Abstract
The widespread outbreak of the monkeypox virus (MPXV) recognized in 2022 poses new challenges for public healthcare systems worldwide. With more than 86,000 people infected, there is concern that MPXV may become endemic outside of its original geographical area leading to repeated human spillover infections or continue to be spread person-to-person. Fortunately, classical public health measures (e.g., isolation, contact tracing and quarantine) and vaccination have blunted the spread of the virus, but cases are continuing to be reported in 28 countries in March 2023. We describe here the vaccines and drugs available for the prevention and treatment of MPXV infections. However, although their efficacy against monkeypox (mpox) has been established in animal models, little is known about their efficacy in the current outbreak setting. The continuing opportunity for transmission raises concerns about the potential for evolution of the virus and for expansion beyond the current risk groups. The priorities for action are clear: 1) more data on the efficacy of vaccines and drugs in infected humans must be gathered; 2) global collaborations are necessary to ensure that government authorities work with the private sector in developed and low and middle income countries (LMICs) to provide the availability of treatments and vaccines, especially in historically endemic/enzootic areas; 3) diagnostic and surveillance capacity must be increased to identify areas and populations where the virus is present and may seed resurgence; 4) those at high risk of severe outcomes (e.g., immunocompromised, untreated HIV, pregnant women, and inflammatory skin conditions) must be informed of the risk of infection and be protected from community transmission of MPXV; 5) engagement with the hardest hit communities in a non-stigmatizing way is needed to increase the understanding and acceptance of public health measures; and 6) repositories of monkeypox clinical samples, including blood, fluids, tissues and lesion material must be established for researchers. This MPXV outbreak is a warning that pandemic preparedness plans need additional coordination and resources. We must prepare for continuing transmission, resurgence, and repeated spillovers of MPXV.
Keywords: Monkeypox, Poxvirus, Vaccine, Anti-virals, Orthopoxvirus vaccine, Smallpox vaccine, mpox
1. Introduction
Historically, Monkeypox (mpox) is a disease caused by a zoonotic poxvirus, likely transmitted from several small rodent reservoirs in Africa.[1] Spillover infections from rodents are not well understood, but human-to-human transmission involves contact and occasionally exposure to larger respiratory droplets[2], [3]. There are distinct virus lineages: the DRC/Central African lineage designated as clade I (with a higher reported Case-Fatality Rate [CFR]) and the West African lineage designated as clade II[4] (which was recently subdivided into IIa and IIb or proposed as clade 3[5]). Clade IIb is the origin from which the current outbreak was seeded. Most people infected with the currently circulating clade IIb monkeypox virus (MPXV) develop non-life-threatening symptoms within three weeks after infection, which may include some or all of the following: headache, fever, respiratory symptoms, swollen lymph nodes with a skin rash or lesions developing 1–4days after symptom onset[2], [3], [6], [7], [8], [9]. In immunocompetent hosts, the lesions typically heal within 4 weeks after disease onset[2], [3], [6], [7], [8], [9]. Serious complications may occur in certain populations, especially in children, pregnant women, older adults, and immunocompromised patients, and may include: non-healing skin lesions, pneumonia, encephalitis, sepsis, loss of vision after eye infection, and adverse pregnancy outcomes. Small studies document case fatality rates reported for monkeypox caused by MPXV clade I in the African region to be between 3 and 11%. Lower case fatality rates were observed during the current outbreak caused by clade II virus. Besides humans, many diverse animal species can become infected with the MPXV, via direct contact or fomites. Wild animal species that become infected with the MPXV can become new reservoirs in a previously unaffected geographic region. Indeed, cases of human transmission to pets have already been reported, but this is not universally accepted.
MPXV has been enzootic in the Democratic Republic of Congo (DRC), where the first, often severe human cases and outbreaks were described since 1970, and in West Africa, where there have been sporadic reports of human disease[10], [11], [12], [13], [14], [15], [16], [17], [18]. Over the years, the incidence of spillovers of virus from animals into humans seems to have increased, but information on the epidemiology and ecology of these spillovers in the African region is limited. Outside of Africa, the first significant outbreak occurred in 2003, resulting in>90 cases in the US[19], [20], [21]. The outbreak was eventually traced to rodents imported from Ghana, infected with Clade II virus. MPXV was transmitted in animal distribution facilities to local rodent species, including prairie dogs (genus Cynomys) sold as pets, and subsequently transmitted to humans [21], [22], [23]. Two children were hospitalized with serious illnesses, but there were no deaths. The outbreak was quickly contained, without sustained human-to-human transmission or establishment of the virus in free-living wild animal species.
