Abstract
Objectives
Lockdown was implemented in many countries during the pandemic, which led to myriad changes in pregnant women's lives. However, the potential impacts of the COVID-19 pandemic on neonatal outcomes remain unclear. We aimed to evaluate the association between the pandemic and neonatal birth weight.
Study design
This was a systematic review and meta-analysis of the previous literature.
Methods
We searched the MEDLINE and Embase databases up to May 2022 and extracted 36 eligible studies that compared neonatal birth weight between the pandemic and the prepandemic period. The following outcomes were included: mean birth weight, low birth weight (LBW), very low birth weight (VLBW), macrosomia, small for gestational age (SGA), very small for gestational age (VSGA), and large for gestational age (LGA). Statistical heterogeneity among studies was assessed to determine whether a random effects model or fixed effects model was conducted.
Results
Of the 4514 studies identified, 36 articles were eligible for inclusion. A total of 1,883,936 neonates during the pandemic and 4,667,133 neonates during the prepandemic were reported. We identified a significant increase in mean birth weight (pooled mean difference [95% confidence interval (CI)] = 15.06 [10.36, 19.76], I2 = 0.0%, 12 studies) and a reduction in VLBW (pooled OR [95% CI] = 0.86 [0.77, 0.97], I2 = 55.4%, 12 studies). No overall effect was identified for other outcomes: LBW, macrosomia, SGA, VSGA, and LGA. There was publication bias for mean birth weight with a borderline significance (Egger's P = 0.050).
Conclusion
Pooled results showed the pandemic was significantly associated with an increase in mean birth weight and a reduction in VLBW, but not for other outcomes. This review provided clues about the indirect effects of the pandemic on neonatal birth weight and more healthcare measures needed to improve neonatal long-term health.
Keywords: COVID-19 pandemic, Birth outcomes, Birth weight, Meta-analysis
Introduction
The outbreak of COVID-19 has profound effects on the global economy, social structures, and health services systems.1 , 2 Then, many governments implemented national or regional blockades and restrictions on free activities, which led to myriad changes in how pregnant women live their lives.3 , 4 Pregnant women are vulnerable to not only the direct effects of infection with COVID-19 but also the indirect effects of disruption of essential healthcare services and restrictions on social interaction. However, the indirect impact of the pandemic on pregnancy outcomes, including neonatal birth weight, remains unclear.
Birth weight, a sensitive indicator of intrauterine growth, is well documented that stress during pregnancy, prenatal care, and change in social life may result in adverse infant birth weight.5 , 6 As the important predictive indicator of neonatal health, the changes of low birth weight (LBW), very low birth weight (VLBW), small for gestational age (SGA), very small for gestational age (VSGA), and other adverse outcomes during the pandemic have drawn more attention, but it is mainly regarded as the secondary outcome in previous studies and with inconsistent results. A large sample study showed a significant increase of 13 g in mean birth weight during the pandemic compared with before the pandemic,7 but there were no differences in other studies.8 , 9 For LBW, there were no significant differences in some countries, such as Ireland,10 China,11 , 12 and Australia,13 whereas the studies from Spain14 and Turkey15 observed a significant increase in the pandemic period. Moreover, the recent two large sample sizes and nationwide studies reported a significant reduction in the rate of SGA during the pandemic (the United States16 and England17), but not for other studies.13 , 18 , 19 The COVID-19 lockdown and population response measures, as well as risk factors for adverse birth outcomes, vary from region to region, which may partly explain the differences between studies.20
Inconsistency among results from previous reports and a lack of evidence prompted us to conduct further exploration of neonatal birth weight changes during the pandemic. We aimed to assess the indirect effects on birth outcomes of the global COVID-19 pandemic.
Methods
We did a meta-analysis of studies on the effects of the pandemic on neonatal birth weight. This review was reported according to the Preferred Reporting Items in Systematic Reviews and Meta-analyses guidelines.21 The study protocol was registered with PROSPERO (No. CRD42022337886).
Search strategy and selection criteria
We searched the MEDLINE and Embase databases and reference lists of included studies up to May 2022 for relevant articles. The keywords used in this study were ‘COVID-19’ or ‘2019-nCoV’ or ‘SARS-COV-2’ or ‘n-COV’ or ‘coronavirus’ and combined them with terms related to outcomes, such as ‘birth outcome,’ ‘neonates,’ ‘bw,’ ‘birth weight,’ ‘LBW,’ ‘VLBW,’ ‘macrosomia,’ ‘SGA,’ ‘VSGA,’ and ‘LGA’ (Table S1). The search strategy was appropriately translated for the database. Studies were included if (1) the following outcomes were compared: mean birth weight, LBW, VLBW, macrosomia, SGA, VSGA, or large for gestational age (LGA) between the pandemic and the prepandemic period; (2) effect size (odds ratios [ORs] or risk ratios or β) with 95% confidence interval (CI) or mean with standard deviation were provided or could be calculated; (3) published in English. We excluded studies that were case reports or not published as full reports, studies with data unextractable or inappropriate design, and studies of only SARS-COV-2–infected women. Two investigators (X.D.Y. and H.J.) independently reviewed all the articles, and disagreements were resolved after discussion with the third author (J.Q.M.).
