 (8.5) | 21 (5.5) | 34 (8.9) | 42 (11.0) | 0.023 |
| HAS-BLED score | 2.4 ± 1.0 | 2.3 ± 0.9 | 2.4 ± 0.9 | 2.4 ± 1.1 | 0.306 |
| CHA2DS2-VASc score | 4.4 ± 1.2 | 4.4 ± 1.2 | 4.3 ± 1.1 | 4.4 ± 1.3 | 0.384 |
| Comorbidities, n (%) | |||||
| Hypertension | 794 (69.6) | 274 (72.1) | 276 (72.6) | 244 (64.0) | 0.015 |
| Diabetes | 304 (26.6) | 104 (27.4) | 88 (23.2) | 112 (29.4) | 0.139 |
| Coronary heart disease | 477 (41.8) | 162 (42.6) | 166 (43.7) | 149 (39.1) | 0.407 |
| Chronic heart failure | 534 (46.8) | 158 (41.6) | 162 (42.6) | 214 (56.2) | <0.001 |
| COPD | 122 (10.7) | 31 (8.2) | 34 (8.9) | 57 (15.0) | 0.004 |
| History of cancer | 146 (12.8) | 44 (11.6) | 47 (12.4) | 55 (14.4) | 0.476 |
| Heart valvular disorders | 55 (4.8) | 16 (4.2) | 17 (4.5) | 22 (5.8) | 0.559 |
| Prior stroke | 376 (33.0) | 116 (30.5) | 127 (33.4) | 133 (34.9) | 0.425 |
| Primary reason of hospital admission, n (%) | <0.001 | ||||
| Atrial fibrillation | 416 (36.5) | 198 (52.1) | 136 (35.8) | 82 (21.5) | |
| Acute heart failure | 151 (13.2) | 41 (10.8) | 58 (15.3) | 52 (13.6) | |
| ACS | 110 (9.6) | 25 (6.6) | 43 (11.3) | 42 (11.0) | |
| Other | 464 (40.7) | 116 (30.5) | 143 (37.6) | 205 (53.8) | |
| Hematological and biochemical variables | |||||
| WBC, 10^9/L | 7.1 ± 3.4 | 5.6 ± 1.6 | 6.7 ± 2.5 | 9.1 ± 4.5 | <0.001 |
| Neutrophil percentage, % | 68.8 ± 11.5 | 57.6 ± 7.6 | 68.9 ± 6.4 | 79.7 ± 7.5 | <0.001 |
| Lymphocyte, 10^9/L | 1.4 ± 0.6 | 1.7 ± 0.6 | 1.4 ± 0.5 | 1.0 ± 0.5 | <0.001 |
| Hemoglobin, g/L | 119.9 ± 21.0 | 125.5 ± 17.8 | 120.7 ± 20.1 | 113.5 ± 22.9 | <0.001 |
| Platelet, 10^9/L | 178.9 ± 65.3 | 171.6 ± 52.6 | 180.2 ± 60.7 | 185.0 ± 79.2 | 0.016 |
| CRP, mg/L | 8.7 (3.3, 27.7) | 4.5 (1.7, 8.9) | 7.3 (3.2, 19.9) | 29.4 (10.1, 66.3) | <0.001 |
| Blood urea, mmol/L | 7.3 (5.4, 9.6) | 6.4 (5.2, 8.3) | 7.0 (5.4, 9.0) | 8.8 (6.1, 12.4) | <0.001 |
| Blood creatine, umol/L | 90.9 (73.3, 116.4) | 87.7 (71.7, 105.6) | 92.0 (76.1, 118.2) | 95.5 (71.6, 135.5) | <0.001 |
| eGFR, mL/min/1.73m2 | 56.6 ± 20.6 | 60.7 ± 16.7 | 55.9 ± 19.1 | 53.4 ± 24.7 | <0.001 |
| Albumin, g/dL | 3.7± 0.5 | 4.1 ± 0.4 | 3.8 ± 0.3 | 3.3 ± 0.4 | <0.001 |
| Total cholesterol, mmol/L | 3.9 ± 1.0 | 4.1 ± 1.0 | 3.9 ± 0.9 | 3.6 ± 1.0 | <0.001 |
| BNP, pg/mL | 332.0 (172.0, 516.0) | 248.5 (126.2, 442.2) | 320.5 (163.8, 494.0) | 473.0 (224.0, 860.0) | <0.001 |
