ABSTRACT
Background and Aims:
Acute pain management in drug addicts is a critical yet understudied topic. Drug addicts have a decreased pain threshold, impairing anaesthetic pain control. This study aimed to evaluate the postoperative quality of recovery in addicts and non-addicts after receiving erector spinae plane block (ESPB) with general anaesthesia.
Methods:
Sixty males, aged 18-60 years, with an American Society of Anesthesiologists physical status I/II, scheduled for elective lumbar decompression surgery, were divided into two equal groups. Group A included 30 addicts and group N included 30 non-addicts. Both groups received bilateral ultrasound-guided ESPB with 20 mL bupivacaine (0.25%) before induction of general anaesthesia. The primary outcome was comparison of the 24-hour postoperative quality of recovery (QoR-15) score. The secondary outcomes were time to first analgesic requirement, postoperative pain scores, morphine consumption, and adverse events.
Results:
The QoR-15 score was higher in group N (median = 128.5, interquartile range = 107-136) than in group A (118 [99-130]), indicating a better recovery in group N. The visual analogue scale pain score was lower in group N than in group A, especially in the first 12 hours postoperatively. Time to first analgesic request was significantly longer in group N than in group A (mean ± standard deviation: 8.67 ± 2.74 and 5.53 ± 1.64 hours, respectively, P =0.001), Morphine consumption was significantly higher in group A than in group N (9.62 ± 3.2 and 7.08 ± 2.57 mg, respectively, P =0.041).
Conclusion:
Drug addicts experienced decreased analgesic efficacy of ESPB compared to non-addicts, with comparable postoperative QoR-15 score following lumbar decompression surgery.
Keywords: Drug addicts, erector spinae block, lumbar decompression, ultrasound, postoperative pain, analgesia, anaesthesia
INTRODUCTION
Worldwide, drug addiction has become an increasingly prevalent issue especially among young urban males. Many patients are discovered to be addicted during regular preoperative history taking.[1] The main classes of abused drugs involve alcohol, opiates, cannabinoids, and stimulants. Chronic substance addiction is associated with substantial psychiatric and somatic disorders, along with additional perioperative challenges, including intravenous cannulation, airway protection, intraoperative management, and postoperative pain control.[2]
Lumbar spine surgery is a common therapeutic option for patients with spine pathology but pain management is challenging following such surgeries. Systemic analgesia is an option; however, regional anaesthesia can provide more advantages than opioids due to better pain relief and less adverse events.[3] The erector spinae plane block (ESBP) is an interfascial plane block with various applications as a perioperative analgesic for back, thorax, and abdominal surgeries. ESPB targets the ventral and dorsal rami of spinal nerves and sympathetic innervation on different levels.[4]
Unlike other medical conditions, patients with drug addiction do not often get satisfactory treatment in the perioperative period. This has been related to under-reporting or underasking.[5] Therefore, evaluating the addicts’ recovery can be difficult. Previous studies have focused on assessment of physical outcomes, recovery durations, and postoperative adverse events in addicts.[6,7] However, it seems more appropriate to focus on the patient’s viewpoint regarding quality of their recovery. The 15-item Quality of Recovery (QoR-15) scale is a frequently used self-assessment questionnaire for the early postoperative period.[8]
To our knowledge, no former study has addressed the issue of the recovery quality in addicts. Moreover, the information that guide the postoperative acute pain management in addicts is inadequate. This encouraged us to conduct this comparative study aiming to estimate the postoperative QoR-15 score in addicts and non-addicts after receiving bilateral ESPB in lumbar decompression surgeries. Present study hypothesises that local anaesthetic (LA) duration and intensity would be reduced by chronic substance addiction, affecting postoperative recovery experience.
METHODS
The institutional ethics committee approved the study and written informed consent was obtained from patients. This prospective double-blinded controlled study was performed in compliance with the Declaration of Helsinki of 1975, as revised in 2013. The patients were recruited during June 2021 to December 2021. The trial was prospectively registered in the clinicaltrials.gov (NCT04943549). Eligible patients were assigned into two equal-sized groups (number = 30).