Data extraction
The characteristics of included studies were extracted based on authors, year of publication, sample size, study population and location, pandemic period definition, prepandemic period definition, effect size with 95% CI or mean with standard deviation, and other related information.
Neonatal birth weight assessed were mean birth weight, LBW, VLBW, macrosomia, SGA, VSGA, and LGA. LBW was defined as <2500 g, VLBW was defined as <1500 g, and macrosomia was defined as >4000 g. SGA was defined as birth weight less than the 10th percentile, VSGA was defined as birth weight less than the 3rd percentile, and LGA was defined as birth weight greater than the 90th percentile by gestational week at birth.22, 23, 24
Quality assessment
Each study was scored according to the Newcastle–Ottawa Scale25 independently by two assessors (X.D.Y. and H.J.). Quality assessment of these studies was based on three domains: selection, comparability, and outcomes. A study that scored 0–3 was considered to have a high risk of bias, 4–6 have a moderate risk of bias, and 7–9 have a low risk of bias. A lower risk of bias denotes higher quality.
Statistical analyses
For binary outcomes, if adjusted OR was not given, crude OR was used. For continuous outcomes, mean difference (MD) with 95% CI was calculated by pooling the mean with standard deviation. Statistical heterogeneity among studies was evaluated using the Chi-squared test, I 2 statistics, and P values. If the homogeneous test was not significant (I 2 < 50% and/or P > 0.10), a fixed effects model was used to obtain a summary OR or MD. Otherwise, a random effects model was used. Publication bias was evaluated by using funnel plots and Egger's test. Then, we conducted a subgroup analysis for factors that could potentially affect COVID-19 pandemic definition, lockdown measures, or neonatal birth weight: effect size (adjusted OR or crude OR), sample size (<10,000 or ≥10,000), study population (single center, multicenter, or regionwide/nationwide), country classification (low-/middle-income or high-income country according to World Bank classifications), prepandemic period definition (equivalent period in previous years or near before the lockdown period), and quality assessment of included studies (moderate or low risk of bias). In addition, we performed sensitivity analyses by omitting each study individually and recalculating the pooled effect size estimates for the remaining studies to assess the effect of individual studies on the pooled results. All statistical analyses were two sided and performed using STATA software (version 11.0; College Station, TX, USA).
Results
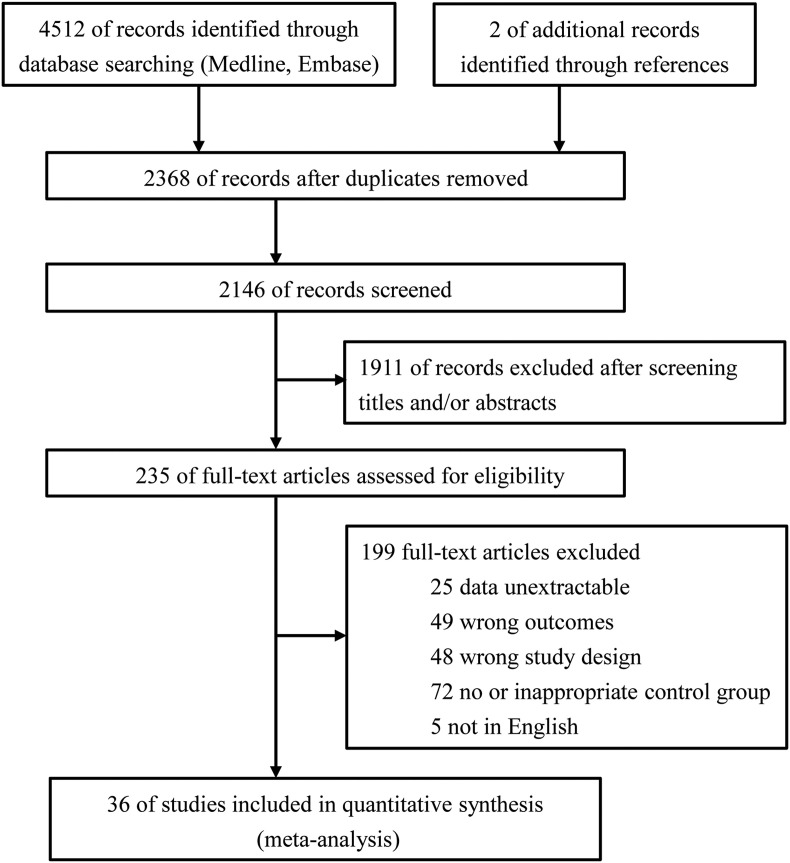
Of the 4514 studies identified, 36 articles were eligible for inclusion with further screening (Fig. 1 ).4 , 5 , 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 , 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Table S2 shows the characteristics of included studies in the quantitative synthesis. All the studies used a historical cohort design. Across the included studies, a total of 1,883,936 neonates during the COVID-19 pandemic and 4,667,133 neonates during the prepandemic were reported. Of the 36 primary studies, 12 reported mean birth weight, 23 reported adverse birth weight (LBW, VLBW, or macrosomia), and nine reported birth weight for gestational age (SGA, VSGA, or LGA). Twenty-two countries were represented, with substantial variation in pandemic mitigation measures among countries. There were 21 reports from single-center studies, four multicenter studies, and six regional reports, and the remaining five were national registries. Total sample sizes varied from 81 to 2,219,914 neonates. The duration of the ‘pandemic period’ studied varied from 1 month to 11 months, and the duration of the ‘prepandemic period’ studied varied from 2 months to 18 years. The scores of quality assessments of the studies ranged from 5 to 9 (Table S3). There were 21 articles with moderate risk of bias and 15 articles with low risk of bias.
