Abstract
Background
The present study aimed to investigate the progression of the SARS-CoV-2 pandemic in Ireland over the first three waves of infection.
Method
A selection of blood donor serum samples collected between February 2020 and December 2021 were analysed by various commercially available serological assays for antibodies to SARS-CoV-2 (n = 15,066).
Results
An increase in seropositivity was observed between wave 1 (February to September 2020) and wave 2 (November and December 2020) of 2.20% to 3.55%. A large increase in estimated seroprevalence to 11.89% was observed in samples collected in February and March 2021 (wave 3 of infection).The rate of seropositivity varied by age group, with the highest rate observed in the youngest donors (18–29 years) peaking at 18.79% in wave 3. The results of spike antibody (anti-S) testing indicated that 44/1009 (4.36%) of seroreactive donors in wave 3 had a serological profile consistent with vaccination. By November 2021, we detected an overall seropositivity of 97.04%.
Conclusions
The present study provides a comprehensive estimation of the level of circulating SARS-CoV-2 antibodies in Irish blood donors, enabling differentiation between vaccination and natural infection, as well as real-time monitoring of the progression of the COVID-19 pandemic in Ireland. Seroepidemiology has a role in determining reliable estimates of transmission, infection fatality rates and vaccine uptake. The continued screening of blood donors for this purpose has the potential to generate important data to assist with the management of future waves of SARS-CoV-2.
Keywords: Ireland, SARS-CoV-2, Epidemiology, Blood, Donor, Vaccination, Serology
Highlights
-
•
Report the progression of the SARS-CoV-2 pandemic in Ireland over the first three waves of infection
-
•
By end of 2021, 97% of Irish blood donors were positive for SARs-CoV-2 antibodies
-
•
Vaccination and infection result in different levels of anti-spike production.
-
•
Seroepidemiology has a key role estimating the extent of SARS-CoV-2 pandemic
Introduction
The World Health Organisation (WHO) issued guidance in May 2020 for population-based age-stratified COVID-19 seroepidemiological investigations recommending suitable populations for studies which included blood donors [1]. Indeed, seroepidemiological assessment of SARS-CoV-2 infection in blood donors assisted with the surveillance and assessment of spread of the COVID-19 pandemic in Ireland [[2], [3], [4]]. We previously reported the detection of SARS-CoV-2 antibodies in Irish blood donors two weeks prior to the first notification of COVID-19 infection in Ireland [5]. Seroprevalence estimates indicate that approximately one third of individuals with detectable SARS-CoV-2 antibodies are asymptomatic [[6], [7], [8]]. Irish COVID-19 surveillance reports indicate that 82% of those with detectable viral RNA were symptomatic at the time of testing over the first year of the pandemic [3]. As a result, and consistent with many infectious disease outbreaks, the full extent of the SARS-CoV-2 pandemic in Ireland is likely under-recorded [7]. Blood donor studies offer a unique opportunity to screen healthy populations for the presence of antibodies to new and emerging infections [[9], [10], [11]].
SARS-CoV-2 infection results in the production of antibodies to many different SARS-CoV-2 antigens, including the viral nucleocaspid (N) and Spike (S) proteins. In contrast, vaccines currently approved in Ireland induce antibody production against the S protein only. Therefore, a combination of anti-N and anti-S antibody screening has been used to distinguish between a serological profile consistent with SARS-CoV-2 infection, and that of immunisation, as anti-N would not develop in response to vaccination alone [1,12]. There are limitations with this approach however, as the lack of a durable anti-N response in some individuals confounding the reporting in many cases [13]. Therefore, the use of serology screening assays as an epidemiological tool for the surveillance of SARS-CoV-2 has had to evolve alongside the pandemic and a comprehensive vaccination programme.
The present study aimed to serologically track the progression of the SARS-CoV-2 pandemic in Ireland over the first three waves of infection and to develop an optimum testing algorithm for differentiating between prior infection and vaccination, thereby adding to the body of epidemiological information employed by public health to respond to an ever-evolving pandemic.
Materials & methods
Study design and ethical approval
A selection of anonymised blood donor samples received by the Irish Blood Transfusion Service (IBTS) between February 2020 and December 2021 were analysed for antibodies to SARS-CoV-2 and categorised according to the time period at which they were collected. Due to operational constraints, time points reflecting the major changes in the COVID-19 landscape in Ireland were selected. Samples collected from February to September 2020 represent Wave 1 of the pandemic (n = 8509), samples from November to December 2020 represent Wave 2 (n = 1014) and samples collected from February to March 2021 represent Wave 3 (n = 1009). An additional cohort of samples from October to December 2021 (n = 4534) were selected to represent the donor population after the vaccination rollout. This study was approved by the National Office for Research Ethics Committee (www.nrecoffice.ie). Limited demographic information was collected and included age, gender and donation clinic. No clinical information, including symptoms of possible COVID-19 infection, or vaccination status were available.
