Abstract
Background
Surgical site infections (SSIs) are wound infections that occur after invasive (surgical) procedures. Preoperative bathing or showering with an antiseptic skin wash product is a well‐accepted procedure for reducing skin bacteria (microflora). It is less clear whether reducing skin microflora leads to a lower incidence of surgical site infection.
Objectives
To review the evidence for preoperative bathing or showering with antiseptics for preventing hospital‐acquired (nosocomial) surgical site infections.
Search methods
For this fifth update we searched the Cochrane Wounds Group Specialised Register (searched 18 December 2014); the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014 Issue 11); Ovid MEDLINE (2012 to December Week 4 2014), Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations December 18, 2014); Ovid EMBASE (2012 to 2014 Week 51), EBSCO CINAHL (2012 to December 18 2014) and reference lists of articles.
Selection criteria
Randomised controlled trials comparing any antiseptic preparation used for preoperative full‐body bathing or showering with non‐antiseptic preparations in people undergoing surgery.
Data collection and analysis
Two review authors independently assessed studies for selection, risk of bias and extracted data. Study authors were contacted for additional information.
Main results
We did not identify any new trials for inclusion in this fifth update. Seven trials involving a total of 10,157 participants were included. Four of the included trials had three comparison groups. The antiseptic used in all trials was 4% chlorhexidine gluconate (Hibiscrub/Riohex). Three trials involving 7791 participants compared chlorhexidine with a placebo. Bathing with chlorhexidine compared with placebo did not result in a statistically significant reduction in SSIs; the relative risk of SSI (RR) was 0.91 (95% confidence interval (CI) 0.80 to 1.04). When only trials of high quality were included in this comparison, the RR of SSI was 0.95 (95%CI 0.82 to 1.10). Three trials of 1443 participants compared bar soap with chlorhexidine; when combined there was no difference in the risk of SSIs (RR 1.02, 95% CI 0.57 to 1.84). Three trials of 1192 patients compared bathing with chlorhexidine with no washing, one large study found a statistically significant difference in favour of bathing with chlorhexidine (RR 0.36, 95%CI 0.17 to 0.79). The smaller studies found no difference between patients who washed with chlorhexidine and those who did not wash preoperatively.
Authors' conclusions
This review provides no clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products, to reduce surgical site infection. Efforts to reduce the incidence of nosocomial surgical site infection should focus on interventions where effect has been demonstrated.
Keywords: Female; Humans; Male; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Baths; Baths/methods; Chlorhexidine; Chlorhexidine/administration & dosage; Chlorhexidine/analogs & derivatives; Disinfection; Disinfection/methods; Preoperative Care; Preoperative Care/methods; Randomized Controlled Trials as Topic; Soaps; Soaps/administration & dosage; Surgical Wound Infection; Surgical Wound Infection/prevention & control
Plain language summary
Preoperative bathing or showering with skin antiseptics to prevent surgical site infection
Surgical site infection is a serious complication of surgery and is usually associated with increased length of hospital stay for the patient, and also higher hospital costs. The use of an antiseptic solution for preoperative bathing or showering is widely practiced in the belief that it will help to prevent surgical site infections from developing. This review identified seven trials, with over 10,000 patients, that tested skin antiseptics (chlorhexidine solution) against normal soap or no presurgical washing. The review of these trials did not show clear evidence that the use of chlorhexidine solution before surgery was better than other wash products at preventing surgical site infections from developing after surgery.
Summary of findings
Summary of findings for the main comparison. Preoperative showering with chlorhexidine 4% compared to placebo.
| pre‐operative showering with Chlorhexidine 4% compared to placebo for surgical patients | ||||||
|
Patient or population: surgical patients Settings: Hospitals Intervention: pre‐operative showering with Chlorhexidine 4% Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | pre‐operative showering with Chlorhexidine 4% | |||||
| Surgical site infection Follow‐up: 1 ‐ 6 weeks1 | Low risk population | RR 0.91 (0.8 to 1.04) | 7791 (4 studies) | ⊕⊕⊕⊕ high2 | ||
| 30 per 1000 | 27 per 1000 (24 to 31) | |||||
| High risk population | ||||||
| 100 per 1000 | 91 per 1000 (80 to 104) | |||||
| Allergic reaction Follow‐up: 1 ‐ 6 weeks1 | Study population | RR 0.89 (0.36 to 2.19) | 3589 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 6 per 1000 | 5 per 1000 (2 to 13) | |||||
| Medium risk population | ||||||
| 3 per 1000 | 3 per 1000 (1 to 7) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Some studies followed patients only until hospital discharge; however, as these studies are over 20 years old, we have assumed 7 days. 2 In one trial, five months into the study, the placebo solution was found to contain a microbiological agent. The solution was changed for the remaining 17 months of the trial. There was a total of over 7,000 participants included in this outcome, so we do not believe that the overall effect estimate would have been substantially altered. 3 Only 19 events were reported. All of these were from one trial.
Summary of findings 2. Chlorhexidine 4% compared with bar soap.
| Chlorhexidine 4% compared to bar soap for Surgical patients | ||||||
|
Patient or population: Surgical patients Settings: Intervention: Chlorhexidine 4% Comparison: bar soap | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| bar soap | Chlorhexidine 4% | |||||
| Surgical site infection | Study population | RR 1.02 (0.57 to 1.84) | 1443 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 136 per 1000 | 139 per 1000 (78 to 250) | |||||
| Medium risk population | ||||||
| 128 per 1000 | 131 per 1000 (73 to 236) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The method of allocation was unclear in some studies and outcome assessment was not blinded. 2 Heterogeneity between trials was evident; this was most probably due to the different types of surgeries and different definitions used for infection. 3 95% confidence interval around the pooled estimate of effect includes both 1) no effect and 2) appreciable benefit or appreciable harm.
Summary of findings 3. Chlorhexadine 4% compared with no wash.
| Chlorhexadine 4% compared to no wash for surgical patients | ||||||
|
Patient or population: surgical patients Settings: Hospital Intervention: Chlorhexadine 4% Comparison: no wash | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no wash | Chlorhexadine 4% | |||||
| Surgical site infection Follow‐up: 1 ‐ 3 weeks1 | Study population | RR 0.82 (0.26 to 2.62) | 1142 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 56 per 1000 | 46 per 1000 (15 to 147) | |||||
| Medium risk population | ||||||
| 46 per 1000 | 38 per 1000 (12 to 121) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Some studies followed patients only until hospital discharge; as the studies were over 20 years old, we have assumed this to be one week. 2 A number of potential biases existed including inadequate allocation concealment and blinding. 3 Hetrogeneity between studies was evident; this was most likely due to different types of surgeries, differences in length of follow‐up, varying sample sizes and ways of defining infection. 4 Wide confidence intervals, low event rate.
Background
Surgical site infections (SSIs) are wound infections that occur after invasive (surgical) procedures. SSI is the third most frequently hospital‐acquired (nosocomial) infection amongst hospital patients (Smyth 2008). The Centers for Disease Control and Prevention (CDC) have used the National Nosocomial Infections Surveillance system (NNIS) to monitor nosocomial infections in acute care hospitals in the United States since 1970. Between 1986 and 1996 the CDC studied approximately 600,000 operations. Surgical site infections developed after three per cent (15,523) of these operations. During the period of data collection, 551 of the 15,523 patients who developed an SSI died, and 77% of deaths were attributed to the infection (Mangram 1999). More recently, summary of data from 30 hospitals in America, Asia, Africa and Europe found an overall SSI incidence of 2.3% (Rosenthal 2013). Apart from the morbidity and mortality associated with surgical site infections, there are significant cost implications. A study, using the NNIS system found that it cost over USD 3000 more to treat a patient with an SSI than a non‐infected patient . These costs were attributable to a greater likelihood of admission to an intensive care unit, a longer than usual post‐operative stay, and an increased rate of hospital re‐admission (Kirkland 1999). In Britain a study over a 2‐year period found the additional median cost attributed to a SSI was £5,239 (UKP)(Jenks 2014). Potential litigation is also a concern (Rubinstein 1999). Consequently, prevention of surgical site infection has become a priority for health care facilities.
An SSI is defined as an infection occurring within 30 days after an operation, and involves either a discharge of pus (purulent discharge), with or without laboratory confirmation; an organism isolated from an aseptically obtained culture; or signs and symptoms of infection, such as localised swelling, redness, or tenderness (Mangram 1999). The CDC have developed a set of standardised criteria for defining SSI in an attempt to make surveillance and rate calculation more accurate and amenable to comparison (Mangram 1999). SSIs are classified as being: superficial incisional (involving only skin or subcutaneous tissues); deep incisional (involving deeper soft tissue and fascia); or organ/space (involving any other part of the anatomy that was opened or manipulated). To help predict the likelihood, or SSI risk, surgical sites can be assessed preoperatively and classified into one of four categories with clear definitions: Class 1 (clean), Class II (clean‐contaminated), Class III (contaminated) and Class IV (dirty/infected) (Mangram 1999). Clean wounds are defined as uninfected surgical wounds in which the respiratory, alimentary, genital or uninfected urinary tract are not present and in which no inflammation is encountered. Non‐clean wounds are defined according to the anatomical area of operation, cause (aetiology) of wound, presence of existing clinical infection, and intra‐operative contamination. Since clean wounds are less likely to become infected, SSIs following clean surgery are usually associated with either: (1) patient risk factors, such as age, nutritional status, diabetes and obesity; (2) risk factors related to the procedure: including incomplete preoperative hand and forearm antisepsis by one of the surgical team, length of surgical procedure and surgical technique; or (3) risk factors associated with preoperative preparation of the patient: for example, antimicrobial prophylaxis, preoperative hair removal and preoperative antiseptic showering (Mangram 1999).
Skin is not sterile. Indeed, thousands of bacteria live permanently on skin and contribute to health by maintaining a steady colony that inhibits establishment of harmful yeast and fungal infections. These bacterial populations are referred to as the 'resident flora'. A number of bacteria are present on the skin for a short period due to transfer from other people or the environment, and these constitute the 'transient flora'. At present, whole body bathing or showering with skin antiseptic to prevent SSIs is a widespread practice before surgery. The aim of washing is to make the skin as clean as possible by removing transient flora and some resident flora. Chlorhexidine 4% in detergent ('Hibiscrub' or 'Hibiclens') or a triclosan preparation is usually used for this purpose, and there is evidence that the numbers of bacteria on the skin are reduced when it is applied (Derde 2012; Koburger 2010; Kaiser 1988). Moreover, use of a skin antiseptic on consecutive days not only reduces microbial counts from baseline measurements, but also reduces the counts progressively over time (Paulson 1993). Although this body of evidence demonstrates the effectiveness of antiseptics as skin cleansing agents, the more important question is whether preoperative bathing or showering with an antiseptic reduces the incidence of SSI. In a 10‐year prospective surveillance study, the SSI rate was lower amongst patients showering with hexachlorophene before surgery than in those who either did not shower or showered using a non‐medicated soap (Cruse 1980). In addition, at least two studies have used a before‐and‐after design to test the effect of introducing preoperative showering with triclosan to control methicillin‐resistant Staphylococcus aureus (MRSA) SSIs. In the first of these, showering before and after surgery was introduced to reduce the MRSA SSI rate. This intervention, however, was only one of a battery of measures introduced, so it was not possible to determine the independent effect of preoperative showering (Brady 1990). In the second, the incidence of MRSA SSI was reduced amongst orthopaedic patients after presurgical showering with triclosan was introduced, however, the patients were also treated with nasal mupirocin for five days before surgery (Wilcox 2003). Finally, a retrospective analysis of the incidence of SSI following a total hip or knee arthroplasty, over a two year period in two hospitals was undertaken (Colling 2014). In one hospital, patients were required to shower or bathe with an antiseptic on the night before and morning of surgery. There was no similar policy in the second hospital. Although there was no difference in the overall SSI incidence during the study, there was a reduction in Staphylococcus aureus and methicillin‐resistant Staphylococcus aureus SSI(Colling 2014). While these observational studies provide some support for the practice of preoperative showering with an antiseptic, the evidence is not definitive.