Fig. 1.
Flowchart of study selection.
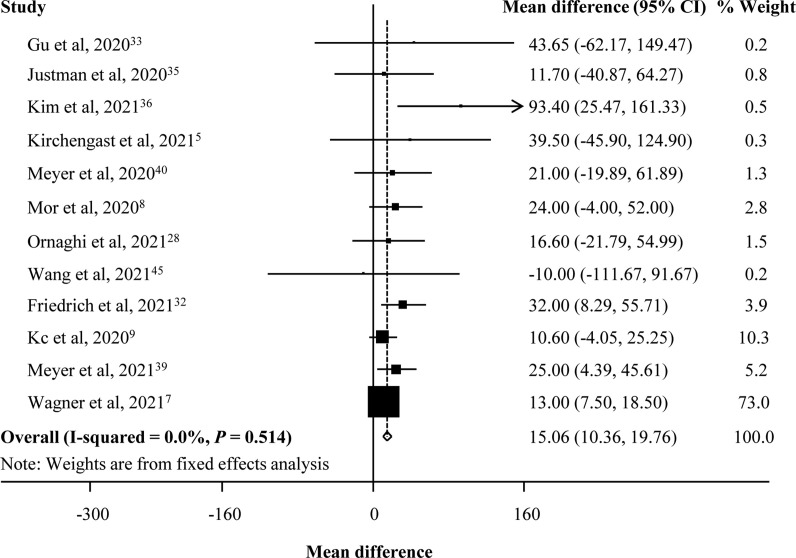
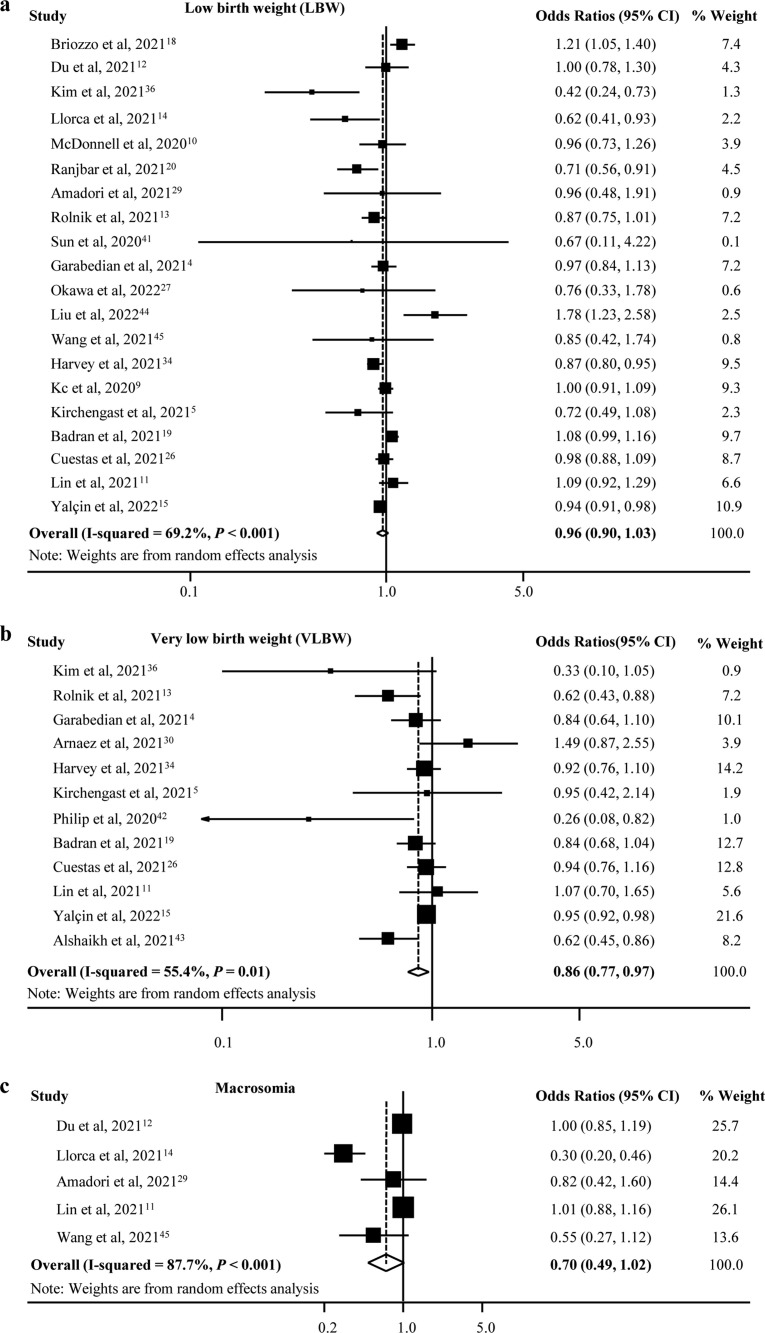
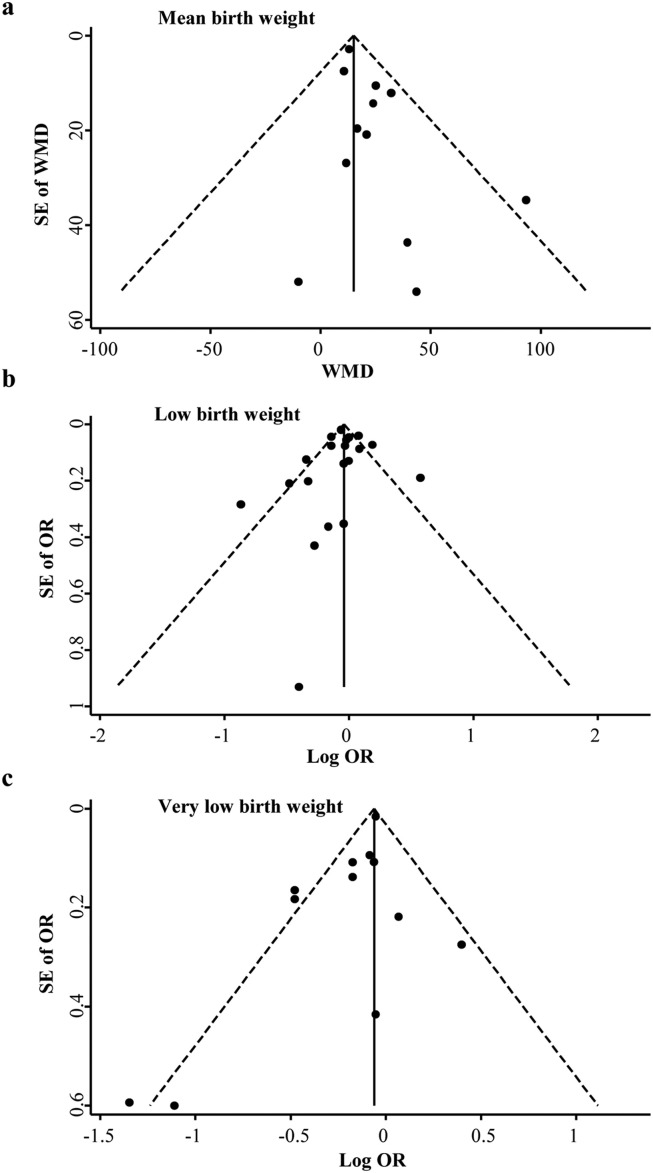
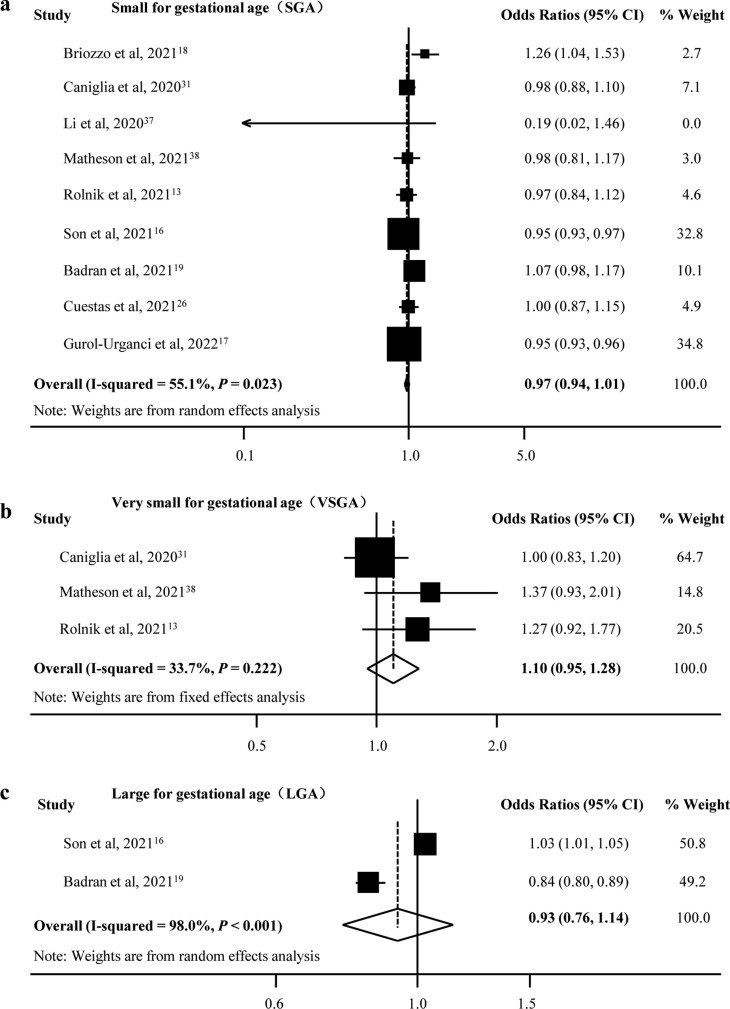
There was an increase in mean birth weight during the pandemic compared with the prepandemic period (pooled MD [95% CI] = 15.06 [10.36, 19.76], I 2 = 0.0%, 12 studies; Fig. 2 ). We only found a significant decrease in the rate of VLBW (pooled OR [95% CI] = 0.86 [0.77, 0.97], I 2 = 55.4%, 12 studies), but there was no difference for LBW and macrosomia during the pandemic compared with the prepandemic period (LBW: pooled OR [95% CI] = 0.96 [0.90, 1.03], I 2 = 69.2%, 20 studies; macrosomia: pooled OR [95% CI] = 0.70 [0.49, 1.02], I 2 = 87.7%, five studies; Fig. 3 ). Moreover, there was publication bias for mean birth weight with a borderline significance (Egger's P = 0.050) but not for LBW (Egger's P = 0.681) and VLBW (Egger's P = 0.071). Fig. 4 shows the funnel plots of the included studies for neonatal birth weight (≥10 studies). In studies with birth weight for gestational age, we found a reduction for SGA in the pandemic period with a borderline significance (SGA: pooled OR [95% CI] = 0.97 [0.94, 1.01], I 2 = 55.1%, nine studies), whereas there was no significant difference for VSGA and LGA (VSGA: pooled OR [95% CI] = 1.10 [0.95, 1.28], I 2 = 33.7%, three studies; LGA: pooled OR [95% CI] = 0.93 [0.76, 1.14], I 2 = 98.0%, two studies; Fig. 5 ).
Fig. 2.
Forest plot for mean birth weight.
Fig. 3.
Forest plot for odds of birth weight.
Fig. 4.
Funnel plots for studies reporting on birth weight.
Fig. 5.
Forest plot for odds of birth weight for gestational age.
Further subgroup analyses were performed to assess the effect of potential confounders on the pooled results. For mean birth weight, there was a significant increase during the pandemic, which was supported by most study subgroups (P < 0.01), except for the data from low-/middle-income countries (Table S4). Although the pooled odds of LBW had no change during the pandemic, the subgroups analysis