Subsequent cases of infections reported outside of Africa were in individuals who traveled to Nigeria and were diagnosed upon their return home. None of these cases are known to have resulted in significant further spread of the virus[10], [11], [12], [13], [14], [15], [16], [17]. In 2022, a case of monkeypox was contracted by a traveler to Nigeria who was diagnosed on return to the United Kingdom (UK). Subsequent cases reported in the UK did not report travel to Africa or contact with the initial case, suggesting community transmission[2], [3], [7].
According to the World Health Organization, https://worldhealthorg.shinyapps.io/mpx_global/#23_Tables>86,000 monkeypox cases have been confirmed as of March 15, 2023; with > 30,000 cases in the US; > 10,000 in Brazil; 7,000 in Spain; and > 3,000 each in Germany, the UK, France, Columbia, Mexico, and Peru, with 110 countries reporting cases (including 99 non-endemic countries). There have been 112 deaths recorded as of March 28, 2023, and the infections are continuing globally with 28 countries reporting cases in the preceding 21 days. Deaths have been associated with encephalitis, uncontrolled infections in patients with advanced HIV, septic shock, and other comorbidities. It is possible that co-infection with various other viruses might also exacerbate the disease. Complete genome sequences from over 1,400 samples have been deposited in open access public databases (e.g., https://www.bv-brc.org/view/Taxonomy/10244#view_tab=overview) to support comparative genomics analysis.
The vast majority of cases reported globally have been observed in males (96.4%), and among cases with known sexual orientation, 85% of the cases have been identified in subgroups of men who have sex with men (MSM). The presence of lesions in genital, perianal, and oral areas is consistent with transmission via sexual contact. Monkeypox is not defined as a sexually transmitted disease because any close contact can cause transmission, not just sexual contact, although MPXV has been detected in semen [24]. Women account for 3.6% of cases, with exposure in the household setting accounting for 42% of cases and via sexual contact for 41% of cases. With continuing human-to-human spread during the current global outbreak, the possibility of widening of the circulation into the rest of the community continues to be a serious concern. However, by March 2023, the transmission of MPXV has decreased significantly to only ∼ 150 cases/week (down from the peak of > 6,000/week). While there is great concern that children would become infected more frequently, only ∼ 1% of cases have been reported in ages 0–17 (patient age was available in 82,000 cases): ∼0.2% of cases in US are age 15 or under. While there is potential for transmission in places with regular close contact between individuals, such as daycare centers, schools, universities, workplaces, places of worship, jails, only 1 school setting transmission was identified. This lack of wider community spread is reassuring considering the fact that the replication-competent vaccine strain, vaccinia virus (a close relative of MPXV), was found to spread and infect several patrons in a martial arts gym[25]. Since MPXV can cause lesions on the hands, it seems MPXV would be even more likely to spread under similar conditions. Evidence from the current outbreak indicates that MPXV is more transmissible in humans than vaccinia virus. Vaccinia virus can also cause multiple or widespread lesions and genital lesions[26], [27], [28], [29], [30], [31], [32], [33], but it has never caused such widespread infection and transmission in humans. The possible spread of MPXV into animal reservoirs remains a great concern because strong evidence suggests that vaccinia virus used as the smallpox vaccine in humans spread into cattle herds, forming endemic reservoirs. These constitute a serious risk to animal and public health, with multiple zoonotic human outbreaks documented[26]. If MPXV finds a foothold in a new animal reservoir, the likelihood of continued outbreaks of MPXV in humans will be greatly increased.
2. Diagnostics and surveillance
An essential pillar of outbreak preparedness is the ability to detect and track cases. It is essential to develop and distribute validated diagnostic assays worldwide to facilitate rapid identification of cases to interrupt chains of transmission. Reliable diagnostics are important because MPXV lesions may be confused with secondary syphilis, herpesvirus (chickenpox or simplex [28], [29]) infections, or molluscum contagiosum (a poxvirus that commonly infects children). Including monkeypox testing in clinics that see patients with sexually transmitted diseases should be considered. The development of rapid point of care tests that can be used by healthcare workers, similar to the home COVID antigen tests may seem straightforward. However, the judicious use of such assays requires in-depth knowledge of the infection kinetics in relation to test performance, not only for case ascertainment but for guiding isolation and quarantine measures.