Patterns of resistance have developed with some antiseptics (Thomas 2000), leading to calls to restrict their use to situations where effectiveness can be demonstrated. In addition, hypersensitivity to chlorhexidine on the part of the patient is not uncommon. Consequently, the potential benefit of bathing or showering with antiseptics needs to be assessed alongside their potential for harm (Beaudounin 2004; Krautheim 2004). As it is unclear whether the use of antiseptics for preoperative bathing or showering leads to lower rates of SSIs, a systematic review is justified to guide practice in this area.
Objectives
To review the evidence for preoperative bathing or showering with antiseptics for the prevention of surgical site infections.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs) that allocate surgical patients individually, or by cluster, comparing any antiseptic preparation used for preoperative full body wash or showering, with non‐antiseptic preparations. Quasi‐randomised trials were not included (i.e. trials that allocate treatment by day of the week, medical record number, sequential admitting order etc.).
Types of participants
Men, women and children undergoing any type of surgery in any setting.
Types of interventions
Any type of antiseptic solution (any strength, any regimen, at any time before surgery) used for preoperative tub‐ or bed‐bathing or showering compared with:
non‐antiseptic soap;
non‐antiseptic soap solution;
no shower or bath.
Antiseptic solutions were defined as liquid soap products containing an antimicrobial ingredient such as chlorhexidine, triclosan, hexachlorophene, povidone‐iodine or benzalkonium chloride. Trials comparing different types of antiseptic with each other would also be compared if preoperative showering with an antiseptic showed evidence of benefit.
Types of outcome measures
Primary outcomes
Surgical site infection. (Note: despite development of standardised criteria for defining SSI, the diagnosis of SSIs continues to vary between studies. We therefore accepted the definition used by the original authors to determine the proportion of patients who develop any SSI before or after discharge from hospital).
Secondary outcomes
Mortality (any cause).
Allergic reactions (e.g. contact dermatitis, anaphylaxis).
Postoperative antibiotic use.
Length of hospital stay.
Re‐admission to hospital.
Cost.
Other serious infection or infectious complication, such as septicaemia or septic shock.
Postoperative fever higher than 38o C on at least two occasions more than four hours apart, excluding the day of surgery.
Secondary outcomes were extracted from trial reports regardless of whether the primary outcome was reported. We excluded studies that measured only skin colonisation.
Search methods for identification of studies
Electronic searches
For an outline of the search methods used in the fourth update of this review see Appendix 1.
For this fifth update we searched the following electronic databases:
The Cochrane Wounds Group Specialised Register (searched 18 December 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 11);
Ovid MEDLINE (2012 to December Week 4 2014);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations December 18, 2014);
Ovid EMBASE (2012 to 2014 Week 51);
EBSCO CINAHL (2012 to 18 December 2014)
The following strategy was used to search CENTRAL: #1 MeSH descriptor: [Skin] explode all trees 3486 #2 MeSH descriptor: [Antisepsis] explode all trees 101 #3 #1 and #2 23 #4 skin next antisep* 62 #5 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees 1691 #6 MeSH descriptor: [Soaps] explode all trees 179 #7 MeSH descriptor: [Povidone‐Iodine] explode all trees 409 #8 MeSH descriptor: [Iodophors] explode all trees 435 #9 MeSH descriptor: [Chlorhexidine] explode all trees 1376 #10 MeSH descriptor: [Alcohols] explode all trees 29960 #11 iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol or alcohols or antiseptic* or soap* or detergent* 18981 #12 MeSH descriptor: [Disinfectants] explode all trees 598 #13 #1 and #12 21 #14 skin near/5 disinfect* 128 #15 MeSH descriptor: [Detergents] explode all trees 299 #16 #1 and #15 51 #17 skin near/5 detergent* 34 #18 #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #13 or #14 or #16 or #17 46631 #19 MeSH descriptor: [Surgical Wound Infection] explode all trees 2837 #20 surg* near/5 infection* 5241 #21 surgical near/5 wound* 4315 #22 (postoperative or post‐operative) near/5 infection* 2386 #23 MeSH descriptor: [Preoperative Care] explode all trees 3539 #24 (preoperative or pre‐operative) next care 3951 #25 MeSH descriptor: [Perioperative Care] explode all trees 10389 #26 (perioperative or peri‐operative) next care 971 #27 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 17697 #28 MeSH descriptor: [Baths] explode all trees 263 #29 shower* or bath* or wash* or cleans* 26111 #30 #28 or #29 26111 #31 #18 and #27 and #30 238
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebrve 2011). This filter is published in the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009]; Section 6.4.11. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). No date or language restrictions were applied.
Searching other resources
Reference lists of all retrieved articles were searched in order to identify additional studies. For the original version of the review, manufacturers of antiseptic products were contacted in order to obtain any unpublished data.
Data collection and analysis
Selection of studies
Both review authors independently assessed the titles and abstracts of references identified by the search strategy. Full reports of all potentially relevant trials were then retrieved for assessment of eligibility based on the inclusion criteria. Reference lists of retrieved studies were screened to identify further studies, which were also retrieved. Differences of opinion were settled by consensus or referral to the editorial base of the Wounds Group.
Data extraction and management
The following data were extracted from each study by both review authors independently using a piloted data extraction sheet: type of study, study setting, number of participants, sex, mean age, predisposing risk factors, type of antiseptic solutions, use of prophylactic antibiotics, procedure and timing for full body wash, period of community follow‐up, all primary and secondary outcome descriptions and outcome measures reported, including infection rates and study authors' conclusions.
Assessment of risk of bias in included studies
Two review authors independently assessed all included studies using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 5 for details of criteria on which the judgement was be based for this review). Blinding and completeness of outcome data were assessed for each outcome separately. We completed a risk of bias table for each eligible study. Once again, disagreements between review authors were resolved by consensus or referral to the editorial base of the Wounds Group. When possible, contact was made with investigators of included trials to resolve any ambiguities.
We presented an assessment of risk of bias using a 'risk of bias summary figure', which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
We defined high quality trials as those receiving a 'Low risk of bias' judgement for the domains of random sequence generation, allocation concealment and for blinding of outcome assessment.
Data synthesis
Analyses were performed using RevMan 5 software. Relative risks (RR) and 95% confidence intervals (CI) were calculated for dichotomous outcomes, and mean differences (MD) and 95% CI calculated for continuous outcomes. Results of comparable trials were pooled using the fixed‐effect model and 95% CI. Heterogeneity was investigated by calculating the I2 statistic (Higgins 2002). If evidence of significant heterogeneity was identified (i.e. a value greater than 50%), potential sources of heterogeneity were explored and a random‐effects approach to the analysis undertaken. A narrative review of eligible studies was conducted where statistical synthesis of data from more than one study was not possible, or considered inappropriate.
One trial used a multi‐centre design, but patients were allocated individually to the treatment or control arm (Rotter 1988). Two trials allocated clusters of patients to each intervention (Hayek 1987; Wihlborg 1987). In this review results were not analysed using the number of clusters as the unit of analysis but analysed as if the allocation was by individual. This was necessary because the authors of the trial did not use the cluster as the unit of analysis. Analysing cluster trials in this way has the potential to over‐estimate the effect of treatment (Mollison 2000).
Subgroup analysis and investigation of heterogeneity
The planned sub‐group analyses (one preoperative bath or shower compared with more than one preoperative bath or shower; and clean surgery compared with clean contaminated surgery) were not conducted due to the format of the reported data.
Sensitivity analysis
We included all eligible trials in the initial analysis and carried out sensitivity analyses to evaluate the effect of trial quality. This was done by excluding those trials most susceptible to bias based on the following quality assessment criteria: those with inadequate allocation concealment; or unblinded outcome assessment; or where blinding of outcome assessment was uncertain.
Results
Description of studies
For a detailed description of the included studies see Characteristics of included studies.
Our original search strategy identified 43 articles. Full‐text assessment was conducted for 16 potentially eligible papers. Seven of these papers were excluded from further review because the studies were not randomised, or were randomised trials evaluating other interventions (e.g. preoperative scrub solutions), or other outcomes (e.g. intraoperative wound colonisation) (Ayliffe 1983; Bergman 1979; Brandberg 1980; Garabaldi 1988; Leigh 1983; Newsom 1988; Wells 1983). The remaining nine citations referred to six trials, which reported outcomes for 10,007 participants, and were included in the review (Byrne 1992; Earnshaw 1989; Hayek 1987; Randall 1983; Rotter 1988; Wihlborg 1987). The results of these six trials were reported in nine publications (Byrne 1992; (Byrne 1994, Lynch 1992); Earnshaw 1989; Hayek 1987 (Hayek 1988); Randall 1983; Rotter 1988; Veiga 2008; Wihlborg 1987). Four authors of included trials (Byrne 1992; Earnshaw 1989; Randall 1983; Wihlborg 1987), and one non‐included trial author (Garabaldi 1988), responded to queries about study methods or requests for additional unpublished information, or both.
During the subsequent updates of this review we completed Risk of Bias and Summary of findings tables. We screened 87 citations for the updates and retrieved 13 full text studies for further assessment. Thirteen additional studies were excluded (Bode 2010; Colling 2014; Edminson 2010; Edmiston 2008; Eiselt 2009; Enjabert 1984; Jakobsson 2010; Kaiser 1988; Kalanter‐Hormozi 2005; Murray 2011; Paulson 1993; Tanner 2011; Veiga 2008). Details of these can be found in the Characteristics of excluded studies table. One trial of 150 participants met the inclusion criteria and was added to the review at the time of the second update (Veiga 2009).
Participants
The age range of the participants in the seven included studies was nine to 90 years old. The trials enrolled men, women and children booked for elective surgery.
Byrne 1992 included clean and potentially infected cases but all other studies were of clean surgery. Two studies included general surgical patients (Byrne 1992; Hayek 1987); one involved participants undergoing general, orthopaedic and vascular surgery (Rotter 1988); one enrolled participants scheduled for plastic surgical procedures (Veiga 2009) and one included biliary tract, inguinal hernia or breast surgery (Wihlborg 1987). The remaining studies involved only one type of surgery (Earnshaw 1989: vascular reconstruction; Randall 1983: vasectomy). Participants in the vasectomy study were day patients (Randall 1983).
Four of the centres in which the studies were conducted were in the United Kingdom (Byrne 1992; Earnshaw 1989; Hayek 1987; Randall 1983); one was in Sweden (Wihlborg 1987); one study was undertaken in Brazil (Veiga 2009); and one included a number of European centres (eight from Denmark, five from the United Kingdom, four from Sweden, two from Austria, and one each from Germany and Italy) (Rotter 1988).
While Veiga 2009 used CDC definitions for surgical site infection, all of the other studies included the presence of pus in their definition of infection. Earnshaw 1989 and Hayek 1987 also included patients with severe cellulitis (although there was only such patient), and Randall 1983 included patients with a discharge of serous fluid in his definition of infection.