showed a significant decrease in the studies from regionwide/nationwide and high-income countries (studies from regionwide/nationwide: pooled OR [95% CI] = 0.92 [0.86, 0.97], I 2 = 29.7%, three studies; studies from high-income countries: pooled OR [95% CI] = 0.85 [0.77, 0.95], I 2 = 37.7%, nine studies; Table S5). Furthermore, we also found a significant reduction for VLBW in most subgroups, including the data from adjusted odds, low-/middle-income or high-income countries, and the studies with moderate risk of bias or total sample size <10,000, but not for other subgroups (Table S6). Different from summary odds of SGA, there was a significant reduction in specific subgroups: the studies from regionwide/nationwide or high-income countries, the studies with a low risk of bias or total sample size ≥10,000, and the prepandemic period defined as equivalent period in previous years (P < 0.01; Table S7).
In sensitivity analysis, most pooled estimates were not significantly different when a study was omitted, whereas the pooled estimate effect became significant for SGA when Briozzo et al.18 or Badran et al.19 were omitted (P < 0.001 and P = 0.002).
Discussion
We have provided quantitative estimates for the associations between the COVID-19 pandemic and neonatal birth weight through a systematic search and comprehensive meta-analysis. The results showed a significant increase of 15 g in mean birth weight in the pandemic period compared with before the pandemic period. We also identified a reduction in the rate of VLBW, but not for LBW and macrosomia. Besides, there was a reduction in the rate of SGA with a borderline significance but not for VSGA and LGA with a few studies included.
Recently, some reviews mainly reported the effects of COVID-19 infection on pregnancy outcomes46, 47, 48 and the major birth outcomes, such as preterm birth and stillbirths.49, 50, 51 This meta-analysis was designed to evaluate the indirect impacts of the COVID-19 pandemic on secondary birth outcomes and excluded studies that reported outcomes of maternal COVID-19 