Patients in group A had a history of drug addiction (one drug or combination of two or more) for more than one year, such as marijuana (cannabis), clonazepam, and/or tramadol. Patients in group N had no history of addiction to any substance (control group). Both groups received bilateral ESPB before general anaesthesia.
The study included 60 males, aged 18 to 60 years, who were eligible for elective lumbar spine decompression surgery under general anaesthesia, with a history of smoking and had an American Society of Anesthesiologists physical status (ASA-PS) I/II. Patients were classified as addicts based on their personal history obtained by the anaesthetist responsible of the preoperative assessment. Patients were required to have at least one year of drug consumption (as a regular habit, not as a prescribed medication) and drug withdrawal symptoms when the substance was discontinued. In contrast, subjects in the control group had no drug addiction history in the past two years. The exclusion criteria were patients with hepatic or renal dysfunction, preoperative communication disability or cognitive disorder, previous back surgery, block puncture site infection, allergy to any drug, alcohol consumption, coagulation disorders, and emergency surgery.
All addicts were encouraged to take their daily dose of the drug before surgery.
The patients were taken to the anaesthesia preparation room, 30 minutes before the procedure. Intravenous (IV) cannula was inserted and standard monitoring (electrocardiogram, noninvasive arterial blood pressure, and pulse oximetry) were applied on the patient and premedications (midazolam 0.02 mg/kg and ranitidine 50 mg) were administered. Patients had bilateral ultrasound-guided ESPBs at the lowest thoracic level (T12).
Each patient was positioned on his left side. A low-frequency, curved, ultrasound transducer (LOGIQ e, GE corporate, general electric company, United States of America) was used. After skin sterilisation, the ultrasound probe was positioned in a longitudinal alignment, 2-3 cm lateral to the midline to locate the transverse process, and identify the erector spinae muscles covering it. After LA infiltration into the superficial tissues, an 8-cm 22-gauge block needle (Perifix, B.BRAUN, Melsungen AG, Germany) was introduced cranio-caudally to make contact with the transverse process, with its tip under the erector spinae muscles. A small bolus of LA was injected to observe the muscle detaching from the transverse process and confirm the correct needle position. Bupivacaine 0.25% (20 mL) was injected deep to the erector spinae muscles. This manoeuver was repeated on the other side. Five minutes following the block, cutaneous sensation was checked by a pinprick test over the patient’s back and repeated every 5 minutes until sensory loss was detected. Lack of sensory loss after 15 minutes on either or both sides was considered as failed block and the patient was excluded from the analysis.
Once the sensory loss was ensured, anaesthesia was induced using propofol 1–2 mg/kg, fentanyl 1–2 μg/kg, and muscle relaxant (cisatracurium 0.2–0.3 mg/kg). Isoflurane was used to maintain anaesthesia. Fentanyl bolus was given if heart rate (HR) or mean arterial pressure (MAP) increased more than 20% of preoperative baseline and cisatracurium booster doses were used for muscle relaxation. After surgery and reversal of muscle relaxant, all patients were moved to the postanaesthesia care unit (PACU) for at least 30 minutes. Both groups received the same postoperative analgesic treatment, including 30 mg of IV ketorolac given 30 minutes before the end of procedure and repeated every 8 hours afterward. When the visual analogue scale (VAS) score exceeded 3, IV morphine was titrated by 2-5 mg increments every 5 minutes, until pain relief (VAS ≤3) or a maximum dose of 20 mg was reached. The anaesthesiologist in charge of the surgery and evaluating physician were uninformed of the group assignment.
The primary outcome was QoR-15 at 24 hours following surgery. This recovery score compromises 15 questions that assess five clinical characteristics: physical status (5-items), emotional well being (4-items), physical independence (2-items), psychological care (2-items), and pain (2-items). Each item is scored on an 11-point numerical rating scale [Figure 1]. The sum of the 15 items’ scores is from 0 to 150 and higher scores represent better recovery.[8] The questionnaire was translated to the patient by the evaluating physician.