Active surveillance is also critical to detect unrecognized cases because transmission can occur from people with mild or inapparent symptoms. Indeed, a US CDC serology study in fall of 2022 found strong evidence for 3 cases of previously undetected monkeypox among a sample of 74 persons (1.4%) experiencing homelessness[34]. Appropriate case tracking is important to notify household, professional, and community contacts of MPXV-infected individuals so that they can seek post-exposure prophylaxis. Many laboratories now have the ability to monitor for the presence of MPXV nucleic acids in sewer water, which is a sensitive means to detect the presence of other viruses (e.g., SARS-CoV-2 or poliovirus) in geographical areas, sometimes even before human infections were apparent[35], [36], [37], [38], [39], [40]. Indeed a Wastewater Scan dashboard is available in the United States for SARS-Co-V2, influenza, human metapneumovirus, RSV, norovirus, and monkeypox https://data.wastewaterscan.org/tracker/?charts=&selectedChartId=8cf27d. Some viruses can be detected in the air in building HVAC systems (e.g., SARS-CoV-2) [41]. For MPXV, detection on surfaces may be of added value since MPXV can cause virus-laden lesions on the hands and palms, and thereby contaminate surfaces. Infectious MPXV particles can persist on fomites for weeks[42], [43]. A child in the Netherlands was infected with MPXV with no human source contact identified, thus suggesting community surface fomite transmission[44].
In addition to increasing local surveillance, it is important to develop an internationally accepted single case definition of monkeypox. The WHO has provided interim guidance in December 2022 for suspected, probable, and confirmed monkeypox cases, https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4. Currently, a single PCR or sequencing positivity is considered sufficient for defining an infection, but such testing must be widely available, especially in low and middle income countries (LMICs), for effective for worldwide surveillance. Importantly, as long as cases of MPXV infection in humans occurs, the likelihood of reverse zoonosis to pets, livestock and eventually wild animals is increasing. Therefore, surveillance of individual animals or animal populations in close contact with affected patients should be performed to detect and possibly prevent establishment of animal reservoirs.
3. Vaccines
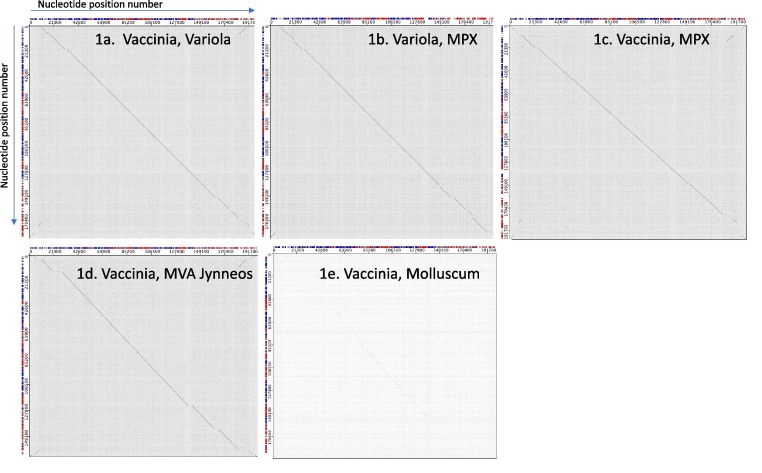
While vaccines were not developed for the prevention of monkeypox per se, the existing licensed smallpox vaccines have been deployed because all three viruses (variola virus [the causative agent of smallpox], MPXV, and the vaccinia virus used as a vaccine) are within the Orthopoxvirus genus[45], [46], and are closely related at both the genome and protein levels, leading to immune responses that may be expected to offer adequate cross-protection. The close genetic relationship between these viruses is demonstrated in bioinformatics DotPlots comparing their genomes (Fig. 1 ). The genomes were analyzed using the tools available at the Viral Bioinformatics Research Centre https://4virology.net/ [46], [47]. A dot appears on the graph at each location where a nucleotide is identical between the genomes, yielding a gray background. If there is substantial identity across an area of the genome, the dots form a visible diagonal line showing the similarity of the genome sequences in that area. Variola virus, MPXV, and vaccinia virus show nearly continuous diagonal lines, with variation and gaps occurring mostly at the ends of the genome (Fig. 1 a-c). This pattern occurs because Orthopoxviruses are relatively conserved in the central region of the genomes and vary more near the termini, which encode gene products more associated with host-range and virulence factors. However, a close genetic relationship between a vaccine virus and a pathogenic virus, does not necessarily mean that adequate cross-protection will always be induced by the vaccine virus. In the case of the immunogenicity of the Dryvax vaccinia virus, T cell responses in samples from recipients showed a high degree of MPXV cross-reactive T cells[48].
Fig. 1.
Dotplots comparing viral genomes at each nucleotide position. In each plot, the first virus name shown is plotted horizontally with the first nucleotide position on the left, and the second viral genome named is plotted vertically with the first nucleotide at the top. Genome sequences are vaccinia ACAM2000, Variola major India 3 1967, MPX monkeypox Cop58, Modified Vaccinia Ankara MVA-1721, and Molluscum contagiosum virus. Genome nucleotide identity is indicated by a diagonal line.
