Abstract
Metformin is a frequently used anti-diabetic drug. In addition to the well-known modulating properties on glyco-metabolic control, metformin reduces cardiovascular (CV) risk partly independently of its anti-hyperglycaemic effect. The use of ‘new’ anti-diabetic drugs, inhibitors of the renal Na-glucose co-transporter (SGLTs-I or ‘gliflozines’) and GLP-1 receptor agonists (GLP1-RAs), has further contributed to challenge the strictly ‘gluco-centric’ view of diabetic CV disease. Several controlled trials have demonstrated that the cardio-renal benefits of gliflozines and GLP1-RAs are present regardless of the presence of metformin as ‘background’ therapy. The impact on the ‘cardio-renal continuum’ exerted by SGLTs-I was also noted in non-diabetic patients with heart failure and reduced or preserved ventricular function and different levels of renal function. These drugs reduced re-hospitalization, CV mortality, and progression to end-stage renal disease. These clinical acquisitions, implemented by Scientific Societies, have led to a change in the therapeutic approach to diabetic cardio-renal disease. Although metformin still represents a valid therapeutic option to be offered particularly to ‘naïve’ diabetic patients without previous cardio-renal events, SGLTs-I and/or GLP1-RAs emerge as ‘first-line’ drugs in diabetic patients with previous CV events, or at high CV risk, without having to request ‘on board’ metformin therapy.
Keywords: Diabetes mellitus, Metformin, Gliflozines
Introduction
Metformin (dimethylbiguanide) is the only member of the biguanide family on the market today. Biguanides are molecules extracted from an herb rich in guanidine, Galega officinalis (‘goat’s rue’ or French lilac), used in the past as a treatment for diabetes mellitus, thanks to the anti-hyperglycaemic properties demonstrated by guanidine. In spite of the other biguanides (e.g. phenformin and buformin), withdrawn from the market following the high incidence of cases of lactic acidosis, metformin is today the most widely used drug in the world in the treatment of Type 2 diabetes mellitus (T2DM).1
How does metformin work?
Metformin activates the enzyme AMPK (AMP-activated protein kinase) in the hepatocytes and striated muscle cells. The enzyme AMPK is normally activated in the presence of energy deficiency, a deficiency expressed by an increase in the concentration of AMP in relation to ATP (increased AMP/ATP ratio). Basically, AMPK is activated when it senses that the liver and muscles need ATP. By activating AMP, the enzyme AMPK generates a ‘cascade’ increase in ATP, which, in turn, increases glucose uptake by the skeletal muscle and inhibits hepatic glucose production (reducing gluconeogenesis and hepatic glycogenolysis), without, however, leading to hypoglycaemia. These two insulin-sensitizing effects largely contribute to the positive modulation of glyco-metabolic control.2,3 Experimental studies, however, demonstrated that metformin exerts some ‘pleiotropic’ effects. In particular, metformin increases the plasma levels and gene expression of the two main intestinal hormones, GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic peptide), also known as ‘incretins’ (i.e. intestinal-derived hormones capable of modulating the endogenous secretion of insulin), as well as improving the ‘sensitivity’ to the ‘incretin’ effect, thus making itself a candidate as a GLP1 ‘enhancer’ and ‘sensitizer’ drug. This activity further justifies the glucodynamic effect of the molecule when used as an ‘add-on’ to incretin drugs.4
Does metformin have clinical trials based on major cardiovascular events?