| Echocardiography | |||||
| LVEF, % | 58.7 ± 7.8 | 60.1 ± 6.6 | 58.8 ± 6.9 | 57.2 ± 9.2 | <0.001 |
| LA, mm | 44.3 ± 6.7 | 45.1 ± 6.7 | 44.4 ± 7.2 | 43.4 ± 6.2 | 0.002 |
| LVIDd, mm | 46.9 ± 6.2 | 47.0 ± 5.8 | 46.9 ± 5.5 | 46.9 ± 7.1 | 0.989 |
| LVIDs, mm | 32.0 ± 6.2 | 31.7 ± 5.5 | 31.9 ± 5.5 | 32.5 ± 7.4 | 0.175 |
| LVSV, mL | 55.3 ± 17.1 | 57.5 ± 16.0 | 55.4 ± 16.3 | 53.0 ± 18.8 | 0.001 |
| LVEDV, mL | 96.2 ± 34.9 | 97.5 ± 33.1 | 95.5 ± 30.7 | 95.5 ± 40.4 | 0.670 |
| Medication, n (%) | |||||
| Statin | 66 (5.8) | 19 (5.0) | 20 (5.3) | 27 (7.1) | 0.406 |
| Beta blocker | 132 (11.6) | 41 (10.8) | 39 (10.3) | 52 (13.6) | 0.291 |
| RAS inhibitors | 248 (21.7) | 78 (20.5) | 91 (23.9) | 79 (20.7) | 0.440 |
| Antiplatelet | 163 (14.3) | 55 (14.5) | 49 (12.9) | 59 (15.5) | 0.589 |
| Anticoagulation | 63 (5.5) | 18 (4.7) | 28 (7.4) | 17 (4.5) | 0.153 |
| Cardiovascular mortality, n (%) | 38 (3.3) | 1 (0.3) | 7 (1.8) | 30 (7.9) | <0.001 |
| All-cause mortality, n (%) | 99 (8.7) | 3 (0.8) | 18 (4.7) | 78 (20.5) | <0.001 |
Notes: Mean ± SD for normally distributed continuous variables, median [Q1, Q3] for non-normally distributed continuous variables: P-value was calculated by one-way ANOVAs. Number () for categorical variables: P-value was calculated by chi-square test. eGFR was defined by the Chronic Kidney Disease Epidemiology Collaboration equation. Heart valvular diseases was defined as the presence of moderate-to-severe mitral stenosis or a mechanical heart valve. ACS included STEMI, non-STEMI and unstable angina.
Abbreviations: AF, atrial fibrillation; NPAR, neutrophil percentage-to-albumin ratio; CHA2DS2-VASc, Cardiac failure or dysfunction, Hypertension, Age ≥ 75 years (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74 years and sex category (Female); HAS-BLED score, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio (INR), Elderly (65 years), Drugs/alcohol concomitantly. COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACS, acute coronary syndrome; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; WBC, white blood cell; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; LA, left atrial diameter; LVIDd, left ventricular interior diameter at end-diastole, LVIDs, left ventricular interior diameter at end-systolic, LVSV, left ventricular stroke volume, LVEDV, left ventricular end diastolic volume.
