Abstract
The mechanisms underlying sudden cardiac death (SCD) in patients with ischaemic heart disease (IHD) caused by coronary atherosclerosis are not yet clarified. For decades, acute coronary causes have been sought as the main triggers of SCD in these patients. In fact, angiographic and pathological studies in cardiac arrest survivors and SCD victims, respectively, consistently show that acute plaque events occur in ∼50% of SCD of patients with IHD. Among the acute events, plaque rupture and erosion triggering acute coronary thrombosis remain the main substrates; however, a significant percentage of plaque haemorrhage (20%) is identified by pathological studies. Its role in acute coronary thrombosis is unknown and deserves future intravascular imaging developments. In the remaining 50% of SCD, the atherosclerotic coronary disease shows the characteristics of structural stability. More recent studies have focused attention not only on the coronary tree and on the search for acute complications of atherosclerotic plaques but also on myocardial tissue, identifying replacement and patchy fibrosis as the most frequent findings in the post-mortem hearts of these patients, a feature followed by cardiac hypertrophy, as assessed by the heart weight, usually associated with fibrosis. The possibility of characterizing myocardial fibrosis in vivo, besides confirming the pathological data, now offers new risk stratification perspectives to prevent SCD in IHD, alongside the consolidated secondary prevention criteria based on left ventricular dysfunction.
Keywords: Sudden cardiac death (SCD), Ischaemic heart disease (IHD), Atherosclerotic plaques, Myocardial fibrosis
Introduction
The risk of sudden cardiac death (SCD) has a central role in secondary prevention pathways in patients with ischaemic heart disease (IHD). The causes of cardiac arrest in this clinical context are still a matter of debate: fatal arrhythmias are generally attributed to the acute destabilization and thrombotic occlusions of an atherosclerotic plaque. However, recent studies have questioned this pathophysiological aspect, placing the emphasis on other causes of ventricular arrhythmias underlying cardiac arrest. The latter can be identified in the heart muscle and not only in the coronary bed.
Pathology studies in sudden cardiac death identify acute coronary lesions in ∼50% of cases
In 1997, Burke et al.1 described the pathology findings of 113 male subjects with coronary artery disease (CAD) who died suddenly. The authors identified acute coronary thrombosis in 59 subjects (52%), and severe coronary narrowing in the absence of thrombosis, indicating stable plaque, in 48% of cases. Furthermore, only, 36% of cases with acute thrombosis had an underlying ruptured vulnerable plaque, while in 16% of cases, the lesion underlying the thrombosis was a plaque erosion. Pathology features of acute myocardial infarction (AMI) were observed in 19% of cases with acute thrombosis, 11% of cases with erosion, and 4% of cases with stable plaques. In the multivariate analysis, cigarette smoking emerged as the only risk factor associated with acute thrombosis.
The study by Burke et al.1 mainly aimed at identifying atherosclerotic plaques as a possible cause of AMI; however, it also highlighted the common association between SCD and ‘stable’ plaques with non-vulnerable morphology. Two years later, the same group investigated the role of effort in the destabilization of atherosclerotic plaque in subjects who died suddenly.2 Plaque rupture was the most common mechanism associated with coronary thrombosis in the presence of intense effort (68%), while rupture was less common in victims of SCD at rest (23%) (P < 0.001).2 Acute myocardial infarction was never diagnosed during exercise and only in 13% of subjects with SCD in resting conditions.
For decades, pathological and imaging studies that explored the causes of SCD in CAD have focused on atherosclerotic plaques and strove to identify ‘acute plaque lesions’ matching the acute clinical event. All past studies emphasized the role of plaque vulnerability as a contributor to the risk of SCD. Whereas it was proved that culprit plaques with acute thrombosis were often ulcerated, had a thin fibrous cap, were lipid-rich, and showed inflammation, it was also proved that in 50% of cases, coronary thrombosis was absent and there was no acute coronary lesion underlying the fatal arrhythmic event. These findings should not be overlooked: in 50% of cases of SCD in IHD, only stable atherosclerotic plaques were present. In these cases, the mechanism of the fatal arrhythmia could not be attributed to an acute occlusive coronary thrombosis; an alternative mechanism had to be considered.