Another smallpox vaccine, the live but replication-deficient Modified Vaccinia Virus Ankara (MVA, in the vaccine Jynneos, Imvamune, Imvanex from Bavarian Nordic), is used in the US and Europe for post-exposure MPXV prophylaxis. In regions that have sufficient vaccine available, Jynneos is used for pre-exposure MPXV prophylaxis in high-risk individuals and communities. The MVA strain, has six major deletion sites with loss of about 20% of the genome[46], visible as gaps in the diagonal line of the dot-plot comparison (Fig. 1d). These deletions are attenuating because of reduced replication capacity in mammalian cells, conferring an excellent safety profile for MVA[49], [50], [51], but the vaccine is missing antigens that are present in both the pathogenic orthopoxviruses and the live-attenuated replicating vaccine ACAM2000 vaccinia strain (derived from the New York Board of Health (NYBOH) strain that was used in the smallpox eradication program in the US) (Fig. 1 a-d). Indeed, the MVA strain fails to induce some major T cell and antibody responses elicited by the replication competent parental vaccinia virus[52], [53], [54], [55]. Both MVA and ACAM2000 vaccines induce protective immunity against MPXV in animal models, but it is noteworthy that the MVA Jynneos vaccine was approved as a 2-dose regimen, while the live-attenuated, replicating ACAM2000 is effective after 1 administration. Fig. 1e demonstrates the genetic distance between vaccinia virus and the human poxvirus molluscum contagiosum virus (Genus Molluscipox), from which the vaccine does not protect. The limited sequence identity is clear. The vaccinia virus vaccine only protects from the orthopoxviruses and not molluscum contagiosum infection because, although both are poxviruses, they belong to different genera and are too distantly related to provide cross-protection.
A study published in 1988 found that subjects that were vaccinated during the smallpox eradication program with Dryvax, the calf-skin-grown vaccinia vaccine from which ACAM2000 was derived, were 85% protected from MPXV infection and disease severity in an endemic setting[56]. However, there is little data on the efficacy of the currently available vaccines in humans against MPXV infection during the present outbreak especially in the setting of transmission occurring largely through sexual contact in MSM. One trial in DRC showed immunogenicity of the Jynneos vaccine, but data on protective efficacy are not yet available[49]. In the current outbreak, the US CDC data on > 200,000 Jynneos MVA vaccinated individuals showed monkeypox incidence among unvaccinated individuals was 7.4 (95% CI = 6.0–9.1) times higher compared to those vaccinated with 1 dose, and 9.6 times (95% CI = 6.9–13.2) higher compared to those who had received 2 doses ≥ 14 days earlier[50]. While the Jynneos MVA vaccine is preferred because of its safety profile, the durability of the vaccine protection is unknown, and recent data show that MVA induces relatively low levels of MPXV neutralizing antibodies[51]. This emphasizes the necessity for continuing human clinical trials to assess its long term efficacy against MPXV infection.
After smallpox eradication, vaccination of the public with live replicating vaccinia (e.g., Dryvax, now ACAM2000) was discontinued due to significant rates of adverse events, including disseminated vaccinia, progressive vaccinia, eczema vaccinatum, and encephalitis[30], [31], [32], [33], [57]. It has been estimated that 25% of the US population would be at a greater risk of adverse event from the live replicating ACAM2000 due to immunosuppression, inflammatory skin conditions (e.g., eczema), or who are pregnant, or have heart abnormalities[31], [32], [57], and it has been predicted that 1 out of every 450 people vaccinated would have a serious adverse reaction resulting in hospitalization[33]. Thus, in the absence of a smallpox exposure event, the use of ACAM2000 is contraindicated for many, however, it is routinely administered to military personnel in the US and other countries because of continuing bioterrorism/biowarfare concerns. Under normal use, an ACAM2000 vaccination causes the occurrence of an oozing lesion in vaccinated patients, which can spread the virus infection to contacts [27], [28], [29], [30]. Because of these problems, ACAM2000 was not routinely used during the current global monkeypox outbreak. Development of a single-dose vaccine with efficacy similar to ACAM200 but with a safety profile like MVA is desirable.
Robust, longer term clinical trials are needed to document the efficacy of ACAM2000 or Jynneos against MPXV infection, as well as to evaluate the route and dose of the vaccine (subcutaneous vs intradermal) in real-world efficacy settings. Trials must include enough persons who may be exposed to the virus for sufficient power in a Phase III trial to demonstrate efficacy. In addition, the ethical selection of an appropriate negative control group is challenging. In preliminary unpublished data from US CDC, the control group for the retrospective study was composed of patients with an incident HIV diagnosis or HIV pre-exposure prophylaxis prescription[50].