The Relationship Between NPAR Values and Mortality in Older AF Patients
For multivariate analyses, patients in the three established NPAR tertiles were compared to determine whether NPAR is associated with 28-day all-cause or cardiovascular mortality outcomes (Table 2, Supplemental Table 1). When using the minimally adjusted model that corrected for sex age, and SBP, higher NPAR values were related to an elevated risk of all-cause mortality (HR 1.15, 95% CI 1.13, 1.17, P < 0.001) and cardiovascular mortality (HR 1.14, 95% CI 1.10, 1.19, P < 0.001). When using a fully adjusted model controlling for sex, age, SBP, history of cancer, AF type, primary reason for hospitalization, hemoglobin levels, BNP levels, urea levels, HAS-BLED scores, total cholesterol levels, eGFR, and LVEF, higher NPAR values remained significantly related to a higher risk of both all-cause mortality (HR 1.13, 95% CI 1.09, 1.16, P < 0.001) and cardiovascular mortality (HR 1.16, 95% CI 1.10, 1.23, P < 0.001). Higher NPAR values were associated with an increased risk of cardiovascular mortality (T2: HR 6.55, 95% CI 0.80, 53.28, T3: HR 28.46, 95% CI 3.85, 210.20, P for trend <0.001) and all-cause mortality (T2: HR 5.62, 95% CI 1.65, 19.08, T3: HR 24.23, 95% CI 7.61, 77.12, P for trend <0.001). These associations were also significant for all-cause mortality (T2: HR 4.43, 95% CI 1.30, 15.18, T3: HR 13.52, 95% CI 4.14, 44.21, P for trend <0.001) and cardiovascular mortality (T2: HR 5.20, 95% CI 0.63, 42.91, T3: HR 20.24, 95% CI 2.63, 155.60, P for trend <0.001) when using the fully adjusted model.
Table 2.
Associations Between NPAR and All-Cause Mortality by Cox Analysis
| The Number of Events (Deaths), n (%) | Unadjusted HR (95 CI, P) | Minimally Adjusted, HR (95 CI, P) | Fully Adjusted, HR (95 CI, P) | |
|---|---|---|---|---|
| NPAR | 99 (8.7) | 1.15 (1.13, 1.17) <0.001 | 1.15 (1.12, 1.17) <0.001 | 1.13 (1.09, 1.16) <0.001 |
| NPAR (tertiles) | ||||
| T1 | 3 (0.8) | Ref | Ref | Ref |
| T2 | 18 (4.7) | 6.08 (1.79, 20.64) 0.004 | 5.62 (1.65, 19.08) 0.006 | 4.43 (1.30, 15.18) 0.016 |
| T3 | 78 (20.5) | 28.89 (9.12, 91.52) <0.001 | 24.23 (7.61, 77.12) <0.001 | 13.52 (4.14, 44.21) <0.001 |
| P for trend | -- | <0.001 | <0.001 | <0.001 |
Notes: Minimally adjusted HR controls for age, sex, SBP. Fully adjusted HR controls for age, sex, SBP, AF type, history of cancer, primary reason of hospital admission, LVEF, CRP, total cholesterol, hemoglobin, eGFR, urea, BNP and HAS-BLED score.
Abbreviations: AF, atrial fibrillation; NPAR, neutrophil percentage-to-albumin ratio; SBP, systolic pressure; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; WBC, white blood cell; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
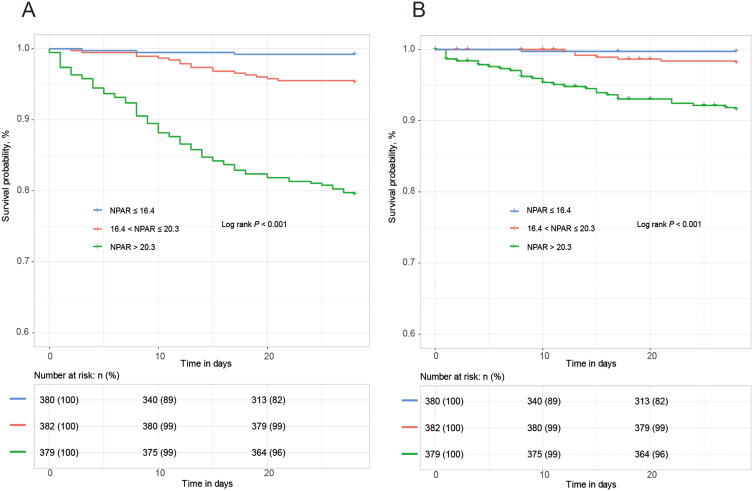
Kaplan-Meier curves generated for patient NPAR tertiles indicated that rates of all-cause and cardiovascular mortality rose with increasing NPAR tertiles such that the T3 group exhibited the highest mortality rates (P < 0.001) (Figure 2). While both neutrophil percentage and albumin levels were significantly related to both of these mortality outcomes, NPAR values exhibited a larger AUC value with respect to both all-cause (AUC 0.81, 95% CI 0.77, 0.85) and cardiovascular mortality (AUC 0.80, 95% CI 0.73, 0.86), indicating that NPAR may be a more robust tool for use when predicting mortality outcomes among older AF patients (Table 3 and Supplemental Table 2).