Interventions
There were inconsistencies in both the interventions and the control procedures between studies. One trial compared a regimen that included three preoperative washes (Byrne 1992), three trials included a two‐wash regimen (Earnshaw 1989; Hayek 1987; Rotter 1988), and participants in three trials had only one wash preoperatively (Randall 1983; Veiga 2009; Wihlborg 1987).
The breakdown of the studies according to timing of bathing was as follows:
One wash on admission, a second on the night before surgery and a third on the morning of surgery (Byrne 1992).
One wash immediately after admission, and a second on the day of surgery (Hayek 1987).
One wash on the day before surgery, and a second on the day of surgery (Rotter 1988).
Two washes preoperatively, timing not specified (Earnshaw 1989).
One wash on the day before surgery only (Wihlborg 1987).
One wash not more than one hour before surgery (Randall 1983).
One wash (with two applications of chlorhexidine detergent) two hours before surgery (Veiga 2009).
Three of the studies had two arms (Byrne 1992; Earnshaw 1989; Rotter 1988), whilst four had three arms (Hayek 1987; Randall 1983; Veiga 2009; Wihlborg 1987). The breakdown of studies according to bathing products was as follows:
4% chlorhexidine gluconate (Hibiscrub/Riohex) detergent solution compared with a matching placebo (i.e. the same detergent without chlorhexidine) (Byrne 1992; Hayek 1987; Rotter 1988; Veiga 2009).
4% chlorhexidine gluconate (Hibiscrub) compared with bar soap (Earnshaw 1989; Hayek 1987; Randall 1983).
Chlorhexidine with no shower or bath (Randall 1983; Veiga 2009; Wihlborg 1987).
Chlorhexidine full body bathing compared with localised washing, i.e. restricted to the part of the body to be subjected to surgery (chlorhexidine used in both arms of trial) (Wihlborg 1987).
Antibiotic prophylaxis was used routinely in only one study (Earnshaw 1989). In three other studies there was no attempt to alter the treating surgeons' usual routine for administering antibiotic prophylaxis but, in these studies, the reported rate of prophylactic antibiotic use was low (1% to 15%) (Byrne 1992; Rotter 1988; Wihlborg 1987). One trial excluded patients who were on antibiotics at the time of surgery but did not control for postoperative antibiotic use, which the trialists said was high (Veiga 2009). Two studies did not mention whether antibiotics were used before surgery (Hayek 1987; Randall 1983).
Outcome measures
Primary outcome
The primary outcome measure for this review, the effectiveness of preoperative washing or showering with an antiseptic in preventing SSI, was reported in all of the studies (Byrne 1992; Earnshaw 1989; Hayek 1987; Randall 1983; Rotter 1988; Veiga 2009; Wihlborg 1987).
Secondary outcomes
The secondary outcomes of the review were reported as follow:
Mortality (any cause) was reported in two studies (Byrne 1992; Earnshaw 1989).
Allergic reactions (e.g. contact dermatitis, anaphylaxis) were reported in two trials (Byrne 1992; Veiga 2009).
Postoperative antibiotic use was not reported in any of the studies.
Length of hospital stay was not reported in any of the studies.
Re‐admission to hospital was not reported in any of the studies.
Cost was reported in one study (Byrne 1992).
Other serious infection or infectious complication, such as septicaemia or septic shock was not reported in any of the studies.
Postoperative fever exceeding 38o C on at least two occasions more than four hours apart, excluding the day of surgery, was not reported in any of the studies
Sample size
None of the trials reported how the sample size was calculated.
Risk of bias in included studies
Two of the seven included studies were assessed as being overall at low risk of bias using the assessment criteria described above (a low risk of bias judgement for the criteria of random sequence generation, allocation concealment and blinding of outcome assessment) (Byrne 1992; Rotter 1988). (See Risk of Bias Figure 1; Figure 2).
1.
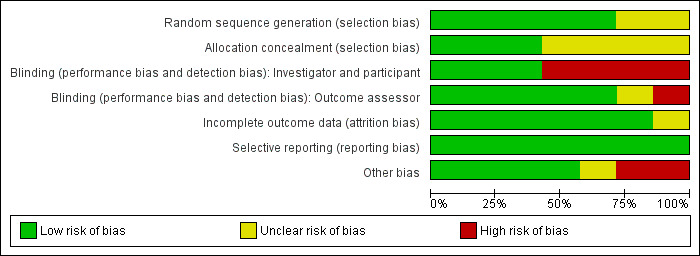
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
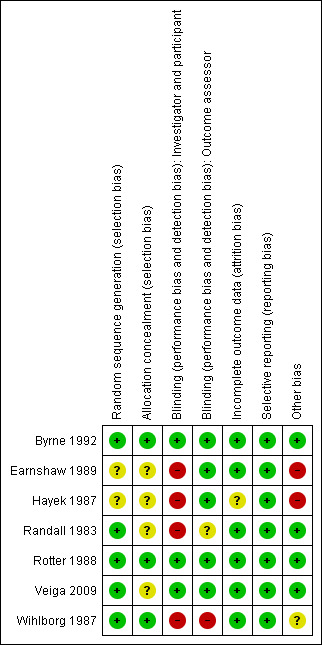
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Generation of random allocation sequence
All studies mentioned a process of randomisation. The method of generating the random allocation sequence was adequate in some studies (Byrne 1992; Randall 1983; Rotter 1988; Veiga 2009; Wihlborg 1987), and unclear in others (Earnshaw 1989; Hayek 1987). In four studies the random sequence was computer‐generated (Byrne 1992; Randall 1983; Rotter 1988; Veiga 2009). One study used block randomisation in groups of six using computer‐generated random numbers (Byrne 1992). A large multi‐centre study used cluster randomisation, whereby randomisation was carried out for each surgical unit in the study by means of computer‐generated numbers (Rotter 1988). Personal correspondence with authors of two of the studies confirmed that they used either computer‐generated random numbers (Randall 1983), or a randomisation list (Wihlborg 1987).
Allocation concealment
As with generation of the allocation sequence, concealment of allocation was adequate in some studies (Byrne 1992; Randall 1983; Rotter 1988; Wihlborg 1987), and unclear in others (Earnshaw 1989; Hayek 1987; Veiga 2009).
Blinding
Blinding of intervention
Blinding of intervention in three studies was by a double‐blind method (Byrne 1992; Rotter 1988; Veiga 2009). In one study there was single‐blinding of the intervention in two arms of the study but no blinding in the third arm of the study (Hayek 1987). In the remaining studies, there was no blinding of intervention (Earnshaw 1989; Randall 1983; Wihlborg 1987).
Blinding of outcome assessment
Five of the trials blinded outcome assessment (Byrne 1992; Earnshaw 1989; Hayek 1987; Rotter 1988; Veiga 2009). In one of the studies there was no blinding of the outcome assessment (Wihlborg 1987 personal communication). In one study it is unclear whether blinding of outcome assessment occurred (Randall 1983).
Incomplete outcome data
In one study analysis by intention‐to‐treat was not done (Byrne 1992). For all of the other studies it could not be determined whether analysis by intention‐to‐treat occurred (Earnshaw 1989; Hayek 1987; Randall 1983; Rotter 1988; Veiga 2009; Wihlborg 1987).
All of the studies reported the status of all people entered into the trials. One study reported only one of the 94 patients as lost to follow‐up (Randall 1983). Byrne 1992 reported a 99.4% completeness of follow‐up. All other studies reported that all patients were followed‐up (Earnshaw 1989; Hayek 1987; Rotter 1988; Veiga 2009; Wihlborg 1987). In one study, 140 patients out of the 2,953 enrolled were withdrawn from the study for several reasons: failure to have two preoperative showers, not meeting inclusion criteria, transferring out of unit, or no identification number on patient protocol (Rotter 1988). Despite this, the study reported on all remaining patients (n = 2813), resulting in 95.2% completeness of reporting.
Two authors recorded SSIs during hospitalisation and then followed patients for six weeks after hospital discharge (Byrne 1992; Hayek 1987), Rotter 1988 followed patients for three weeks, Randall 1983 for seven days, Veiga 2009 for 30 days; Wihlborg 1987 monitored SSIs that occurred in hospital and among those returning for an outpatient visit, and Earnshaw 1989 reviewed patients twice weekly until hospital discharge.
Effects of interventions
See: Table 1; Table 2; Table 3
This review includes outcome data from seven trials with a total of 10,157 participants. Six comparisons were undertaken: chlorhexidine 4% versus placebo (Analysis: 01) (Byrne 1992; Hayek 1987; Rotter 1988; Veiga 2009); chlorhexidine 4% versus bar soap (Analysis: 02) (Earnshaw 1989; Hayek 1987; Randall 1983); chlorhexidine versus no bath or shower (Analysis: 03) (Randall 1983; Veiga 2009; Wihlborg 1987); whole body wash with chlorhexidine versus washing only that part of the body to be submitted to surgery (Analysis: 04) (Wihlborg 1987); more than one wash versus one wash (Analysis: 05) (Byrne 1992; Hayek 1987; Randall 1983; Rotter 1988); and one post hoc comparison, individual allocation versus cluster allocation (Analysis: 06) (Byrne 1992; Earnshaw 1989; Hayek 1987; Randall 1983; Rotter 1988; Wihlborg 1987). A random‐effect meta‐analysis was used when significant heterogeneity was present (i.e. where the I2 value was greater than 50%).
Chlorhexidine compared with placebo
This comparison includes four trials of 7791 participants and includes four outcomes (SSI, allergic reactions, mortality and cost) (Byrne 1992; Hayek 1987; Rotter 1988; Veiga 2009).
Surgical site infection
Participants in three of the trials had more than one wash (Byrne 1992; Hayek 1987; Rotter 1988); but in the Veiga 2009 trial participants had only one shower. Hayek 1987, Rotter 1988 and Veiga 2009 included patients having elective surgery, whereas Byrne 1992 included patients undergoing "clean or potentially infected surgery". It should be noted that in the Hayek 1987 trial the placebo was found to contain antimicrobial properties and was changed during the study. None of the individual trials found that washing with chlorhexidine had a statistically significant effect on SSI. All of the trials were included in the meta‐analysis. When compared with placebo, bathing with chlorhexidine did not result in a statistically significant reduction in the SSI rate (chlorhexidine 9.1%, placebo 10.0%); the relative risk (RR) was 0.91 (95% confidence interval (CI) 0.80 to 1.04) (Analysis 1.1).
1.1. Analysis.
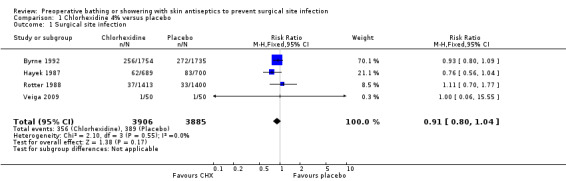
Comparison 1 Chlorhexidine 4% versus placebo, Outcome 1 Surgical site infection.
Surgical site infection ‐ high quality trials
For this outcome we conducted a separate analysis of trials rated as high quality by the criteria described in the 'Methods of the Review' section ‐ namely Byrne 1992 and Rotter 1988 ‐ and obtained a similar result, the RR was 0.95 (95% CI 0.82 to 1.10) (Analysis 1.2). The event rate was 9.3% for the chlorhexidine group and 9.7% for the placebo group.