Despite its wide use, metformin has been scarcely investigated in outcome-based controlled clinical trials. More than 25 years ago, in the UKPDS study, conducted in 753 patients with no prior cardiovascular (CV) events, with newly diagnosed T2DM, and followed up for ∼10 years, metformin significantly reduced the risk of myocardial infarction (−39%), coronary death (−50%), and cerebral stroke (−41%).5 These results were partly maintained (reduction of heart attack and mortality) over the following 10 years of observation, in a context no more than initial treatment, but ‘free’ with various anti-diabetic drugs (‘legacy’ effect).6
Other observational studies have confirmed the potential beneficial effect of metformin on major CV events.7,8 Essentially on the basis of the results of the UKPDS study (both in terms of the initial randomized study and in terms of a 10-year ‘free’ follow-up), the joint guidelines of the American Diabetes Association/European Association for the study of Diabetes (ADA/EASD) recommend metformin as a drug of first choice in the presence and absence of high risk and previous CV events.9,10
The ESC (European Society of Cardiology)/EASD guidelines had recommended metformin in primary prevention in patients with T2DM who are overweight and in the absence of previous major CV events (Class IIa recommendation and Level of Evidence C).1 In high-risk patients, i.e. with a history of previous CV events, the ESC/EASD guidelines placed both metformin and the ‘new’ antidiabetic drugs (gliflozines and GLP1-RAs) as first-line drugs, as discussed below.1
Subsequent ESC guidelines on Cardiovascular Prevention, published in 2021, ‘raised’ the strength of the recommendation for metformin in the ‘majority of patients without major cardiovascular events, renal insufficiency or heart failure’ to I B, adding a IIa B recommendation to the use of metformin in patients with previous CV events.11
The Joint Guidelines of the Italian Society of Diabetology and the Association of Diabetologists (SID/AMD) suggest, in their recommendation 5.1, ‘the use of metformin as a drug of first choice for long-term treatment in patients with type 2 diabetes without previous cardiovascular events’. SGLT-2I and GLP-1 RAs are recommended as second-line drugs. Conversely, metformin, GLP1-RAs, and SGLT-2I are recommended as drugs of first choice in patients with previous CV events but without heart failure, while gliflozines are recommended as drugs of first choice, while GLP1-RAs and metformin as second-choice drugs, in patients with Type 2 diabetes and heart failure (https://snlg.iss.it/wp-content/uploads/2021/07/LG_379_diabete_2.pdf).
Metformin and renal failure
Since metformin is extensively eliminated by the kidney, its use in patients with chronic renal insufficiency could trigger some undesirable effects, including lactic acidosis. However, this effect has been scaled down by the Food and Drug Administration, which removed in year 2016 the ban on metformin in patients with an estimated glomerular filtration rate (GFR) between 30 and 59 mL/min (Stages 3A and 3B). The currently recommended12 metformin dose is the following:
Estimated GFR 45–59 cc/min (Stage 3A): 1500 mg (500 mg in the morning and 1000 mg in the evening).
Estimated GFR 30–44 cc/min (Stage 3B): 1000 mg (500 mg in the morning and 500 mg in the evening).
Estimated GFR 15–29 cc/min (Stage 4): 500 mg/day.12
Metformin should not be discontinued in case of interventional cardiac procedures, except in patients with renal insufficiency, and renal function should be monitored in the hours following the procedure, with transient discontinuation if renal function deteriorates.11
We will later discuss the use of metformin in relation to new anti-diabetic drugs, essentially gliflozines, in patients with high-risk Type 2 diabetes or with previous CV events.
The gliflozins
The class of SGLTs-I or gliflozins includes different molecules (e.g. empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin) that act by increasing urinary glucose excretion by inhibiting its reabsorption in the renal tubule. The normal kidney filters ∼180 L of plasma and 180 g of glucose each day; 90% of the filtered glucose is reabsorbed at the level of the first segment of the proximal tubule by a high capacity and low-affinity receptor system (Type 2 receptors or SGLT-2). The remaining 10% is reabsorbed at a more distal level of the proximal tubule by a with low capacity and high affinity (Type 1 receptors or SGLT-1). On the luminal side of the tubular cells, at the level of the SGLT-2 receptors, glucose is reabsorbed with an active mechanism against the concentration gradient, together with sodium, using the energy produced by an Na/K/ATP-ase system. Once inside the tubular cell, glucose is expelled to the blood via the GLUT-2 co-transporter along a concentration gradient. SGLT-1 receptors are also expressed in the intestine (intestinal villi) and in the heart, while SGLT-2 receptors, mainly localized in the kidney, are also partially present in pancreatic beta cells.
From a biochemical point of view, gliflozines are derivatives of phlorizine, a substance extracted from the root of the apple tree that cannot be used from a therapeutic point of view in humans as it is associated with important gastrointestinal side effects (diarrhoea and abdominal pain).