Figure 2.
Kaplan–Meier survival curve analysis of all-cause mortality (A) and cardiovascular mortality (B) across the tertiles of the NPAR value (NPAR: ≤ 16.4, 16.4 to 20.3, and > 20.3).
Table 3.
The Predictive Values of NPAR, Neutrophil Percentage or Albumin for All-Cause Mortality
| HR (95% CI) | P-value | AUC | 95% CI | |
|---|---|---|---|---|
| NPAR | 1.13 (1.09, 1.16) | <0.001 | 0.81 | (0.77, 0.85) |
| Neutrophil percentage, | 1.08 (1.05, 1.10) | <0.001 | 0.77 | (0.72, 0.82) |
| Albumin, g/dL | 0.34 (0.22, 0.53) | <0.001 | 0.71 | (0.66, 0.76) |
Note: The Cox proportional model adjusted HR controls for age, sex, SBP, AF type, history of cancer, primary reason of hospital admission, LVEF, CRP, total cholesterol, hemoglobin, eGFR, urea, BNP and HAS-BLED score.
Abbreviations: AF, atrial fibrillation; NPAR, neutrophil percentage-to-albumin ratio; SBP, systolic pressure; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; WBC, white blood cell; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
The Relationship Between NPAR Values and Mortality Rates in AF Patient Subgroups
Subgroup analyses were further used to explore how NPAR and 28-day all-cause or cardiovascular mortality outcomes are linked in these older AF patients (Supplemental Figure 1 and Supplemental Figure 2). In the majority of the analysed subgroups, including male and female patients, those with and without diabetes, hypertensive people, patients with and without chronic heart failure, patients with and without CHD, patients with and without a history of cancer, patients with eGFR values < 60 or ≥ 60 mL/min/1.73 m2, and patients admitted primarily because of AF, acute CHD, or other causes, a higher NPAR value was significantly related to all-cause mortality Notably, diabetes and cardiovascular mortality were the only variables that substantially interacted (P for interaction 0.005).
Discussion
This study is the first we are aware of to explore the relationships between NPAR values and 28-day rates for cardiovascular or all-cause deaths among hospitalized patients with AF ≥ 80 years of age. All-cause and cardiovascular death rates were shown to be significantly positively correlated with NPAR levels in this older AF patient sample. Once multiple potential confounders were taken into account, an increase of one unit in NPAR levels was linked to an increase of 13% in all-cause mortality and an increase of 16% in cardiovascular mortality. Therefore, the NPAR index may be an excellent predictor of short-term mortality outcomes for this cohort of patients.