Donor SARS-CoV-2 antibody screening
Three antibody screening algorithms were used. The testing strategies were adapted to reflect the increasing availability of commercial assays and the commencement of the national vaccination programme. All testing was carried out as per manufacturer's instructions.
Algorithm 1
Donor serum samples screened during wave 1 and wave 2 were tested with the SARS-CoV-2 IgG assay (Abbott Diagnostics) to detect anti-N, SARS-CoV-2 IgG II Quantitative assay (Abbott Diagnostics) targeting anti-S and SARS-CoV-2 Total Antibody assay (Abbott Diagnostics) targeting anti-N and anti-S antibodies. A selection of weakly reactive samples or samples with discordant results (n = 40) were referred to the University College Dublin National Virus Reference Laboratory for further analysis as per Butler et al [5].
Algorithm 2
Samples from Wave 3, that were reactive with both the Abbott SARS-CoV-2 IgG assay and the Abbott SARS-CoV-2 IgG II Quantitative assay (S+/N+), were classified as indicative of previous infection. In response to evidence in the literature indicating a decline in the sensitivity of the Abbott Architect SARS-CoV-2 IgG assay over time, samples that were anti-S positive and anti-N negative (S+/N-) were further classified according to anti-S quantitative results.
Algorithm 3
Samples from October–December 2021 were screened using the Abbott SARS-CoV-2 IgG II Quantitative Assay, which detects Anti-S. Reactive specimens were referred for testing at Central Pathology Laboratory (CPL), St. James's Hospital using the Roche Elecsys® Anti-SARS-CoV-2 IgG assay which is a highly sensitive assay for the qualitative detection of Anti-N. This work was carried out for the purposes of surveillance work in collaboration with the Health Protection Surveillance Centre.
Statistical analysis
Statistical analyses were performed using the statistical software package IBM SPSS (Version 27) and MedCalc (www.medcalc.org). Assay performance characteristics were calculated based on the samples from Wave 1 (Algorithm 1) according to the testing algorithm described by Butler et al [5]. Seroprevalence rates were adjusted for age and sex to reflect the Irish population demographics using the 2016 CENSUS data as per Lewin et al [14].
The Chi-Square test and confidence intervals were used to assess associations between donor demographic variables.
Results
The changing SARS-CoV-2 seroepidemiological profile during three waves of infection
The SARS-CoV-2 seroprevalence in Irish blood donors after the three waves of infection in Ireland was estimated. Overall, a total of 343/10,533 donor samples were deemed positive for the presence of SARS-CoV-2 antibodies between February 2020 and March 2021. An increase in seropositivity was observed between wave 1 (February to September 2020) and wave 2 (November and December 2020) of 2.20% [95% CI; 1.89 to 2.54] to 3.55% [95% CI; 2.49 to 4.92]. A large increase in estimated seroprevalence to 11.89% [95% CI; 9.89 to 13.89] was observed in samples collected in Wave 3. Wave 3 seroepidemiology was influenced by the vaccination programme which began on the 29th December 2020, starting with healthcare workers and those over 80 years old. Quantitative anti-S testing indicates that 44/1009 (4.36%) of donors in wave 3 had serological profiles consistent with vaccination. SARS-CoV-2 antibody detection rates for donor demographics and time periods are listed in Table 1.
Table 1.
Donor sample population demographics and SARS-CoV-2 antibody detection for waves 1, 2 and 3.