infection. In this review, pooled results showed that LBW did not significantly change during the COVID-19 pandemic, which was supported by the previous review.50 , 51 However, we identified a significant decrease for LBW in the data from regionwide/nationwide and high-income countries. Yang et al.49 found a reduction in LBW during the pandemic using regional/national data, but there was no overall difference in LBW and VLBW. In contrast, there was a significant decrease in the rate of VLBW during the pandemic compared with the prepandemic period in our study. Nevertheless, we also found substantial heterogeneity and discordant results among the subgroups. Inconsistency among conclusions from different studies possibly be attributed to variations in sample sizes, location of the population, socio-economic status, lengths or definition of the pandemic and prepandemic periods, and different blockade measures between countries.
The previous meta-analyses mainly reported the effects of the COVID-19 pandemic on preterm, stillbirths, maternal mortality, and LBW.49, 50, 51 As a more sensitive indicator of neonatal health, SGA infants are more likely to have intensive care therapy, morbidity due to perinatal fetal distress and neonatal asphyxia, and perinatal mortality compared with appropriate for gestational age.52 However, the effect of the pandemic on SGA has not received much attention in the previous review. To improve this deficiency, our study investigated the association between SGA and the pandemic. Although we found no difference in the rate of SGA during the pandemic compared with the prepandemic period, there was a significant decrease in the data from regionwide/nationwide, high-income countries, and the studies with low risk of bias. Besides, we found a significant change when Briozzo et al.18 or Badran et al.19 were omitted, and the studies were from single-center and low-/middle-income countries. Explanations for these results may be related to substantial variation in pandemic mitigation measures and population responses among countries.