In prior studies, AF was reported to be related to higher rates of both short- and long-term mortality.4,20 In one population-based retrospective analysis, AF was estimated to impact 34% of older adults, with these AF patients exhibiting a 17% higher in-hospital mortality rate as compared to patients without AF.4 Fauchier et al have further reported a 9.4% short-term mortality rate among AF patients with a mean age of 78.0 ± 11.4 years,21 in line with the rate observed in this study (8.7%). The RE-LY trial, in contrast, found that younger AF patients (mean age 71.5 ± 9 years) exhibited a somewhat lower annual mortality rate (3.8%).22 Given that advanced age was associated with overall higher rates of death,23 the differences in study population ages may account for these varying mortality rates. Older hospitalized individuals were also more likely to suffer from various comorbidities likely to contribute to higher rates of mortality.4,21 Patients in the T3 group had a higher likelihood of being older and suffering from comorbidities like diabetes, chronic heart failure, a history of cancer, heart valvular disease, and prior stroke, while those with higher NPAR values were also associated with lower serum albumin and hemoglobin levels (Table 1), consistent with potential malnourishment. It is possible that the increased all-cause and cardiovascular death rates seen in the older with high NPAR were attributable to the presence of these complicated comorbid variables.
The combined clinical consequences of changes in neutrophil percentages and serum albumin levels may explain the association between NPAR values and rates of all-cause or cardiovascular death. Neutrophils, serving as innate immune cells, could mediate inflammatory responses and induce humoral and cellular immune responses.24,25 Meanwhile, neutrophils have increasingly been linked to both atherothrombosis and all-cause mortality.26,27 In cases of myocardial ischemia, NADPH oxidase, myeloperoxidase, and lipoxygenase release from neutrophils can contribute to more severe oxidative stress and endothelial dysfunciton.28 High levels of neutrophil influx in the ischemic injured myocardium can also potentially cause extensive collateral damage that impairs the healing of the myocardium, thereby shaping patient prognostic outcomes.26,28 Neutrophil counts have also been found to be strongly positively related to the incidence of cardiovascular conditions including heart failure, peripheral artery disease, and sudden coronary death.29 These suggest that nuetrophil-related inflammation may be related to the incidence of adverse events, highlight possible preventative or therapeutic targets associated with these forms of disease.30
Serum albumin is a plasma protein that has several roles, including regulating inflammation, maintaining normal colloidal osmotic pressure, and transporting many substances.31 Previous studies have found the low albumin levels was linked to increased rates of cardiovascular disease or mortalities.32–35 In addition, Peter et al had reported the hypoalbuminemia was associated with inflammation.36 Considering the role of hypoalbuminemia in inflammatory response, the older AF patients in the T3 group may thus be closely related to adverse events. Furthermore, lower serum albumin levels reflected patient malnutrition,37 further accounting for the higher rates of all-cause and cardiovascular mortality observed for patients in the T3 group.
The fact that NPAR values integrate both neutrophil percentage and serum albumin levels may make this a more representative biomarker capable go gauging the nutritional status and inflammatory conditions in patients with various diseases. Recent studies have shown that the elevated NPAR levels are closely correlated with adverse outcomes in patients with cardiovascular diseases. The higher NPAR values were independently associated with short-term mortality among patients with acute myocardial infarction.38,39 Moreover, Hu et al found the NPAR values predicted the length of hospital stay and mortality in heart failure patients.14 The admission NPAR was also reported to be positively related to the short- or long-term mortality in patients with cardiogenic shock.40,41 Considering the function above, the NPAR may be a potentially valuable measure for risk categorization of older individuals with AF. Further supporting the value of NPAR as a predictor of clinical risk when attempting to predict short-term all-cause or cardiovascular mortality risk in this patient population, the AUC values were higher when using NPAR as a predictor of mortality compared to using the neutrophil percentage or albumin levels alone.
The results of the subgroup analysis also showed a significant association between higher NPAR levels and an increased risk of all-cause or cardiovascular death at 28 days. While the HR value for older patients without hypertension did not achieve significance, it was nonetheless > 1 such that the association between NPAR values and mortality rates is likely to remain intact in this patient subgroup. When patients were separated into groups depending on the major reason for hospital admission, NPAR was substantially positively linked with all-cause death rates for all patients except those hospitalized owing to heart failure (Supplemental Figure 1). In terms of 28-day all-cause mortality rates, there was no statistically significant correlation between NPAR levels and the primary reason for hospitalization (as determined by the P-value for the interaction between NPAR values and the primary reason for hospital admission). Although the P-value for the interaction between NPAR levels and 28-day cardiovascular mortality was significant among patients with diabetes (P for interaction 0.005, Supplemental Figure 2), caution is warranted when considering whether diabetes status can influence the relationship between NPAR values and cardiovascular mortality, and prospective work will be needed to validate these findings. Altogether, these results lent credence to the idea that NPAR is a reliable and accurate means of predicting short-term prognostic outcomes in patients over the age of 80 with AF and several co-morbidities.