| Wave 1 |
Wave 2 |
Wave 3 (past infection) |
Wave 3 (Vaccinated) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SARS-CoV-2 Ab detected | Crude Seroprevalence % (95% CI) | Adjusted Seroprevalence % (95% CI) | Total | SARS-CoV-2 Ab detected | Crude Seroprevalence % (95% CI) | Adjusted Seroprevalence % (95% CI) | Total | SARS-CoV-2 Ab detected | Crude Seroprevalence % (95% CI) | Adjusted Seroprevalence % (95% CI) | Total | SARS-CoV-2 Ab detected | Crude Seroprevalence % (95% CI) | Adjusted Seroprevalence % (95% CI) | ||
| Total | 8509 | 187 | 2.20% [1.89–2.54] |
2.48% [2.16–2.84] |
1014 | 36 | 3.55% [2.49–4.92] |
6.69% [5.21–8.50] |
1009 | 76 | 7.53% [5.94–9.43] |
10.76% [8.87–13.03] |
1009 | 44 | 4.36% [3.17–5.85] |
5.40% [4.11–7.10] |
|
| Gender | |||||||||||||||||
| Male | 4842 | 104 | 2.15% [1.76–2.60] |
1.86% [1.50–2.29] |
584 | 19 | 3.25% [1.96–5.08] |
2.79% [1.57–4.45] |
580 | 46 | 7.93% [5.81–10.58] |
6.82% [4.93–9.39] |
580 | 8 | 1.38% [0.60–2.72] |
1.19% [0.49–2.49] |
|
| Female | 3667 | 83 | 2.26% [1.80–2.81] |
2.43% [2.15–3.23] |
430 | 17 | 3.95% [2.30–6.33] |
4.71% [2.84–7.18] |
429 | 30 | 6.99% [4.72–9.98] |
8.32% [5.88–11.62] |
429 | 36 | 8.39% [5.88–11.62] |
10.02% [7.25–13.50] |
|
| Age (years) | |||||||||||||||||
| 18–29 | 1133 | 46 | 4.06% [2.97–5.41] |
5.3% [4.04–6.82] |
137 | 7 | 5.11% [2.05–10.53] |
6.58% [3.00–12.47] |
149 | 18 | 12.08% [7.16–19.09] |
14.23% [8.72–21.54] |
149 | 10 | 6.71% [3.22–12.34] |
8.05% [4.16–14.07] |
|
| 30–39 | 1664 | 46 | 2.76% [2.02–3.69] |
3.55% [2.70–4.57] |
195 | 7 | 3.59% [1.44–7.40] |
4.65% [2.11–8.76] |
197 | 10 | 5.08% [2.43–9.34] |
6.60% [3.51–11.28] |
197 | 9 | 4.57% [2.09–8.67] |
5.58% [2.79–9.99] |
|
| 40–49 | 2271 | 39 | 1.72% [1.22–2.35] |
1.50% [1.04–2.09] |
264 | 6 | 2.27% [0.47–4.07] |
5.49% [2.51–10.42] |
278 | 19 | 6.83% [4.12–10.67] |
5.77% [3.29–9.35] |
278 | 8 | 2.88% [1.24–5.67] |
2.52% [1.01–5.19] |
|
| 50–59 | 2241 | 37 | 1.65% [1.16–2.28] |
1.21% [0.79–1.75] |
276 | 9 | 3.26% [1.49–6.19] |
2.32% [0.80–4.73] |
228 | 17 | 7.46% [4.34–11.94] |
6.58% [3.68–10.85] |
228 | 10 | 4.39% [2.10–8.07] |
3.95% [1.81–7.49] |
|
| ≥60 | 1200 | 19 | 1.58% [0.95–2.47] |
1.67% [1.02–2.57] |
142 | 7 | 4.93% [1.98–10.16] |
5.31% [2.43–11.10] |
157 | 12 | 7.64% [3.48–11.80] |
9.42% [5.02–16.11] |
138 | 7 | 5.07% [2.04–10.45] |
5.80% [2.50–11.42] |
|
| Province | |||||||||||||||||
| Leinster | 4955 | 136 | 2.74% [2.30–3.25] |
2.60% [2.17–3.09] |
589 | 23 | 3.90% [2.48–5.86] |
3.72% [2.34–5.66] |
562 | 52 | 9.25% [6.85–11.65] |
12.33% [9.44–15.92] |
485 | 25 | 5.15% [3.34–7.61] |
5.98% [4.00–8.59] |
|
| Munster | 2257 | 37 | 1.64% [1.15–2.26] |
1.68% [1.19–2.31] |
305 | 12 | 3.93% [2.03–6.87] |
3.61% [1.80–6.45] |
323 | 17 | 5.26% [3.07–8.43] |
4.42% [2.37–7.27] |
323 | 17 | 5.26% [3.07–8.43] |
4.33% [2.37–7.27] |
|
| Connacht | 649 | 5 | 0.77% [0.25–1.80] |
1.23% [0.53–2.43] |
34 | 1 | 2.94% [0.07–16.39] |
8.82% [1.82–25.79] |
38 | 4 | 10.53% [2.87–26.95] |
32.5% [16.32–55.16] |
38 | 0 | 0.00% [0.00–0.00] |
0.00% [0.00–0.00] |
|
| Ulster | 648 | 9 | 1.39% [0.63–2.63] |
1.08% [0.43–2.22] |
86 | 0 | 0.00% [0.00–0.00] |
0.00% [0.00–0.00] |
86 | 3 | 3.49% [0.72–10.20] |
2.33% [0.28–8.40] |
86 | 2 | 2.33% [0.28–8.40] |
1.70% [0.03–6.48] |
|
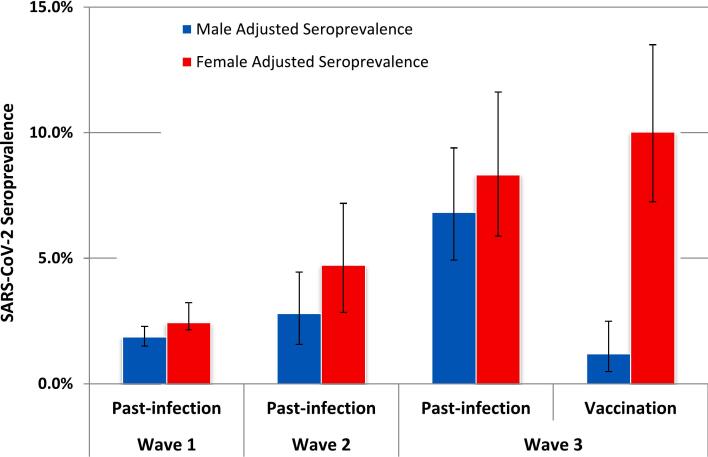
There was no difference in the rate of seropositivity between males and females throughout wave 1 and wave 2. However, a higher rate of vaccination was detected in females of 8.39% [95% CI; 5.88–11.62] compared to males, at 1.38% [95% CI; 0.60–2.72] following roll-out of the vaccination programme in wave 3 (Fig. 1). Seroprevalence varied by age group, with the highest rate consistently detected in the youngest age category of 18–29 year olds, peaking at 18.79% [95% CI; 12.52–25.06] in wave 3.