Gliflozins exert their glucodynamic effect independently of insulin by reducing the tubular reabsorption threshold of glucose with a consequent increase in its elimination in the urine (about 60–80 g/day). The glycosuric effect of these molecules is therefore a direct function of the GFR. The increase in glycosuria induces a loss of energy with a negative caloric balance, an aspect that justifies the weight loss of 2–3 kg observed during treatment. Studies that have evaluated body composition have shown that about two-thirds of weight loss is secondary to the loss of abdominal fat mass, an aspect of no small clinical relevance for CV prevention. In addition to the metabolic effect, gliflozines exert a unique diuretic effect since the diuresis induced by SGLTs-I is ‘glucose-driven’ i.e. mainly induced by an osmotic effect due to the reduction of sodium and glucose renal reabsorption. Consequently, there is a very limited reduction of circulating plasma volume (unlike ‘traditional’ diuretics), accompanied by a loss of the interstitial fluid (an effect that accounts for the protective haemodynamic action in heart failure). It is therefore appropriate to attribute to these molecules a unique ‘metabolic-diuretic’ effect that cannot be replicated by other antidiabetic or CV drugs available so far.
At the myocardial level, gliflozines improve the use of ketone bodies, a sort of ‘super-fuel’ for the myocardium in relation to the ability to induce ATP production more efficiently than other substrates such as glucose and free fatty acids. The myocardium is a heavy consumer of ketone bodies, whose consumption, however, decreases in the presence of insulin, which blocks lipolysis and therefore reduces its formation. The effect of SGLT-2/1-I on lipolysis induces a mild hyperketonaemia, which could increase the energy efficiency of the myocardium particularly in the presence of ischaemia. However, the increase in ketogenesis, if not suitably contrasted by the insulin effect (state of absolute insulinopenia) could lead to a picture of diabetic ketoacidosis, with potential negative consequences on the haemodynamic state.
Among the undesirable effects of SGLTs-I, the following should be considered: (i) genitourinary infections (candidiasis) due to the glycosuric effect. Females and elderly patients appear to be particularly susceptible. To prevent genitourinary infections it is important to take care of personal hygiene and increase the water intake; (ii) although an increased risk of minor foot amputations was reported in the CANVAS study (0.6%/year vs. 0.3%/year with placebo), this finding has not been confirmed in other studies either with the same molecule (canagliflozin study CREDENCE) than with other molecules of the same class.
The inspection of the feet is, however, suggested in every patient treated with SGLT2-I even if recent real-world data and on subpopulations of the various CardioVascular Outcomes Trials (CVOTs) demonstrate the ‘protective’ effect (lower risk of major adverse limb events) of the SGLT2-I in patients with T2DM and ischaemic diabetic foot syndrome; (iii) pathological fractures, perhaps linked to the risk of falls (from hypovolaemia and hypotension), to an increase in parathyroid hormone and to the reabsorption of phosphates in the kidney. There was also an excess risk for this side effect in the canagliflozin study, not confirmed in other studies; (iv) normoglycaemic diabetic ketoacidosis, favoured by infections, low caloric intake, surgical interventions, and linked to hyper-glucagonemia, which increases the production of ketone bodies. It is important to measure plasma and urinary ketone bodies at the beginning of treatment and in case of symptoms (nausea, vomiting, dyspnoea, and malaise), regardless of blood sugar levels; (v) acute renal failure, rare and probably favoured by hypovolaemia and concomitant nephrotoxic agents (contrast media, NSAIDs). The hidden fear that these drugs could represent potential ‘nephrotoxic’ effects has been radically subverted by the results of recent dedicated clinical trials (CREDENCE, DAPA-CKD, EMPA-Kidney), where in patients with clinically overt nephropathy, both with and without history of T2DM, SGLTs-I have been shown to prevent major renal events (including end-stage renal disease); (vi) Fournier’s gangrene. Very rare but severe necrotizing infection of the perineum, external genitalia, and peri-anal region. Fifty-five cases were identified (source FDA) between 2013 and 2019.
