Abstract
The 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure (HF) have abandoned the sequential approach for optimal drug therapy and propose four drug classes (enzyme inhibitors conversion agents, angiotensin receptor antagonists, beta-blockers, and sodium–glucose cotransporter inhibitors 2) to be initiated and titrated in all patients with an ejection fraction <35%. This new approach offers advantages such as rapid introduction and titration, better tolerability, and early instrumental re-evaluation. In the VICTORIA study, the molecule vericiguat, a soluble guanylate cyclase activator, was shown to reduce the composite outcome of death from cardiovascular causes and first hospitalization for HF in a high-risk population. An additional randomized clinical trial (VICTOR) is ongoing to evaluate the efficacy and safety of vericiguat in a population with HF on optimized therapy and with no recent episodes of stabilization.
Keywords: Heart failure, Sodium–glucose cotransporter inhibitors, Ejection fraction <35%
The guidelines (GL) for the diagnosis and treatment of acute and chronic heart failure (HF) of the European Society of Cardiology (ESC)1 of 2021 differ significantly from the previous GL of 2012 and 2016 because they abandon the sequential approach to the optimal pharmacological therapy (OMT) initiation for patients with HF.2,3 The initiation of therapy and subsequent titration to target dose of angiotensin-converting enzyme inhibitors (ACE inhibitors, ACE-i) or angiotensin receptor blockers and beta-blockers previously preceded the introduction of aldosterone antagonists (MRAs), and the replacement of ACE-i with angiotensin receptor neprilysin inhibitors was reserved for the portion of patients who were still symptomatic and with persistent left ventricular dysfunction, despite full titration of the first three drug classes.
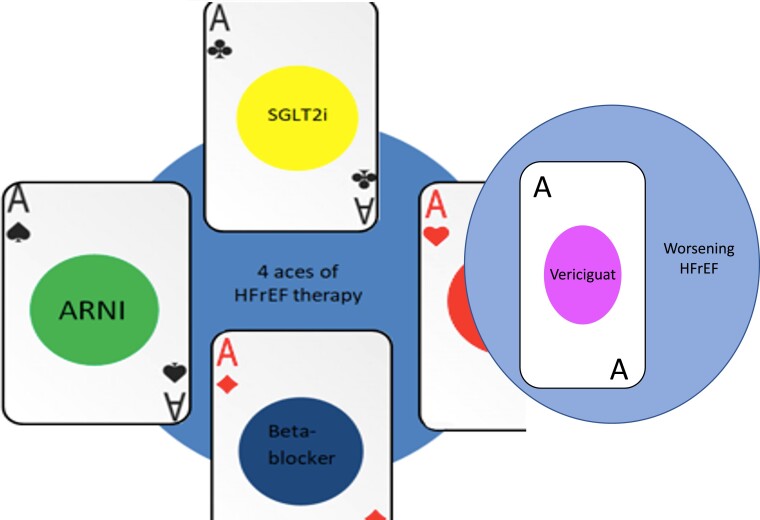
This sequence, based on the historical series of randomized clinical trials rather than on solid scientific evidence, has been challenged and overcome by the 2021 GL. The flowchart in which titration to target dose was followed by clinical and instrumental reassessment and modification of therapy makes way for a more sober statement of Class I A evidence. Four drug classes, consisting of the four fundamental pillars of HF, are therefore proposed to be initiated and titrated simultaneously in all patients with ejection fraction (EF) <35%. No longer a hierarchy between molecules, or an obligate sequence of steps, but an additional drug class (sodium–glucose cotransporter 2 inhibitors—SGLT-2 inhibitors), and ample space for the initiative of clinicians and researchers, in order to shape and optimize a new treatment paradigm for this complex syndrome.
The potential advantages of the new approach seem to be:
fast introduction of each drug class;
fast titration to target dose;
better tolerability of individual drug classes (more evident side effects at higher doses);
early instrumental reassessment (echocardiographic or cardiac magnetic resonance) of the contractile response of the heart muscle (reverse remodelling); and
better timing in the timing of defibrillator placement in patients with persistently reduced EF despite OMT.