Fig. 1.
SARS-CoV-2 antibody detection rates in Irish Blood Donations adjusted to National demographic distributions recorded in the 2016 CENSUS.
Error bars represent 95% confidence intervals.
Evaluation of the SARS-CoV-2 serological screening assays
Performance characteristics of the SARS-CoV-2 assays in the analysis of 8509 anonymised donor specimens from Wave 1 of the pandemic are reported in Table 2. Samples were classified as ‘true’ or ‘false’ positive or negative as per the testing algorithm and based on the result concordance between all assays as per Butler et al [5]. Samples that were negative by all three screening assays were considered truly negative, those that were positive on at least two out of three screening assays were considered positive. Inconclusive results were referred for additional testing at the National Virus Reference Laboratory using the Fortress Diagnostics Wantai Total antibody assay [5]. Due to the emerging nature of SARS-CoV-2 assays and the absence of a gold standard for SARS-CoV-2 serology, the limitations of the testing algorithms in classifying samples as ‘true’ positive and ‘true’ negative was negated by the inclusion of multiple assays targeting different antibody classes and from different manufacturers.
Table 2.
SARS-CoV-2 antibody screening assay performance evaluation in healthy blood donors.
| Assay | Negative | Grey zone | Positive | Sensitivity % [+/− 95% CI] |
Specificity % [+/− 95% CI] |
AUC [+/− 95% CI] |
PPV % [+/− 95% CI] |
NPV % [+/− 95% CI] |
|
|---|---|---|---|---|---|---|---|---|---|
| Abbott SARS-CoV-2 IgG | 8051 | 341 | 117 | ||||||
| aw/o Grey zone | 8051 | – | 117 | 75.6 [67.0 to 82.9] | 99.7 [99.6 to 99.8] | 0.877 [0.869 to 0.884] | 79.5 [72.0 to 85.4] | 99.6 [99.5 to 99.7] | |
| bGrey zone negative | 8392 | – | 117 | 55.7 [47.8 to 63.4] | 99.7 [99.6 to 99.8] | 0.777 [0.768 to 0.786] | 79.5 [71.8 to 85.5] | 99.1 [99.0 to 99.3] | |
| cGrey zone positive | 8051 | – | 458 | 75.6 [67.0 to 82.9] | 95.7 [95.2 to 96.1] | 0.856 [0.849 to 0.864] | 20.3 [18.1 to 22.7] | 99.6 [99.5 to 99.7] | |
| Abbott SARS-CoV-2 IgGII Quant | 8303 | N/A | 206 | 98.8 [95.7 to 99.9] | 99.5 [99.3 to 99.6] | 0.992 [0.989 to 0.993] | 80.1 [74.8 to 84.5] | 100.0 [99.9 to 100.0] | |
| Abbott SARS-CoV-2 Total Ab | 8315 | N/A | 194 | 96.8 [93.1 to 98.8] | 99.8 [99.7 to 99.9] | 0.983 [0.980 to 0.986] | 93.3 [89.0 to 96.0] | 99.9 [99.8 to 100.0] | |
| Final ‘True’ Result | 8322 | N/A | 187 | – | – | – | – | – | |
Grey zone results were excluded from assay performance calculations.
Grey zone results were classified as negative for assay performance calculations.
Grey zone results were classified as positive for assay performance calculations.