In vivo studies in cardiac arrest survivors exclude an acute coronary causes in ∼50% of cases
More recently, in vivo studies performed in cardiac arrest survivors have expanded the pathophysiological aspects of SCD in IHD. The COACT study3 randomized 552 patients to investigate the results of an aggressive strategy based on an immediate coronary angiography procedure vs. a wait-and-see solution based on a deferred coronary angiography. Immediate coronary angiography had no clinical benefit. The coronary angiography results agreed with the pathological observations of Burke et al.1,2 Coronary angiography, performed in 97% of cases, showed thrombotic coronary occlusion in only 3.4% of subjects in the group with immediate coronary angiography vs. 7.6% of patients in the group with deferred coronary angiography. Overall, coronary thrombosis occurred in a very low percentage of cases (8.1%). In line with this observation, angiographically unstable coronary lesions were found in 15% of cases, while severe CAD was common and observed in 66% of cases. Therefore, most subjects showed angiographically severe but stable atherosclerotic disease.
These ‘negative’ clinical results excluded the benefit deriving from an immediate coronary angiography with possible revascularization and were explained by the low rate of coronary thrombosis. In fact, the reason why primary angioplasty is effective in AMI is the recanalization of the occluded coronary artery, i.e. the removal of the occluding intracoronary thrombus.
The conclusions of the COACT3 were confirmed by the TOMAHAWK study.4 This randomized study, with a design similar to the COACT,3 compared an aggressive strategy with immediate angiography with a more conservative solution in cardiac arrest survivors. A culprit lesion potentially responsible for the arrhythmic event was only observed in 40% of cases.
Beyond coronary arteries: the myocardial fibrosis and hypertrophy
Pathological and angiographic studies concordantly show that AMI caused by the destabilization of atherosclerotic plaques is not the unique pathophysiological mechanism underlying SCD in subjects with IHD. Therefore, the open question is what the other causes are. The recent study by Holmström et al.5 helps to clarify these aspects. The study was performed on 600 subjects who died of SCD in the presence of CAD and included histological analysis of both coronary arteries and myocardial tissue. Heart weight was higher than normal values in 78% of subjects, and fibrosis was present in 93% of cases. In 58% of cases, the fibrosis was substitutive and attributable to prior healed AMI. In 56% of cases, however, there was an AMI, a percentage higher than those previously reported in subjects with SCD and CAD.1–4
The latter finding agrees with the rate of thrombosis and/or plaque ulceration observed in autopsy studies. In fact, the study of the coronary arteries showed signs of plaque rupture or erosion in 24% of cases, plaque haemorrhage in 24%, and stable plaque in the remaining 52.3% of cases. In confirmation of previous studies, ‘plaque destabilization’ was present in less than half of the cases. Finally, plaque rupture and erosion, which commonly trigger acute coronary thrombosis, were more frequent in subjects with sudden death that occurred during exercise. The pathological study of the myocardium has explored alternative mechanisms to plaque destabilization as a trigger for fatal arrhythmias: myocardial fibrosis was present in over 93% of cases. Fibrosis was of a substantial degree in 13% of cases and of a moderate degree (patchy) in 68% of cases.
In addition to the fibrosis, a relevant finding was the presence of hypertrophy (weight above normal values) in 78% of cases. Only 2.7% of the victims had neither fibrosis nor ventricular hypertrophy. In the subgroup of patients with stable atherosclerotic plaques, i.e. in the absence of plaque rupture, erosion, or haemorrhage, fibrosis was found in 92% of cases and was substantial in 11.8% and patchy in 66.2%. Hypertrophy was rarely an isolated finding and was observed in association with fibrosis in almost all cases.