Figure 1.

The quality of recovery (QoR-15) questionnaire.[6]
The secondary outcomes included the onset of sensory blockade (defined as the time elapsed from the end of final injection till complete disappearance of pinprick sensation of all distributions of the nerve), intraoperative hemodynamic parameters (MAP and HR), and number of additional fentanyl boluses. Postoperative pain evaluation using VAS, during rest and movement, at PACU admission, 2, 4, 8, 12, and 24 hours, and the first time to request morphine and its dosage were also recorded. Finally, any side effects related to the block such as pruritus, nausea, vomiting, agitation, and delirium were noted.
Statistical analysis was carried out using Statistical Package for Social Sciences version 25 (International Business Machines, Armonk, New York, United States). The Shapiro-Wilk test and Q–Q plots were used to determine the normality of quantitative variables. Quantitative variables were summarized as mean ± standard deviation (SD) or median and interquartile range. Unpaired t-test was used to compare normally distributed data between the study arms, while the Mann-Whitney U test was used to compare non-normally distributed variables. Qualitative variables were summarized as numbers and percentages and compared using the Chi-squared test. P values <0.05 indicated statistical significance.
The sample size was calculated using G*power software version 3.1.9.2 (Heinrich Heine University, Düsseldorf, Germany) based on difference in QOR-15 between addicts and non-addicts. With a large expected effect size (d = 0.8), the sample size was 52 patients (26 per group). Alpha and power were adjusted at 0.05 and 0.8, respectively. The study included 60 patients to account for possible dropouts.
RESULTS
Sixty eight patients were assessed for eligibility. Eight were excluded due to refusal to participate (n = 4), meeting the exclusion criteria (n = 3), and failed block (n = 1). The remaining 60 patients were included in the analysis (30 in each group) [Figure 2].
Figure 2.
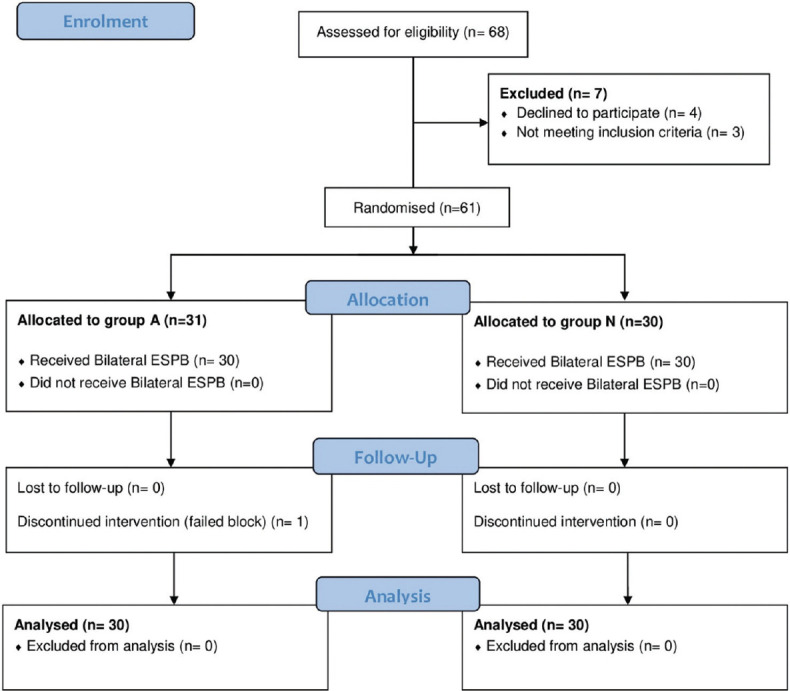
Consort flow diagram of the study cases.
No significant differences were reported between both groups regarding demographic characteristics (age, body mass index, and ASA PS) and operative data (length of the procedure, type and level of surgical intervention, average time to perform the block, and onset of the block) [Table 1].
Table 1.