Given that most previous AF-centered research has been on younger patients and studies of adults ≥ 80 years of age with AF have been scarce, the inclusion of a large cohort of older adult patients with AF is a critical feature of our study. This study, to the best of our knowledge, is the first to investigate the link between NPAR and cardiovascular or all-cause mortality in older individuals with AF. However, these findings are subject to certain limitations. First, owing to the nature of retrospective analysis, the causation cannot be confirmed. Second, this was a single-center analysis and it remains to be determined whether these findings can be generalized to other institutions. Third, the coronary heart disease was acquired by a synthesis of self-reported physician diagnoses or standardized medical condition inquiries, which might overestimate the prevalence. And the lack of SYNTAX scores compromised the complexity of coronary artery disease. Fourth, older patients with AF were likely to exhibit a range of comorbidities, and it was not possible to fully exclude the potential effect of these comorbidities on mortality outcomes. Last, the values used to calculate the NPAR were based on single measurements within 24 h post-admission, thus potentially overlooking the effects of dynamic changes in these parameters with time. Further research examining the association between changes in NPAR and mortality outcomes throughout hospitalization is thus warranted.
Conclusion
In conclusion, this study shows that higher NPAR levels are related positively to higher risks of all-cause and cardiovascular mortality in the older patients with AF. Validation of these results, however, would need more large-scale observational study.
Funding Statement
The financial support by the Shanghai Municipal Health Commission (202140237) and Fudan University Zhongshan Hospital, Qingpu Branch (QY2022-04).
Ethics Statement
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Biomedical Research Ethics Committee at Huadong Hospital (Shanghai, China; Ethical code: 2021K174).
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article had been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen M, Li C, Liao P, et al. Epidemiology, management, and outcomes of atrial fibrillation among 30 million citizens in Shanghai, China from 2015 to 2020: a medical insurance database study. Lancet Reg Health West Pac. 2022;23:100470. doi: 10.1016/j.lanwpc.2022.100470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 3.Singh SM, Abdel-Qadir H, Pang A, et al. Population trends in all-cause mortality and cause specific-death with incident atrial fibrillation. J Am Heart Assoc. 2020;9(19):e016810. doi: 10.1161/JAHA.120.016810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Terwangne C, Lelubre C, Hanotier P, et al. Prevalence and impact of atrial fibrillation on intra-hospital mortality in patients aged ≥75 years. Am J Cardiol. 2022;177:40–47. doi: 10.1016/j.amjcard.2022.04.050 [DOI] [PubMed] [Google Scholar]
- 5.Narasimhan B, Patel N, Chakraborty S, et al. Impact of atrial fibrillation on acute coronary syndrome-analysis of in-hospital outcomes and 30-day readmissions. Curr Probl Cardiol. 2021;46(4):100764. doi: 10.1016/j.cpcardiol.2020.100764 [DOI] [PubMed] [Google Scholar]
- 6.Shen X-F, Cao K, Jiang J-P, Guan W-X, Du J-F. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21(9):1687–1697. doi: 10.1111/jcmm.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park I, Kim M, Choe K, et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur Respir J. 2019;53:3. doi: 10.1183/13993003.00786-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. 2020;148:e87. doi: 10.1017/S0950268820000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolis AA, Manolis TA, Melita H, Mikhailidis DP, Manolis AS. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med. 2022;102:24–39. doi: 10.1016/j.ejim.2022.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Chen C, Zhang L, et al. The association between the prognostic nutritional index and 28-day mortality among atrial fibrillation patients in China over 80 years of age. NMCD. 2022;32(6):1493–1501. doi: 10.1016/j.numecd.2022.03.013 [DOI] [PubMed] [Google Scholar]
