Abstract
Background:
Shortened telomeres are associated with several different subtypes of interstitial lung disease (ILD), although studies of telomere length and ILD in rheumatoid arthritis (RA) are lacking.
Methods:
Within the Veterans Affairs Rheumatoid Arthritis (VARA) registry, we performed cross-sectional and case-control studies of prevalent and incident ILD, respectively. We randomly selected a subset of RA patients with ILD and individually matched them to RA patients without ILD according to age, sex, and VARA enrollment date. Telomere length was measured on peripheral blood leukocytes collected at registry enrollment using quantitative PCR (T/S ratio). Short telomeres were defined as a T/S ratio in the lowest 10th percentile of the cohort.
Results:
Our cross-sectional study cohort was comprised of 54 RA-ILD patients and 92 RA-non-ILD patients. T/S ratios significantly differed between patients with and without prevalent ILD (1.56 [IQR 1.30, 1.78] vs. 1.96 [IQR 1.65, 2.27], p<0.001). Similarly, prevalence of ILD was significantly higher in patients with short vs. normal-length telomeres (73.3% vs. 32.8%, p=0.002). Short telomeres were independently associated with an increased odds of prevalent ILD compared to normal-length telomeres (adjusted OR 6.60, 95% CI 1.78–24.51, p=0.005). In our case-control analysis, comprised of 22 incident RA-ILD cases and 36 RA-non-ILD controls, short telomeres were not associated with incident RA-ILD (adjusted OR 0.90, 95% CI 0.06–13.4, p=0.94).
Conclusion:
Short telomeres were strongly associated with prevalent but not incident ILD among patients with RA. Additional studies are needed to better understand telomere length dynamics among RA patients with and without ILD.
Keywords: Interstitial lung disease, rheumatoid arthritis, rheumatoid arthritis-associated interstitial lung disease, telomere length, telomeres, T/S ratio
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune rheumatic disease characterized by symmetric, inflammatory polyarthritis of the peripheral joints that leads to cartilaginous and bony destruction.1 Extraarticular involvement of RA is common and includes a variety of pulmonary manifestations.2,3 Clinically significant interstitial lung disease (ILD) is seen in up to 10% of patients with RA and strongly contributes to morbidity and mortality.4,5 In addition, interstitial changes on computed tomography (CT) have been described in up to 60% of RA patients.6 Despite a growing recognition of ILD in RA (hereafter, RA-ILD), identifying at-risk patients remains a challenge.
Unlike other connective tissue diseases, usual interstitial pneumonia (UIP) – the radiographic and histopathologic pattern associated with idiopathic pulmonary fibrosis (IPF) – is the most common pattern seen in RA-ILD7 and is associated with faster disease progression and higher risk for mortality.8,9 Notably, RA-ILD and IPF have many shared risk factors, including male sex, history of smoking, and older age.10,11 Telomeres are the protective “caps” on the ends of chromosomes, which, when sufficiently long, prevent the activation of DNA damage responses that result in cellular senescence or apoptosis. Telomeres tend to shorten with age and various cellular injuries, and shortened telomeres have been associated with both familial and sporadic IPF.12,13 Although the association between telomere length and RA-ILD remains unknown, telomere shortening is not unique to IPF and has been described in a variety of ILD subtypes, including connective tissue disease-associated ILD (CTD-ILD).14–17
Our primary objective was to determine whether telomere shortening is associated with prevalent and incident ILD in RA patients. Given the radiographic and histopathologic similarities between RA-ILD and IPF, we hypothesized that telomere shortening at baseline would be associated with prevalent RA-ILD and would confer greater risk for the development of incident RA-ILD.
Study Design and Methods
Study Design and Patient Population
We performed both a cross-sectional study of prevalent RA-ILD and a nested case-control study of incident RA-ILD within the Veterans Affairs Rheumatoid Arthritis (VARA) registry. Initiated in 2003, the VARA registry is a multicenter prospective registry of U.S. veterans with RA fulfilling the 1987 American College of Rheumatology criteria.18 Prevalent RA-ILD was defined as ILD diagnosed prior to or within one year of registry enrollment, whereas incident RA-ILD was defined as ILD diagnosed at least 1 year but less than 6 years after registry enrollment. Our incident ILD definition took into account available longitudinal data within our cohort and recent data demonstrating a median time to ILD onset after RA diagnosis of 2.3 years.19
For our cross-sectional study, we randomly selected a subset of patients with RA-ILD (n=54) and individually matched them to RA patients without ILD (n=92) based on age, sex, and VARA registry enrollment date. We individually matched on the basis of age and sex to account for population-based age- and sex-related telomere length differences.20 RA-ILD cases and RA-non-ILD controls were matched in a 1:2 ratio whenever possible, although a limited number of RA-ILD patients could only be matched 1:1. Our case-control study population was derived from the same 92 subjects without baseline RA-ILD examined in our cross-sectional analysis. For this study, cases were identified according to our incident RA-ILD definition (n=22), whereas subjects were included as controls if they had at least 6 years of follow-up from registry enrollment (n=36). Follow-up occurred through December 31, 2018.
Data on patient socio-demographics, comorbidities, medications, smoking status (current, former, or never), and date of RA disease onset were collected at enrollment, in addition to disease activity and functional status metrics such as the Disease Activity Score in 28 Joints (DAS28)21 and the Multidimensional Health Assessment Questionnaire (MDHAQ).22 All patients provided informed consent prior to enrollment. In addition, all 13 participating sites obtained local institutional review board approval. The present study was approved by the VARA Scientific Ethics and Advisory Committee.