What impact can the speed of titration of therapy have, in terms of hard outcomes? This is a question that has rarely been addressed previously, but the recently published STRONG-HF (Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure) randomized, multicentre, open-label clinical trial attempted to answer it. A population of 1087 patients admitted for acute HF enrolled in 87 hospitals in 14 different countries, in home therapy not titrated to target doses, underwent ‘standard treatment’ vs. ‘intensive treatment’. In the active treatment arm, therapy was titrated to the target dose (100% of the recommended dose) within 2 weeks of discharge. The results of this study show that the primary endpoint of all-cause death and HF hospitalization at 180 days was markedly lower in the high-intensity arm than in the standard treatment (15.2 vs. 23.3%, P = 0.021).
In view of the significantly higher number of adverse events at 90 days in the ‘high-intensity’ arm, the study still leaves the debate open regarding the optimal sequential drug timing, and paves the way for further investigations. After this study, the maladaptive neuro-humoral response of the HF falls to all effects among the ‘time dependent’ conditions, in which the precociousness of the pharmacological intervention modifies the outcome in the vulnerable phase of the disease.
Baseline risk, residual risk, worsening heart failure
Recent advances in polytherapy in HF have led to a significant reduction in mortality and rehospitalizations of HF patients, but the risk of adverse events of an increasingly complex pharmacological therapy is not negligible. It is important to consider the risk of mortality and hospitalization not as a static phenomenon, but as a variable that is constantly evolving over the course of the disease’s clinical history. To simplify and facilitate better communication between clinicians and researchers, it is helpful to divide the disease into distinct stages, characterized by different relative risks of progression and death.
In this regard, it is important to distinguish residual risk from the risk of a patient with HF in the ‘worsening’ phase. The first is the risk of a patient in a relatively stable clinical phase, in which therapy has been titrated to the maximum tolerated doses. Patients in the treatment arms of the DAPA-HF4 (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) and EMPEROR-REDUCED5 (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction) trials represent the best-described cohorts for this risk.
For ‘worsening’ HF, we mean any clinical instability (hospitalization for HF, access to emergency room for HF, intensification of diuretic therapy at home) in a known HF patient, not at the first episode of instability. An important rule, especially in order to create homogeneous groups of patients in randomized clinical trials or observational studies, is to count in this category only patients who present symptomatic worsening during the course of optimized background therapy.
On the basis of the risks and the stages of the disease, it can be stated that the vast majority of randomized clinical trials on which the ‘four pillars’ of HF therapy are based have been conducted with the aim of reducing the residual risk. In the ‘stable’ patient, with background therapy titrated to the maximum tolerated doses, the addition of MRA [EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) and EMPHASIS (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) trials], ARNI [PARADIGM (Prospective Comparison of ARNI with angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial], or SGLT-2i reduced the residual risk of mortality or rehospitalization, and therefore, the progression to the worsening HF.
The fifth card
Vericiguat is a direct stimulator of soluble guanylate cyclase (sGC), a cytosolic enzyme involved in the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). At the vascular level, the binding of cGMP to protein kinase G leads to the release of calcium and the consequent relaxation of smooth muscles. In physiology, sGC activation is secondary to the binding of the haeme group of the enzyme to nitric oxide (NO), which is in turn produced by the enzyme endothelial cell synthetase (eNOS) and regulated by wall stress and laminar flow. The role of the NO-sGC-cGMP axis and its dysfunction in the pathogenesis of IC have been recognized for long time.6 Endothelial dysfunction, common to many cardiovascular conditions, results in a reduced concentration of NO, both due to a reduced production by the enzyme eNOS and to an accelerated consumption with the formation of peroxynitrite from oxidative stress, as a consequence of tissue hypoperfusion.
The attempts to restore the function of this pathway have long focused on the first link in the chain, nitrates, and the last one, with increased cGMP through inhibition of the phosphodiesterase 5 (PDE5) enzyme, responsible for the degradation of cGMP to GMP.
Despite positive evidence on the reduction of pulmonary pressure,7 the reverse remodelling of left ventricular hypertrophy,8 improvement in diastolic and systolic function,9 nitrates and PDE5 inhibitors have so far not provided convincing results in terms of strong outcomes,10 both in the setting of acute and chronic HF.
Soluble guanylate cyclase stimulators are responsible for activating the enzyme, regardless of the bond with NO. In addition to vericiguat, cinaciguat, and riociguat (the latter already approved for the treatment of Type 1 and Type 4 pulmonary hypertension) belong to this pharmacological class.
The VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) trial enrolled 5050 patients between September 2016 and December 2018.
Enrolment criteria included:
FE < 45%;
BNP > 300 pg/mL or NTproBNP > 1000 pg/mL if in sinus rhythm (RS);
BNP > 500 pg/mL or NTproBNP > 1600 pg/mL if in atrial fibrillation.
Evidence of worsening HF (defined as hospitalization for HF within 6 months prior to randomization or administration of IV furosemide with no hospitalization within the previous 3 months) NYHA Class II–IV; eGFR > 15 mL/min. The primary outcome was a composite of death from cardiovascular causes and first hospitalization for HF.
First of all, it is interesting to note the differences between the population enrolled in this trial and that of the recent registration trials of sacubitril/valsartan (PARADIGM-HF),11 dapaglifozin (DAPA-HF),4 and empaglifozin (EMPEROR-REDUCED).5 The criterion of evidence of a worsening HF episode and the highest natriuretic peptide values in the VICTORIA trial inevitably selected a population at higher risk of events. The median NTproBNP values (2816 pg/mL in VICTORIA, 1608 pg/mL in PARADIGM-HF, 1437 pg/mL in DAPA-HF) and the proportion of patients in NYHA Class III and IV (41% in VICTORIA, 25% in PARADIGM-HF, 32% in DAPA-HF) justify, at least in part, the occurrence of the primary outcome in 33.6%/year in the treated population. A rate which is more than double than that observed in the comparative trials (10.5 and 11.6 in the active treatment population in PARADIGM-HF and DAPA-HF, respectively).12
Knowledge of these data is essential for the interpretation of the trial results. The primary outcome was achieved after a median follow-up of 10.8 months, a shorter observation period than 27 months for PARADIGM-HF and 18 months for DAPA-HF. This outcome, whose individual components did not reach statistical significance, occurred in 35.5% of patients on treatment vs. 38.5% in the placebo group [relative risk (RR), 0.90; 95% confidence interval (CI), 0.82–0.98; P = 0.02]; 16.4% of patients on treatment and 17.5% of patients in the placebo arm died of cardiovascular causes (RR, 0.93; 95% CI, 0.81 to 1.06); 27.4% of patients on treatment and 29.6% in the placebo group were hospitalized for HF (RR, 0.90; 95% CI, 0.81–1.00).
Despite achieving statistical significance, a 10% reduction in a composite outcome may seem insignificant in absolute terms.13 In particular, the comparison with the successes on the same outcome obtained by the recent trials on ARNI and SGLT-2i generates a misleading perception. The 20% reduction in PARADIGM-HF, 26% in DAPA-HF, and 25% in EMPEROR-REDUCED refer to the RR. To evaluate the real efficacy of one treatment compared with another, it is essential to consider not only the RR but also the overall risk of events in the study population and the duration of follow-up. The annualized event rate parameter (the number of events per 100 patient-years at risk) allows us to calculate the absolute risk reduction, expressed in terms of events avoided/100 patient-years.
In the VICTORIA trial, characterized as we have seen by a high event rate and short follow-up duration, the 10% reduction in the primary outcome translates into an absolute risk reduction of 4.2 events/100 patients/year. In the PARADIGM-HF and DAPA-HF trials (lower event rate and longer follow-up duration), the same number is 2.7 and 4015, respectively.
In the light of this evidence, the positive results of the VICTORIA trial allowed the drug to be included in the ESC 2021 GL, with a Class II B indication for patients with recent episodes of worsening HF during OMT (Figure 1).
Figure 1.
Guidelines directed therapy for patients with worsening heart failure during optimal medical treatment.
The history of this molecule and its use in HFrEF (heart failure with reduced ejection fraction) is still in its early stages. The decision to enrol a high-risk population in the first Phase III trial was probably based on economic reasons (the need for a short follow-up), rather than a limited efficacy assumption for this subgroup. Preclinical data and the mechanism of action suggest that it is precisely the residual risk population that could benefit the most from this treatment.
