Abstract
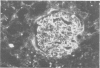
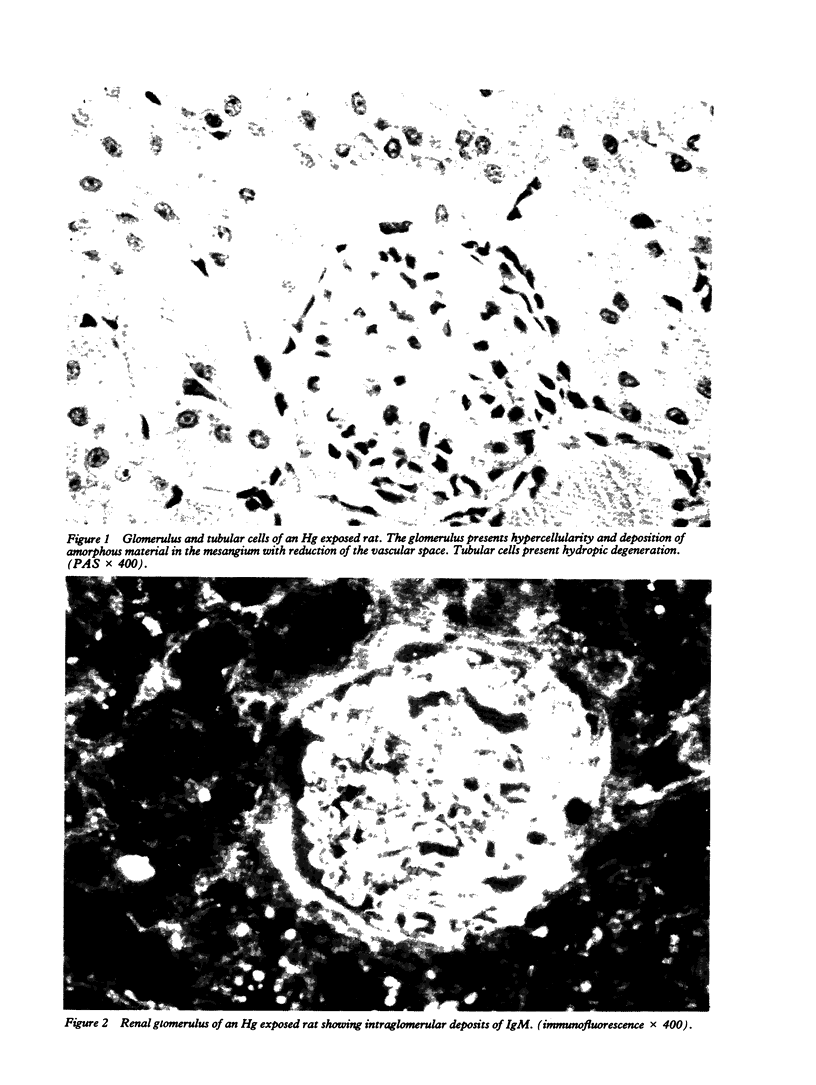
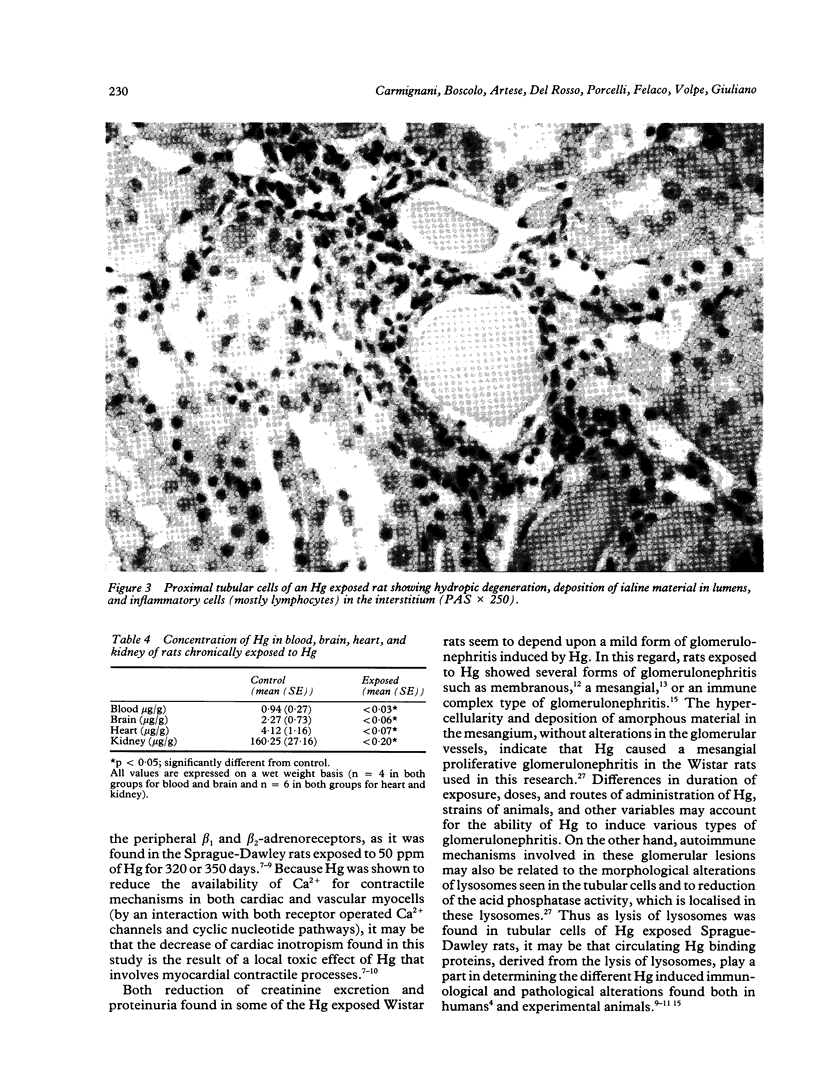
Male weanling Wistar rats received 200 micrograms/ml of mercury (Hg), as HgCl2, in drinking water for 180 days. At the end of the treatment, systemic arterial blood pressure was augmented, cardiac inotropism was reduced, and heart rate was unchanged. Light and electron microscopical studies of the kidney showed a mesangial proliferative glomerulonephritis in about 80% of the glomeruli. Tubular cells showed reduction of the acid phosphatase activity, which was related to functional abnormalities of the lysosomes. In the 24 hour urine samples of the Hg exposed rats, there was slight reduction of kallikrein activity, but evident proteinuria was not present in all samples. Plasma renin activity was reduced, that of angiotensin I-converting enzyme was augmented, and plasma aldosterone concentrations were unchanged. Mercury was accumulated mostly in the kidney of the Hg treated animals; and the content of Hg in the heart was higher than in the brain. These data show that chronic exposure to Hg acts on the kidney with complex mechanisms of toxicity; these contribute to modify systemic haemodynamics.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bariety J., Druet P., Laliberte F., Sapin C. Glomerulonephritis with - and 1C-globulin deposits induced in rats by mercuric chloride. Am J Pathol. 1971 Nov;65(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Barregård L., Hultberg B., Schütz A., Sällsten G. Enzymuria in workers exposed to inorganic mercury. Int Arch Occup Environ Health. 1988;61(1-2):65–69. doi: 10.1007/BF00381609. [DOI] [PubMed] [Google Scholar]
- Bernard A., Roels H. A., Buchet J. P., Lauwerys R. R. Comparison, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, of urinary proteins excreted by workers exposed to cadmium, mercury or lead. Toxicol Lett. 1980 Mar;5(3-4):219–222. doi: 10.1016/0378-4274(80)90063-6. [DOI] [PubMed] [Google Scholar]
