Abstract
Sudden cardiac death is a leading cause of mortality, claiming millions of lives every year. Recent advances in cardiac arrhythmia mapping have demonstrated that the ventricular epicardial region has a critical arrhythmogenic role in some inherited cardiogenetic diseases. Historically, electroanatomic abnormalities have been identified in the ventricular epicardium of patients with arrhythmogenic right ventricular cardiomyopathy. More recently, epicardial pathological substrates have been identified also in electrical disease (Brugada syndrome, early repolarization syndrome) and currently in long QT syndrome. In light of these findings, the epicardial area has emerged as an important determinant in sudden cardiac death-related cardiomyopathies.
Keywords: Percutaneous cardiac ablation, Long QT syndrome, Arrhythmogenic left ventricular cardiomyopathy
Introduction
Despite advancements in prevention and treatment, sudden cardiac death (SCD) remains a leading cause of mortality, claiming millions of lives every year. While most cases of SCD occur in older patients as a consequence of ischaemic heart disease, many apparently healthy individuals suffer episodes of ventricular fibrillation (VF) ending in SCD. Hereditary primary electrical disorders, historically known as cardiac channelopathies, account for a large proportion of SCD in otherwise healthy individuals. Among these, Brugada syndrome (BrS), long QT syndrome (LQTS), and early repolarization syndrome (ERS) more commonly predispose to SCD in the absence of apparently structural heart disease (SHD). Currently, echocardiography, computed tomography, and magnetic resonance imaging are used to assess morphological and functional abnormalities in patients suspected of SHD. Because no structural anomalies are detected by these conventional imaging techniques, BrS, LQTS, and ERS have been historically considered primary electric cardiac diseases. In contrast to structural inherited cardiomyopathies [such as arrhythmogenic right ventricular cardiomyopathy (ARVC), hypertrophic cardiomyopathy, or dilated cardiomyopathy], which are mainly caused by mutations in structural genes, these electrical disorders are secondary to mutations in genes encoding cardiac ion channels and/or proteins modifying their function. Indeed, the alteration in the cardiac action potential has been considered the driving functional dynamic substrate leading to electrical instability at cellular, regional, and global levels (favouring local and/or diffuse electrical inhomogeneity), predisposing to life-threatening ventricular arrhythmias. However, recent advances in invasive and non-invasive electroanatomic imaging, mapping, and ablation techniques have consistently shown that a reproducible anatomic substrate can be identified in a large proportion of symptomatic individuals with hereditary cardiac diseases and otherwise healthy heart. Moreover, there is overwhelming evidence that symptomatic patients with inherited diseases without obvious structural abnormalities have an epicardial, more than endocardial, arrhythmogenic substrate.
The epicardial arrhythmogenic substrate in cardiogenetic diseases
Since its introduction more than 20 years ago, percutaneous catheter-based epicardial mapping and ablation have become widely adopted by cardiac electrophysiologists around the world.1 Recent developments in mapping and ablation techniques have shown that the epicardial region of the heart is a key player in the occurrence of ventricular arrhythmic events in several cardiac diseases.2 The epicardial area extends between the epicardium, the outer mesothelial layer of the heart, and the myocardium (subepicardial myocardial layer). It contains connective tissue, coronary vessels, mesenchymal cells, inflammatory cells, fibroblasts, nerves, and the epicardial adipose tissue (EAT). The EAT is tightly associated with the subepicardial myocardium, exchanging with its paracrine factors and modulating its metabolism. The adipose tissue can also secrete cytokines and adipokines that can modulate the cardiac electrical properties. The subepicardial myocardium has its distinct electrophysiological properties: it has a shorter action potential and a much higher density of the fast component of the voltage-dependent outward current (Ito), compared to the subendocardial myocardium. Due to the heterogeneous and complex arrangement of the muscle fibres, the subepicardial myocardium (especially that of the right ventricle) is more prone to conduction delay and block. Moreover, the right ventricle outflow tract (RVOT) has a reduced conduction reserve, due to the low expression levels of connexins and cardiac sodium channel protein-α subunit (Scn5a), and consequently an increased susceptibility for arrhythmia compared to the rest of the myocardium.3
The ARVC is the archetype of the diseases with an anatomical epicardial substrate, as the myocardium is progressively replaced by fibro-fatty infiltrates derived from the EAT (starting from the subepicardium and then progressively extending to the sub-endocardium). Indeed, since the early stages of disease, there is a predominance of low-voltage areas, fractionated electrograms, and late potentials in the epicardial layer of the RV (and less commonly of left ventricle). By infiltrating subepicardial myocardial layers, adipocytes and fibrosis disrupt myocyte–myocyte coupling leading to areas of scar and block of electric conduction, thus prompting the formation of re-entry circuits, the substrate for the ventricular tachycardias in these patients.4