1.2. Analysis.
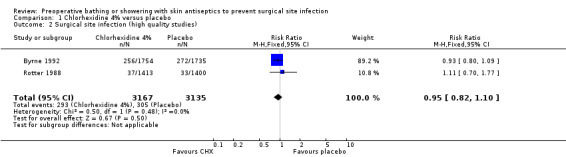
Comparison 1 Chlorhexidine 4% versus placebo, Outcome 2 Surgical site infection (high quality studies).
Mortality (any cause)
One trial in this comparison reported mortality data (Byrne 1992). A total of 23 patients died in the study period, but these were not reported in groups.
Allergic reaction
Two trials included allergic reaction as an outcome (Byrne 1992; Veiga 2009). No adverse reactions were reported by Veiga 2009. In the Byrne 1992 study 19 events were reported, nine (0.5%) in the chlorhexidine group and 10 (0.6%) in the placebo group. There was no evidence of a statistically significant difference in allergy rate (RR 0.89, 95% CI 0.36 to 2.19) (Analysis 1.3).
1.3. Analysis.
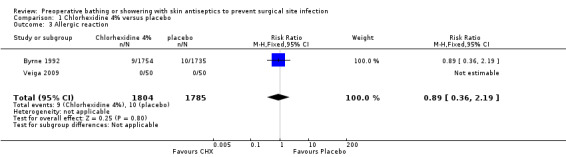
Comparison 1 Chlorhexidine 4% versus placebo, Outcome 3 Allergic reaction.
Cost
There was an estimate of cost in one study (Byrne 1992). The average total cost (based on drug costs, hotel costs, dressing costs and outpatients' costs) of patients washing with chlorhexidine was UK £936 compared with UK £897 when patients washed with a placebo. Standard deviations were not reported but, according to the authors, the difference was not statistically significant.
Chlorhexidine compared with bar soap
Three trials compared washing with chlorhexidine with washing with bar soap (Earnshaw 1989; Hayek 1987; Randall 1983). These included 1443 participants and reported on two outcomes (SSI and mortality). Due to small numbers in two of the trials (Earnshaw 1989; Randall 1983), and methodological inconsistencies in the Hayek 1987 trial, estimates of effect are imprecise and need to be interpreted with caution. Heterogeneity was high for this comparison (P value 0.08, I2 = 60%), so we used a random‐effects model for the meta‐analysis. There are two possible explanations for heterogeneity. First, different types of surgery were conducted in each trial; Earnshaw 1989 included patients undergoing vascular reconstruction, while Hayek 1987 included patients booked for routine elective surgery and Randall 1983 included only vasectomy patients. Alternatively, a different definition of SSI was used by Randall 1983, who included patients with a wound which discharged pus or serous fluid, whereas Earnshaw 1989 and Hayek 1987 defined SSI as the discharge of pus.
Surgical site infection
Two trials that compared washing with chlorhexidine with washing with soap found no difference between the treatments in postoperative SSI rate (Earnshaw 1989; Randall 1983). The largest trial (Hayek 1987), however, reported significantly fewer SSIs when patients washed preoperatively with chlorhexidine compared with those who washed with soap (RR 0.70, 95% CI 0.51 to 0.96) (Analysis 2.1). When results of the three trials were combined there was no statistically significant difference (RR 1.02, 95% CI 0.57 to 1.84) (Analysis 2.1), the event rate was 10.9% for chlorhexidine and 13.6% for bar soap.
2.1. Analysis.
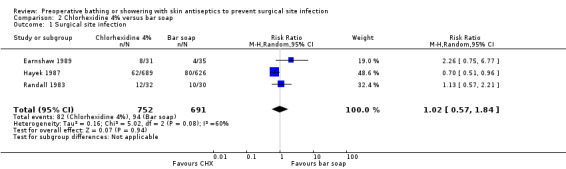
Comparison 2 Chlorhexidine 4% versus bar soap, Outcome 1 Surgical site infection.
Mortality (any cause)
Two patients died in the Earnshaw 1989 trial, but these were not reported by group.
Chlorhexidine compared with no wash
Three trials compared washing with chlorhexidine with no specific washing instructions (Randall 1983; Veiga 2009; Wihlborg 1987). These included 1142 patients and reported on SSI only. There was significant statistical heterogeneity between the three trials (P value < 0.03), and clinical heterogeneity (outpatient surgery versus inpatient surgery; different types of included patients). Randall 1983 enrolled patients undergoing vasectomy, Veiga 2009 included patients having plastic surgery and Wihlborg 1987 included patients undergoing elective surgery of the biliary tract, inguinal hernia or breast cancer. In addition, Randall 1983 defined SSI as a wound which discharged pus or serous fluid, Wihlborg 1987 defined SSI as the discharge of pus and Veiga 2009 defined SSI using Centers for Disease Control criteria for wound infection and wound classification.
Surgical site infection
Randall 1983 found no difference in the postoperative SSI rate between patients who washed with chlorhexidine compared with patients who did not wash preoperatively (12/32 (37.5%) patients in the chlorhexidine group developed an infection compared with 9/32 (28.1%) in the no wash group. In the Veiga 2009 trial, none of the patients in the no wash group developed a surgical site infection (0%) compared to one (0.2%) in the chlorhexidine group (RR 3.00; 95% CI 0.13 to 71.92). In the larger trial, Wihlborg 1987 found that a chlorhexidine wash when compared with no wash resulted in a statistically significant reduction in the number of patients with a SSI; (9 of the 541 (1.7%) patients in the chlorhexidine group developed an infection compared with 20 of 437 (4.6%) in the no wash group (RR 0.36; 95% CI 0.17 to 0.79) (Analysis 3.1). Although patients in the no‐wash groups were given no instructions to shower or bathe preoperatively, it is unclear whether any did so.
3.1. Analysis.
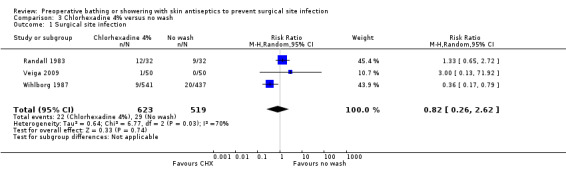
Comparison 3 Chlorhexadine 4% versus no wash, Outcome 1 Surgical site infection.
Chlorhexidine full wash compared with partial wash
One trial compared washing the whole body with chlorhexidine with a partial localised wash with chlorhexidine soap (Wihlborg 1987). This trial included 1093 participants and assessed one outcome; SSI.
Surgical site infection
Data from one trial making this comparison (Wihlborg 1987) showed a statistically significant reduction in SSIs when whole body washing (1.7%) was compared with partial localised washing (4.1%) (RR 0.40, 95% CI 0.19 to 0.85) (Analysis 4.1).
4.1. Analysis.
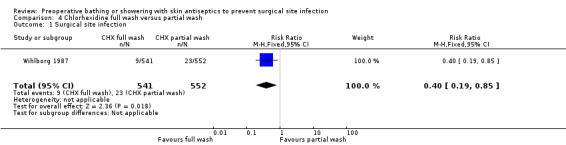
Comparison 4 Chlorhexidine full wash versus partial wash, Outcome 1 Surgical site infection.
Summary of findings tables
To assess the overall body of evidence, we developed three Summary of findings' tables (4% chlorhexidine gluconate verus placebo; 4% chlorhexidine gluconate versus bar soap and 4% chlorhexidine gluconate versus no bath or shower), using GRADEprofiler. The quality of the body of evidence was assessed against five principle domains 1) limitations in design and implementation; 2) indirectness of evidence or generalisability of findings; 3) inconsistency of results ‐ for example unexplained heterogeneity and inconsistent findings; 4) imprecision of results where confidence intervals are wide; and 5) other potential biases, for example publication bias or high manufacturer involvement (Schunermann 2011).
Discussion
Widespread use of preoperative antiseptic washing agents to prevent SSI continues. This review summarises trial data from over 10,000 patients, that compared washing with chlorhexidine with either a placebo solution, or a bar soap, or no preoperative washing at all. There was no clear evidence that washing with chlorhexidine reduced the incidence of SSI. The results of the review are strengthened by the heterogeneous nature of the participants; the trials included men, women and children undergoing a range of surgeries that were either clean or potentially infected, and undertaken in both inpatient and outpatient settings. These studies were published over a 26‐year period between 1983 and 2009. The product used in the trials (chlorhexidine 0.4%) remains unchanged and the quality of the two largest trials (that included over 6,000 participants) was high, concealing the randomisation process and blinding the interventions. Both of these trials also included community follow‐up.
One of the limitations of the review was the quality of some of the studies. Community follow‐up was attempted in only four studies, only one of the authors provided justification for their sample sizes (Byrne 1992), and in both studies where a cluster design was used, analysis was conducted as if participants had been allocated individually. Nonetheless, the high quality trials and trials where participants were allocated individually, showed no statistically significant reduction in SSIs when chlorhexidine was used for preoperative washing.
Only one of the trials provided data for other important outcomes. Byrne 1992 assessed complications or undesirable effects attributable to the use of an antiseptic. In this trial patients assigned to chlorhexidine use were no more likely to suffer an adverse reaction than those assigned to the placebo group. There were no comparisons with bar soap for this outcome. Byrne 1992 also assessed the cost of washing with chlorhexidine compared with placebo and found a non‐significant cost reduction in the placebo group. Costs included length of hospital stay, so, even though the SSI rate was 1.1% higher in the placebo group, using a placebo still resulted in an overall cost benefit.
Our findings are consistent with two recent reviews of preoperative skin antiseptics for preventing surgical site infection. The first, which investigated any type of antiseptic preparation, included a section on pre‐operative body washing. The authors accepted both RCTs and non‐randomised comparative studies and found that showering with an antiseptic reduced skin flora but the effect on SSIs remained inconclusive (Kamel 2012). In the second review, Chlebicki 2013 included RCTs, quasi RCTs and pre/post intervention studies. A total of 16 clinical trials met their inclusion criteria and were incorporated in a meta analysis with two subgroups (clean surgery and clean contaminated/contaminated surgery). No benefit for chlorhexidine showering was found in either of the subgroups or in the overall estimate of effect; RR 0.90 (95% CI 0.77 to 1.05) (Chlebicki 2013).
Authors' conclusions
Implications for practice.
This review provides no clear evidence of a benefit in reduced SSI rates associated with preoperative showering or bathing with chlorhexidine compared with other wash products. Efforts to reduce the incidence of nosocomial SSI should focus on interventions where effect has been demonstrated.
Implications for research.
Any future trials designed to assess the effectiveness of chlorhexidine as a preoperative body wash to prevent surgical site infection should:
Follow the CONSORT statement when designing and reporting the trial.
Conduct a priori sample size calculations based on results of this review.
Document preoperative and intra‐operative antibiotic use.
Include a follow‐period of at least 4 weeks.
Include clinically‐relevant secondary endpoints (mortality, adverse effects, cost effectiveness, length of hospital stay).
Feedback
Molnlycke feedback
Summary
A detailed letter was received from Mölynlycke Health Care along with comments from a statistical consultant. Responses to the feedback are detailed below.
Reply
Response of J Webster and S Osborne to Comments received from Mölynlycke Healthcare
Firstly we would like to thank Mölynlycke Healthcare and Mr P N Lee for submitting comments and for helping to improve the contents of the Cochrane Library.
We have carefully considered the Comments made and respond to each individually below; we also consulted with Gill Worthy, recently appointed Statistical Editor of the Cochrane Wounds Group in compiling our response.