- Boscolo P., Carmignani M. Neurohumoral blood pressure regulation in lead exposure. Environ Health Perspect. 1988 Jun;78:101–106. doi: 10.1289/ehp.8878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo P., Carmignani M., Sacchettoni-Logroscino G., Rannelletti F. O., Artese L., Preziosi P. Ultrastructure of the testis in rats with blood hypertension induced by long-term lead exposure. Toxicol Lett. 1988 May;41(2):129–137. doi: 10.1016/0378-4274(88)90086-0. [DOI] [PubMed] [Google Scholar]
- Boscolo P., Porcelli G., Carmignani M., Finelli V. N. Urinary kallikrein and hypertension in cadmium-exposed rats. Toxicol Lett. 1981 Jan;7(3):189–194. doi: 10.1016/0378-4274(81)90066-7. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Bernard A., Lauwerys R. Assessment of renal function of workers exposed to inorganic lead, calcium or mercury vapor. J Occup Med. 1980 Nov;22(11):741–750. [PubMed] [Google Scholar]
- Campbell D. J. Circulating and tissue angiotensin systems. J Clin Invest. 1987 Jan;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignani M., Boscolo P. Cardiovascular homeostasis in rats chronically exposed to mercuric chloride. Arch Toxicol Suppl. 1984;7:383–388. doi: 10.1007/978-3-642-69132-4_66. [DOI] [PubMed] [Google Scholar]
- Carmignani M., Boscolo P., Preziosi P. Renal ultrastructural alterations and cardiovascular functional changes in rats exposed to mercuric chloride. Arch Toxicol Suppl. 1989;13:353–356. doi: 10.1007/978-3-642-74117-3_68. [DOI] [PubMed] [Google Scholar]
- Carmignani M., Finelli V. N., Boscolo P. Mechanisms in cardiovascular regulation following chronic exposure of male rats to inorganic mercury. Toxicol Appl Pharmacol. 1983 Jul;69(3):442–450. doi: 10.1016/0041-008x(83)90267-3. [DOI] [PubMed] [Google Scholar]
- Davidson D. M., Covell J. W., Malloch C. I., Ross J., Jr Factors influencing indices of left ventricle contractility in the conscious dog. Cardiovasc Res. 1974 May;8(3):299–312. doi: 10.1093/cvr/8.3.299. [DOI] [PubMed] [Google Scholar]
- Druet P., Pelletier L., Hirsch F., Rossert J., Pasquier R., Druet E., Sapin C. Mercury-induced autoimmune glomerulonephritis in animals. Contrib Nephrol. 1988;61:120–130. doi: 10.1159/000415242. [DOI] [PubMed] [Google Scholar]