Characterization of RA-ILD within the VARA Registry
As described in prior studies, RA-ILD status was determined through standardized medical record adjudication among patients who screened positive for one or more International Classification of Diseases, 9th Revision (ICD-9) or 10th Revision (ICD-10) codes previously proposed to ascertain ILD status.23 Specifically, RA-ILD classification required a provider diagnosis of ILD and either (1) radiographic ILD features on chest CT (e.g., honeycombing, reticulation, traction bronchiectasis, groundglass opacities, centrilobular nodules, or cysts, among others) or (2) histopathologic ILD features on lung biopsy reports (UIP, nonspecific interstitial pneumonia, organizing pneumonia, desquamative interstitial pneumonia, etc. or more generically “pulmonary fibrosis”). Of note, all patterns of ILD were included in our RA-ILD definition, in accordance with an existing ILD classification schema.24 Patterns of RA-ILD were based on clinical reads of chest CTs, with only approximately 50% of cases reporting a specific ILD phenotypic pattern, in which UIP was most common. The vast majority of RA-ILD cases (more than 95%) were diagnosed based on CT findings. Imaging reports were reviewed by three rheumatologists with clinical expertise in RA-ILD who trained against each other in pilot abstraction. To assess the sensitivity of our screening approach, a random sample of 243 VARA registry subjects without ILD diagnostic codes was reviewed by applying the same methodology, of which only 7 (2.9%) were ultimately classified as having RA-ILD.23
Telomere Length Measurements
We extracted genomic DNA from peripheral blood collected at time of VARA registry enrollment using the QIAamp Blood DNA Midi Kit (QIAGEN), and DNA concentrations were quantified using Qubit fluorometry (Invitrogen). Relative telomere length was measured using a high-throughput monoplex real-time quantitative polymerase chain reaction (qPCR) assay, which quantifies a ratio of telomeric repeat copy signal (T) and a reference single-copy gene (human beta globin) signal (S), thus generating a T/S ratio for each individual subject.25 Assays were performed as previously described26 except that they were carried out in 384-well plates using 3 ng of genomic DNA per reaction on a Roche LightCycler 480 using the second derivative maximum method. Each sample was measured in triplicate and in a blinded fashion, and ΔCt value used to determine T/S was the cycle threshold (Ct) obtained for the telomere qPCR reaction subtracted from that obtained for the single copy gene. To ensure that the qPCR Ct values reflected linear responses to input DNA and that the samples were therefore free of contaminants that could skew measurements, triplicate reactions were also carried out using 6 ng of DNA, and only samples that yielded the same T/S ratios as those obtained using 3 ng were used for further analysis. To account for inter-run variability, experimental sample T/S values were normalized to the mean T/S ratio obtained for six standard samples included in each run.
In an external cohort of 13 patients with ILD, we validated our qPCR method against telomere length (kb) measured in peripheral blood lymphocytes and granulocytes via fluorescent in situ hybridization-flow cytometry (flow-FISH) by the Department of Pathology at Johns Hopkins Hospital, as described previously.27 T/S ratios compared to granulocyte and lymphocyte telomere lengths measured using flow-FISH showed significant correlation (p<0.01 for both comparisons, Figure 1). Unlike flow-FISH, there are no population-based age- or sex-established norms when using qPCR methods to calculate telomere length based on T/S ratio, thus necessitating that we perform within-cohort comparisons. To account for age- and sex-related telomere length differences, our cohort was derived by individually matching patients with and without RA-ILD on these parameters.
Figure 1:
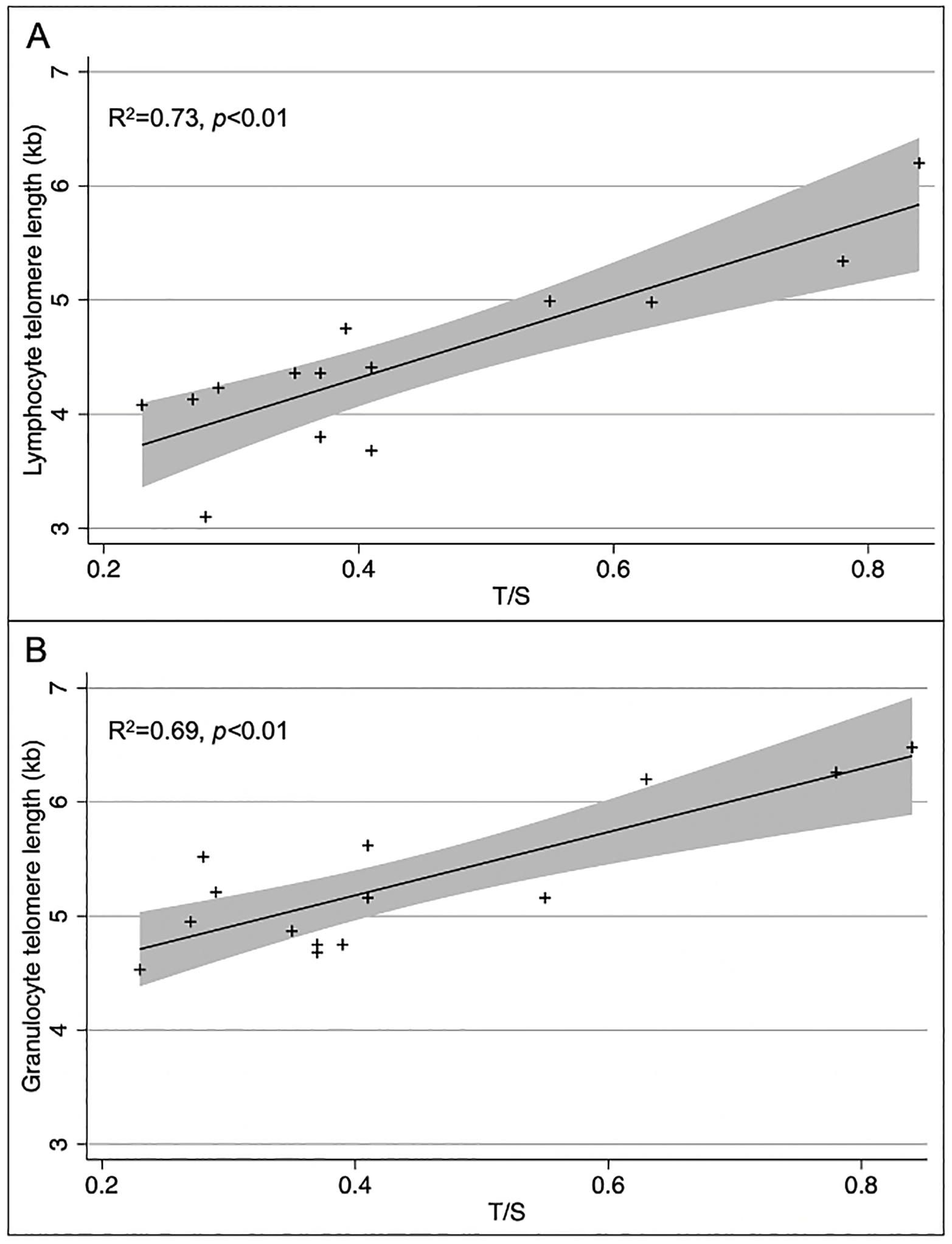
Comparison of telomere length (TL) measurements from lymphocytes and granulocytes using flow-FISH vs. TL measurements from whole blood-extracted DNA using qPCR ΔCt methods. (A) Lymphocyte TL by flow-FISH vs. ΔCt qPCR algorithm; and (B) granulocyte TL by flow-FISH vs. ΔCt qPCR algorithm.