Demographic and clinical criteria of the studied groups
| Group A (n=30) | Group N (n=30) | P | |
|---|---|---|---|
| Age (y) | 37±9.71 | 36.1±9.74 | 0.721 |
| BMI (kg/m2) | 25.9±4.51 | 25.56±3.54 | 0.752 |
| ASA (n%) | |||
| I | 23 (76.7) | 21 (70) | 0.559 |
| II | 7 (23.3) | 9 (30) | |
| Duration of surgery (min) | 121.5±25.47 | 123.17±20.82 | 0.782 |
| Type of surgery (n) | |||
| Laminectomy | 20 (66.7) | 17 (56.7) | 0.426 |
| Discectomy | 10 (33.3) | 13 (43.3) | |
| Level of surgery (n) | |||
| I | 18 (60) | 21 (70) | 0.417 |
| II | 12 (40) | 9 (30) | |
| Time to perform the block (min) | 14.18±3.21 | 14.92±3.5 | 0.107 |
| Onset of sensory block (min) | 15.77±1.87 | 14.8±2.2 | 0.0721 |
Data are presented as mean±standard deviation (SD) and number (n) (%). BMI, body mass index; ASA, American society of Anesthesiologists.
No significant differences were noted regarding the lowest and highest MAP or HR recorded during the surgery. The rates of intraoperative hypotension requiring intervention and postoperative nausea vomiting were low and comparable in both the groups. In addition, both groups had no other block complications (pruritus, respiratory depression, agitation, or delirium) [Table 2].
Table 2.
Intra-operative findings and adverse events in the studied groups
| Group A (n=30) | Group N (n=30) | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | ||
| Highest HR recorded (beat/min) | 93.3 | 4.72 | 91.4 | 3.31 | 0.076 |
| Lowest HR recorded (beat/min) | 59.8 | 6.25 | 61.6 | 6.34 | 0.272 |
| Highest MAP recorded (mm Hg) | 143.36 | 6.11 | 141.57 | 7.27 | 0.672 |
| Lowest MAP recorded (mm Hg) | 86.16 | 3.83 | 84.57 | 3.18 | 0.083 |
| Additional Intraoperative Fentanyl (µg) | 56.33 | 8.80 | 54.5 | 10.2 | 0.459 |
| Hypotension requiring intervention, (n) | 2 (6.6) | 1 (3.33) | 0.553 | ||
| PONV (n) | 5 (16.7) | 3 (10) | 0.447 | ||
| Reported block complications (n) | 0 | 0 | 0.99 | ||
Data are presented as mean±standard deviation (SD) and number (n) (%). HR, heart rate; MAP, mean arterial pressure; PONV, postoperative nausea and vomiting.
Regarding perioperative narcotics received by the patients, the addict group was comparable to the non-addict group in fentanyl consumption intraoperatively (56.33 ± 8.8 and 54.5 ± 10.2 mg, respectively) [Table 2]. However, a significant difference was observed in the mean duration of the first analgesic requirement (8.67 ± 2.74 and 5.53 ± 1.64 hours in the non-addict and addict groups, respectively) (P =0.001). Furthermore, the overall postoperative morphine dose was significantly increased in addict group than non-addict group (9.62 ± 3.20 and 7.08 ± 2.57 mg, respectively) (P =0.041) [Table 3].
Table 3.
Postoperative findings in the studied groups
| Group A | Group N | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | ||
| Time to first analgesic request (h) | 5.53 | 1.64 | 8.67 | 2.74 | 0.001* |
| Total morphine consumption in 24 h (mg) | 9.62 | 3.20 | 7.08 | 2.57 | 0.041* |
| Time to ambulation (h) | 18.03 | 6.14 | 16.83 | 4.57 | 0.394 |
| Duration of hospital stay (days) | 2.8 | 0.81 | 2.63 | 0.72 | 0.401 |
| QoR-15 score | 118 (99-130) | 128.5 (107-136) | 0.067 | ||
Data are presented as mean±standard deviation and median (inter quartile range). *Statistically significant (P<0.05).