The SARS-CoV-2 IgG II Quantitative assay demonstrated the best overall performance with a sensitivity and specificity of 98.8% [95% CI; 95.7 to 99.9] and 99.5% [95% CI; 99.3 to 99.6], respectively and a higher area-under-the-curve value (0.992 [95% CI 0.989 to 0.993], p < 0.05) compared to the other screening assays, (Table 2). The SARS-CoV-2 Total Ab assay also performed well with a higher positive predictive value than the other screening assays, and a calculated sensitivity and specificity of >96%. However, the interpretation of the Abbott SARS-CoV-2 IgG was challenging owing to the greyzone category. Indeed, reference testing using the Fortress Diagnostics Wantai total antibody assay suggested all 50 ‘greyzone –only’ results (i.e. samples without reactivity in any other assay), were falsely reactive. Therefore, for consistency and simplicity ‘greyzone–only’ samples were categorised as antibody negative. We do acknowledge however, that equivocal results such as these may indicate ‘true’ declining antibody levels in the donor sample and further highlight the limitations in the clinical utility of serological analysis using this assay. Inferring all greyzone detections as negative, reduced assay sensitivity to only 55.7% [95% CI; 47.8 to 63.4]. ‘Greyzone-only’ results were excluded from the final calculations of sensitivity and specificity, which were 75.6% [95% CI; 67.0 to 82.9] and 99.7% respectively [95% CI; 99.6 to 99.8].
Quantitative serological differentiation between COVID-19 vaccination and past infection
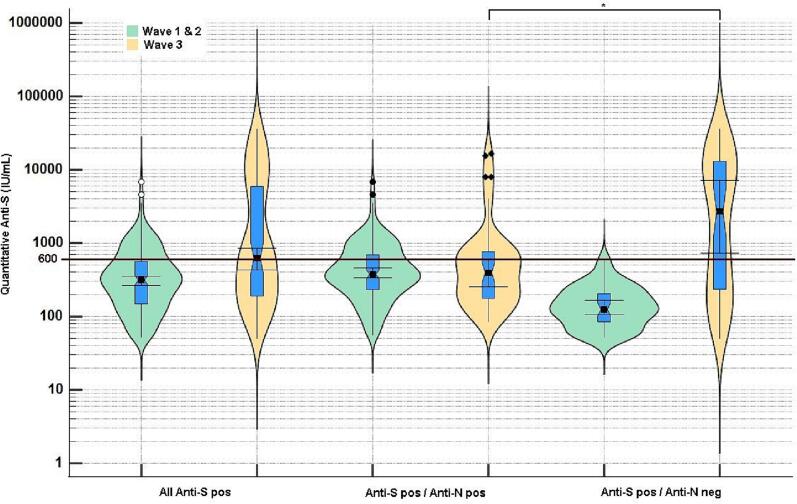
In light of the suboptimal sensitivity of the Abbott SARS-CoV-2 IgG assay, an alternative method to discriminate between vaccine-derived immunity and antibody production following infection that would not rely on the sensitivity of the anti-N assay was adopted. The incorporation of the Abbott SARS-CoV-2 IgG II quantitative assay into the testing algorithm enabled quantitative detection of the anti-S antibody concentration. Lower anti-S IgG quantitative values were observed in donor specimens tested prior to the rollout of national COVID-19 vaccination programme. Donor samples that were negative for anti-N, but positive for anti-S, had average quantitative values during wave 1 and 2 of <600 AU/mL (mean: wave 1504.5; wave 2485.2); however, in wave 3, donors with this antibody profile, had higher anti-S quantitative values, with an average value of 5053.1 AU/mL (Fig. 2). The higher SARS-CoV-2 IgG II quantitative result observed in S+/N- donors in samples from wave 3, allowed for the estimation of an anti-S concentration cut off of 600 AU/mL which, in the absence of anti-N reactivity, was suggestive of a vaccine-specific immune response (testing algorithm 2).
Fig. 2.
Quantitative Anti-Spike antibody levels in Irish Blood donors grouped according to time of collection and serological profile. Marked differences were observed before and after vaccination and quantitative values >600 IU/mL were indicative of vaccination.
The Impact of vaccination on the seroprevalence of SARS-CoV-2 antibodies
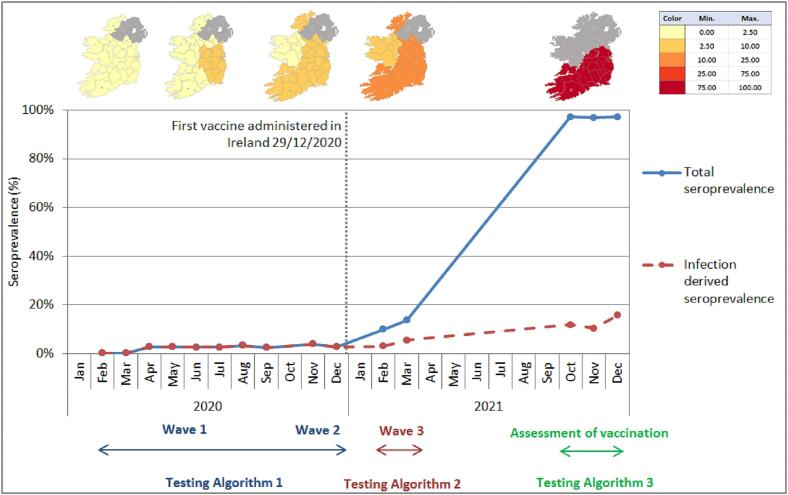
By the end of December 2021, the first two doses of COVID-19 vaccinations had been administered to 94.4% of the adult population, and the vaccination booster programme was on-going with over 2 million booster doses administered by the end of December [4]. We report an overall seropositivity of 96.93% [95% CI; 96.17–97.70] in November 2021 compared to 3.41% [95% CI; 1.95–4.85] in November 2020. In contrast the rate of antibody detection due to natural infection during this time was only 12.59% [95% CI; 11.62–13.56] compared to 3.55% [95% CI; 2.49–4.92] one year previously in November and December 2020 (Fig. 3).