Statistical Analysis
For comparing baseline socio-demographic and clinical covariates, patients were divided according to whether their T/S ratio fell within or above the lowest 10th percentile of the cohort (i.e., “short” vs. “normal” telomeres), as described in prior clinical studies.28,29 Differences in patient characteristics were analyzed using the Mann-Whitney U test, chi-squared test, or Fisher’s exact test, as appropriate. The distribution of T/S ratios was logarithmically transformed given its rightward skewness.
For our cross-sectional study, we performed univariate and multivariable conditional logistic regression analyses to assess the associations between prevalent RA-ILD and telomere length (both dichotomized and continuous log-transformed, as defined above). The following covariates were selected a priori for inclusion in our multivariable model based on preexisting mechanistic and biological knowledge: age at enrollment, sex, race (Caucasian, African American, or other), smoking history (current, former, or never), RA disease duration, and baseline severity of articular disease as measured by the DAS28. Other covariates such as MDHAQ scores and use of biologics, specifically anti-TNF therapies, were not included in our multivariable model given concern they measure overlapping constructs relative to the DAS28. In addition, increased adiposity has not been directly linked to higher rates of interstitial lung disease, which is why we opted to exclude BMI from our multivariable models.
For our case-control study, we similarly performed univariate and multivariable conditional logistic regression analyses to assess the associations between incident RA-ILD and telomere length as both a binary and continuous variable. Similar covariates were included in our multivariable model. As a planned sensitivity analysis, we performed additional univariate and multivariable regressions in which an incident RA-ILD case was defined as diagnosed at least 1 year but less than 5 years after registry enrollment (whereby shorter follow-up increased the total number of subjects eligible for inclusion but reduced the number of incident RA-ILD cases) and as diagnosed at least 1 year but less than 7 years after registry enrollment (whereby longer follow-up decreased the total number of subjects eligible for inclusion but increased the number of incident RA-ILD cases).
According to our power calculations, an estimated 108 patients (36 cases and 94 controls) would be required to achieve 80% power at a two-sided alpha of 0.05 to detect a significant difference in risk for ILD between RA patients with and without short telomeres, assuming a 10% difference in average telomere length between RA-ILD and RA-non-ILD patients. Statistical significance was defined as p<0.05. All analyses were performed using Stata/MP, version 17 (College Station, TX).
Results
Telomere Length and Prevalent RA-ILD
The cross-sectional study sample was comprised of 146 patients: 54 RA-ILD patients and 92 matched controls (median age 66 years, 94.5% male). Patients were stratified according to whether or not they had short telomeres, as previously defined. There were no differences noted in age, sex, race, ethnicity, or smoking history between patients with and without short telomeres (Table 1). In addition, RA disease duration and baseline DAS28 and MDHAQ scores were similar. Patients with short telomeres had significantly higher BMIs. Although prednisone use was higher in patients with short telomeres, there was similar usage of methotrexate and anti-TNF therapies.
Table 1:
Baseline socio-demographic and clinical characteristics of cross-sectional study participants, stratified by telomere length above or below the 10th percentile
| Overall | Telomere Length Above 10th Percentile | Telomere Length Below 10th Percentile | P value | ||
|---|---|---|---|---|---|
| N | 146 | 131 | 15 | ||
| Age, median (IQR) | 66 (61, 73) | 66 (61, 72) | 69 (58, 76) | 0.56 | |
| Male (%) | 138 (94.5) | 123 (93.9) | 15 (100.0) | 0.32 | |
| Race (%) | White or Caucasian | 110 (75.3) | 98 (74.8) | 12 (80.0) | 0.52 |
| Black or African American | 23 (15.8) | 22 (16.8) | 1 (6.7) | ||
| Other | 13 (8.9) | 11 (8.4) | 2 (13.3) | ||
| Ethnicity (%) | Hispanic or Latino | 6 (4.1) | 6 (4.6) | 0 (0.0) | 0.70 |
| Not Hispanic or Latino | 131 (89.7) | 117 (89.3) | 14 (93.3) | ||
| Missing | 9 (6.2) | 8 (6.1) | 1 (6.7) | ||
| BMI, median (IQR) | 28.0 (24.4, 31.1) | 27.6 (24.2, 31.0) | 30.9 (27.6, 36.8) | 0.03 | |
| Smoking history (%) | Never | 25 (17.1) | 23 (17.6) | 2 (13.3) | 0.54 |
| Former | 33 (22.6) | 31 (23.7) | 2 (13.3) | ||
| Current | 88 (60.3) | 77 (58.8) | 11 (73.3) | ||
| Hypertension (%) | 11 (7.5) | 11 (8.4) | 0 (0.0) | 0.24 | |
| Cardiac disease (%) | 8 (5.5) | 6 (4.6) | 2 (13.3) | 0.16 | |
| Diabetes mellitus (%) | 12 (8.2) | 11 (8.4) | 1 (6.7) | 0.82 | |
| COPD or asthma (%) | 4 (2.7) | 2 (1.5) | 2 (13.3) | 0.008 | |
| RA disease duration, years, median (IQR) | 8.3 (1.9, 19.6) | 7.9 (1.8, 19.2) | 9.9 (4.5, 26.6) | 0.41 | |
| DAS28, median (IQR) | 3.6 (2.5, 5.1) | 3.6 (2.5, 5.1) | 3.7 (2.4, 5.1) | 0.92 | |
| MDHAQ, median (IQR) | 0.9 (0.5, 1.3) | 0.8 (0.5, 1.3) | 1.3 (0.5, 1.5) | 0.13 | |
| Prednisone use (%) | 69 (47.3) | 57 (43.5) | 12 (80.0) | 0.007 | |
| Methotrexate use (%) | 80 (54.8) | 74 (56.5) | 6 (40.0) | 0.22 | |
| Anti-TNF use (%) | 55 (37.7) | 48 (36.6) | 7 (46.7) | 0.45 | |
Abbreviations: IQR = interquartile range; BMI = body mass index; COPD = chronic obstructive pulmonary disease; RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints; MDHAQ = Multidimensional Health Assessment Questionnaire; TNF = tumor necrosis factor.