The evidence accumulated from preclinical studies on drugs that act on the NO-sGC-cGMP pathway involves a reduction of fibrosis at the cardiac and renal level, improved perfusion at the cardiac, renal, and peripheral level, and a reduction in myocardial hypertrophy.10 These pathophysiological processes have a long-term effect on hard outcomes: this is demonstrated by the data from the VICTORIA study, which showed that the three quartiles with the lowest baseline natriuretic peptide levels benefited the most from treatment.14
The cohort in the VICTOR trial (A Study of Vericiguat in Participants with Chronic Heart Failure With Reduced Ejection Fraction) will represent the first steps in a new Phase III randomized clinical trial world. The previously mentioned trials have a low proportion of patients on quadruple therapy in common: the PARADIGM-HF and the VICTORIA came before the SGLT-2i, but even in the DAPA-HF and EMPEROR-REDUCED trials, the proportion of patients on ARNI therapy in the active treatment arm only reached 10.5 and 18.3% of the enrolled population, respectively. These are therefore small numbers, and a residual risk that we currently have little data to quantify.
In addition, vericiguat can be used with a glomerular filtration rate of up to 15 mL/min. In accordance with the design of the VICTORIA study, the proportion of patients with an eGFR between 15 and 30 mL/min was limited to 15% of the total. This is, therefore, the first molecule approved for the treatment of patients with an eGFR <20 mL/min (the exclusion criteria limits were 30 mL/min in the PARADIGM-HF, 25 mL/min in the DAPA-HF, and 20 mL/min in the EMPEROR-REDUCED).
The result is that a significant portion of patients who have been excluded from approved treatments for a long time could find an appropriate therapy. This very significant finding allows us to begin focusing on the individual phenotypic treatment of patients with HFrEF and reduced EF, from the early stages of the disease.
Contributor Information
Giulio Balestrieri, Cardiovascular Department, Pope John XXIII Hospital, Bergamo, Italy.
Edoardo Sciatti, Cardiovascular Department, Pope John XXIII Hospital, Bergamo, Italy.
Salvatore D'isa, Cardiovascular Department, Pope John XXIII Hospital, Bergamo, Italy.
Emilia D'elia, Cardiovascular Department, Pope John XXIII Hospital, Bergamo, Italy.
Michele Senni, Cardiovascular Department, Pope John XXIII Hospital, Bergamo, Italy; Cardiology Unit, Milan Bicocca University, Milan, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. McDonagh TA, Metra M, Adamo M; ESC Scientific Document Group et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD; ESC Scientific Document Group et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SDet al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJV, Solomon SD, Inzucchi SE; DAPA-HF Trial Committees and Investigators et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Anker SD, Butler J; EMPEROR-Reduced Trial Investigators et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 6. Boerrigter G, Lapp H, Burnett JC. Modulation of cGMP in heart failure: a new therapeutic paradigm. Handb Exp Pharmacol 2009;191:485–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X, Yang T, Zhou Q, Li Set al. Additional use of a phosphodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: a meta-analysis. Eur J Heart Fail 2014;16:444–453. [DOI] [PubMed] [Google Scholar]
- 8. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 2011;4:8–17. [DOI] [PubMed] [Google Scholar]
- 9. Kim KH, Kim HK, Hwang ICet al. PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; a 12-week, randomized, double-blind, placebo-controlled trial. Am Heart J 2015;169:813–822.e3. [DOI] [PubMed] [Google Scholar]
- 10. Emdin M, Aimo A, Castiglione Vet al. Targeting cyclic guanosine monophosphate to treat heart failure: JACC review topic of the week. J Am Coll Cardiol 2020;76:1795–1807. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJ, Packer M, Desai AS; PARADIGM-HF Investigators and Committees et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 12. Butler J, Anstrom KJ, Armstrong PW. Comparing the benefit of novel therapies across clinical trials: insights from the VICTORIA trial. Circulation 2020;142:717–719. [DOI] [PubMed] [Google Scholar]
- 13. Wearden J, Hough A, Kaiser S. Vericiguat in heart failure with reduced ejection fraction. N Engl J Med 2020;383:1496–1498. [DOI] [PubMed] [Google Scholar]
- 14. Butler J, Usman MS, Anstrom KJet al. Soluble guanylate cyclase stimulators in patients with heart failure with reduced ejection fraction across the risk spectrum. Eur J Heart Fail 2022;24:2029–2036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.