Myocardial imaging confirms the role of fibrosis in sudden cardiac death
Studies performed with cardiac magnetic resonance (CMR) imaging (MRI) have correlated the extent of fibrosis with ventricular arrhythmias or the risk of SCD. Zegard et al.6 performed CMR in 979 patients with IHD. The presence of fibrosis on MRI was significantly associated with the risk of SCD [hazard ratio (HR): 10.1; confidence interval (CI): 1.42–1278.9] and the composite arrhythmic endpoint of SCD, resuscitated cardiac arrest, sustained ventricular tachycardia, ventricular fibrillation, or appropriate defibrillator shock (HR: 28.0; 95% CI: 4.07–3,52). The presence of significant myocardial fibrosis, defined as fibrosis on visual assessment and greyzone fibrosis (GZF) > 5 g on CMR, was associated with the risk of SD and the arrhythmic endpoint (respectively sHR: 10.8; 95%, CI: 3.74–30.9 and sHR: 7.40; 95% CI: 4.29–12.8). Furthermore, the burden of fibrosis was more closely associated with SCD than was observed for reduced ejection fraction. The data, obtained in a population including patients with preserved left ventricular systolic function, confirm other studies carried out in patients with reduced ejection fraction that correlated the extent of fibrosis on CMR expressed as GZF with the risk of SCD.7–9
A dual and different mechanism may trigger sudden cardiac death in ischaemic heart disease
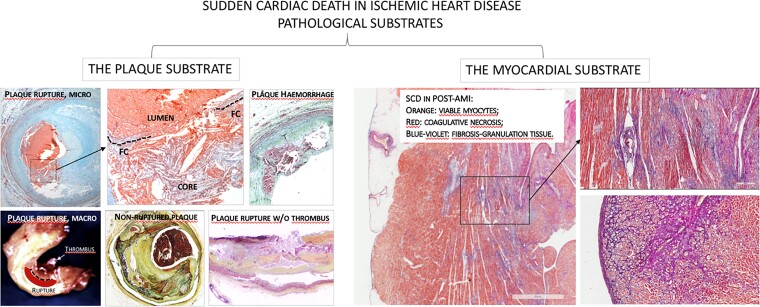
Overall, pathological, angiographic, and CMR studies of SCD in IHD consistently dichotomize the causes into coronary plaque complications and myocardial scarring, in roughly similar proportions (Figure 1). Investigation of plaque vulnerability in secondary prevention remains a key clinical issue. There is no doubt that recurrences of MI and cardiac death increase significantly in subjects with ‘vulnerable’ plaques with thin fibrous caps, large lipid pools, and inflammation. This mechanism, however, accounts for no more than 50% of SCD in IHD. The remaining 50% is likely attributable to myocardial scars. Each mechanism requires targeted prevention and treatment strategies. In the former, the mechanism is likely the acute thrombotic occlusion complicating a vulnerable plaque. In the latter, the most likely mechanism is ventricular arrhythmias due to re-entrant circuits with arrhythmogenic potential in border zones (electrically active) between the transmural infarct scar (electrically silent) and spared myocardium. Similarly, non-transmural ischaemia may also trigger ventricular arrhythmias whose pathological substrate is that of border scar areas. Preferential sites of arrhythmogenesis in post-AMI are the conducting channels in the border zones of scars, where fibrosis is mixed with still viable myocytes and adipocytes.10 Cardiac magnetic resonance is the key diagnostic tool for myocardial fibrosis. Finally, hypertrophy can play a role; however, the hypertrophy as an isolated finding does not seem to be essential or sufficient to trigger fatal arrhythmias in IHD; in the series by Holmström et al.,5 isolated hypertrophy was present in a low percentage (2% of cases) of cases of SCD and stable atherosclerosis.
Figure 1.
Dual etiopathogenesis of sudden cardiac death in ischaemic heart disease: acute plaque pathology (left panels) vs. myocardial scarring in post-acute myocardial infarction (right panels).
Questions more than answers
Prior imaging and pathology evidence and the recent study by Holmström et al.5 call for new considerations and questions in SCD of patients with IHD:
-
Is the burden, localization, and structural 3D conformation of fibrosis synergistic (or alternative) with contractile dysfunction in decision-making for secondary prevention in patients with IHD?
The guidelines still suggest ICD when the ejection fraction falls below 35%, regardless of the presence or absence of IHD. Prospective clinical trials and large registries will better clarify the role of fibrosis as detected by CMR in arrhythmogenic risk stratification.
What is the role of acute ischaemia as a cause of SCD in patients with coronary atherosclerosis? It can be hypothesized that acute myocardial ischaemia due to abrupt interruption of coronary flow acts as a trigger for arrhythmic events. This hypothesis partially contrasts with the most recent data that seem to exclude a benefit from coronary angioplasty in cardiac arrest survivors.11–13 Even the study of coronary flow [fractional flow reserve (FFR) or instantaneous wave-free ratio (IFR)] excluded that the functional evaluation reduces major cardiac events such as cardiac death or myocardial infarction.11–13 It is possible that cardiac arrest from fatal arrhythmia in this clinical setting is a rare event, difficult to prevent by removing significant narrowing.