In summary whilst on reflection we feel that several of the points raised have merit and we have amended the review accordingly (by amending the conclusion to "no clear evidence of benefit", by removing the subgroup analyses, and by not synthesising the data from the "no wash" comparison) we do not agree with many of the points raised, including several of those raised in the letter from Mölynlycke.
Detailed Responses to Letter from Milt Hinsch, Technical Services Director, Mölynlycke Healthcare, dated 30 August 2006.
"the conclusion [of the review] is inaccurate" We agree that the conclusion that there is "evidence of no benefit for preoperative showering or bathing with chlorhexidine…" is badly worded and in the next update of the Library this has been changed to read "no clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products".
"in our view, the erroneous conclusion was reached by reliance on data of poor quality and diversity, and statistical analysis errors and omissions. For example the study states that antibiotic prophylaxis routinely was used in Earnshaw study (n = 66), but the antibiotic prophylaxis was not administered using a standardized protocol" The quality of the many of the studies reviewed was poor and this was clearly acknowledged in the review (e.g. Discussion, paragraph 2) and is typical of research quality in many areas of medical and health care. Unfortunately reviewers can only deal with the data that exists and this is frequently different from the data one would wish to have. Nevertheless, it is highly worthwhile to review even poor quality data as it allows identification of unanswered research questions.
As you have not referenced specifics, we are not sure what you mean by statistical analysis errors and omissions. This statement does not reflect the critique of the review made by your statistician, Mr Lee who did not highlight major statistical analysis errors and omissions. We do not understand the point being made about the Earnshaw study nor how you think this affected the results and conclusions. All participants in the Earnshaw study received antibiotic prophylaxis irrespective of which arm they were allocated to and therefore no bias is introduced as both arms received the same co‐interventions. They did in fact receive standardized prophylaxis, viz. 3 perioperative doses of intravenous amoxicillin/clavulanic acid (or erythromycin in the case of sensitivity to the former). Since many patients do receive antibiotic prophylaxis in real life, inclusion of this study, if anything, increases external validity.
"in the Byrne (n = 3733) , Rotter (n = 2813), and Wihlborg (n = 1530) studies the prophylaxis rate was only 1‐15%. Hayek (n = 1989) and Randall (n = 94) studies did not mention antibiotic usage. One cannot pool these study patients when it is known that antibiotic prophylaxis can have an effect on surgical site infections (SSI)". The decision about which studies are pooled together is a matter of judgment, not fact. Byrne, Hayek and Rotter were pooled together and the amount of statistical heterogeneity was low at only 4.6%. There was more heterogeneity evident when Earnshaw, Hayek and Randall were pooled (60%) and therefore a more conservative random‐effects model was applied, which takes account of between‐study as well as within‐study variance. Pooling studies which may have different antibiotic usage rates is not "wrong" nor does it introduce bias, since both arms within trials were treated the same; the only impact may be to reduce precision.
"…the omission of the SSI rate for the different types of surgeries at each institution makes it impossible to understand the significance of the data. A lower SSI rate requires a greater number of subjects to discern the differences between antiseptic and placebo. The placebo effect needs better explanation, because review of the clinical outcomes of SSI for the placebo controlled studies shows that the SSI rate for placebo ranges from 2.4 to 33.3%. The great variation in the SSI rate for placebo calls into question the sensitivity of the method in the hands of the authors and the ability of the method to detect differences between antiseptic and placebo". Again, if we understand the point being made here correctly, it is the same one as in the previous paragraph, and our response is the same; whilst there may be variation in the baseline infection rates, pooling these studies does not introduce bias and does not therefore threaten internal validity. The rates quoted are not placebo rates since the 33.3% figure relates to the "shower with normal soap" arm of the Randall study. Furthermore we have reported a relative measure of outcome rather than absolute differences (which are more greatly affected by variations in baseline event rates).
"…many of the studies are underpowered to detect differences between antiseptic and placebo…Few of the studies provided information about calculation of sample size. For this reason it appears that they pooled data from multiple references to try and provide a sufficient sample size to draw inferences of effect". This paragraph succinctly explains the whole rationale of meta‐analysis.
Response of J Webster and S Osborne to Comments received from Mr PN Lee (Independent Statistician) forwarded by Mölynlycke Healthcare
Much of Mr Lee's 15 page document is merely a description of the review so we will confine our response to his substantive criticisms:
"It should be noted that in the Hayek 1987 study the placebo used was found, 5 months into the 2 year study, to have some antimicrobial activity and was subsequently changed… in the Cochrane review, both the discussion on chlorhexidine vs bar soap and the detail of the characteristics of the Hayek study wrongly state that the soap, and not the placebo, originally used… was changed". (p3) Thank you for spotting this error. We have amended this and drawn attention to it, although it does not materially change anything and we cannot amend the analysis as we do not know how many participants were affected.
"…it certainly seems that many of the studies will be considerably underpowered to detect any plausible true level of risk reduction". (p5) We agree and the small sample sizes and frequent lack of sample size calculations were discussed in the review.
"There is uncertainty as to how valid either estimate is, given the heterogeneity. The dubious nature of the random effects estimate…together with its wide CI, provides little evidence against chlorhexidine actually reducing risk of SSI" (p6) We agree, there is a great deal of uncertainty around the individual study estimates and hence the pooled estimates however we are not claiming that chlorhexidine increases the risk of SSI. We believe that amending the review conclusions to "no clear evidence of benefit" from "evidence of no benefit" will deal with this issue.
"For the two studies where the comparison is with no wash both (pooled) estimates… show a non significant reduction in risk of SSI… the data are difficult to interpret, because there are two widely differing estimates…both with very wide CIs". (p7) We agree that there is great heterogeneity here and it is probably not sensible to pool these studies; we have amended the review and removed the pooling. This does not, however, materially affect the overall conclusions.
"It would seem not unreasonable to carry out an additional analysis using data for each study comparing the chlorhexidine whole body group with the combined results for each study with no chlorhexidine…" (p7) We don't see the rationale for this additional analysis; it was not pre‐planned in the protocol (unlike all the analyses presented) and it would not alter the conclusions.
"I find the conclusions of the authors of the Cochrane review … to be surprising and misleading… even if they did mean "evidence of no benefit" it does not seem justified bearing in mind that i) two of the six studies showed a statistically significant advantage to chlorhexidine ii) all the meta analyses (with the minor exception of the dubious random effects analysis for soap) provided estimates less than 1.00 i.e., an advantage to chlorhexidine, and iii) the meta analyses based on the largest numbers of SSIs showed a near significant advantage to chlorhexidine.. My own conclusion is that the data suggest a possible advantage to chlorhexidine but that more studies are needed." (p8) We agree that the conclusions are erroneously worded and have been amended to "no clear evidence of benefit for preoperative showering or bathing with chlorhexidine…" We note that: i) the two studies that showed a statistically significant advantage associated with chlorhexidine were not of high quality (by pre‐specified quality criteria). ii) none of the meta analyses showed a statistically significant advantage in favour of chlorhexidine. iii) Mr Lee's own analysis (which is post hoc unlike the pre‐planned analyses presented in the Cochrane review) is the most favourable to chlorhexidine but still not significant.
Other issues:
"It is stated on page 5 that for four of the included trials (Byrne 1992, Earnshaw 1989, Randall 1983, Wihlborg 1987) the authors 'responded to queries about study methods and/or requests for additional unpublished information.' It is interesting to note that in various places in the review there is reference to information from some of these studies as unknown or unclear. Were the original authors not asked about this, or did they no longer remember or have records of the relevant details?" The authors were unable to recall or obtain these details.
"The assessment of quality is in fact inconsistently described. In the section 'Methodological quality assessment' on p4 it is stated that trials were coded on six criteria…it is then stated that trials were defined as "high quality" based only on receiving an A rating for criteria 2 and 3, making one wonder why criteria 1, 4, 5 and 6 were coded at all… However, in the 'Data synthesis' section on p5 it was stated that the effect of trial quality was carried out based on excluding those trials most susceptible to bias based on three criteria; two (2 and 4) essentially as defined above and another not mentioned before… the fact that the authors have been inconsistent in their definitions is not crucial to the selection of which studies are considered to be of high quality." The other criteria were coded to facilitate full discussion of all aspects of quality. We have amended the inconsistency in the explanation of how trial quality was used in sensitivity analysis and agree that this does not affect the results or conclusions.
"On page 15, comparison 05 'More than one wash versus one wash' is stated to have an effect size of 0.92 (95% CI 0.80‐1.04). This is totally misleading and the relative risk has nothing whatsoever to do with how risk of SSI depends on the number of washes! Similar problems relate to the comparison of individual versus cluster randomisation." We agree and have removed these subgroup analyses, however, this does not materially affect the overall results or conclusions.
Contributors
Joan Webster and Sonja Osborne, authors of the review. Gill Worthy, Statistical Editor Cochrane Wounds Group. Nicky Cullum, Coordinating Editor Cochrane Wounds Group. PN Lee, Statistics and Computing Ltd (Independent Statistical Consultant to Mölynlycke Health Care). Milt Hinsch, Technical Services Director, Mölynlycke Health Care.
Full text of the letter from Mölynlycke Health Care and the report of Mr Lee are available from the Editorial base, please contact Sally Bell‐Syer (email: sally.bell‐syer@york.ac.uk).
What's new
| Date | Event | Description |
|---|---|---|
| 19 February 2015 | New search has been performed | Fifth update, new search, no new studies identified for inclusion. |
| 19 February 2015 | New citation required but conclusions have not changed | One new excluded study (Colling 2014) and an updated reference for a study that was previously published ahead of print Chlebicki 2013). No change to conclusions. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 20 July 2012 | New citation required but conclusions have not changed | Two new excluded studies (Murray 2011; Tanner 2011). One study moved from awaiting classification to excluded study (Bode 2010). No change to conclusions. |
| 20 July 2012 | New search has been performed | Fourth update, new search, no new studies identified for inclusion. |
| 30 August 2011 | Amended | republish, update affiliations, risk of bias terminology |
| 13 November 2010 | New search has been performed | Third update. New search, no new studies added. Three new excluded studies (Edminson 2010; Eiselt 2009; Jakobsson 2010. No change to conclusions. |
| 16 March 2009 | New search has been performed | Second update. One new study added (Veiga 2009). Three new excluded studies (Veiga 2008; Kaiser 1988; Enjabert 1984). 'Risk of Bias' and 'Summary of Findings' tables added. |
| 23 July 2008 | Amended | Converted to new review format. |
| 6 February 2007 | New citation required and conclusions have changed | Substantive amendment. Feedback added and authors' response added. |
Acknowledgements
The authors would like to thank the Managing Editor of the Wounds Group (Sally Bell‐Syer), Cochrane Review Wounds Group referees (Peggy Edwards, Miles Maylor, Vicky Whittaker, Amy Zelmer) and Editors (Nicky Cullum, Andrea Nelson, Raj Mani) for their comments to improve the review. In addition the copy editor Elizabeth Royle who copy edited the third update.