Beyond the ARVC, recent studies have shown the high incidence of epicardial microstructural cardiomyopathic areas in symptomatic patients (sustained ventricular arrhythmias or aborted cardiac arrest) with inherited cardiac diseases. These subclinical alterations, which may act as the substrate of VF, can be identified using high-density invasive endo-/epicardial mapping. It was clearly demonstrated that symptomatic BrS patients have a well-defined anatomic and electrophysiological substrate characterized by abnormal fragmented prolonged low-frequency ventricular electrograms (Figure 1). Combined extensive left and right endo-/epicardial mapping localized the substrate exclusively on the epicardial anterior RVOT and RV anterior free wall [reflecting the position-related appearance of the echocardiogram (ECG) pattern], and ajmaline administration was able to delineate its extension and distribution as a suitable target for successful ablation.5,6 Indeed, the complete elimination of the substrate by radiofrequency ablation (RFA), as confirmed by post-ablation remapping and provocation testing, resulted in disappearance of typical BrS ECG pattern and no inducibility of ventricular arrhythmias in the patients. Nowadays, there is unequivocal evidence indicating the presence of a structural substrate in the epicardial RV of BrS patients based on the presence of conduction slowing (by invasive epicardial mapping) and interstitial fibrosis by pathological examinations (mainly localized at the RVOT).7 These ultrastructural alterations, combined with a pathological and genetically codified susceptibility to external factors that influence the electric propagation (such as the sodium channel blockers), delineate a region of delayed activation and conduction blocks.8 The collocation of myocardial late potentials and fibrosis in the RVOT, the exquisitely selective and highly reproducible localization of the pathological substrate of the disease in the epicardial RV, and the effects of RFA on outcomes make the BrS a cardiomyopathy with a definite ultrastructural arrhythmogenic substrate amenable of ablation.
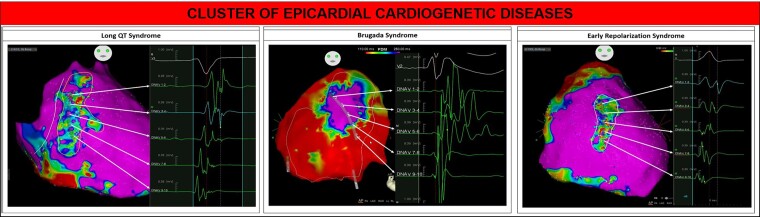
Figure 1.
Example of abnormal epicardial ventricular electrograms typically discovered in patients with inherited cardiogenetic diseases [Brugada syndrome (BrS), long-QT syndrome (LQTS), and early repolarization syndrome (ERS)], treated in our institution. Left panel shows an example of epicardial mapping in a LQTS patient. The abnormal signals present a low amplitude (<1.5 mV, bipolar voltage) and are characterized by multiple fragmented and double components. The multiple components could be the expression of local conduction delay and lines of block. The distribution of abnormal signals extended from the epicardial RVOT to the infero-lateral peritricuspid region including the anterior wall. Middle panel shows an example of epicardial mapping in a BrS patient. The potential duration map identifies an area exhibiting delayed and prolonged signals. These electrical abnormalities show a concentric ‘onion-like’ distribution in the RVOT and anterior epicardial wall. Traditionally, the core region displays the most delayed electrical activity (most prolonged signal in DNAV 5–6), with a less prolongation degree at the periphery (less prolonged activity in DNAV 3–4, 1–2, and 7–8; normal signal in DNAV 9–10). Right panel shows an example of epicardial mapping in an ERS patient. The anterior wall of the epicardial RV exhibits low-amplitude (<1 mV) and fragmented abnormal electrograms. These regions may harbour microstructural abnormalities and fibrosis ultimately causing the low-voltage and the electrogram fractionation.
Compared to BrS, the pathophysiological mechanisms underlying the ERS is less definite.9 The inferolateral ER is a subtle ECG phenotype that may associate with SCD in patients with no apparent SHD. Its occurrence at the QRST junction on the surface ECG can be the expression of a local delayed activation abnormality in the myocardium of the right and/or left ventricle. Indeed, myocardial ultrastructural discontinuities may lead to disturbances in electrical propagation, which in turn give rise to J-waves, less or more pronounced on different ECG leads according to the location of the pathological substrate.10 These ultrastructural alterations cause unidirectional conduction blocks and re-entrant circuits, thus prompting an anatomical substrate for initiating and maintaining VF. In our experience, the extensive endo-/epicardial mapping of patients with ERS symptomatic for recurrent episodes of VF showed consistently the presence of localized areas of slow-conducting myocardium characterized by multi-components or low-voltage and fragmented electrograms, located exclusively in the epicardial layers of the RV and/or LV (Figure 1). RFA of these substrates made all patients free of ventricular arrhythmic events at follow-up and reduced or abolished the J-waves at the surface ECG (data not published). Identifying these anatomic arrhythmogenic substrates by high-density electroanatomic mapping could significantly improve the risk stratification and prognosis of symptomatic patients, by offering them the epicardial RFA as a therapeutic approach.11
Recently, Pappone et al. provided new insights into the presence of electrophysiological structural abnormalities also in patients with LQTS. In this study,12 11 patients with LQTS symptomatic for spontaneous malignant arrhythmias underwent an extensive endo-/epicardial mapping procedure of right and left ventricles. All subjects showed clear evidence of local electroanatomic abnormalities, characterized by delayed low-amplitude signals with multiple fragmented components, localized exclusively in the epicardial RV, extending from the epicardial RVOT to the infero-lateral peritricuspid region including the anterior wall (Figure 1). Looking at the reproducible substrate localization, it might correspond to the anatomical distribution of sympathetic nervous system in the RV subepicardium; as a result, imbalanced innervation density and chronically increased adrenergic tone can lead to functional and structural remodelling and thus be responsible for the ultrastructural abnormalities. Indeed, the pathological electrograms reflect microstructural myocardial heterogeneities favouring re-entry and probably acting as the substrate of VF. As RFA of the trigger in LQTS patients often is not feasible as the premature ventricular contractions triggering the TdP are rarely mappable, RFA of the anatomic substrate can be an innovative therapeutic approach. In this series, RFA abolished recurrence of VF in all cases. Surprisingly, the authors also reported a stable QT shortening after elimination of the pathological substrate, possibly due to the distal denervation that impairs repolarization of the entire heart. These results suggest that localized epicardial structural abnormalities may underlie a significant subset of high-risk LQTS patients, and these regions could serve as a target for ablation treatment.