- 11.Tingle SJ, Severs GR, Goodfellow M, Moir JA, White SA. NARCA: a novel prognostic scoring system using neutrophil-albumin ratio and Ca19-9 to predict overall survival in palliative pancreatic cancer. J Surg Oncol. 2018;118(4):680–686. doi: 10.1002/jso.25209 [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Li D, Cheng B, Ying B, Gong Y. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute kidney injury. Biomed Res Int. 2020;2020:5687672. doi: 10.1155/2020/5687672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Wu T, Tian X, Lyu P, Wang J, Cao Y. High neutrophil percentage-to-albumin ratio can predict occurrence of stroke-associated infection. Front Neurol. 2021;12:705790. doi: 10.3389/fneur.2021.705790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z, Wang J, Xue Y, et al. The neutrophil-to-albumin ratio as a new predictor of all-cause mortality in patients with heart failure. J Inflamm Res. 2022;15:701–713. doi: 10.2147/JIR.S349996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Cong HL, Zhang JX, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi: 10.1186/s12933-020-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 19.Jaddoe VWV, de Jonge LL, Hofman A, Franco OH, Steegers EAP, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 21.Fauchier L, Samson A, Chaize G, et al. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015;2(1):e000290. doi: 10.1136/openhrt-2015-000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marijon E, Le Heuzey J-Y, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–1056. doi: 10.1001/jama.290.8.1049 [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Hou H, Li L, et al. Neutrophil percentage-to-albumin ratio: a good parameter for the evaluation of the severity of anti-NMDAR encephalitis at admission and prediction of short-term prognosis. Front Immunol. 2022;13:847200. doi: 10.3389/fimmu.2022.847200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol. 2013;35(4):377–394. doi: 10.1007/s00281-013-0374-8 [DOI] [PubMed] [Google Scholar]
- 26.Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. doi: 10.1093/eurheartj/ehaa1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Eliot M, Koestler DC, Wu W-C, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the duffy antigen variant. JAMA Cardiol. 2018;3(6):455–462. doi: 10.1001/jamacardio.2018.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a CALIBER cohort study. J Am Coll Cardiol. 2017;69(9):1160–1169. doi: 10.1016/j.jacc.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J. 2013;40(1):17–29. [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. doi: 10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 32.González-Pacheco H, Amezcua-Guerra LM, Sandoval J, et al. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–958. doi: 10.1016/j.amjcard.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 33.Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol. 2016;219:20–24. doi: 10.1016/j.ijcard.2016.05.067 [DOI] [PubMed] [Google Scholar]
- 34.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 35.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477 [DOI] [PubMed] [Google Scholar]
- 36.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2(8677):1434–1436. doi: 10.1016/S0140-6736(89)92042-4 [DOI] [PubMed] [Google Scholar]
- 38.Dai K, Li Z, Luo Y, et al. Neutrophil percentage-to-albumin ratio and monocyte-to-lymphocyte ratio as predictors of free-wall rupture in patients with acute myocardial infarction. J Clin Lab Anal. 2022;36(1):e24136. doi: 10.1002/jcla.24136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui H, Ding X, Li W, Chen H, Li H. The neutrophil percentage to albumin ratio as a new predictor of in-hospital mortality in patients with ST-segment elevation myocardial infarction. Med Sci Monit. 2019;25:7845–7852. doi: 10.12659/MSM.917987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Liu Y, Ling X, et al. The neutrophil percentage-to-albumin ratio as a new predictor of all-cause mortality in patients with cardiogenic shock. Biomed Res Int. 2020;2020:7458451. doi: 10.1155/2020/7458451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y, Xue Y, Wang J, et al. Association between neutrophil-to-albumin ratio and mortality in patients with cardiogenic shock: a retrospective cohort study. BMJ Open. 2020;10(10):e039860. doi: 10.1136/bmjopen-2020-039860 [DOI] [PMC free article] [PubMed] [Google Scholar]