Appendices
Appendix 1. Search strategy for the fourth review update ‐ 2012
For this fourth update we searched the following electronic databases:
The Cochrane Wounds Group Specialised Register (searched 29 June 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 6);
Ovid MEDLINE (2010 to June Week 3 2012);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations June 27, 2012);
Ovid EMBASE (2010 to 2012 Week 25);
EBSCO CINAHL (1982 to 21 June 2012)
The following strategy was used to search CENTRAL: #1 MeSH descriptor Skin explode all trees #2 MeSH descriptor Antisepsis explode all trees #3 (#1 AND #2) #4 skin NEXT antisep* #5 MeSH descriptor Anti‐Infective Agents, Local explode all trees #6 MeSH descriptor Soaps explode all trees #7 MeSH descriptor Povidone‐Iodine explode all trees #8 MeSH descriptor Iodophors explode all trees #9 MeSH descriptor Chlorhexidine explode all trees #10 MeSH descriptor Alcohols explode all trees #11 iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol or alcohols or antiseptic* or soap* #12 MeSH descriptor Disinfectants explode all trees #13 (#1 AND #12) #14 skin NEAR/5 disinfect* #15 MeSH descriptor Detergents explode all trees #16 (#1 AND #15) #17 skin NEAR/5 detergent* #18 (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #13 OR #14 OR #16 OR #17) #19 MeSH descriptor Surgical Wound Infection explode all trees #20 surg* NEAR/5 infection* #21 surgical NEAR/5 wound* #22 (postoperative or post‐operative) NEAR/5 infection* #23 MeSH descriptor Preoperative Care explode all trees #24 (preoperative or pre‐operative) NEXT care #25 MeSH descriptor Perioperative Care explode all trees #26 (perioperative or peri‐operative) NEXT care #27 (#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 MeSH descriptor Baths explode all trees #29 shower* or bath* or wash* or cleans* #30 (#28 OR #29) #31 (#18 AND #27 AND #30)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format. This filter is published in the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009]; Section 6.4.11. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN). No date or language restrictions were applied.
Searching other resources
Reference lists of all retrieved articles were searched in order to identify additional studies. For the original version of the review, manufacturers of antiseptic products were contacted in order to obtain any unpublished data.
Searching other resources
Reference lists of all retrieved articles were searched for additional studies. Manufacturers of antiseptic products were contacted in order to obtain any unpublished data.
Appendix 2. Ovid MEDLINE search strategy
1 exp Skin/ 2 exp Antisepsis/ 3 and/1‐2 4 skin antisep*.tw. 5 exp Anti‐Infective Agents, Local/ 6 exp Soaps/ 7 exp Povidone‐Iodine/ 8 exp Iodophors/ 9 exp Chlorhexidine/ 10 exp Alcohols/ 11 (iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol*1 or antiseptic* or soap*).tw. 12 exp Disinfectants/ 13 1 and 12 14 (skin adj5 disinfect*).tw. 15 exp Detergents/ 16 1 and 15 17 (skin adj5 detergent*).tw. 18 or/3‐11,13‐14,16‐17 19 exp Surgical Wound Infection/ 20 (surgical adj5 infection*).tw. 21 (surgical adj5 wound*).tw. 22 ((post‐operative or postoperative) adj5 wound infection*).tw. 23 exp Preoperative Care/ 24 ((preoperative or pre‐operative) adj care).tw. 25 exp Perioperative Care/ 26 ((perioperative or peri‐operative) adj care).tw. 27 or/19‐26 28 exp Baths/ 29 (shower* or bath* or wash* or cleans*).tw. 30 or/28‐29 31 18 and 27 and 30
Appendix 3. Ovid EMBASE search strategy
1 exp Skin/ 2 exp Antisepsis/ 3 1 and 2 4 skin antisep*.tw. 5 exp Antiinfective Agent/ 6 exp Soap/ 7 exp Povidone Iodine/ 8 exp Iodophor/ 9 exp Chlorhexidine/ 10 exp Alcohol/ 11 (iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol*1 or antiseptic* or soap*).tw. 12 exp Disinfectant Agent/ 13 1 and 12 14 (skin adj5 disinfect*).tw. 15 exp Detergents/ 16 1 and 15 17 (skin adj5 detergent*).tw. 18 or/3‐11,13‐14,16‐17 19 exp Surgical Infection/ 20 (surgical adj5 infection*).tw. 21 (surgical adj5 wound*).tw. 22 ((post‐operative or postoperative) adj5 wound infection*).tw. 23 exp Preoperative Care/ 24 ((preoperative or pre‐operative) adj care).tw. 25 exp Perioperative Care/ 26 ((perioperative or peri‐operative) adj care).tw. 27 or/19‐26 28 exp Bath/ 29 (shower* or bath* or wash* or cleans*).tw. 30 or/28‐29 31 18 and 27 and 30
Appendix 4. EBSCO CINAHL search strategy
S29 S15 and S25 and S28 S28 S26 or S27 S27 TI ( shower* or bath* or wash* or cleans* ) or AB ( shower* or bath* or wash* or cleans* ) S26 (MH "Bathing and Baths") S25 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 TI ( perioperative care or peri‐operative care ) or AB (perioperative care or peri‐operative care ) S23 (MH "Perioperative Care+") S22 TI ( pre‐operative care or preoperative care ) or AB ( pre‐operative care or preoperative care ) S21 (MH "Preoperative Care+") S20 TI post‐operative wound infection* or AB post‐operative wound infection* S19 TI postoperative wound infection* or AB postoperative wound infection* S18 TI surgical N5 wound* or AB surgical N5 wound* S17 TI surgical N5 infection* or AB surgical N5 infection* S16 (MH "Surgical Wound Infection") S15 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S11 or S12 or S13 or S14 S14 (MH "Skin Preparation, Surgical") S13 TI skin N5 antisep* or AB skin N5 antisep* S12 TI skin N5 disinfect* or AB skin N5 disinfect* S11 S7 and S10 S10 (MH "Disinfectants") S9 S7 and S8 S8 (MH "Detergents+") S7 (MH "Skin+") S6 TI ( iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol* or antiseptic* or soap*) and AB (iodophor* or povidone‐iodine or betadine or chlorhexidine or triclosan or hexachlorophene or benzalkonium or alcohol* or antiseptic* or soap* ) S5 (MH "Alcohols+") S4 (MH "Chlorhexidine") S3 (MH "Povidone‐Iodine") S2 (MH "Soaps") S1 (MH "Antiinfective Agents, Local+")
Appendix 5. Risk of bias assessment definitions
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Chlorhexidine 4% versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 4 | 7791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| 2 Surgical site infection (high quality studies) | 2 | 6302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 3 Allergic reaction | 2 | 3589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.36, 2.19] |
Comparison 2. Chlorhexidine 4% versus bar soap.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 3 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
Comparison 3. Chlorhexadine 4% versus no wash.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.26, 2.62] |
Comparison 4. Chlorhexidine full wash versus partial wash.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 1 | 1093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Byrne 1992.
| Methods | RCT. Power calculation: yes. Follow‐up period: 6 weeks after discharge. | |
| Participants | 3733 patients undergoing elective or potentially contaminated surgery. Exclusion criteria: patients undergoing day surgery, emergency surgery, re‐operation or contaminated surgery and those unable to comply with the washing procedure, or with a known allergy to chlorhexidine or having more than the standard prophylactic antibiotic regimen. Baseline comparability: age, sex, type of surgery, ASEPSIS score. | |
| Interventions | All patients showered 3 times (on admission, the night before surgery and the morning of surgery) using 50 ml of either: Group 1: 4% chlorhexidine, or Group 2: a placebo. Written instructions were provided to all participants. | |
| Outcomes | Primary outcome: Wound infection was defined as discharge of pus from a wound for inpatients or outpatients, or an ASEPSIS score greater than 10. Group 1: 256/1754 (14.6%); Group 2: 272/1735 (15.7%). Secondary outcomes: Death, allergic reactions, cost. | |
| Notes | Data were extracted from 3 papers reporting results from 1 study (see Lynch 1992 & Byrne 1994). There were minor discrepancies in numbers reported between the 3 studies. The version reported is the definitive study (personal correspondence with author). The abstract stated there were 1753 patients in the placebo group but this should have been 1735 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 6. Randomisation in each group of 6 was by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Personal communication with author. Allocation in sealed envelopes. |
| Blinding (performance bias and detection bias) Investigator and participant | Low risk | 2 bottles of either 4% chlorhexidine detergent solution or a physically‐identical placebo detergent were given to the patient. Neither the investigators nor the patient were aware of the allocation. Outcome blinding was not specifically mentioned, however, it seems likely that those assessing infection status would have been unaware of the allocation. |
| Blinding (performance bias and detection bias) Outcome assessor | Low risk | Outcome assessment blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 3733 patients randomised, 244 (6.5%) were excluded from the outcome analysis. The majority of these, 219 (5.9%) were because the operation was cancelled after randomisation. 4 patients in the chlorhexidine group and 9 patients in the control group were excluded for protocol violations. However, as these numbers are extremely small (0.4% of those randomised), it is unlikely that results were compromised. |
| Selective reporting (reporting bias) | Low risk | The study reported on all of the stated outcomes. |
| Other bias | Low risk | The trial was supported by ICI Pharmaceuticals. However, as results did not support the use of chlorhexidine, it is unlikely that results were compromised. |
Earnshaw 1989.
| Methods | RCT. Power calculation: no. Follow‐up period: until hospital discharge. | |
| Participants | 66 patients undergoing vascular reconstruction surgery. Exclusion: none reported. Baseline comparability: stated that groups were similar, no data. | |
| Interventions | All patients had 2 baths. Group 1: painted entire body with undiluted 4% chlorhexidine followed by rinsing in the bath. Precise instructions given; Group 2: non‐medicated soap used. No specific instructions provided. | |
| Outcomes | Primary outcome: Wound infection was defined as discharge of pus from a wound; 1 patient with severe cellulitis was also included. Group 1: 8/31 (26%); Group 2: 4/35, (11.4%). Secondary outcome: Death. | |
| Notes | Different washing information provided to participants in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Investigator contacted ‐ no further information available. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Investigator contacted ‐ no further information available. |
| Blinding (performance bias and detection bias) Investigator and participant | High risk | Both the investigator and the participant were aware of the allocation. Postoperatively wounds were reviewed blind until patients left hospital. |
| Blinding (performance bias and detection bias) Outcome assessor | Low risk | Outcome assessment blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were available for all those enrolled. No drop outs or exclusions were described. |
| Selective reporting (reporting bias) | Low risk | The study reported on wound infection. A number of post hoc analyses were also conducted. |
| Other bias | High risk | Baseline data tables were not provided but the author stated that "clinical details of the two groups were similar". Different washing information provided to participants in each group. Hibiscrub (chlorhexidine) was provided by ICI. However, as results favoured the use of soap, it is unlikely that the involvement of the pharmaceutical company influenced results. |
Hayek 1987.
| Methods | Cluster RCT. Power calculation: no. Follow‐up period: until hospital discharge. | |
| Participants | 2015 patients undergoing routine surgery. Exclusion: those receiving antibiotics or with an existing infection. Baseline comparability: age, sex, preoperative skin preparation, wound classification, proportion who washed their hair. | |
| Interventions | All patients had either a shower or bath on the day before and morning of their operation. Group 1: chlorhexidine 4%. Instruction card for washing provided. Group 2: placebo. Instruction card for washing provided (5 months into the study, the placebo was found to have antimicrobial properties and was changed). Group 3: bar soap. No washing instructions provided. | |
| Outcomes | Primary outcome: Wound infection was defined as either discharge of pus from a wound, or erythema, or swelling considered to be greater than expected. Group 1: 62/689 (9.0%); Group 2: 83/700 (11.7%); Group 3: 80/626 (12.8%). | |
| Notes | Data were extracted from 2 papers reporting results from 1 study (Hayek 1988). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Investigator and participant | High risk | 3 arms to the study. "None of the ward staff or those assessing the wounds were aware of whether placebo or active compound was being used as these were issued in identical coded sachets, though no form of double blind was possible for the bar soap group". |
| Blinding (performance bias and detection bias) Outcome assessor | Low risk | Outcome assessment blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It appears from the text that results were available from all patients who entered the study. However, as patients were followed up for 6 weeks postoperatively; it seems unusual that none were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | High risk | No baseline data presented. The authors state that "The three groups are comparable except for one interesting facet; only 14% washed their hair with bar soap against 28% with either of the liquids. Specific washing instructions were provided to the chlorhexidine and placebo groups but not to the bar soap group. 5 months into the study, the placebo was found to have antimicrobial properties and was changed. It is unclear how this large study was funded. No competing interests were declared. |
Randall 1983.