The researchers have proposed that COVID-19–related lockdown may cause maternal behavioral modifications, potential reduction in work-related stresses, optimal opportunities for rest and sleep, reduced exposure to infections, and improved opportunities for nutritional support and exercise.19 , 42 While the pandemic has caused disruption to healthcare systems, economic crises, and rising unemployment, people with high income and people in developed countries have experienced faster restructuring of healthcare systems and timely increased access to care through telehealth.53 In addition, their family assets may ease the burden of unemployment and allow them to spend more time enjoying life. However, for low-income families, it may have exacerbated socio-economic inequalities in health, limitation of health services, family conflicts, perinatal anxiety, and depression.26 , 54 In low-income countries, where remote consultations are less feasible, financial or employment issues are prominent, and maternity staff shortages exist, resulting in reduced access to preventive antenatal care and nutritional support for pregnant women.55 As a result, pregnant women can suffer negative physical and mental health outcomes. Stress, worries, and anxieties during pregnancy are often associated with LBW.56 Therefore, the effect of the pandemic on neonatal adverse birth weight is a double-edged sword. COVID-19 is not only a pandemic and global health crisis but also a psychosocial and economic disaster. The potential influence of a multitude of biological, physical, and environmental factors could cumulatively modify the birth outcomes of neonates.
The strength of this review was the inclusion of large populations from 22 countries and the synthesis of a broad range of articles. We mainly investigate the potential effects of the COVID-19 pandemic on secondary birth outcomes, and deep subgroup analysis was conducted to clarify the source of heterogeneity. Nevertheless, our study had some limitations. First, the included studies all used a retrospective design, and the heterogeneity of study countries, the definitions of comparable groups, and statistical methodology all affected the comparability of results. Besides, we only reported the non–COVID-19 infection population, and more birth outcomes during the pandemic should be further assessed.
Conclusion
In this study, we found the COVID-19 pandemic was significantly associated with an increase in mean birth weight and a reduction in VLBW, but not for other outcomes. However, subgroup analysis showed that LBW and SGA had a significant reduction in specific subgroups. This review provided clues about the indirect effects of the pandemic on neonatal birth weight. Further studies should be conducted to clarify the underlying mechanisms of the findings, and more healthcare measures needed to improve neonatal long-term health.
Author statements
Author contributions
J.W. initiated, conceived, and supervised the study. X.Y., H.J., J.M., and Y.L. did data collection and performed the data analysis. All authors approved the final format of the submitted manuscript.
Ethical approval
None sought.
Funding
This work was supported by Jiangsu provincial key research and development program (BE2020626).
Competing interests
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2023.04.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Temesgen Z.M., DeSimone D.C., Mahmood M., Libertin C.R., Varatharaj Palraj B.R., Berbari E.F. Health care after the COVID-19 pandemic and the influence of telemedicine. Mayo Clinic Proc. 2020;95:S66–S68. doi: 10.1016/j.mayocp.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y.N., Chen Y., Wang Y., Li F., Pender M., Wang N., et al. Reduction in healthcare services during the COVID-19 pandemic in China. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao C., Jin F., Hao C., Zhang X., Xie L., Zhang Y., et al. Evaluation of the effects on uninfected pregnant women and their pregnancy outcomes during the COVID-19 pandemic in Beijing, China. Front Med. 2022;9 doi: 10.3389/fmed.2022.842826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garabedian C., Dupuis N., Vayssière C., Bussières L., Ville Y., Renaudin B., et al. Impact of COVID-19 lockdown on preterm births, low birth weights and stillbirths: a retrospective cohort study. J Clin Med. 2021;10:5649. doi: 10.3390/jcm10235649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchengast S., Hartmann B. Pregnancy outcome during the first COVID 19 lockdown in Vienna, Austria. Int J Environ Res Public Health. 2021;18:3782. doi: 10.3390/ijerph18073782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reneker J.C., Zhang Y., Young D.K., Liu X., Lutz E.A. Use of telehealth services for prenatal care in Mississippi: comparison of pre-COVID-19 pandemic and pandemic obstetric management. Int J Clin Pract. 2022;2022 doi: 10.1155/2022/3535700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner M., Falcone V., Neururer S.B., Leitner H., Delmarko I., Kiss H., et al. Perinatal and postpartum care during the COVID-19 pandemic: a nationwide cohort study. Birth. 2022;49:243–252. doi: 10.1111/birt.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mor M., Kugler N., Jauniaux E., Betser M., Wiener Y., Cuckle H., et al. Impact of the COVID-19 pandemic on excess perinatal mortality and morbidity in Israel. Am J Perinatol. 2021;38:398–403. doi: 10.1055/s-0040-1721515. [DOI] [PubMed] [Google Scholar]