Fig. 3.
SARS-CoV-2 antibody detection rate in Irish Blood Donors in 2020 and 2021.
Discussion
Serology has played a key role in both retrospective and real-time modelling of the spread of SARS-CoV-2 infection and is useful in tracking asymptomatic transmission in ‘healthy’ individuals, such as healthcare workers and blood donors [1,4,11,15]. The results of the present study are consistent with previous findings on the performance of SARS-CoV-2 antibody diagnostic assays [16]. However, the majority of performance evaluation studies of SARS-CoV-2 serology assays have typically assessed sensitivity using samples from SARS-CoV-2 PCR positive patients at known time points after the onset of symptoms. We describe a healthy blood donor population, in which the Abbott SARS-CoV-2 IgG assay yielded inconsistent results. Donor results that were scattered throughout the entire “greyzone” range of the Abbott SARS-CoV-2 IgG assay were classified as either serologically positive or negative, following repeat testing using another assay. It is possible that limiting the greyzone range may improve assay specificity but conversely may impact the identification of true positive samples in the clinical setting. The sensitivity of the Abbott SARS-CoV-2 IgG assay has been reported to reduce to 71% at >81 days post diagnosis [17]. In addition, 10–30% of healthcare workers in the United Kingdom with mild SARS-CoV-2 infection have had negative results with this assay, yielding a reported sensitivity of 79.3% in mildly symptomatic individuals [18], and we conclude is not sufficiently sensitive as screening tool for asymptomatic infections in blood donors.
The “Adapting UNITY study protocols to COVID-19 vaccine” recommends using two highly sensitive serological assays to accurately determine the proportion of the population that have vaccine-induced antibodies to SARS-CoV-2 and those with serological profiles consistent with prior infection [19]. Our results provide evidence that the Abbott SARS-CoV-2 IgG II quantitative assay is highly sensitive and specific, and is a suitable assay for this application. A recent comprehensive evaluation of this assay demonstrated its potential capacity for monitoring the immune response to natural infection and its ability to detect antibodies in patients with two SARS-CoV-2 new variants of concern [20]. The detection of SARS-CoV-2 anti-spike antibodies using the Abbott SARS-CoV-2 IgG II assay correlates well with the presence of functional neutralizing antibodies and is highly sensitive for the quantitative detection of vaccine specific antibody responses [21]. Therefore, quantitative anti-S screening may also have a role in evaluating the efficacy of the various SARS-CoV-2 vaccines. The reason for the notable difference in the detection of anti-S between men and women remains unclear and may relate to the preponderance of females healthcare workers in Ireland. In addition, blood donation eligibility criteria has changed throughout the pandemic and many healthcare workers working in high-risk wards, were initially deferred from donation or advised not to attend.
Throughout 2021, further doses of COVID-19 vaccines were administered at various rates in different age groups, concurrent with the emergence of viral variants of concern and cases of reinfection. The WHO published enhanced guidelines to assist in the differentiation of vaccine-derived immunity versus infection-derived immunity and recommended the inclusion of a sensitive anti-N screening assay as a more robust method for the differentiation of antibody responses [1]. The present study introduced the Roche Elecsys Anti-SARS-CoV-2 immunoassay in response to this recommendation, which detects total antibodies (including IgG) against the SARS-CoV-2 N antigen and has reported better performance compared to the Abbott SARS-CoV-2 IgG assay in a number of studies [22,23]. The higher sensitivity in detecting antibodies to the N antigen could be in part because the Roche assay detects multiple classes of antibodies compared to the Abbott assay, which exclusively detects IgG antibodies. In addition to this the Roche assay has demonstrated a superior ability in maintained detection of antibodies up to at least 7 months compared to the Abbott assay, making it more suitable for identifying infection-derived immunity [24].