| Methods | RCT. Power calculation: no. Follow‐up period: 1 week after discharge. | |
| Participants | 94 patients undergoing vasectomy. Exclusion: none stated. Baseline comparability: none stated. | |
| Interventions | Group 1: 1 preoperative shower with chlorhexidine 4%, Group 2: 1 shower with normal soap. Group 3: no shower. | |
| Outcomes | Primary outcome: Wound infection was defined as discharging either purulent or serous fluid. Group 1: 12/32 (37.5%); Group 2: 10/30 (33.3%); Group 3: 9/32 (28.1%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with study authors confirmed that trialists used computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) Investigator and participant | High risk | Blinding of the intervention was not possible. |
| Blinding (performance bias and detection bias) Outcome assessor | Unclear risk | It is unclear if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All but 1 enrolled patient were assessed 7 days postoperatively; 83 returned to the ward, 10 were contacted at home and 1 was lost to follow‐up (it is unclear which group this patient was in); results were presented for the total number enrolled. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | No baseline data presented. No competing interests declared. |
Rotter 1988.
| Methods | Cluster RCT. Power calculation: no. Follow‐up period: 3 weeks after discharge. | |
| Participants | 2953 patients undergoing elective clean surgery. Exclusion: patients with a temperature of 37.5oC on the day of or day before surgery, infection remote from operation site, antibiotics given within 7 days prior to surgery for infection, incarcerated inguinal hernia, radical mastectomy. Baseline comparability: age, sex, type of surgery, antibiotic prophylaxis, hair washed, hair removal method, wound drainage. | |
| Interventions | All patients had 2 showers;1 the day before surgery and 1 on the day of surgery. Group 1: used 50 ml of chlorhexidine 4% for each shower; Group 2: placebo. Special application instructions were provided to all participants. | |
| Outcomes | Primary outcome: Wound infection was defined in the report as "inflammation of the surgical wound with discharge of pus, spontaneous and/or after surgical intervention that occurs during hospitalisation or during routine follow‐up" Group 1: 37/1413 (2.6%); Group 2: 33/1400 (2.4%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out for each surgical unit by means of computer‐generated patient trial numbers. |
| Allocation concealment (selection bias) | Low risk | Bottles of solution were contained in boxes of 10, each holding 5 chlorhexidine and 5 placebo in random sequence. Unless the code was broken, it was impossible to know which preparation the patient had used. |
| Blinding (performance bias and detection bias) Investigator and participant | Low risk | Neither patients nor investigators were aware of group allocation. |
| Blinding (performance bias and detection bias) Outcome assessor | Low risk | Outcome assessment was by routine surveillance methods employed by individual hospitals (infection control nurse or surgeon). They were unaware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 140 (4.7%) patients were withdrawn after randomisation either because the patient was not operated on (62 patients), had had 1 bath only (26), operation not stated 'clean' (14) or had a current infection (38). The withdrawals were evenly distributed between the chlorhexidine and placebo groups. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | ICI supplied the bathing materials. However, as results favoured the placebo group, this is unlikely to have affected results. |
Veiga 2009.
| Methods | RCT. Power calculation: no. Follow‐up period: 30 days. | |
| Participants | Adult patients, scheduled for plastic surgery at a University‐affiliated hospital in Brazil. Exclusions: hypersensitivity to chlorhexidine, presence of skin lesions, antibiotic use at time of surgery, diabetes, heavy smoking, immunosuppression. |
|
| Interventions | Group 1: shower with liquid‐based detergent containing 4% chlorhexidine; Group 2: shower with the same liquid‐based detergent, without chlorhexidine; Group 3: no preoperative showering instructions were given. Patients in Groups 1 and 2 were asked to rinse thoroughly, lather with the antiseptic, rinse, lather and rinse again. | |
| Outcomes | Primary outcome:
Surgical site infection (defined using Centers for Disease Control criteria for wound infection and wound classification)
Group 1: 1/50 (2%);
Group 2: 1/50 (2%);
Group 3: 0/50 (0%). Secondary outcome: Adverse reactions: no adverse reactions were reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Investigator and participant | Low risk | Surgeons and patients were all blinded to the allocation group. |
| Blinding (performance bias and detection bias) Outcome assessor | Low risk | Outcome assessors and microbiologists were all blinded to the allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed for the nominated 30 days. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported. |
| Other bias | Low risk | The placebo solution was provided by Rioquimica Industria Farmaceutica, the manufacturer of the intervention product. However, as results in different groups were similar, this is unlikely to have affected results. |
Wihlborg 1987.
| Methods | RCT. Power calculation: no. Follow‐up period: until hospital discharge. | |
| Participants | 1530 patients undergoing elective surgery of the biliary tract, inguinal hernia and breast cancer. Exclusion: none stated. Baseline comparability: age, duration of surgery > 2 hours, steroids, diabetes, malignancy (other than breast cancer), type of surgery. | |
| Interventions | Group 1: patients washed their entire body with chlorhexidine on the day before surgery using 2 consecutive applications followed by rinsing under the shower; Group 2: washed only that part of the body to be submitted to surgery with chlorhexidine soap; Group 3: No chlorhexidine wash. | |
| Outcomes | Primary outcome: Wound infection was defined as a definite collection of pus emptying itself spontaneously or after incision: Group 1: 9/541 (1.7%); Group 2: 23/552 (4.2%); Group 3: 20/437 (4.6). | |
| Notes | This study was conducted over a 7 year period from 1978 to 1984. It was unclear from the text whether patients allocated to the 'no chlorhexidine wash' group had any preoperative shower. 3 patients died and were not included in the analysis. Strength of wash solution was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with study authors confirmed that they used a randomisation list. |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to 1 of 3 wards in which different preoperative preparation schedules were used. The author states that "The randomisation was done by the Chief surgeon in such a way that he did not know the identity of the patients allocated to each ward." |
| Blinding (performance bias and detection bias) Investigator and participant | High risk | Blinding would have been impossible because of the allocation method. |
| Blinding (performance bias and detection bias) Outcome assessor | High risk | Outcome assessment was not blinded (personal correspondence with study authors). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients died in the few days following surgery, these were not identified by group. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Unclear risk | Baseline risk factors were similar across groups. No competing interests declared. |
Abbreviations
> = more than ASEPSIS = a scoring method for postoperative wounds infections for use in clinical trials of antibiotic prophylaxis (Wilson 1986). RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ayliffe 1983 | Not a randomised controlled trial. |
| Bergman 1979 | No data on wound infection. Not a randomised controlled trial. |
| Bode 2010 | Co‐intervention (mupirocin and chlorhexidine soap). Also included patients who did not undergo surgery. |
| Brandberg 1980 | Not a randomised controlled trial. Local wash versus full body wash with chlorhexidine. |
| Colling 2014 | Retrospective review comparing two hospitals. One hospital had a pre‐operative showering policy using an antiseptic solution; the other hospital did not. |
| Edminson 2010 | Not a clinical trial. |
| Edmiston 2008 | Healthy volunteers used to compare skin concentration levels of chlorhexidine gluconate (CHG) following washing with various CHG products. |
| Eiselt 2009 | Not a randomised controlled trial. Used a pre and post intervention design. |
| Enjabert 1984 | Not a randomised controlled trial. |
| Garabaldi 1988 | No no‐antiseptic group. Did not report infection rates by group. |
| Jakobsson 2010 | Systematic review. |
| Kaiser 1988 | Did not report infection rates by group. |
| Kalanter‐Hormozi 2005 | Patients in both groups showered with water. The trial compared methods of skin preparation prior to surgery. |
| Leigh 1983 | Not a randomised controlled trial. |
| Murray 2011 | 2% chlorhexidine gluconate cloths were used to wipe over the entire body one hour after showering. |
| Newsom 1988 | Not a randomised controlled trial. Patients were allocated by month. |
| Paulson 1993 | Assessed reduction in microbial loads in healthy volunteers. |
| Tanner 2011 | Trial of healthy volunteers |
| Veiga 2008 | Assessed the influence of povidone‐iodine preoperative showers on skin colonization. Rates of wound infection not reported. |
| Wells 1983 | Not a randomised controlled trial. Did not report infection rates by group. |
Contributions of authors
JW conceived, designed, coordinated the review and conducted the initial literature search. The protocol was jointly written by JW and SO. JW and SO separately reviewed the abstracts and selected papers for review. JW and SO separately reviewed and scored the trials. Both authors contributed to the final version of the review. JW lead the updates of the review.