It will also be desirable to evaluate the use of vaccines both pre- and post-exposure. Availability of the preferred, safer non-replicating MVA vaccine, produced by Bavarian Nordic has been limited. To allow vaccination of more people, intradermal injection of a lower dose has been widely used in the U.S, and preliminary data show that the subcutaneous and intradermal administration both provided at least short-term protection[50]. Furthermore, 'ring vaccination' strategies, in which exposed individuals and close contacts are vaccinated, are a practical and immediate approach to preventing MPXV transmission. Governments worldwide will have to promote the quick manufacturing and approval of doses sufficient to quell this outbreak and prepare for resurgence and additional spillover events. Deployment of vaccines must include African countries where MPXV is endemic. If widespread vaccination against MPXV is made available in Africa, it will not only provide protection there, but also significantly protect the rest of the world from future monkeypox outbreaks.
4. Therapeutics
Similar to vaccine development, therapeutics have also been developed to treat the closely related smallpox (variola) virus, which while eradicated, remains a major bioterrorism class A agent threat to humans. Specific therapeutic antiviral agents against MPXV have not been included in the approval process as this virus was until recently considered a pathogen of lesser importance for humans. However, antiviral drugs developed for smallpox may also be effective against the MPXV infection because of the close relationship and sequence similarity between the viruses (Fig. 1). In 2018, the US FDA approved TPOXX, also known as tecovirimat or ST-246, specifically to treat smallpox. The decision was based exclusively on animal data showing protection from both smallpox and monkeypox[58], [59], [60], [61]. TPOXX is currently being used in the U.S. under an Expanded Access IND investigational new drug (EA-IND) protocol for treatment of monkeypox[62]. This drug targets the maturation of variola virus and related poxviruses by blocking the viral F13 protein, also known as OPG057 or VP37 in vaccinia, a component of the viral outer envelope required for the spread of virus particles within an animal or human [63]. Since a single mutation can confer resistance to TPOXX there are concerns that with widespread use, the efficacy of this drug could be lost, especially with long-term treatment in immunocompromised individuals. Drug combinations should be considered to delay or prevent MPXV mutants from being selected during prolonged single drug treatment. >6,800 US monkeypox patients have received TPOXX (including 13 age 5 or under, 173 females, 4,869 males, and 113 transgender) https://www.cdc.gov/poxvirus/mpox/response/2022/demographics-TPOXX.html March 8, 2023). Anecdotal evidence suggests good efficacy in humans with monkeypox, but placebo controlled human clinical trials are ongoing in the United States (STOMP trial, https://actgnetwork.org/studies/a5418-study-of-tecovirimat-for-human-monkeypox-virus-stomp/), in the Democratic Republic of Congo (https://clinicaltrials.gov/ct2/show/record/NCT05559099), and in Brazil https://clinicaltrials.gov/ct2/show/NCT05597735?term=tecovirimat&cond=monkeypox&draw=2&rank=4.
Another drug approved for smallpox that can be used against MPXV is Brincidofovir, (Tembexa), a lipid-conjugated prodrug of cidofovir, an analog of 2′-deoxycytidine that inhibits the viral DNA polymerase[64], [65], [66], [67]. However, liver function needs to be monitored carefully in person receiving this drug, especially when co-administered with HIV drugs cobicistat and fostemsavir. Fortunately, Brincidofovir does not cause nephrotoxicity like cidofovir, its parent molecule[67], [68]. While this drug was not widely available during the height of the monkeypox outbreak, in the U.S. it is available under an EA-IND for monkeypox treatment[69]. Efficacy data for Brincidofovir are lacking, but it is currently used as off-label drug treatment in monkeypox patients [70]. Brincidofovir and cidofovir have a range of undesirable side effects that limit their use in the clinic. Finally, and most critically, the availability of these drugs is currently very limited for global use. The MPXV outbreak argues for immediate action by government agencies to coordinate with drug manufacturers to produce and make available adequate quantities of MPXV therapeutics and to develop agreements with pharmaceutical companies, including prices compatible with massive deployment during public health emergencies. The plans for drug availability must also make provisions for delivering sufficient quantities to Africa, to decrease infections and transmission in endemic areas. The next set of priorities will be, as for vaccines, to gather data on safety and efficacy in human trials. In addition, it will be necessary to assess which drugs can be used for monkeypox prophylaxis to limit the spread of MPXV in family and workplace contacts and especially in health care facilities. In the medium- to long-term, the development of new antiviral compounds continues to be a pressing, unmet need as drug resistance may develop. Here too, government agencies can do much to facilitate both basic and applied research toward new antiviral remedies. While monoclonal antibodies can be effective in treating viral diseases and are often evaluated as therapeutic agents in emergency situations, their use will not always be practical, especially in nations with less advanced health care systems. There is a report of the polyclonal human antibody vaccinia immune globulin (VIG-IV) having therapeutic value [71]. However, priority should be given to small molecule antivirals which could be widely used. Given the limited number of current infections and geographical distribution of cases, pharmaceutical companies are unlikely to fund the development of new drugs. Therefore, government agencies should support the discovery and development of new therapeutics.