T/S ratios significantly differed between patients with and without prevalent RA-ILD (1.56 [IQR 1.30, 1.78] vs. 1.96 [IQR 1.65, 2.27], p<0.001, Figure 2). Similarly, patients with short telomeres were more likely to have RA-ILD compared to those with normal telomeres (73.3% vs. 32.8%, p=0.002). Patients with short telomeres had increased odds of prevalent RA-ILD compared to patients with normal telomeres in both unadjusted (OR 5.63, 95% CI 1.69–18.71, p=0.005) and adjusted (OR 6.60, 95% CI 1.78–24.51, p=0.005) analyses (Table 3). In addition, continuous log-transformed T/S ratio was highly associated with prevalent RA-ILD in both our univariate (OR per 1 standard deviation [SD] decrease 2.70, 95% CI 1.72–4.22, p<0.001) and multivariable (OR per 1 SD decrease 3.30, 95% CI 1.99–5.47, p<0.001) analyses (Table 3), where lower values of log-transformed T/S ratio (i.e., shorter telomeres) conferred greater risk of having RA-LD.
Figure 2:
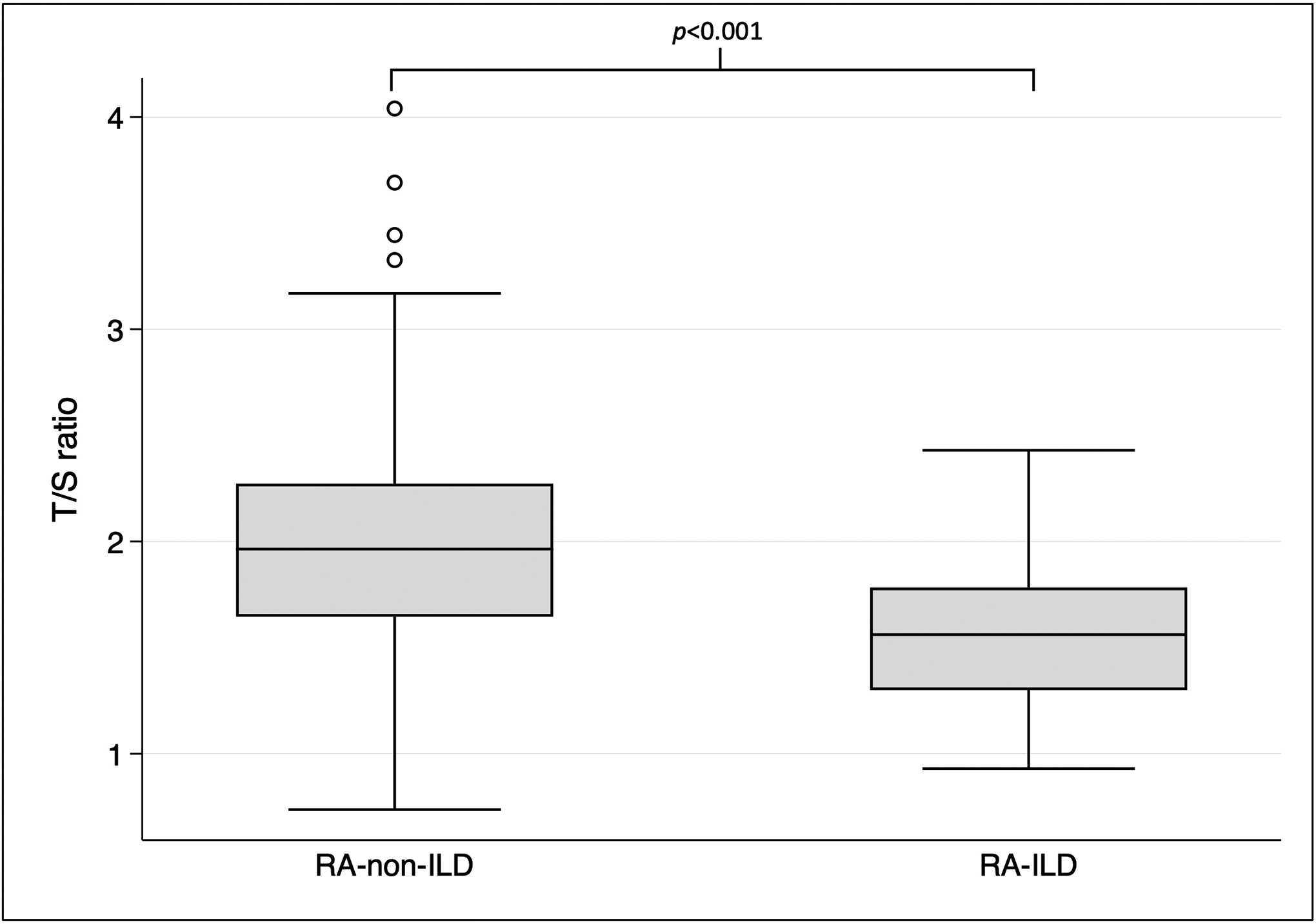
Comparison of T/S ratios among RA-non-ILD and RA-ILD subjects within the cross-sectional study population.
Table 3:
Univariate and multivariable conditional logistic regression analyses of the cross-sectional study sample
| Univariate | Multivariable * | |||||||
|---|---|---|---|---|---|---|---|---|
| No. exposed | No. with ILD (%) | OR | 95% CI | P value | OR | 95% CI | P value | |
| Binary Telomere Length Analysis | ||||||||
| T/S ratio above 10th percentile | 131 | 43 (32.8) | Ref. | - | - | Ref. | - | - |
| T/S ratio below 10th percentile | 15 | 11 (73.3) | 5.63 | 1.69–18.71 | 0.005 | 6.60 | 1.78–24.51 | 0.005 |
| Continuous Log-transformed Telomere Length Analysis | ||||||||
| Per 1 SD decrease in ln(T/S ratio) | - | - | 2.70 | 1.72–4.22 | <0.001 | 3.30 | 1.99–5.47 | <0.001 |
Multivariable models are adjusted for age at enrollment, sex, race (Caucasian, African American, or other), smoking history, RA disease duration, and baseline articular disease severity (DAS28).
Abbreviations: ILD = interstitial lung disease; OR = odds ratio; CI = confidence interval; ln = natural log; RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints. SD??
Telomere Length and Incident ILD
The case-control sample was comprised of 58 patients: 22 incident cases of RA-ILD and 36 controls. There were no statistically significant differences noted in age, sex, race, ethnicity, BMI, smoking history, RA disease duration, baseline DAS28 and MDHAQ scores, or use of pharmacologic treatments for RA (Table 4) between patients with and without incident RA-ILD, nor were any differences noted between patients with short telomers and normal telomeres across all measured baseline socio-demographic and clinical covariates (data not shown). Among cases, median time from registry enrollment to ILD diagnosis was 3.1 years (IQR 2.2, 4.4 years).