High concentrations of circulating anti-S, detected in our donors after the initiation of the vaccination programme, were used as a secondary factor to distinguish between COVID-19 vaccination as opposed to past infection, prior to the publication of the revised WHO testing algorithm and the implementation of the more sensitive Roche Elecsys anti-N assay. The finding that vaccines induce higher quantitative antibody levels compared to natural infection has been reported in numerous studies to date [25,26]. One item for consideration is that of hybrid immunity, which is defined as the immune protection in individuals who have had one or more doses of a COVID-19 vaccine, and experienced at least one SARS-CoV-2 infection before or after the initiation of vaccination [27]. A single booster dose given to previously infected patients raised an antibody response significantly higher than two doses given to naïve individuals. In addition heterologous vaccination generated a robust persistent antibody response at high levels, steady up to three months after administration [27]. It was not possible to distinguish hybrid immunity from those that were previously vaccinated only in the anonymised donor sample population, and therefore some hybrid immunity may have been misclassified as vaccine-derived immunity if the anti-N assay failed to detect their anti-N antibody due to poor assay sensitivity.
The use of serology for diagnosis and surveillance of respiratory viruses has limitations and is not routinely performed for other viral infections. In addition, blood donors are likely to differ from the general population in terms of sociodemographic, behavioural and health-related factors. Indeed, the exposure to various strains of the seasonal influenza virus, reinfection, antigenic drift and vaccination leads to complex individual antibody profiles [28,29]. However, Pandemic Preparedness and Response Planning includes seroepidemiology as a key surveillance initiative that is required at the start of any pandemic as it has a role in determining reliable estimates of transmission, tracing, infection fatality rates and impact of emerging pathogens [10,19,30]. Serological studies can inform age-related morbidity and mortality modelling, supporting estimates for the provision of vaccines and antiviral medications [10,19,30]. Due to the developing nature of SARS-CoV-2 serology assays and the absence of a gold standard for SARS-CoV-2 serology, the testing algorithms used in this study to classify samples as truly positive or truly negative was limited by the sensitivity and specificity of the assays used. We attempted to account for this by using multiple assays targeting various antibody classes and viral targets. However it must be noted that classifying S + N- as indicative of vaccination and non-exposure to the SARS-Cov-2 Virus is not a perfect method as it may exclude previously infected individuals where anti-N has declined rapidly compared to anti-S, and is potentially no longer detected by a screening assay [[31], [32], [33]].
The present study clearly demonstrated the remarkable impact that vaccination had in increasing the total seropositivity by November 2021 and is consistent with the vaccine uptake estimated for the general Irish population. Overall, a total of 93.4% Irish adults were estimated to be fully vaccinated at the end of November 2021, which is one of the highest vaccine uptake rates in the European Union [3]. The continued screening of blood donors has the potential to generate important data, assisting with the management of future waves of infection and supporting the development of appropriate national vaccination strategies.
Financial support
This work was supported by the Irish Blood Transfusion Service internal Research and Development Fund, and by Abbott Diagnostics, United States. Abbott diagnostics provided reagents for testing; however, has had no involvement in study design; sample collection, analysis and interpretation of data; writing of the report; and in the decision to submit the article for publication.
CRediT authorship contribution statement
Dermot Coyne: Methodology, Data curation, Supervision, Funding acquisition. Dearbhla Butler: Methodology, Data curation, Formal analysis, Writing – original draft. Adrienne Meehan: Methodology, Data curation. Evan Keogh: Methodology, Data curation. Pádraig Williams: Resources, Project administration. Alex Carterson: Methodology, Funding acquisition. Tor Hervig: Funding acquisition. Niamh O'Flaherty: Conceptualization, Methodology, Writing – review & editing, Project administration, Supervision. Allison Waters: Data curation, Writing – original draft, Project administration, Formal analysis.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
This work was supported by the Irish Blood Transfusion Service internal research and development funding, and by Abbott Diagnostics. Abbott diagnostics provided reagents for testing; however, has had no involvement in study design; sample collection, analysis and interpretation of data; writing of the report; and in the decision to submit the article for publication.
Acknowledgements
The IBTS is indebted to our dedicated donors who have continued to attend blood donor clinics during these challenging times. We also kindly acknowledge the contribution of our colleagues at the UCD National Virus Reference Laboratory and St. James's Hospital for their time and help with confirmation SARS-CoV-2 antibody testing. We also acknowledge the contribution of Abbott Laboratories for to the testing protocol methodology and part funding of this study.