Conclusions
Recent advances in cardiac arrhythmia mapping have demonstrated that the epicardial region has a critical arrhythmogenic role. Indeed, the identification of electroanatomic abnormalities has established the ventricular epicardium as an area of interest in several inherited cardiac diseases. In light of these findings, the epicardial area has emerged as an important determinant in SCD-related cardiomyopathies, evolving from a neglected region to a virtual fifth chamber of the heart (Figure 1). There is overwhelming evidence that patients with inherited diseases without obvious structural abnormalities (BrS, ERS, LQTS, and ARVC) have an epicardial arrhythmogenic substrate. These manifestations differ mainly in the localization and distribution of epicardial abnormalities. Moreover, the broad clinical spectrum of these disorders may also overlap, suggesting a possible pathophysiological link in a specific subgroup of patients yet to be identified.
Contributor Information
Carlo Pappone, Cardiology Unit, Vita-Salute San Raffaele University, Milan, Italy; Arrhythmia and Electrophysiology Center, IRCCS Policlinico San Donato, Milan, Italy.
Antonio Boccellino, Cardiology Unit, Vita-Salute San Raffaele University, Milan, Italy; Arrhythmia and Electrophysiology Center, IRCCS Policlinico San Donato, Milan, Italy.
Giuseppe Ciconte, Cardiology Unit, Vita-Salute San Raffaele University, Milan, Italy; Arrhythmia and Electrophysiology Center, IRCCS Policlinico San Donato, Milan, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Aryana A, Tung R, d'Avila A. Percutaneous epicardial approach to catheter ablation of cardiac arrhythmias. JACC Clin Electrophysiol 2020;6:1–20. [DOI] [PubMed] [Google Scholar]
- 2. Chaumont C, Suffee N, Gandjbakhch E, Balse E, Anselme F, Hatem SN. Epicardial origin of cardiac arrhythmias: clinical evidences and pathophysiology. Cardiovasc Res 2022;118:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boukens BJ, Sylva M, de Gier-de Vries Cet al. . Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res 2013;113:137–141. [DOI] [PubMed] [Google Scholar]
- 4. Cheng WH, Chung FP, Lin YJet al. . Arrhythmogenic right ventricular cardiomyopathy: diverse substrate characteristics and ablation outcome. Rev Cardiovasc Med 2021;22:1295–1309. [DOI] [PubMed] [Google Scholar]
- 5. Brugada J, Pappone C, Berruezo Aet al. . Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol 2015;8:1373–1381. [DOI] [PubMed] [Google Scholar]
- 6. Pappone C, Brugada J, Vicedomini Get al. . Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053. [DOI] [PubMed] [Google Scholar]
- 7. Nademanee K, Raju H, de Noronha SVet al. . Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol 2015;66:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haissaguerre M, Nademanee K, Sacher Fet al. . Multisite conduction block in the epicardial substrate of Brugada syndrome. Heart Rhythm 2022;19:417–426. [DOI] [PubMed] [Google Scholar]
- 9. Haissaguerre M, Nademanee K, Hocini Met al. . Depolarization versus repolarization abnormality underlying inferolateral J-wave syndromes: new concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm 2019;16:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boukens BJ, Potse M, Coronel R. Fibrosis and conduction abnormalities as basis for overlap of Brugada syndrome and early repolarization syndrome. Int J Mol Sci 2021;22:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bisson J, Scheinman M, Hadjis A. Epicardial substrate ablation in early repolarization syndrome patient with recurrent ventricular fibrillation. HeartRhythm Case Rep 2021;7:731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pappone C, Ciconte G, Anastasia Let al. . Right ventricular epicardial arrhythmogenic substrate in long-QT syndrome patients at risk of sudden death. Europace 2023;25:948-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.