Table 4:
Baseline socio-demographic and clinical characteristics of case-control study participants, stratified by incident RA-ILD case status
| Overall | Incident ILD Cases | Controls | P value | ||
|---|---|---|---|---|---|
| N | 58 | 22 | 36 | ||
| Age, median (IQR) | 65 (60, 69) | 65 (64, 70) | 65 (59.5, 69) | 0.77 | |
| Male (%) | 54 (93) | 20 (91) | 34 (94) | 0.61 | |
| Race (%) | White or Caucasian | 43 (74) | 16 (73) | 27 (75) | 0.52 |
| Black or African American | 10 (17) | 5 (23) | 5 (14) | ||
| Other | 5 (9) | 1 (5) | 4 (11) | ||
| Ethnicity (%) | Hispanic or Latino | 3 (5) | 1 (5) | 2 (6) | 0.84 |
| Not Hispanic or Latino | 51 (88) | 20 (91) | 31 (86) | ||
| Missing | 4 (7) | 1 (5) | 3 (8) | ||
| BMI, median (IQR) | 28.8 (25.0, 31.1) | 29.1 (24.6, 31.1) | 28.7 (25.7, 31.0) | 0.76 | |
| Smoking history (%) | Never | 11 (19) | 3 (14) | 8 (22) | 0.14 |
| Former | 11 (19) | 7 (32) | 4 (11) | ||
| Current | 36 (62) | 12 (55) | 24 (67) | ||
| Hypertension (%) | 6 (10) | 3 (8) | 3 (14) | 0.52 | |
| Cardiac disease (%) | 1 (2) | 1 (3) | 0 (0) | 0.43 | |
| Diabetes mellitus (%) | 8 (14) | 3 (8) | 5 (23) | 0.12 | |
| COPD or asthma (%) | 2 (3) | 1 (3) | 1 (5) | 0.72 | |
| RA disease duration, years, median (IQR) | 10.5 (1.8, 17.4) | 8.0 (1.8, 15.3) | 10.8 (3.2, 19.0) | 0.30 | |
| DAS28, median (IQR) | 3.0 (2.3, 4.2) | 2.9 (2.4, 3.8) | 3.2 (2.2, 4.5) | 0.67 | |
| MDHAQ, median (IQR) | 0.7 (0.4, 1.2) | 0.8 (0.4, 1.1) | 0.7 (0.4, 1.2) | 0.76 | |
| Prednisone use (%) | 27 (47) | 11 (50) | 16 (44) | 0.68 | |
| Methotrexate use (%) | 33 (57) | 15 (68) | 18 (50) | 0.17 | |
| Anti-TNF use (%) | 20 (34) | 8 (36) | 12 (33) | 0.81 | |
Abbreviations: RA-ILD = rheumatoid arthritis-associated interstitial lung disease; IQR = interquartile range; BMI = body mass index; COPD = chronic obstructive pulmonary disease; RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints; MDHAQ = Multidimensional Health Assessment Questionnaire; TNF = tumor necrosis factor.
Cases and controls had similar T/S ratios (2.14 [IQR 1.65, 2.35] vs. 2.00 [IQR 1.67, 2.25], p=0.38). In addition, the proportions of subjects with incident RA-ILD did not statistically significantly differ between the short telomere and normal telomere groups (16.7% vs. 40.4%, p=0.42). The odds ratios of short telomeres for case status were OR 0.30, 95% CI 0.03–2.71, p=0.28 in our unadjusted analysis and OR 0.90, 95% CI 0.06–13.43, p=0.94 in our adjusted analysis (Table 5). In addition, log-transformed T/S ratio was not associated with incident RA-ILD in both our univariate (OR per 1 SD decrease 0.69, 95% CI 0.39–1.23, p=0.21) and multivariable (OR per 1 SD decrease 0.80, 95% CI 0.36–1.76, p=0.58) analyses (Table 5). Similar findings were noted when the incident RA-ILD definition was modified as diagnosed at least 1 year but less than 5 years after registry enrollment (adjusted OR per 1 SD decrease 0.67, 95% CI 0.31–1.47, p=0.32) and as diagnosed at least 1 year but less than 7 years after registry enrollment (adjusted OR per 1 SD decrease 0.92, 95% CI 0.43–2.00, p=0.84).
Table 5:
Univariate and multivariable conditional logistic regression analyses of the case-control study sample
| Univariate | Multivariable * | |||||||
|---|---|---|---|---|---|---|---|---|
| No. exposed | No. with ILD (%) | OR | 95% CI | P value | OR | 95% CI | P value | |
| Binary Telomere Length Analysis | ||||||||
| T/S ratio above 10th percentile | 52 | 21 (40.4) | Ref. | - | - | Ref. | - | - |
| T/S ratio below 10th percentile | 6 | 1 (16.7) | 0.30 | 0.03–2.71 | 0.28 | 0.90 | 0.06–13.43 | 0.94 |
| Continuous Log-transformed Telomere Length Analysis | ||||||||
| Per 1 SD decrease in ln(T/S ratio) | - | - | 0.69 | 0.39–1.23 | 0.21 | 0.80 | 0.36–1.76 | 0.58 |
Multivariable models are adjusted for age at enrollment, sex, race (Caucasian, African American, or other), smoking history, RA disease duration, and baseline articular disease severity (DAS28).
Abbreviations: ILD = interstitial lung disease; OR = odds ratio; CI = confidence interval; ln = natural log; RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints.
Discussion
In this study, patients with short telomeres were more likely to have RA-ILD at entry into the VARA registry compared to patients with normal telomeres. However, patients with short telomeres at baseline did not have a higher risk of developing incident RA-ILD during the ensuing 5–7 years. To our knowledge, this is the largest study of an RA-specific cohort examining associations between telomere length and ILD.