- 9.Kc A., Gurung R., Kinney M.V., Sunny A.K., Moinuddin M., Basnet O., et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8:e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonnell S., McNamee E., Lindow S.W., O'Connell M.P. The impact of the Covid-19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin T.-T., Zhang C., Chen L., Jin L., Lin X.-H., Pan J.-X., et al. COVID-19 lockdown increased the risk of preterm birth. Front Med. 2021;8 doi: 10.3389/fmed.2021.705943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du M., Yang J., Han N., Liu M., Liu J. Association between the COVID-19 pandemic and the risk for adverse pregnancy outcomes: a cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolnik D.L., Matheson A., Liu Y., Chu S., McGannon C., Mulcahy B., et al. Impact of COVID-19 pandemic restrictions on pregnancy duration and outcome in Melbourne, Australia. Ultrasound Obstet Gynecol. 2021;58:677–687. doi: 10.1002/uog.23743. [DOI] [PubMed] [Google Scholar]
- 14.Llorca J., Lechosa-Muñiz C., Frank de Zulueta P., López-Gómez S., Orallo V., Alonso-Molero J., et al. Results of pregnancy control before and during the COVID-19 pandemic: a comparison of two cohorts. Int J Environ Res Public Health. 2021;18:8182. doi: 10.3390/ijerph18158182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yalcin S.S., Boran P., Tezel B., Sahlar T.E., Ozdemir P., Keskinkilic B., et al. Effects of the COVID-19 pandemic on perinatal outcomes: a retrospective cohort study from Turkey. BMC Pregnancy Childbirth. 2022;22:51. doi: 10.1186/s12884-021-04349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son M., Gallagher K., Lo J.Y., Lindgren E., Burris H.H., Dysart K., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy outcomes in a U.S. population. Obstet Gynecol. 2021;138:542–551. doi: 10.1097/AOG.0000000000004547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurol-Urganci I., Waite L., Webster K., Jardine J., Carroll F., Dunn G., et al. Obstetric interventions and pregnancy outcomes during the COVID-19 pandemic in England: a nationwide cohort study. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briozzo L., Tomasso G., Viroga S., Nozar F., Bianchi A. Impact of mitigation measures against the COVID 19 pandemic on the perinatal results of the reference maternity hospital in Uruguay. J Matern Fetal Neonatal Med. 2022;35:5060–5062. doi: 10.1080/14767058.2021.1874911. [DOI] [PubMed] [Google Scholar]
- 19.Badran E.F., Darwish R.M., Khader Y., AlMasri R., Al Jaberi M., AlMasri M., et al. Adverse pregnancy outcomes during the COVID-19 lockdown. A descriptive study. BMC Pregnancy Childbirth. 2021;21:761. doi: 10.1186/s12884-021-04221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranjbar F., Allahqoli L., Ahmadi S., Mousavi R., Gharacheh M., Eshraghi N., et al. Changes in pregnancy outcomes during the COVID-19 lockdown in Iran. BMC Pregnancy Childbirth. 2021;21:577. doi: 10.1186/s12884-021-04050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villar J., Giuliani F., Fenton T.R., Ohuma E.O., Ismail L.C., Kennedy S.H. Intergrowth-21st very preterm size at birth reference charts. Lancet. 2016;387:844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 23.Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 24.Fenton T.R. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GA Wells, B Shea, D O'Connell, J Peterson, V Welch, M Losos, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [accessed 16 October 2022].
- 26.Cuestas E., Gomez-Flores M.E., Charras M.D., Peyrano A.J., Montenegro C., Sosa-Boye I., et al. Socioeconomic inequalities in low birth weight risk before and during the COVID-19 pandemic in Argentina: a cross-sectional study. Lancet Reg Health Am. 2021;2 doi: 10.1016/j.lana.2021.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okawa S., Hosokawa Y., Nanishi K., Zaitsu M., Tabuchi T. Threatened abortion, threatened premature labor, and preterm birth during the first state of emergency for COVID-19 in 2020 in Japan. J Obstet Gynaecol Res. 2022;48:1116–1125. doi: 10.1111/jog.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornaghi S., Fumagalli S., Guinea Montalvo C.K., Beretta G., Invernizzi F., Nespoli A., et al. Indirect impact of SARS-CoV-2 pandemic on pregnancy and childbirth outcomes: a nine-month long experience from a university center in Lombardy. Int J Gynaecol Obstet. 2022;156:466–474. doi: 10.1002/ijgo.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amadori R., Aquino C.I., Colagiorgio S., Osella E., Surico D., Remorgida V. What may happen if you are pregnant during Covid-19 lockdown? A retrospective study about peripartum outcomes. Minerva Obstet Gynecol. 2022;74:319–324. doi: 10.23736/S2724-606X.21.04878-8. [DOI] [PubMed] [Google Scholar]