5. Action Plan: What needs to be done
Surveillance. The rapid decline of monkeypox cases in many countries should not induce complacency. MPXV has shown its propensity for spillover into humans from animal reservoirs and sustained human-to-human spread. Resurgence and continuing spillover events are likely, so surveillance is key. Health care facilities must remain vigilant to detect monkeypox disease, especially when caring for elderly and immunocompromised patients, who together with young children, have the highest risk of severe complications of monkeypox. Underserved communities will have to be monitored closely: virus transmission may go undetected in populations that have little or no access to health care[34]. We must ensure that LMICs have the needed diagnostics and ability to perform surveillance. Wastewater screening for early detection of viral threats emerging in populations offers great potential for cost effective surveillance. In addition, we need to assess whether MPXV is becoming established in new animal reservoirs. MPXV can become enzootic or endemic in any area with a potential animal reservoir, creating a continuing risk of outbreaks through spillover infections. Veterinarians must be aware of clinical signs of MPXV infection characterized by muco-cutaneous pustules and ulcers in animal species. Continued genetic monitoring of the virus is needed, as orthopoxviruses evolve, and MPXV may mutate to become more adapted to humans[5] and change phenotypes such as transmissibility, host range, and virulence.
Vaccination/treatment. Vaccination has helped to blunt the spread of MPXV in many countries, but governments must work to increase production and availability of existing vaccines and continue to test these vaccines for efficacy in pre- and post-exposure prophylaxis, as well as monitor the durability of protection. Vaccination in endemic regions of DRC and West Africa should be provided to protect both the local and the global population from spillover. Development of a single dose vaccine with efficacy similar to ACAM200, but with a safety profile similar to that of MVA, is desirable. Continued evaluation of existing therapeutics for safety and efficacy in the treatment of MPXV infection and for prevention is necessary, while developing new candidates.
6. Conclusions
We strongly urge governments and agencies to take note that viral outbreaks and pandemics will keep occurring, as they have been occurring throughout the history of our species, often with devastating consequences. If anything, climate change, decreased separation between human and animal species, and intensive animal husbandry will increase the potential for emerging and re-emerging pathogens to cause outbreaks and pandemics. Therefore, pandemic preparedness should not be seen as a contingency, but as a necessary sustained effort worldwide. We should learn from the current outbreak.
The Global Virus Network (GVN), with its 70 centers of excellence, 11 affiliate centers, and hundreds of virologists worldwide, is ready to assist in international studies to meet this challenge toward ending the MPXV scourge.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rachel Roper reports financial support was provided by East Carolina University. Rachel Roper reports a relationship with East Carolina University that includes: employment and non-financial support. Rachel Roper has patent #Patent No 8202521 issued to East Carolina Univeristy. Roper serves as a reviewer for Vaccine, but not in an editorial role. (R Roper) Tartaglia, James is an employee of Sanofi and holds stock options.
Acknowledgments
We would like to thank Drs. Ming Fan at East Carolina University and Dara Wambach and her team at Johnson & Johnson for critically reviewing the manuscript.
Data availability
Data will be made available on request.
References
- 1.Falendysz E.A., Lopera J.G., Doty J.B., et al. Characterization of Monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl Trop Dis. 2017;11(8):e0005809. doi: 10.1371/journal.pntd.0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 3.Harris E. What to Know About Monkeypox. JAMA. 2022;327(23):2278–2279. doi: 10.1001/jama.2022.9499. [DOI] [PubMed] [Google Scholar]
- 4.Chen N., Li G., Liszewski M.K., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isidro J., Borges V., Pinto M., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28(8):1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarin-Vicente E.J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monkeypox symptoms https://www.cdc.gov/poxvirus/monkeypox/symptoms.html.