Broadly speaking, human telomere disease consists of a wide spectrum of disorders linked to neoplastic, pulmonary, hepatic, and bone marrow abnormalities.14,30 Telomere erosion is a hallmark feature of immunosenescence and has been demonstrated in both lymphoid and myeloid cells in RA patients.31 This accelerated aging of the immune system, as well as many other organ systems, is often characteristic of RA.32 Subsequent studies have linked telomere shortening to more severe articular disease and accelerated atherosclerosis in a subset of patients with RA.33,34 Furthermore, additional studies have demonstrated that telomere shortening is associated with worse outcomes in other subtypes of ILD.17,35
Patients with RA have approximately a nine-fold increased risk for ILD in comparison to the general population.36,37 Previous studies have estimated that roughly 10% of RA patients have clinically significant ILD.4,38–40 Furthermore, radiographic interstitial changes on chest CT are present in 30–60% of patients with RA,6 although it is unclear what proportion of these patients subsequently progress to more advanced disease. Risk of mortality among RA-ILD patients is three times higher in comparison to RA patients without ILD,41 with one recent study reporting significant differences in one- and five-year mortality rates (14% vs. 4% and 39% vs. 18%, respectively).5 As such, identifying those most at risk for ILD is a crucial aspect of providing comprehensive medical care to patients with RA. While telomere length alone may not be sufficient to quantify ILD risk, additional studies are needed to determine whether its use with alternative biomarkers or in combination with screening for clinical symptoms of ILD such as cough and dyspnea may result in earlier identification and treatment, particularly among RA patients with other established ILD risk factors.
Prior studies have only examined telomere length and RA-ILD to a limited extent. Newton et al. recently demonstrated a more rapid decline in lung function among CTD-ILD patients with short telomeres.17 This cohort consisted of CTD patients with established ILD (among which only 25% had RA-ILD) and did not specifically address the association of telomere length and ILD risk in RA. Juge et al. demonstrated a higher prevalence of mutations in the TERT gene among RA-ILD patients relative to the general population.42 This gene encodes for a subunit of telomerase, an enzyme responsible for maintaining the length of telomeres by adding guanine-rich repetitive sequences thereby preventing chromosomal degradation. In addition, they noted shorter telomere lengths among RA-ILD patients relative to a limited number of healthy controls.42 Although beyond the scope of this project, it would be of value to study whether there is a higher prevalence of telomerase gene mutations in RA-ILD patients compared to RA-non-ILD patients (as opposed to in comparison to healthy non-RA controls), although this would require a much larger sample size. Regardless, existing evidence suggests telomere length may play an important role in the pathogenesis of RA, even in the absence of ILD.31–34
Results from our cross-sectional and case-control studies differed. One possible explanation is that the development of critically short telomeres only predates the onset of pulmonary fibrosis by several weeks to months, as has been shown in several murine models.43,44 In addition, RA patients have been demonstrated to have telomere shortening even in the absence of ILD, which may have limited our ability to detect differences in T/S ratios between pre-ILD patients and non-ILD controls. Another possibility is that our case-control study was underpowered. We attempted to account for this by analyzing T/S ratios as both a binary and a continuous variable. In addition, we performed several sensitivity analyses using different time periods to define incident ILD. One final potential explanation for the discrepancies is a “depletion of susceptibles” phenomenon given subjects with short telomeres had a longer median duration of RA prior to VARA registry enrollment, thus providing more time “at risk” for developing ILD prior to or within the first year of study enrollment. Collectively, our findings highlight the importance of a longitudinal study of RA patients without baseline ILD in which serial measurements of telomere length are acquired at regular intervals while closely monitoring for new-onset ILD.
Within our cross-sectional study population, rates of smoking were similar between patients with and without prevalent RA-ILD, as well as between patients with and without shorter telomeres, thus making this an unlikely explanation as why patients with shorter telomeres were more likely to have prevalent RA-ILD. Although prednisone use did not differ between patients with and without prevalent RA-ILD, there was a higher proportion of prednisone use among patients with shorter telomeres. This may, in part, be due to the fact that telomere dysfunction correlates with articular disease severity.33 Lastly, patients with shorter telomeres had significantly higher BMIs, in line with findings from a recent meta-analysis in which obesity was shown to accelerate leukocyte telomere length shortening in healthy adults.45 Increased adiposity, however, has not been previously directly linked to a higher risk of ILD, which is why we opted to exclude BMI from our multivariable models.
Our study has limitations. The male-predominant nature of our veteran cohort, as well as its high rates of cigarette smoking and additional potential unique inhalant exposures through military service46 compared to other modern-day RA cohorts may affect generalizability. However, because RA-ILD is more common in men than in women4,41 and given almost 70% of patients with RA-ILD have smoked,10 our study population is uniquely enriched with ILD risk factors, thus better enabling us to investigate associations of telomere length and other biomarkers with prevalent and incident ILD. In addition, men with RA tend to have more aggressive disease in comparison to women, characterized by more severe articular damage, a higher prevalence of extraarticular manifestations, and greater disease-related mortality.47,48 Lastly, the VA represents the largest integrated healthcare system in the U.S. and thus provides an opportunity to study a uniquely vulnerable RA population while minimizing confounding that arises from significant socioeconomic barriers to healthcare.
Given a significant proportion of RA patients have subclinical radiographic interstitial changes and because our patients were not uniformly screened for ILD, we may have underestimated the prevalence and incidence of ILD within our study population. However, such ILD misclassification should have biased our results towards the null. Furthermore, we utilized qPCR to determine telomere length. Although this method has previously been applied for studying ILD and other pulmonary diseases,49–53 flow-FISH is thought to be the gold standard.12,13 However, we demonstrated significant correlation between the two methods in a pilot study of patients with ILD. Because pulmonary function test (PFT) data were obtained as part of routine care and retrospectively extracted from medical records, the variability in timing and availability of PFT data in relationship to telomere length measurements limited our ability to assess associations between telomere length and degree of physiologic impairment. Finally, we were not able to assess associations between telomere length and RA-ILD pattern (e.g., UIP vs. nonspecific interstitial pneumonia vs. other) given the low frequency with which the specific ILD phenotypic pattern was reported.
Conclusion
We found telomere length to be highly associated with prevalent ILD among patients with RA. However, in the immediate years prior to ILD onset, telomere length was not meaningfully shorter among RA patients who would later develop ILD. Additional longitudinal studies are needed to better characterize telomere dynamics among RA patients with and without ILD.