References
- 1.WHO . World Health Organisation; 2020. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection | COVID-19: surveillance, case investigation and epidemiological protocols. [Google Scholar]
- 2.Allen N., Riain U.N., Conlon N., Ferenczi A., Carrion Martin A.I., Domegan L., et al. Prevalence of antibodies to SARS-CoV-2 in Irish hospital healthcare workers. Epidemiol Infect. 2021;149 doi: 10.1017/S0950268821000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HPSC . Health Protection Survellance Centre; 2021. COVID-19 vaccination uptake in Ireland 2021 weekly reports. [Google Scholar]
- 4.HPSC . Health Protection Surveillance Centre; 2022. Epidemiology of COVID-19 in Ireland. [Google Scholar]
- 5.Butler D., Coyne D., Pomeroy L., Williams P., Holder P., Carterson A., et al. Confirmed circulation of SARS-CoV-2 in Irish blood donors prior to first national notification of infection. J Clin Virol. 2022;146 doi: 10.1016/j.jcv.2021.105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 7.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic : a systematic review. Ann Intern Med. 2021;174:655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien S.F., Lieshout-Krikke R.W., Lewin A., Erikstrup C., Steele W.R., Uzicanin S., et al. Research initiatives of blood services worldwide in response to the covid-19 pandemic. Vox Sang. 2021;116:296–304. doi: 10.1111/vox.12995. [DOI] [PubMed] [Google Scholar]
- 10.Erikstrup C., Hother C.E., Pedersen O.B.V., Molbak K., Skov R.L., Holm D.K., et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2021;72:249–253. doi: 10.1093/cid/ciaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slot E., Hogema B.M., Reusken C.B.E.M., Reimerink J.H., Molier M., Karregat J.H.M., et al. 2020. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak. [Google Scholar]
- 12.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutukina M., Kaznadzey A., Kireeva M., Mazo I. IgG antibodies develop to spike but not to the nucleocapsid viral protein in many asymptomatic and light COVID-19 cases. Viruses. 2021:13. doi: 10.3390/v13101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewin A., Therrien R., De Serres G., Gregoire Y., Perreault J., Drouin M., et al. SARS-CoV-2 seroprevalence among blood donors in Quebec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health. 2021;112:576–586. doi: 10.17269/s41997-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S., et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020:9. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jugwanth S., Gededzha M.P., Mampeule N., Zwane N., David A., Burgers W.A., et al. Performance of the Abbott SARS-CoV-2 IgG serological assay in south African 2 patients. PloS One. 2022;17 doi: 10.1371/journal.pone.0262442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J Infect Dis. 2021;223(3):389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyre D.W., Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., et al. Stringent thresholds in SARS-CoV-2 IgG assays lead to under-detection of mild infections. BMC Infect Dis. 2021;21:187. doi: 10.1186/s12879-021-05878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergeri I., Lewis H.C., Subissi L., Nardone A., Valenciano M., Cheng B., et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID-19 pandemic. Influenza Other Respi Viruses. 2022;16:7–13. doi: 10.1111/irv.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English E., Cook L.E., Piec I., Dervisevic S., Fraser W.D., John W.G. Performance of the Abbott SARS-CoV-2 IgG II quantitative antibody assay including the new variants of concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and first steps towards global harmonization of COVID-19 antibody methods. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00288-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkmann T., Mucher P., Perkmann-Nagele N., Radakovics A., Repl M., Koller T., et al. The comparability of anti-spike SARS-CoV-2 antibody tests is time-dependent: a prospective observational study. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01402-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller L., Ostermann P.N., Walker A., Wienemann T., Mertens A., Adams O., et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur J Clin Microbiol Infect Dis. 2021;40:1063–1071. doi: 10.1007/s10096-021-04169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkmann T., Perkmann-Nagele N., Breyer M.K., Breyer-Kohansal R., Burghuber O.C., Hartl S., et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66:1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen N., Brady M., Carrion Martin A.I., Domegan L., Walsh C., Houlihan E., et al. SARS-CoV-2 antibody testing in health care workers: a comparison of the clinical performance of three commercially available antibody assays. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.00391-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schipani M.C., Tomassetti F., Polidori I., Ricci P., Frassanito M.L., Seraceni S., et al. Evaluation of natural and vaccine-induced anti-SARS-CoV-2 immunity: a comparative study between different groups of volunteers. Diseases. 2022:10. doi: 10.3390/diseases10020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Esposito D., Kang Z., Lu J., Remaley A.T., De Giorgi V., et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali H., Alahmad B., Al-Shammari A.A., Alterki A., Hammad M., Cherian P., et al. Previous COVID-19 infection and antibody levels after vaccination. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.778243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucharski A.J., Lessler J., Cummings D.A.T., Riley S. Timescales of influenza A/H3N2 antibody dynamics. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B., Lessler J., Zhu H., Jiang C.Q., Read J.M., Hay J.A., et al. Life course exposures continually shape antibody profiles and risk of seroconversion to influenza. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5:223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terpos E., Stellas D., Rosati M., Sergentanis T.N., Hu X., Politou M., et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021;89:87–96. doi: 10.1016/j.ejim.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Elslande J., Gruwier L., Godderis L., Vermeersch P. Estimated half-life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin Infect Dis. 2021;73:2366–2368. doi: 10.1093/cid/ciab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Elslande J., Oyaert M., Lorent N., Vande Weygaerde Y., Van Pottelbergh G., Godderis L., et al. Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection. Diagn Microbiol Infect Dis. 2022;103 doi: 10.1016/j.diagmicrobio.2022.115659. [DOI] [PMC free article] [PubMed] [Google Scholar]