The main clinical trials (CVOTs)
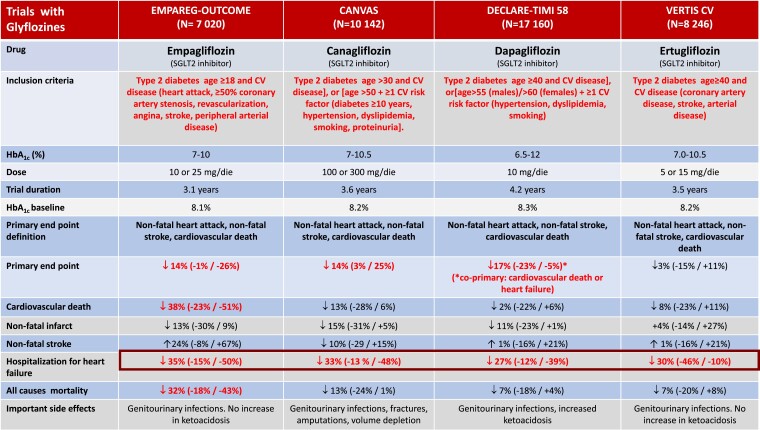
As seen in Figure 1, major CVOTs performed with gliflozines showed a significant reduction in the risk of the pre-specified primary endpoint (three criteria MACE: death from CV causes, non-fatal myocardial infarction, non-fatal stroke). In the empagliflozin study, the reduction in the primary endpoint was driven by a 38% reduction in CV mortality, but not by a reduction in myocardial infarction or stroke. Of note, the large reduction of hospitalizations for heart failure (−35%) noted in this study was comparable to that observed in studies with canagliflozin (−33%), dapagliflozin (−27%), and ertugliflozin (−30%).
Figure 1.
The main controlled clinical trials with gliflozines in patients with Type 2 diabetes mellitus.
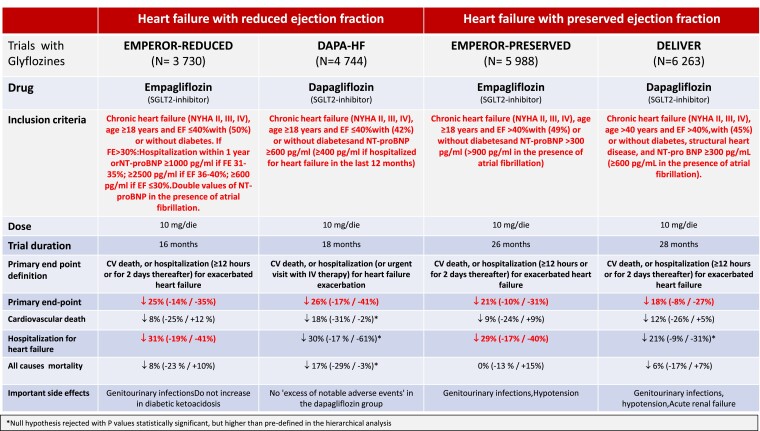
Following these impressive results, particularly in terms of reduced hospitalization for heart failure, gliflozines were specifically studied in patients with heart failure with reduced or preserved ventricular function, with or without diabetes (Figure 2). Although this is not the place to analyse the results of these important CVOTs in detail, the literature now appears unequivocal in indicating SGLTs-I as drugs of choice in patients with heart failure.13
Figure 2.
Main controlled clinical trials with gliflozines (empagliflozin and dapagliflozin) in patients with chronic heart failure with reduced or preserved ejection fraction, either diabetic or non-diabetic.
Position of the guidelines and practical implications
In summary, as indicated in Table 1, the ADA/EASD guidelines continue to recommend metformin as first-choice drug in patients with T2DM, either newly diagnosed or long-lasting, at high or low risk, with or without previous major CV events.10,14
Table 1.