Table 2:
Baseline socio-demographic and clinical characteristics of cross-sectional study participants, stratified by prevalent RA-ILD case status
| Without Prevalent ILD | With Prevalent ILD | P value | ||
|---|---|---|---|---|
| N | 92 | 54 | ||
| Age, median (IQR) | 66 (61, 72) | 68.5 (62, 75) | 0.27 | |
| Male (%) | 87 (95) | 51 (94) | 0.98 | |
| Race (%) | White or Caucasian | 69 (75) | 41 (76) | 0.64 |
| Black or African American | 16 (17) | 7 (13) | ||
| Other | 7 (8) | 6 (11) | ||
| Ethnicity (%) | Hispanic or Latino | 4 (4) | 2 (4) | 0.95 |
| Not Hispanic or Latino | 82 (89) | 49 (91) | ||
| Missing | 6 (7) | 3 (6) | ||
| BMI, median (IQR) | 28.4 (24.4, 31.1) | 27.8 (24.2, 31.3) | 0.82 | |
| Smoking history (%) | Never | 20 (22) | 5 (9) | 0.12 |
| Former | 18 (20) | 15 (28) | ||
| Current | 54 (59) | 34 (63) | ||
| Hypertension (%) | 8 (9) | 3 (6) | 0.49 | |
| Cardiac disease (%) | 2 (2) | 6 (11) | 0.02 | |
| Diabetes mellitus (%) | 11 (12) | 1 (2) | 0.03 | |
| COPD or asthma (%) | 2 (2) | 2 (4) | 0.58 | |
| RA disease duration, years, median (IQR) | 8.8 (2.2, 18.1) | 8.0 (1.5, 21.8) | 0.95 | |
| DAS28, median (IQR) | 3.5 (2.4, 4.8) | 3.9 (2.6, 5.7) | 0.06 | |
| MDHAQ, median (IQR) | 0.7 (0.5, 1.2) | 1.1 (0.5, 1.5) | 0.10 | |
| Prednisone use (%) | 39 (42) | 30 (56) | 0.12 | |
| Methotrexate use (%) | 51 (55) | 29 (54) | 0.84 | |
| Anti-TNF use (%) | 31 (34) | 24 (44) | 0.20 | |
Abbreviations: RA-ILD = rheumatoid arthritis-associated interstitial lung disease; IQR = interquartile range; BMI = body mass index; COPD = chronic obstructive pulmonary disease; RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints; MDHAQ = Multidimensional Health Assessment Questionnaire; TNF = tumor necrosis factor.
Key Points.
Patients with shorter telomeres were more likely to have prevalent rheumatoid arthritis-associated interstitial lung disease (RA-ILD) at entry into the Veterans Affairs Rheumatoid Arthritis registry compared to patients with longer telomeres.
Patients with shorter telomeres at baseline did not have a higher risk of developing incident RA-ILD during the ensuing 5–7 years.
Telomere length measurements on peripheral blood leukocytes using quantitative PCR (T/S ratio) significantly correlated with telomere length measurements on peripheral blood lymphocytes and granulocytes via fluorescent in situ hybridization-flow cytometry (flow-FISH). Thus, quantitative PCR may provide a suitable alternative to flow-FISH for measuring telomere length in studies of ILD.
Acknowledgements
The authors thank all of the patients for their military service and for their contributions to this work. They also thank the Veterans Affairs Rheumatoid Arthritis registry site coordinators.
Funding:
This work was supported by the National Heart, Lung, and Blood Institute, grant numbers T32HL007891 (JGN) and K24HL103844 (SMK), VA Clinical Science Research and Development, grant numbers CX001703 (JFB) and CX002203 (BRE), VA Biomedical Laboratory Research and Development, grant number BX004600 (TRM), U.S. Department of Defense, grant number PR200793 (BRE), and the National Institute of General Medical Sciences, grant number U54GM115458 (BRE, TRM).
Abbreviation List
- BMI
Body mass index
- CI
Confidence interval
- CT
Computed tomography
- Ct
Cycle threshold
- CTD
Connective tissue disease
- CTD-ILD
Connective tissue disease-associated interstitial lung disease
- DAS
Disease Activity Score in 28 Joints
- DNA
Deoxyribonucleic acid
- Flow-FISH
Fluorescent in situ hybridization-flow cytometry
- ICD
International Classification of Diseases
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- IQR
Interquartile range
- MDHAQ
Multidimensional Health Assessment Questionnaire
- OR
Odds ratio
- PFT
Pulmonary function test
- qPCR
Quantitative polymerase chain reaction
- RA
Rheumatoid arthritis
- RA-ILD
Rheumatoid arthritis-associated interstitial lung disease
- SD
Standard deviation
- T/S ratio
Telomeric repeat copy signal to reference single-copy gene (human beta globin) signal ratio
- TNF
Tumor necrosis factor
- UIP
Usual interstitial pneumonia
- U.S.
United States
- VA
Veterans Affairs
- VARA registry
Veterans Affairs Rheumatoid Arthritis registry
Footnotes
Declarations of interest: None to report. Specifically, JGN, JFB, QC, NS, TDM, PR, GMT, BCS, TRM, and FBJ and have no relevant conflicts of interest. BRE has received consulting fees from Boehringer Ingelheim unrelated to this project. SMK has received consulting fees from Acceleron Pharma, United Therapeutics Corporation, and VIVUS unrelated to this project and holds stock in AbbVie.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365(23):2205–19. DOI: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 2003;62(8):722–7. (In eng). DOI: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanoue LT. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med 1998;19(4):667–85, viii. (In eng). [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183(3):372–8. DOI: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017;76(10):1700–1706. DOI: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 6.Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156(2 Pt 1):528–35. DOI: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 7.Kim EJ, Collard HR, King TE, Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009;136(5):1397–1405. (In eng). DOI: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47(2):588–96. (In eng). DOI: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35(6):1322–8. (In eng). DOI: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 10.Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–82. (In eng). DOI: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 11.Assayag D, Lubin M, Lee JS, King TE, Collard HR, Ryerson CJ. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014;19(4):493–500. (In eng). DOI: 10.1111/resp.12234. [DOI] [PubMed] [Google Scholar]
- 12.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007;356(13):1317–26. (In eng). DOI: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 13.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America 2008;105(35):13051–6. (In eng). DOI: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George G, Rosas IO, Cui Y, et al. Short telomeres, telomeropathy, and subclinical extrapulmonary organ damage in patients with interstitial lung disease. Chest 2015;147(6):1549–1557. (In eng). DOI: 10.1378/chest.14-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley B, Newton CA, Arnould I, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med 2017;5(8):639–647. (In eng). DOI: 10.1016/s2213-2600(17)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snetselaar R, van Moorsel CHM, Kazemier KM, et al. Telomere length in interstitial lung diseases. Chest 2015;148(4):1011–1018. (In eng). DOI: 10.1378/chest.14-3078. [DOI] [PubMed] [Google Scholar]
- 17.Newton CA, Oldham JM, Ley B, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J 2019;53(4) (In eng). DOI: 10.1183/13993003.01641-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31(3):315–24. DOI: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Zhuo J, Zhang Q, Knapp K, et al. Examination of interstitial lung disease in patients with rheumatoid arthritis – prevalence, time to onset, and clinical characteristics. Annals of the Rheumatic Diseases 2020;79(Suppl 1):24–25. DOI: 10.1136/annrheumdis-2020-eular.1189. [DOI] [Google Scholar]