- 10.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease C, Prevention. Human monkeypox -- Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. MMWR Morb Mortal Wkly Rep 1997;46(49):1168-71. [PubMed]
- 12.Vaughan A., Aarons E., Astbury J., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durski K.N., McCollum A.M., Nakazawa Y., et al. Emergence of Monkeypox - West and Central Africa, 1970–2017. MMWRMorb Mortal Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan A., Aarons E., Astbury J., et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erez N., Achdout H., Milrot E., et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong S.E.F., Ng O.T., Ho Z.J.M., et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yinka-Ogunleye A., Aruna O., Dalhat M., et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 19.Anderson M.G., Frenkel L.D., Homann S., Guffey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr Infect Dis J. 2003;22(12):1093–1096. doi: 10.1097/01.inf.0000101821.61387.a5. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease C, Prevention. Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep 2003;52(23):537-40. [PubMed]
- 21.Centers for Disease C, Prevention. Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep 2003;52(27):642-6. [PubMed]
- 22.Guarner J., Johnson B.J., Paddock C.D., et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10(3):426–431. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed K.D., Melski J.W., Graham M.B., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 24.Lapa D., Carletti F., Mazzotta V., et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis. 2022;9:1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes C.M., Blythe D., Li Y., et al. Vaccinia virus infections in martial arts gym, Maryland, USA, 2008. Emerg Infect Dis. 2011;17(4):730–733. doi: 10.3201/eid1704.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matos A.C.D., Rehfeld I.S., Guedes M., Lobato Z.I.P. Bovine Vaccinia: Insights into the Disease in Cattle. Viruses. 2018;10(3):120–132. doi: 10.3390/v10030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzny C.A., King H., Byers P., et al. Vulvar vaccinia infection after sexual contact with a smallpox vaccinee. Am J Med Sci. 2009;337:289–291. doi: 10.1097/MAJ.0b013e3181821978. [DOI] [PubMed] [Google Scholar]
- 28.Egan C., Kelly C.D., Rush-Wilson K., Davis S.W., Samsonoff W.A., Pfeiffer H., et al. Laboratory-confirmed transmission of vaccinia virus infection through sexual contact with a military vaccinee. J Clin Microbiol. 2004 Nov;42(11):5409–5411. doi: 10.1128/JCM.42.11.5409-5411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease C, Prevention. Vaccinia virus infection after sexual contact with a military smallpox vaccinee -Washington, 2010. MMWR Morb Mortal Wkly Rep 2010;59(25):773-5. [PubMed]
- 30.Lederman E., Miramontes R., Openshaw J., et al. Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: an investigation of potential fomites in the home environment. Vaccine. 2009;27(3):375–377. doi: 10.1016/j.vaccine.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Eckart R.E., Love S.S., Atwood J.E., et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44(1):201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Kemper A.R., Davis M.M., Freed G.L. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5(2):84–90. [PubMed] [Google Scholar]
- 33.Casey C.G., Iskander J.K., Roper M.H., et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005;294(21):2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 34.Waddell CJ, Filardo TD, Prasad N et al. Possible Undetected Mpox Infection Among Persons Accessing Homeless Services and Staying in Encampments — San Francisco, California, October–November 2022. MMWR Morb Mortal Wkly Rep: 72(9):227-231. [DOI] [PMC free article] [PubMed]
- 35.Wannigama D.L., Amarasiri M., Hongsing P., et al. Multiple traces of monkeypox detected in non-sewered wastewater with sparse sampling from a densely populated metropolitan area in Asia. Sci Total Environ. 2023;858(Pt 1) doi: 10.1016/j.scitotenv.2022.159816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Rosa G., Mancini P., Veneri C., Ferraro G.B., Lucentini L., Iaconelli M., et al. Detection of Monkeypox Virus DNA in Airport Wastewater, Rome. Italy Emerg Infect Dis. 2023 Jan;29(1):193–196. doi: 10.3201/eid2901.221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wurtzer S., Levert M., Dhenain E., et al. First detection of Monkeypox virus genome in sewersheds in France: The Potential of Wastewater-Based Epidemiology for Monitoring Emerging Disease. Environ Sci Technol Lett. 2022;9(11):991–996. [Google Scholar]
- 38.de Jonge E.F., Peterse C.M., Koelewijn J.M., van der Drift A.R., van der Beek R.F.H.J., Nagelkerke E., et al. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci Total Environ. 2022 Dec;15(852) doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfe M.K., Yu A.T., Duong D., Rane M.S., Hughes B., Chan-Herur V., et al. Use of Wastewater for Mpox Outbreak Surveillance in California. N Engl J Med. 2023 Feb 9;388(6):570–572. doi: 10.1056/NEJMc2213882. [DOI] [PubMed] [Google Scholar]
- 40.Girón-Guzmán I., Díaz-Reolid A., Truchado P., Carcereny A., García-Pedemonte D., Hernáez B., et al. Spanish wastewater reveals the current spread of Monkeypox virus. Water Res. 2023;231 doi: 10.1016/j.watres.2023.119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousan S., Fan M., Outlaw K., Williams S., Roper R.L. SARS-CoV-2 Detection in air samples from inside heating, ventilation, and air conditioning (HVAC) systems- COVID surveillance in student dorms. Am J Infect Control. 2022;50(3):330–335. doi: 10.1016/j.ajic.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffer J.A., Collingwood A., Rider L.E., et al. High-Contact Object and Surface Contamination in a Household of Persons with Monkeypox Virus Infection - Utah, June 2022. MMWRMorb Mortal Wkly Rep. 2022;71(34):1092–1094. doi: 10.15585/mmwr.mm7134e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan CN, Whitehill F, Doty JB, et al. Environmental Persistence of Monkeypox Virus on Surfaces in Household of Person with Travel-Associated Infection, Dallas, Texas, USA, 2021. Emerg Infect Dis 2022;28(10). [DOI] [PMC free article] [PubMed]
- 44.Tutu van Furth AM, van der Kuip M, van Els AL, et al. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Euro Surveill 2022;27(29). [DOI] [PMC free article] [PubMed]
- 45.Moss B, Senkevich TG. Orthopoxvirus‡. In: Tidona C, Darai G, eds. The Springer Index of Viruses. New York, NY: Springer New York; 2011: 1485-94.