QoR-15 score at 24 hours postoperatively was higher (better recovery) in the non-addict group (median = 128.5, interquartile range = 107–136) than in the addict group (118, 99–130) but without statistical significance (P =0.067). Also, no significant difference was reported between both the groups concerning the first ambulation time or duration of hospital stay (P > 0.05) [Table 3].
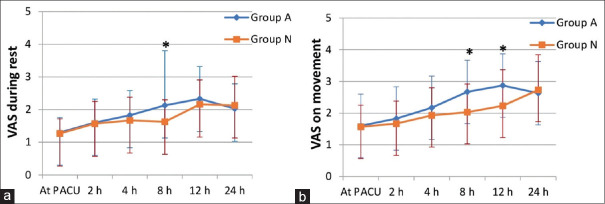
The VAS scores were lower in non-addicts than addicts in the first 12 hours postoperatively with significant values at 8 and 12 hours (P < 0.05). At 8 hours, it was significantly lower in non-addicts during rest and movement, while at 12 hours it was significantly lower only during movement. However, after 12 hours, no observed differences were found between both the groups [Figure 3].
Figure 3.
Visual analog scale (VAS) pain scores at rest (a) and on movement (b) in postanaesthesia care unit (PACU) during the first 24 hours.
DISCUSSION
Anaesthesiologists encounter difficulties while dealing with patients having drug abuse. Multimodal analgesia concepts apply equally well to addicts and non-addicts. Yet, regional anaesthesia has long been recommended for addicts by many anaesthesia practitioners, as a reliable anaesthetic and postoperative pain control strategy, for various procedures.[9]
This clinical trial evaluated the effectiveness of ESPB in substance abusers via the patient-centered outcome measurement (QoR-15). The study revealed a comparable recovery quality at 24 hours postoperatively between addicts and non-addicts. However, ESPB prolonged the time to first opioid request, decreased its consumption, and reduced VAS scores in non-addicts compared to addicts following lumbar decompression surgery. No significant differences were noted regarding onset of block and intraoperative or postoperative complications in both the groups.
Many studies that have investigated the effect of LA for postoperative analgesic profile in opium abusers concur with our results. Azimaraghi et al.[6] performed brachial plexus block on addicts undergoing upper extremity surgery. They concluded that chronic opium abusers had a shorter sensory and motor blockade duration than non-abusers. Another Iranian study reported a shorter length of anaesthesia in opium abusers who underwent suturing for hand lacerations when using lidocaine for digital block.[7] Several studies were conducted on addicts who received spinal anaesthesia for lower abdominal and lower extremity surgeries. They have demonstrated shorter sensory and motor blockade durations after intrathecal LA.[10,11]
In contrast, Majidi et al.[12] did not find any difference in pain reduction or duration of action between addicts and non-addicts when lidocaine was used as an anaesthetic agent for suturing skin lacerations.
The results of present study are concomitant with a retrospective study performed by Liu et al.,[13] who noted that cannabis users had greater pain scores and poorer sleep quality than non-users in the initial postoperative period following orthopaedic surgery. Also, a prospective study conducted by Jefferson et al.[14] reported higher pain scores in cannabis users and suggested increasing the postoperative pethidine doses following elective surgical procedures.
A retrospective study, conducted by Rishel et al.,[15] concurred with our results regarding the impact of long-term preoperative benzodiazepine usage on opioid utilization postoperatively. They reported the need for higher opioid doses in such patients.