Position of the guidelines of the main Scientific Societies on the use of metformin and the ‘new’ antidiabetic drugs (gliflozines and incretins) in patients with Type 2 diabetes mellitus
| European Society of Cardiology (ESC)11 |
|
|
|
|
|
|
|
| American Diabetes Association/European Association for the study of Diabetes (ADA/EASD)10,14 |
|
|
|
|
| Italian Society of Diabetology and Association of Diabetologists (SID/AMD) |
|
|
|
If heart failure or chronic kidney disease prevail in the individual patient, the ADA/EASD guidelines recommend to associate gliflozines, with GLP1-RAs as an alternative only if gliflozines are contraindicated or not well tolerated.10 In contrast, if the high CV risk prevails in the individual patient (which in ADA/EASD is defined exactly by at least one of the following criteria: age >55 years, left ventricular hypertrophy or carotid, coronary, or peripheral arterial stenosis >50%) or there are previous CV events (in the absence of heart failure or nephropathy), the ADA/EASD guidelines recommend to associate a weekly administration of GLP1-RAs, considering the use of gliflozines only if such molecules are contraindicated or not well tolerated.10
The position of the European Guidelines on Cardiovascular Prevention11 differs from the North American one in some respects. In patients with Type 2 diabetes without prior major CV events, renal insufficiency or heart failure, metformin is recommended as the drug of first choice ‘in the majority of patients’, with strength of recommendation I B. In this kind of patients, GLP1-RAs and gliflozines can still be considered, albeit with less strength of recommendation (IIa B), in patients predictably at greater CV risk due to the coexistence of other risk factors or organ damage. Unlike the North American Guidelines, the European ones do not exactly define the criteria for defining high risk.
According to the European Guidelines,11 metformin still remains a drug to be taken into consideration if well tolerated and not contraindicated in patients with T2DM and previous major CV events (IIa B). However, in this type of patient, GLP1-RAs and gliflozines that have demonstrated a benefit on prognosis are recommended with a greater strength of recommendation (I A). In patients with T2DM and signs of organ damage, GLP1-RAs and SGLT2-I that have demonstrated a prognostic benefit remain recommended with the strength of recommendation IIb B. Thus, SGLTs-I and GLP1-RAs are recommended more ‘strongly’ in patients with previous CV events than in patients with no prior events and organ damage ‘only’.
Gliflozines remain strongly recommended (I A) in patients with T2DM and chronic renal failure, as well as in heart failure patients with impaired left ventricular function.
The position of the Italian Society of Diabetology and the Association of Diabetologists appears more in line with the North American one: Metformin is the drug of first choice in patients with Type 2 diabetes without previous CV events, gliflozines and incretins are second choice drugs. Furthermore, metformin, SGLT2-I, and/or GLP1-RAs are drugs of first choice in patients with previous CV events and without heart failure. In the presence of heart failure, gliflozines are first choice drugs, and GLP1-RAs and metformin are choice drugs (https://snlg.iss.it/wp-content/uploads/2021/07/LG_379_diabete_2.pdf).
The information acquired from the different CVOTs leads us to hypothesize that the effect of SGLTs-I and GLP1-RAs on CV risk could be independent of the concomitant use of metformin which, in such a clinical scenario, would lose its role as a potential ‘effect modifier’ of CV benefit. Obviously, in the absence of randomized trials comparing metformin with SGLTs-I or GLP1-RAs, evidence is not conclusive. Recent subgroup analyses demonstrate that, for both SGLT2-I and GLP1-RAs, there are no significant differences in terms of MACE reduction, mortality due to CV causes, hospitalization for heart failure or reduction in renal outcomes among treated patients on metformin and those not using metformin.14 Although data derived from subgroup analyses should be interpreted with caution, the uniformity of results obtained from different randomized controlled trials appears to be robust, also from a statistical point of view. The conclusions, also approved by recent ADA guidelines, are that in subjects with T2DM and diagnosis of heart failure, chronic kidney disease, established CV disease, or in the presence of multiple CV risk factors, the decision of using an SGLT2-I or a GLP1-RA with proven clinical benefit should be fully independent of the concomitant use of metformin.
Finally, although the favourable effect of SGLT2-I on heart failure outcomes is poorly correlated to the anti-hyperglycaemic action, adequate glyco-metabolic control remains a goal for CV prevention in patients with T2DM. In other words, the ‘modern’ cardiologist and diabetologist are today forced to integrate their mutual knowledge in order to apply a ‘holistic’ and multifactorial approach in the clinical management of the cardio-metabolic continuum.