- 20.Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proceedings of the National Academy of Sciences of the United States of America 2018;115(10):E2358–e2365. (In eng). DOI: 10.1073/pnas.1720427115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heijde DM, van ‘t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20(3):579–81. (https://www.ncbi.nlm.nih.gov/pubmed/8478878). [PubMed] [Google Scholar]
- 22.Pincus T, Swearingen C, Wolfe F. Toward a Multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum 1999;42(10):2220–30. (In eng). DOI: . [DOI] [PubMed] [Google Scholar]
- 23.England BR, Roul P, Mahajan TD, et al. Performance of administrative algorithms to identify interstitial lung disease in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2019; doi: 10.1002/acr.24043. (In eng). DOI: 10.1002/acr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27(150) (In eng). DOI: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30(10):e47. (In eng). DOI: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joglekar MV, Satoor SN, Wong WKM, Cheng F, Ma RCW, Hardikar AA. An Optimised Step-by-Step Protocol for Measuring Relative Telomere Length. Methods Protoc 2020;3(2) (In eng). DOI: 10.3390/mps3020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 2006;1(5):2365–76. (In eng). DOI: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 28.Tempaku P, Hirotsu C, Mazzotti D, et al. Long Sleep Duration, Insomnia, and Insomnia With Short Objective Sleep Duration Are Independently Associated With Short Telomere Length. J Clin Sleep Med 2018;14(12):2037–2045. (In eng). DOI: 10.5664/jcsm.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kachuri L, Helby J, Bojesen SE, et al. Investigation of Leukocyte Telomere Length and Genetic Variants in Chromosome 5p15.33 as Prognostic Markers in Lung Cancer. Cancer Epidemiol Biomarkers Prev 2019;28(7):1228–1237. (In eng). DOI: 10.1158/1055-9965.Epi-18-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haycock PC, Burgess S, Nounu A, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA oncology 2017;3(5):636–651. (In eng). DOI: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colmegna I, Weyand CM. Haematopoietic stem and progenitor cells in rheumatoid arthritis. Rheumatology (Oxford) 2011;50(2):252–60. (In eng). DOI: 10.1093/rheumatology/keq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol 2014;26(1):93–100. (In eng). DOI: 10.1097/bor.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamal RM, Hammam N, Zakary MM, et al. Telomere dysfunction-related serological markers and oxidative stress markers in rheumatoid arthritis patients: correlation with diseases activity. Clin Rheumatol 2018;37(12):3239–3246. (In eng). DOI: 10.1007/s10067-018-4318-5. [DOI] [PubMed] [Google Scholar]
- 34.Ormseth MJ, Solus JF, Oeser AM, et al. Telomere Length and Coronary Atherosclerosis in Rheumatoid Arthritis. J Rheumatol 2016;43(8):1469–74. (In eng). DOI: 10.3899/jrheum.151115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart BD, Lee JS, Kozlitina J, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med 2014;2(7):557–65. (In eng). DOI: 10.1016/s2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esposito AJ, Chu SG, Madan R, Doyle TJ, Dellaripa PF. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med 2019;40(3):545–560. (In eng). DOI: 10.1016/j.ccm.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59(6):762–84. (In eng). DOI: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 38.Bartels CM, Bell CL, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United State veterans over 20 years. Rheumatology (Oxford) 2010;49(9):1670–5. (In eng). DOI: 10.1093/rheumatology/keq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019;58(11):2031–2038. (In eng). DOI: 10.1093/rheumatology/kez177. [DOI] [PubMed] [Google Scholar]
- 40.Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 2019;46(4):360–369. (In eng). DOI: 10.3899/jrheum.171315. [DOI] [PubMed] [Google Scholar]
- 41.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62(6):1583–91. DOI: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juge PA, Borie R, Kannengiesser C, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J 2017;49(5) (In eng). DOI: 10.1183/13993003.02314-2016. [DOI] [PubMed] [Google Scholar]
- 43.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with Pulmonary Fibrosis Driven by Telomere Dysfunction. Cell Rep 2015;12(2):286–99. (In eng). DOI: 10.1016/j.celrep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Naikawadi RP, Disayabutr S, Mallavia B, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 2016;1(14):e86704. (In eng). DOI: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khosravaniardakani S, Bokov DO, Mahmudiono T, et al. Obesity Accelerates Leukocyte Telomere Length Shortening in Apparently Healthy Adults: A Meta-Analysis. Front Nutr 2022;9:812846. (In eng). DOI: 10.3389/fnut.2022.812846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Ebel AV, Lutt G, Poole JA, et al. Association of Agricultural, Occupational, and Military Inhalants With Autoantibodies and Disease Features in US Veterans With Rheumatoid Arthritis. Arthritis Rheumatol 2021;73(3):392–400. (In eng). DOI: 10.1002/art.41559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum 1998;41(5):817–22. (In eng). DOI: . [DOI] [PubMed] [Google Scholar]
- 48.Jawaheer D, Lum RF, Gregersen PK, Criswell LA. Influence of male sex on disease phenotype in familial rheumatoid arthritis. Arthritis Rheum 2006;54(10):3087–94. (In eng). DOI: 10.1002/art.22120. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Chung MP, Ley B, et al. Peripheral blood leucocyte telomere length is associated with progression of interstitial lung disease in systemic sclerosis. Thorax 2021;76(12):1186–1192. (In eng). DOI: 10.1136/thoraxjnl-2020-215918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arias-Salgado EG, Galvez E, Planas-Cerezales L, et al. Genetic analyses of aplastic anemia and idiopathic pulmonary fibrosis patients with short telomeres, possible implication of DNA-repair genes. Orphanet J Rare Dis 2019;14(1):82. (In eng). DOI: 10.1186/s13023-019-1046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P, Leung J, Lam A, et al. Lung transplant recipients with idiopathic pulmonary fibrosis have impaired alloreactive immune responses. J Heart Lung Transplant 2021. (In eng). DOI: 10.1016/j.healun.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faust HE, Golden JA, Rajalingam R, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax 2017;72(11):1052–1054. (In eng). DOI: 10.1136/thoraxjnl-2016-209897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtwright AM, Lamattina AM, Takahashi M, et al. Shorter telomere length following lung transplantation is associated with clinically significant leukopenia and decreased chronic lung allograft dysfunction-free survival. ERJ Open Res 2020;6(2) (In eng). DOI: 10.1183/23120541.00003-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