Various mechanisms explain drug tolerance development in opium abusers. Downregulation of the number of receptors reduces the affinity for agonists and hence reduces the response to the drug.[16] The receptors of LA in different parts of the body are structurally and functionally similar to opioid receptors, hence they gain tolerance with long-term exposure to opioids.[17] Also, exposure to excessive exogenous medications causes changes in function and release of the endogenous peptides, decreasing pain threshold and increasing the response to painful stimulation.[18]
On the other hand, cannabinoid receptors (CB1, CB2) are also involved in pain modulation process. Desensitization and downregulation of the number of receptors has been reported with long-term exposure to cannabinoids.[19] Although activation of cannabinoid receptors causes inhibitory effect to pain response, these antinociceptive effects play a better role in chronic pain than acutely induced pain. In vitro studies have revealed that the binding of endocannabinoids to CB1 receptors cause pain sensitization unpredictably and even increase the risk of chronic pain.[20] Finally, studies on neuro-anatomical distribution of opioid and cannabinoid receptors revealed overlap in their locations in the central nervous system that are engaged in painful stimuli processing.[21]
ESPB has proved its ability to enhance the quality of recovery in various studies., Yao et al.[22] performed a preoperative ESPB with ropivacaine in patients undergoing modified radical mastectomy and reported an improvement in the QoR-15 score compared to the controls. Also, Finnerty et al. compared ESPB with serratus anterior plane block, in minimally invasive surgeries in thorax, and noted better scores with ESPB.[23] Furthermore, Yao et al.[24] adopted the QoR-40 score (the older version) to assess the influence of ESPB on recovery after video-assisted thoracic surgery and confirmed the same results.
The present study had some limitations. The sample size was small relative to the frequency of addiction. Also, the recruited patients were addicted to more than one drug, so diagnosing and defining failure was challenging. We suggest that several separate studies should be conducted to investigate efficacy of regional anaesthesia among addicts on a sample population that is only addicted to a single drug. Finally, patients’ preoperative QoR-15 scores were not assessed. Thus, we had no baseline against which postoperative results could be compared. However, the QoR-15 was developed to be used after surgery and due to patients’ exhaustion and surgery-related stress,[25,26] its efficacy to provide a reliable baseline in the preoperative period has been questioned. Furthermore, we used it similarly in both the groups.
CONCLUSION
Although the QoR-15 score at 24 hours postoperatively was comparable in both the groups, addicts receiving preoperative bilateral ESPB required postoperative analgesia earlier and had increased opioid consumption than non-addicts following lumbar decompression surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jacobus J, Bava S, Cohen-Zion M, Mahomood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–65. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran S, Isa J, Steinemann S. Perioperative management in the patient with substance abuse. Surg Clin North Am. 2015;95:417–28. doi: 10.1016/j.suc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Chen YC, Lee CY, Chen SJ. Narcotic addiction in failed back surgery syndrome. Cell Transplant. 2019;28:239–47. doi: 10.1177/0963689718796072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 5.Kork F, Neumann T, Spies C. Perioperative management of patients with alcohol, tobacco and drug dependency. Curr Opin Anaesthesiol. 2010;23:384–90. doi: 10.1097/ACO.0b013e3283391f79. [DOI] [PubMed] [Google Scholar]
- 6.Azimaraghi O, Marashi SM, Khazaei N, Pourhassan S, Movafegh A. The effect of adding sufentanil to 0.5% hyperbaric bupivacaine on duration of brachial plexus blockade in chronic opium abusers: A randomized clinical trial. Anesth Pain Med. 2015;5:e21960. doi: 10.5812/aapm.21960v2. doi:10.5812/aapm. 21960v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemian AM, Omraninava A, Kakhki AD, Sharifi MD, Ahmadi K, Masoumi B, et al. Effectiveness of local anesthesia with lidocaine in chronic opium abusers. J Emerg Trauma Shock. 2014;7:301–4. doi: 10.4103/0974-2700.142765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: The QoR-15. Anesthesiology. 2013;118:1332–40. doi: 10.1097/ALN.0b013e318289b84b. [DOI] [PubMed] [Google Scholar]