- 30.Arnaez J., Ochoa-Sangrador C., Caserío S., Gutiérrez E.P., Jiménez M.D.P., Castañón L., et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur J Pediatr. 2021;180:1997–2002. doi: 10.1007/s00431-021-03984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caniglia E.C., Magosi L.E., Zash R., Diseko M., Mayondi G., Mabuta J., et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. 2021;224:615.e1–615.e12. doi: 10.1016/j.ajog.2020.12.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedrich L., Levin G., Maixner N., Bart Y., Tsur A., Yinon Y., et al. Hematologic adaptation to mask-wearing among pregnant women and obstetrical outcome during the coronavirus disease 2019 pandemic. Int J Gynaecol Obstet. 2021;154:297–303. doi: 10.1002/ijgo.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu X.X., Chen K., Yu H., Liang G.Y., Chen H., Shen Y. How to prevent in-hospital COVID-19 infection and reassure women about the safety of pregnancy: experience from an obstetric center in China. J Int Med Res. 2020;48 doi: 10.1177/0300060520939337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey E.M., McNeer E., McDonald M.F., Shapiro-Mendoza C.K., Dupont W.D., Barfield W., et al. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr. 2021;175:635–637. doi: 10.1001/jamapediatrics.2020.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justman N., Shahak G., Gutzeit O., Ben Zvi D., Ginsberg Y., Solt I., et al. Lockdown with a price: the impact of the COVID-19 pandemic on prenatal care and perinatal outcomes in a tertiary care center. Isr Med Assoc J. 2020;22:533–537. [PubMed] [Google Scholar]
- 36.Kim S.Y., Kim S.Y., Kil K., Lee Y. Impact of COVID-19 mitigation policy in South Korea on the reduction of preterm or low birth weight birth rate: a single center experience. Children. 2021;8:332. doi: 10.3390/children8050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Yin H., Jin Z., Zhang H., Leng B., Luo Y., et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheson A., McGannon C.J., Malhotra A., Palmer K.R., Stewart A.E., Wallace E.M., et al. Prematurity rates during the coronavirus disease 2019 (COVID-19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. 2021;137:405–407. doi: 10.1097/AOG.0000000000004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer R., Bart Y., Tsur A., Yinon Y., Friedrich L., Maixner N., et al. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224:234–237. doi: 10.1016/j.ajog.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer R., Levin G., Hendin N., Katorza E. Impact of the COVID-19 outbreak on routine obstetrical management. Isr Med Assoc J. 2020;22:483–488. [PubMed] [Google Scholar]
- 41.Sun S.Y., Guazzelli C.A.F., de Morais L.R., Dittmer F.P., Augusto M.N., Soares A.C., et al. Effect of delayed obstetric labor care during the COVID-19 pandemic on perinatal outcomes. Int J Gynaecol Obstet. 2020;151:287–289. doi: 10.1002/ijgo.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philip R.K., Purtill H., Reidy E., Daly M., Imcha M., McGrath D., et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alshaikh B., Cheung P.Y., Soliman N., Brundler M.A., Yusuf K. Impact of lockdown measures during COVID-19 pandemic on pregnancy and preterm birth. Am J Perinatol. 2022;39:329–336. doi: 10.1055/s-0041-1739357. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Dai M., Tang S. Effect of initial COVID-19 outbreak during first trimester on pregnancy outcome in Wuxi, China. BMC Pregnancy Childbirth. 2022;22:54. doi: 10.1186/s12884-022-04395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Wang Y., He M.Y., Li Y.X., Cheng X., Yang X., et al. Maternal and infant outcomes during the COVID-19 pandemic: a retrospective study in Guangzhou, China. Reprod Biol Endocrinol. 2021;19:126. doi: 10.1186/s12958-021-00807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L., et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J., D'Souza R., Kharrat A., Fell D.B., Snelgrove J.W., Shah P.S. COVID-19 pandemic and population-level pregnancy and neonatal outcomes in general population: a living systematic review and meta-analysis (Update#2: November 20, 2021) Acta Obstet Gynecol Scand. 2022;101:273–292. doi: 10.1111/aogs.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chmielewska B., Barratt I., Townsend R., Kalafat E., van der Meulen J., Gurol-Urganci I., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaccaro C., Mahmoud F., Aboulatta L., Aloud B., Eltonsy S. The impact of COVID-19 first wave national lockdowns on perinatal outcomes: a rapid review and meta-analysis. BMC Pregnancy Childbirth. 2021;21:676. doi: 10.1186/s12884-021-04156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi Y., Wang X., Mao J. Quantitative assessment of cerebral metabolism and hemodynamics in small-for-gestational-age (SGA) newborns. Quant Imaging Med Surg. 2021;11:2321–2332. doi: 10.21037/qims-20-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madden N., Emeruwa U.N., Friedman A.M., Aubey J.J., Aziz A., Baptiste C.D., et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York city: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005–1014. doi: 10.1055/s-0040-1712939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamadani J.D., Hasan M.I., Baldi A.J., Hossain S.J., Shiraji S., Bhuiyan M.S.A., et al. Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: an interrupted time series. Lancet Glob Health. 2020;8:e1380–e1389. doi: 10.1016/S2214-109X(20)30366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goyal M., Singh P., Singh K., Shekhar S., Agrawal N., Misra S. The effect of the COVID-19 pandemic on maternal health due to delay in seeking health care: experience from a tertiary center. Int J Gynaecol Obstet. 2021;152:231–235. doi: 10.1002/ijgo.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobel C.J., Goldstein A., Barrett E.S. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51:333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.