- 46.Upton C., Slack S., Hunter A.L., Ehlers A., Roper R.L. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J Virol. 2003;77(13):7590–7600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brodie R., Roper R.L., Upton C. JDotter: a Java interface to multiple dotplots generated by dotter. Bioinformatics. 2004;20(2):279–281. doi: 10.1093/bioinformatics/btg406. [DOI] [PubMed] [Google Scholar]
- 48.Grifoni A., Zhang Y., Tarke A., et al. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe. 2022;30(12):1662–1670. doi: 10.1016/j.chom.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Priyamvada L., Carson W.C., Ortega E., et al. Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine. 2022;40(50):7321–732748. doi: 10.1016/j.vaccine.2022.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne AB, Ray LC, Cole MM, et al. Reduced Risk for Mpox After Receipt of 1 or 2 Doses of JYNNEOS Vaccine Compared with Risk Among Unvaccinated Persons - 43 U.S. Jurisdictions, July 31-October 1, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(49):1560-1564. [DOI] [PMC free article] [PubMed]
- 51.Zaeck LM, Lamers MM, Verstrepen BE, et al. Low levels of monkeypox virus neutralizing antibodies after MVA-BN vaccination in healthy individuals. medRxiv 2022: 2022.08.31.22279414. [DOI] [PMC free article] [PubMed]
- 52.Tscharke D.C., Karupiah G., Zhou J., et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201(1):95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jing L., Chong T.M., McClurkan C.L., Huang J., Story B.T., Koelle D.M. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J Immunol. 2005;175(11):7550–7559. doi: 10.4049/jimmunol.175.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies D.H., Liang X., Hernandez J.E., et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102(3):547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones-Trower A., Garcia A., Meseda C.A., et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343(1):128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 57.Upfal M.J., Cinti S. Smallpox vaccination and adverse cardiac events. Emerg Infect Dis. 2004;10(5):961–962. doi: 10.3201/eid1005.030967. discussion 2. [DOI] [PubMed] [Google Scholar]
- 58.Mucker E.M., Goff A.J., Shamblin J.D., et al. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (Smallpox) Antimicrob Agents Chemother. 2013;57(12):6246–6253. doi: 10.1128/AAC.00977-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laudisoit A., Tepage F., Colebunders R. Oral Tecovirimat for the Treatment of Smallpox. N Engl J Med. 2018;379(21):2084–2085. doi: 10.1056/NEJMc1811044. [DOI] [PubMed] [Google Scholar]
- 60.Huggins J., Goff A., Hensley L., et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53(6):2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jordan R., Goff A., Frimm A., et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53(5):1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Information for Healthcare Providers on Obtaining and Using TPOXX (Tecovirimat) for Treatment of Monkeypox https://www.cdc.gov/poxvirus/monkeypox/clinicians/obtaining-tecovirimat.html.
- 63.Roper R.L., Moss B. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J Virol. 1999;73(2):1108–1117. doi: 10.1128/jvi.73.2.1108-1117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quenelle D.C., Collins D.J., Wan W.B., Beadle J.R., Hostetler K.Y., Kern E.R. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48(2):404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quenelle D.C., Prichard M.N., Keith K.A., et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51(11):4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker S., Touchette E., Oberle C., et al. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77(1):39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Painter W., Robertson A., Trost L.C., Godkin S., Lampert B., Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56(5):2726–2734. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tippin T.K., Morrison M.E., Brundage T.M., Mommeja-Marin H. Brincidofovir Is Not a Substrate for the Human Organic Anion Transporter 1: A Mechanistic Explanation for the Lack of Nephrotoxicity Observed in Clinical Studies. Ther Drug Monit. 2016;38(6):777–786. doi: 10.1097/FTD.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treatment Information for Healthcare Professionals https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#anchor_1655488353796.
- 70.Brincidofovir (also known as CMX001 or Tembexa) https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#anchor_1655488353796.
- 71.Thet AK, Kelly PJ, Kasule, SN. The use of vaccinia immune globulin in the treatment of severe mpox virus infection in HIV/AIDS Clin Infect Dis. 2022 Dec 26;ciac971. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.