In a consistent percentage of patients (20%) in the Holmström et al. series, SCD was associated with plaque haemorrhage. This is a ‘black hole’ also with intracoronary imaging, which is increasingly important in the in vivo intravascular imaging of coronary atherosclerosis. The role of plaque haemorrhage in plaque rupture and thrombosis remains to be clarified. New intracoronary imaging techniques are needed because even studies carried out with Optical Coherence Tomography are not sufficient to clarify whether this structural aspect can be reliably detectable in vivo.14
Acknowledgements
Studies on coronary atherosclerotic plaques, risk factors and ischemic heart disease are supported by CLI Foundation (F.P. and F.B.) and by grants from the Ministry of Health to the National IRCCS Cardiology Network (RCR-2022-23682288) and from FRRB grant CP2_14/2018, INTESTRAT-CAD, Lombardia Region, Italy (E.A.).
Contributor Information
Francesco Prati, CLI Foundation Onlus, Cli Foundation, Rome, Italy; UniCamillus—Saint Camillus International University of Health Sciences, Rome, Italy.
Giovanni Gurguglione, Dipartimento di Medicina Clinica e Sperimentale, Università di Foggia, Foggia, Italy.
Flavio Biccire, CLI Foundation Onlus, Cli Foundation, Rome, Italy; UniCamillus—Saint Camillus International University of Health Sciences, Rome, Italy.
Luigi Cipolloni, Dipartimento di Medicina Clinica e Sperimentale, Università di Foggia, Foggia, Italy.
Michela Ferrari, Centre for Inherited Cardiovascular Diseases, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Alessandro Di Toro, Centre for Inherited Cardiovascular Diseases, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Eloisa Arbustini, Centre for Inherited Cardiovascular Diseases, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 2. Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 1999;281:921–926. [DOI] [PubMed] [Google Scholar]
- 3. Lemkes JS, Janssens GN, van der Hoeven NWet al. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med 2019;380:1397–1407. [DOI] [PubMed] [Google Scholar]
- 4. Desch S, Freund A, Graf Tet al. Immediate unselected coronary angiography versus delayed triage in survivors of out-of-hospital cardiac arrest without ST-segment elevation: design and rationale of the TOMAHAWK trial. Am Heart J 2019;209:20–28. [DOI] [PubMed] [Google Scholar]
- 5. Holmström L, Juntunen S, Vähätalo Jet al. Plaque histology and myocardial disease in sudden coronary death: the Fingesture study. Eur Heart J 2022;43:4923–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zegard A, Okafor O, de Bono Jet al. Myocardial 1 fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 2021;77:29–41. [DOI] [PubMed] [Google Scholar]
- 7. Acosta J, Fernandez-Armenta J, Borras Ret al. Scar characterization to predict life threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. J Am Coll Cardiol Img 2018;11:561–572. [DOI] [PubMed] [Google Scholar]
- 8. de Haan S, Meijers TA, Knaapen P, Beek AM, van Rossum AC, Allaart CP. Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart 2011;97:1951–1956. [DOI] [PubMed] [Google Scholar]
- 9. Zeidan-Shwiri T, Yang Y, Lashevsky Iet al. Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm 2015;12:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arbustini E, Kramer CM, Narula J. Arrhythmogenic potential of border zone after myocardial infarction: scar is more than just a healed wound…. JACC Cardiovasc Imaging 2018;11:573–576. [DOI] [PubMed] [Google Scholar]
- 11. Prati F, Arbustini E, Alfonso F. Skating on thin ice: searching for vulnerable plaques. Eurointerv 2022;18:705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prati F, Biccirè FG, Budassi S. Present and future of coronary risk assessment. Eur Heart J 2021;23:E123–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puymirat E, Cayla E, Tabassome S; for the flower-MI trial . Multivessel PCI guided by FFR or angiography for myocardial infarction. N Engl J Med 2021;385:297–308. [DOI] [PubMed] [Google Scholar]
- 14. Prati F, Regar E, Mintz GSet al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.