Sources of support
Internal sources
Royal Brisbane and Women's Hospital, Australia.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Byrne 1992 {published data only}
- Byrne DJ, Lynch W, Napier A, Davey P, Malek M, Cuschieri A. Wound infection rates: the importance of definition and post‐discharge wound surveillance. Journal of Hospital Infection 1994;26(1):37‐43. [DOI] [PubMed] [Google Scholar]
- Byrne DJ, Napier A, Cuschieri A. The value of whole body disinfection in the prevention of postoperative wound infection in clean and potentially contaminated surgery. A prospective, randomised, double‐blind, placebo‐controlled clinical trial. Surgical Research Communications 1992;12(1):43‐52. [Google Scholar]
- Lynch W, Davey PG, Malek M, Byrne DJ, Napier A. Cost‐effectiveness analysis of the use of chlorhexidine detergent in preoperative whole‐body disinfection in wound infection prophylaxis. Journal of Hospital Infection 1992;21(3):179‐91. [DOI] [PubMed] [Google Scholar]
Earnshaw 1989 {published data only}
- Earnshaw JJ, Berridge DC, Slack RC, Makin GS, Hopkinson BR. Do preoperative chlorhexidine baths reduce the risk of infection after vascular reconstruction?. European Journal of Vascular Surgery 1989;3(4):323‐6. [DOI] [PubMed] [Google Scholar]
Hayek 1987 {published data only}
- Hayek LJ, Emerson JM. Preoperative whole body disinfection‐‐a controlled clinical study. Journal of Hospital Infection 1988;11(supplement B):15‐9. [DOI] [PubMed] [Google Scholar]
- Hayek LJ, Emerson JM, Gardner AM. A placebo‐controlled trial of the effect of two preoperative baths or showers with chlorhexidine detergent on postoperative wound infection rates. Journal of Hospital Infection 1987;10(2):165‐72. [DOI] [PubMed] [Google Scholar]
Randall 1983 {published and unpublished data}
- Randall PE, Ganguli L, Marcuson RW. Wound infection following vasectomy. British Journal of Urology 1983;55(5):564‐7. [DOI] [PubMed] [Google Scholar]
Rotter 1988 {published data only}
- Rotter ML, Larsen SO, Cooke EM, Dankert J, Daschner F, Greco D, et al. A comparison of the effects of preoperative whole‐body bathing with detergent alone and with detergent containing chlorhexidine gluconate on the frequency of wound infections after clean surgery. The European Working Party on Control of Hospital Infections. Journal of Hospital Infection 1988;11(4):310‐20. [DOI] [PubMed] [Google Scholar]
Veiga 2009 {published data only}
- Veiga DF, Damasceno CA, Veiga‐Filho J, Figueiras RG, Vieira RB, Garcia ES, et al. Randomized controlled trial of the effectiveness of chlorhexidine showers before elective plastic surgical procedures. Infection Control and Hospital Epidemiology 2009;30(1):77‐9. [DOI] [PubMed] [Google Scholar]
Wihlborg 1987 {published and unpublished data}
- Wihlborg O. The effect of washing with chlorhexidine soap on wound infection rate in general surgery. A controlled clinical study. Annales Chirurgiae et Gynaecologiae 1987;76(5):263‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Ayliffe 1983 {published data only}
- Ayliffe GA, Noy MF, Babb JR, Davies JG, Jackson J. A comparison of pre‐operative bathing with chlorhexidine‐detergent and non‐medicated soap in the prevention of wound infection. Journal of Hospital Infection 1983;4(3):237‐44. [DOI] [PubMed] [Google Scholar]
Bergman 1979 {published data only}
- Bergman BR, Seeberg S. A bacteriological evaluation of a programme for preoperative total body‐washing with chlorhexidine gluconate performed by patients undergoing orthopaedic surgery. Archives of Orthopedic Trauma Surgery 1979;95(1):59‐62. [DOI] [PubMed] [Google Scholar]
Bode 2010 {published data only}
- Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke‐Grauls CM, Roosendaal R, et al. Preventing surgical‐site infections in nasal carriers of Staphylococcus aureus. New England Journal of Medicine 2010;362(1):9‐17. [DOI] [PubMed] [Google Scholar]
Brandberg 1980 {published data only}
- Brandberg A, Holm J, Hammarsten J, Schersten T. Post‐operative wound infections in vascular surgery ‐ effect of pre‐operative whole body disinfection by shower‐bath with chlorhexidine soap. Royal Society of Medicine International Congress an Symposium Series 1980;23:71‐5. [Google Scholar]
Colling 2014 {published data only}
- Colling K, Statz C, Glover J, Banton K, Beilman G. Pre‐operative antiseptic shower and bath policy decreases the rate of S. aureus and methicillin‐resistant S. aureus surgical site infections in patients undergoing joint arthroplasty. Surgical Infections 2014;Nov 18. [Epub ahead of print]:1‐9. [DOI] [PubMed] [Google Scholar]
Edminson 2010 {published data only}
- Edmiston CE Jr, Okoli O, Graham MB, Sinski S, Seabrook GR. Evidence for using chlorhexidine gluconate preoperative cleansing to reduce the risk of surgical site infection. AORN Journal 2010;92:509‐18. [DOI] [PubMed] [Google Scholar]
Edmiston 2008 {published data only}
- Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission?. Journal of the American College of Surgeons 2008;207(2):233‐9. [DOI] [PubMed] [Google Scholar]
Eiselt 2009 {published data only}
- Eiselt D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Orthopaedic Nursing 2009;28(3):141‐5. [DOI] [PubMed] [Google Scholar]
Enjabert 1984 {published data only}
- Enjalbert L, Levade Y, Marchetti P. Comparison of 2 antiseptic soaps used for preoperative showers. Pathologie‐biologie (Paris) 1984;32:604‐6. [PubMed] [Google Scholar]
Garabaldi 1988 {published data only}
- Garibaldi RA, Skolnick D, Lerer T, Poirot A, Graham J, Krisuinas E, et al. The impact of preoperative skin disinfection on preventing intraoperative wound contamination. Infection Control and Hospital Epidemiology 1988;9(3):109‐13. [DOI] [PubMed] [Google Scholar]
Jakobsson 2010 {published data only}
- Jakobsson J, Perlkvist A, Wann‐Hansson C. Searching for evidence regarding using preoperative disinfection showers to prevent surgical site infections: a systematic review. World Views on Evidence‐Based Nursing 2010;Sep 28. doi: 10.1111/j.1741‐6787.2010.00201:1‐10. [DOI] [PubMed] [Google Scholar]
Kaiser 1988 {published data only}
- Kaiser AB, Kernodle DS, Barg NL, Petracek MR. Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers. Annals of Thoracic Surgery 1988;45:35‐8. [DOI] [PubMed] [Google Scholar]
Kalanter‐Hormozi 2005 {published data only}
- Kalantar‐Hormozi AJ, Davami B. No need for preoperative antiseptics in elective outpatient plastic surgical operations: a prospective study. Plastic and Reconstructive Surgery 2005;116(2):529‐31. [DOI] [PubMed] [Google Scholar]
Leigh 1983 {published data only}
- Leigh DA, Stronge JL, Marriner J, Sedgwick J. Total body bathing with 'Hibiscrub' (chlorhexidine) in surgical patients: a controlled trial. Journal of Hospital Infection 1983;4(3):229‐35. [DOI] [PubMed] [Google Scholar]
Murray 2011 {published data only}
- Murray MR, Saltzman MD, Gryzlo SM, Terry MA, Woodward CC, Nuber GW. Efficacy of preoperative home use of 2% chlorhexidine gluconate cloth before shoulder surgery. Journal of Shoulder and Elbow Surgery 2011;20:928‐33. [DOI] [PubMed] [Google Scholar]
Newsom 1988 {published data only}
- Newsom SW, Rowland C. Studies on perioperative skin flora. Journal of Hospital Infection 1988;11(Supplement B):21‐6. [DOI] [PubMed] [Google Scholar]
Paulson 1993 {published data only}
- Paulson DS. Efficacy evaluation of a 4% chlorhexidine gluconate as a full‐body shower wash. American Journal of Infection Control 1993;21:205‐9. [DOI] [PubMed] [Google Scholar]
Tanner 2011 {published data only}
- Tanner J, Gould D, Jenkins P, Hilliam R, Mistry N, Walsh S. A fresh look at preoperative body washing. Journal of Infection Prevention 2011;13:11‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veiga 2008 {published data only}
- Veiga DF, Damasceno CAV, Filho JV, Silva RV Jr, Cordeiro DL, Vieira AM, et al. Influence of povidone‐iodine preoperative showers on skin colonization in elective plastic surgery procedures. Reconstructive and Plastic Surgery 2008;121(1):115‐8. [DOI] [PubMed] [Google Scholar]
Wells 1983 {published data only}
- Wells FC, Newsom SW, Rowlands C. Wound infection in cardiothoracic surgery. Lancet 1983;1(8335):1209‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Beaudounin 2004
- Beaudouin E, Kanny G, Morisset M, Renaudin JM, Mertes M, Laxenaire MC, et al. Immediate hypersensitivity to chlorhexidine: literature review. Allergie et Immunologie (Paris) 2004;36:123‐6. [PubMed] [Google Scholar]
Brady 1990
- Brady LM, Thomson M, Palmer MA, Harkness JL. Successful control of MRSA in a cardiothoracic surgical unit. Medical Journal of Australia 1990;152:240‐5. [DOI] [PubMed] [Google Scholar]
Chlebicki 2013
- Chlebicki MP, Safdar N, O'Horo JC, Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta‐analysis. American Journal of Infection Control 2013;41:167‐73. [DOI] [PubMed] [Google Scholar]
Cruse 1980
- Cruse PJ, Foord R. The epidemiology of wound infection. A 10‐year prospective study of 62,939 wounds. Surgical Clinics of North America 1980;60(1):27‐40. [DOI] [PubMed] [Google Scholar]
Derde 2012
- Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial‐resistant bacteria in intensive care units: a systematic review. Intensive Care Medicine 2012;38:931‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies.. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: Wiley and Sons Ltd.
Jenks 2014
- Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. Journal of Hospital Infection 2014;86:24‐33. [DOI] [PubMed] [Google Scholar]
Kamel 2012
- Kamel C, McGahan L, Polisina J, Miezwinski‐Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: A systematic review. Infection Control and Hospital Epidemiology 2012;33:608‐17. [DOI] [PubMed] [Google Scholar]
Kirkland 1999
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical‐site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection Control and Hospital Epidemiology 1999;20(11):725‐30. [DOI] [PubMed] [Google Scholar]
Koburger 2010
- Koburger T, Hübner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP‐iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. Journal of Antimicrobial Chemotherapy 2010;65:1712‐9. [DOI] [PubMed] [Google Scholar]
Krautheim 2004
- Krautheim AB, Jermann TH, Bircher AJ. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis 2004;50:113‐6. [DOI] [PubMed] [Google Scholar]
Lefebrve 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infection Control and Hospital Epidemiology 1999;20:250‐78. [DOI] [PubMed] [Google Scholar]
Mollison 2000
- Mollison J, Simpson JA, Campbell MK, Grimshaw JM. Comparison of analytical methods for cluster randomised trials: an example from a primary care setting. Journal of Epidemiology and Biostatistics 2000;5:339‐48. [PubMed] [Google Scholar]
Rosenthal 2013
- Rosenthal VD, Richtmann R, Singh S, Apisarnthanarak A, Kübler A, Viet‐Hung N, Ramírez‐Wong FM, Portillo‐Gallo JH, Toscani J, Gikas A, Dueñas L, El‐Kholy A, Ghazal S, Fisher D, Mitrev Z, Gamar‐Elanbya MO, Kanj SS, Arreza‐Galapia Y, Leblebicioglu H, Hlinková S, Memon BA, Guanche‐Garcell H, Gurskis V, Alvarez‐Moreno C, Barkat A, Mejía N, Rojas‐Bonilla M, Ristic G, Raka L, Yuet‐Meng C. Surgical site infections, International Nosocomial Infection Control Consortium (INICC) report, data summary of 30 countries, 2005‐2010. Infection Control and Hospital Epidemiology 2013;34:597‐604. [DOI] [PubMed] [Google Scholar]
Rubinstein 1999
- Rubinstein E. Infectious diseases and litigation. Journal of Hospital Infection 1999;43:Suppl:S165‐7. [DOI] [PubMed] [Google Scholar]
Schunermann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. Available from http://www.sign.ac.uk/methodology/filters.html#random 2011.
Smyth 2008
- Smyth ETM, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, Davies E. Newcombe RG. Spencer RC. Four Country Healthcare Associated Infection Prevalence Survey 2006: overview of the results. Journal of Hospital Infection 2008;69:230‐48. [DOI] [PubMed] [Google Scholar]
Thomas 2000
- Thomas L, Maillard JY, Lambert RJ, Russell AD. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a "residual" concentration. Journal of Hospital Infection 2000;46:297‐304. [DOI] [PubMed] [Google Scholar]
Wilcox 2003
- Wilcox MH, Hall J, Pike H, Templeton PA, Fawley WN, Parnell P, et al. Use of perioperative mupirocin to prevent methicillin‐resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. Journal of Hospital Infection 2003;54:196‐201. [DOI] [PubMed] [Google Scholar]
Wilson 1986
- Wilson APR, Treasure T, Sturridge MF, Gruneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;February:311‐2. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Webster 2006
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004985.pub3] [DOI] [PubMed] [Google Scholar]
Webster 2007
- Webster J, Osborne S. Systematic review and meta‐analysis of the use of antiseptics for pre‐operative showering to prevent surgical site infection. British Journal of Surgery 2006;93:1335‐41. [DOI] [PubMed] [Google Scholar]