- Gobbato F., Chiesura P. La nefropatia da piombo. Minerva Nefrol. 1968 Jan-Apr;15(1):12–24. [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Makker S. P., Aikawa M. Mesangial glomerulonephropathy with deposition of IgG, IgM, and C3 induced by mercuric chloride: a new model. Lab Invest. 1979 Jul;41(1):45–50. [PubMed] [Google Scholar]
- Morita T., Kato H., Iwanaga S., Takada K., Kimura T. New fluorogenic substrates for alpha-thrombin, factor Xa, kallikreins, and urokinase. J Biochem. 1977 Nov;82(5):1495–1498. doi: 10.1093/oxfordjournals.jbchem.a131840. [DOI] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Buchet J. P., Bernard A. M., Lijnen P., Van Houte G. Urinary kallikrein activity in workers exposed to cadmium, lead, or mercury vapour. Br J Ind Med. 1990 May;47(5):331–337. doi: 10.1136/oem.47.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicli A. G., Carretero O. A. Renal kallikrein-kinin system. Kidney Int. 1986 Jan;29(1):120–130. doi: 10.1038/ki.1986.14. [DOI] [PubMed] [Google Scholar]
- Soter N. A., Wasserman S. I., Austen K. F. Cold urticaria: release into the circulation of histamine and eosinophil chemotactic factor of anaphylaxis during cold challenge. N Engl J Med. 1976 Mar 25;294(13):687–690. doi: 10.1056/NEJM197603252941302. [DOI] [PubMed] [Google Scholar]
- Stonard M. D., Chater B. V., Duffield D. P., Nevitt A. L., O'Sullivan J. J., Steel G. T. An evaluation of renal function in workers occupationally exposed to mercury vapour. Int Arch Occup Environ Health. 1983;52(2):177–189. doi: 10.1007/BF00405421. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Goto S., Hotta R. An epidemiological study on retinopathy due to carbon disulfide: CS2 exposure level and development of retinopathy. Int Arch Occup Environ Health. 1976 Apr 28;37(1):1–8. doi: 10.1007/BF00409360. [DOI] [PubMed] [Google Scholar]
- Thorlacius-Ussing O., Møller Graabaek P. Simultaneous ultrastructural demonstration of heavy metals (silver, mercury) and acid phosphatase. Histochem J. 1986 Nov-Dec;18(11-12):639–646. doi: 10.1007/BF01675299. [DOI] [PubMed] [Google Scholar]
- Victery W. Evidence for effects of chronic lead exposure on blood pressure in experimental animals: an overview. Environ Health Perspect. 1988 Jun;78:71–76. doi: 10.1289/ehp.887871. [DOI] [PMC free article] [PubMed] [Google Scholar]