- 9.Rudra A, Bhattacharya A, Chatterjee S, Sengupta S, Das T. Anaesthetic implications of substance abuse in adolescent. Indian J Anaesth. 2008;52:132–9. [Google Scholar]
- 10.Tabatabaei SM, Malekmakan L, Izadpanahi N, Mansourian A. Comparative study of duration of spinal anesthesia with marcaine and lidocaine plus fentanyl between opium abuse and non-nonuse patients. Int J Pharmac Res. 2018;10:341–5. [Google Scholar]
- 11.Razavi M, Bameshki A, Jarahi L, Saghari M. Comparison of spinal anesthesia quality between patients addicted and not addicted to opium. J Perianesth Nurs. 2019;34:1169–75. doi: 10.1016/j.jopan.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Majidi A, Shahhosseini T, Mahmoudi S. Performance of local anesthesia with lidocaine among opium addicts and non-addicts;a case control study. Emergency. 2018;6:e35. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CW, Bhatia A, Buzon-Tan A, Walker S, Ilangomaran D, Kara J, et al. Weeding out the problem:The impact of preoperative cannabinoid use on pain in the perioperative period. Anesth Analg. 2019;129:874–81. doi: 10.1213/ANE.0000000000003963. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson DA, Harding HE, Cawich SO, Jackson-Gibson A. Postoperative analgesia in the Jamaican cannabis user. J Psychoactive Drugs. 2013;45:227–232. doi: 10.1080/02791072.2013.803644. [DOI] [PubMed] [Google Scholar]
- 15.Rishel CA, Zhang Y, Sun EC. Association between preoperative benzodiazepine use and postoperative opioid use and health care costs. JAMA Netw Open. 2020;3:e2018761. doi: 10.1001/jamanetworkopen.2020.18761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie MJ. Cellular neuroadaptations to chronic opioids:Tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–96. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu HE, Thompson J, Sun HS, Leitermann RJ, Fujimoto JM, Tseng LF. Nonopioidergic mechanism mediating morphine induced antianalgesia in the mouse spinal cord. J Pharmacol Exp Ther. 2004;310:240–6. doi: 10.1124/jpet.104.065334. [DOI] [PubMed] [Google Scholar]
- 18.Myles PS, Boney O, Botti M, Cyna AM, Gan TJ, Jensen MP, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (StEP) initiative: Patient comfort. Br J Anaesth. 2018;120:705–11. doi: 10.1016/j.bja.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 19.hlienz NJ, Budney AJ, Lee DC, Vandrey R. Cannabis withdrawal: A review of neurobiological mechanisms and sex differences. Curr Addict Rep. 2017;4:75–81. doi: 10.1007/s40429-017-0143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–4. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Befort K. Interactions of the opioid and cannabinoid systems in reward:Insights from knockout studies. Front Pharmacol. 2015;6:6. doi: 10.3389/fphar.2015.00006. doi:10.3389/fphar.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Li H, He Q, Chen T, Wang Y, Zheng X. Efficacy of ultrasound-guided erector spinae plane block on postoperative quality of recovery and analgesia after modified radical mastectomy:Randomized controlled trial. Reg Anesth Pain Med. 2020;45:5–9. doi: 10.1136/rapm-2019-100983. [DOI] [PubMed] [Google Scholar]
- 23.Finnerty DT, McMahon A, McNamara JR, Hartigan SD, Griffin M, Buggy DJ. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery:A randomised clinical trial. Br J Anaesth. 2020;125:802–10. doi: 10.1016/j.bja.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Yao Y, Fu S, Dai S, Yun J, Zeng M, Li H, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: A prospective, randomized, controlled trial. J Clin Anesth. 2020;63:109783. doi: 10.1016/j.jclinane.2020.109783. doi: 10.1016/j.jclinane.2020.109783. Epub 2020 Mar 13. PMID: 32179393. [DOI] [PubMed] [Google Scholar]
- 25.Chazapis M, Walker E, Rooms M, Kamming D, Moonesinghe SR. Measuring quality of recovery-15 after day case surgery. Br J Anaesth. 2016;116:241–8. doi: 10.1093/bja/aev413. [DOI] [PubMed] [Google Scholar]
- 26.Sharma R, Moied S, Raikwar S, Gupta V. Functional outcomes and quality of recovery after anaesthesia and surgery–Outreaching towards protracted goals. Indian J Anaesth. 2022;66:133–6. doi: 10.4103/ija.ija_356_22. [DOI] [PMC free article] [PubMed] [Google Scholar]