Conclusions
As underlined by Sattar et al.15 in their Editorial, the ADA/EASD recommendations differ from those issued by ESC with regard to some important points:
Metformin is recommended as first-line drug treatment by the ADA/EASD but not by the ESC.
The ADA/EASD guidelines precisely establish the criteria for defining a condition of high CV risk in primary prevention (age >55 years, left ventricular hypertrophy or carotid, coronary, or peripheral arterial stenosis >50%).
The ADA/EASD guidelines formalize the concept that a condition of greater risk for major CV events makes the choice of incretins prevalent, while a condition of greater risk for heart failure makes the choice of gliflozines prevalent.
Sattar et al.15 concludes that cardiologists, diabetologists, and nephrologists should seek a more ‘unified’ approach in order to reduce the negative perception of poor agreement among ‘experts’ in interpreting clinical evidence.
In summary, we might answer the question posed in the title of this paper by saying: ‘Yes, it is reasonable to use less metformin and more (if not almost always) gliflozines’. The use of metformin should not be considered a ‘must’ if the patient is already taking a gliflozine, particularly during conditions such as heart failure.
Contributor Information
Paolo Verdecchia, Umbra Heart and Hypertension Foundation-ONLUS and Department of Cardiology, S. Maria della Misericordia Hospital, Perugia, Italy.
Giuseppe Murdolo, Department of Internal Medicine, Endocrine and Metabolic Sciences (MISEM), S. Maria della Misericordia Hospital, Perugia Hospital, Perugia, Italy.
Stefano Coiro, Department of Cardiology, S. Maria della Misericordia Hospital, Perugia, Italy.
Andrea Santucci, Department of Cardiology, S. Maria della Misericordia Hospital, Perugia, Italy.
Francesco Notaristefano, Department of Cardiology, S. Maria della Misericordia Hospital, Perugia, Italy.
Fabio Angeli, Department of Medicine and Surgery, University of Insubria, Varese, Italy; Department of Cardiopulmonary Medicine and Rehabilitation, Maugeri IRCCS Clinical Scientific Institutes, Tradate, Italy.
Claudio Cavallini, Department of Cardiology, S. Maria della Misericordia Hospital, Perugia, Italy.
Funding
This article is supported in part by the Fondazione Umbra Cuore e Ipertensione-ONLUS, Perugia, Italy.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Cosentino F, Grant PJ, Aboyans Vet al. ; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 2. Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabet Med 2010;27:1097–1106. [DOI] [PubMed] [Google Scholar]
- 3. Zhou G, Myers R, Li Yet al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho YM, Kieffer TJ. New aspects of an old drug: metformin as a glucagon-like peptide 1 (GLP-1) enhancer and sensitiser. Diabetologia 2011;54:219–222. [DOI] [PubMed] [Google Scholar]
- 5. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–865. [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Maruthur NM, Tseng E, Hutfless Set al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–751. [DOI] [PubMed] [Google Scholar]
- 8. Scheen AJ, Paquot N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab 2013;39:179–190. [DOI] [PubMed] [Google Scholar]
- 9. Arnett DK, Blumenthal RS, Albert MAet al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buse JB, Wexler DJ, Tsapas Aet al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020;63:221–228. [DOI] [PubMed] [Google Scholar]
- 11. Visseren FLJ, Mach F, Smulders YMet al. ; ESC National Cardiac Societies; ESC Scientific Document Group . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 12. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care 2018;41:547–553. [DOI] [PubMed] [Google Scholar]
- 13. Rao S. Use of sodium-glucose cotransporter-2 inhibitors in clinical practice for heart failure prevention and treatment: beyond type 2 diabetes. A narrative review. Adv Ther 2022;39:845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies MJ, Aroda VR, Collins BSet al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45:2753–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sattar N, McMurray JJ, Cheng AY. Cardiorenal risk reduction guidance in diabetes: can we reach consensus? Lancet Diabetes Endocrinol 2020;8:357–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.