Abstract
Nationwide data of the COVID-19 pandemic's impact on heart failure (HF) hospitalizations is lacking. We conducted this study to elucidate the impact of the COVID-19 pandemic on HF hospitalizations. Additionally, we assessed the differences in hospitalization characteristics during the pandemic and the impact that a concurrent diagnosis of COVID-19 has on various outcomes and predictors of inpatient mortality among patients admitted for HF. The National Inpatient Sample (NIS) database was queried for all hospitalizations with a primary diagnosis of HF between 2017 and 2020. Monthly HF hospitalizations were trended longitudinally over this period. Beginning April 1, 2020, concurrent COVID-19 infections were identified. Subsequently, we stratified HF hospitalizations between April 2020 and December 2020 (HF-2020) based on if concomitant COVID-19 was diagnosed, forming the HF-COVID+ve and HF-COVID–ve groups respectively. HF-2020 was also compared with prepandemic HF hospitalizations between April 2019 and December 2019 (HF-2019). Baseline characteristics were compared, and adjusted outcomes were obtained. During the initial COVID-19 surge in April 2020, HF admissions were reduced by 47% compared to January 2020. Following this decline, HF hospitalizations increased but did not reach prepandemic levels. HF-2020 admissions had an increased complication burden compared to HF-2019, including acute myocardial infarction (8.9% vs 6.6%, P < 0.005) and pulmonary embolism (4.1% vs 3.4%, P < 0.005) indicating a sicker cohort of patients. HF-COVID+ve hospitalizations had 2.9 times higher odds of inpatient mortality compared to HF-COVID−ve and an increased adjusted length of stay by 2.16 days (P < 0.005). A pandemic of the same magnitude as COVID-19 can overwhelm even the most advanced health systems. Early resource mobilization and preparedness is essential to provide care to a sick cohort of patients like acute HF, who are directly and indirectly effected by the consequences of the pandemic which has worsened hospitalization outcomes.
Introduction
The COVID-19 pandemic rapidly presented an unprecedented global health challenge that crippled health systems worldwide. The downstream impact on major diseases is yet to be well characterized and understood to inform resilient changes. Although primarily a respiratory pathogen, the viral infection is known to effect multiple organ systems.1 Many investigations have determined a link between COVID-19 infections and cardiovascular disease, including de novo manifestations and exacerbation of preexisting chronic disease.2, 3, 4, 5 The preponderance of angiotensin-converting enzyme-2 (ACE2) receptors on cardiac tissue facilitates viral penetrance that leads to cardiac injury during active infection. With the large morbidity of cardiovascular diseases like heart failure (HF) in the U.S. population, the broad implications of the cardiac involvement from a COVID-19 infection and the impact of the COVID-19 pandemic on overall healthcare delivery and HF hospitalizations in the United States on a national scale need improved characterization.
Studies from countries like the United Kingdom and Italy reported that during the initial surge of COVID-19 infections, HF admissions were reduced by 47%-49%.6 , 7 Estimates from regional centers in the United States reported a furthermore 62 +/− 7% decrease in acute HF hospitalizations.8 While there were reports of reduced HF hospitalizations, COVID-19 was found to be associated with a 45% higher hazard of incident HF after a COVID-19 infection.9 This discrepancy suggests an imbalance between the increased healthcare need expected from more HF cases and healthcare delivery through hospitalizations which needs to be understood.
This study aims to address this gap in the literature pertaining to national data in the United States by utilizing the National Inpatient Sample (NIS). First, we studied the impact of the COVID-19 pandemic on the trends of HF hospitalizations to ascertain a surrogate for the necessity of care in these patients. Next, we compared the profile of HF hospitalizations during the COVID-19 pandemic in 2020 to that of HF hospitalizations before the pandemic, that is, 2019 to analyze the differences that hospitalization during the initial surge of the pandemic had on outcomes. For the HF hospitalizations during the pandemic, we studied the impact of a concurrent diagnosis. We also studied the predictors of inpatient mortality in HF exacerbation hospitalizations with concomitant COVID-19 admissions. This information can be helpful to identify individuals at high risk of worsened outcomes so that appropriate resources can be allocated to mitigate adverse prognoses.
Methods
We used hospital-reported data from the NIS database, which is the largest publicly available, all-payer database of hospitalizations in the United States covering more than 97% of the U.S. population. The NIS approximates a 20% stratified sample of all discharges from U.S. community hospitals, excluding rehabilitation and long-term acute care hospitals. Each observation in the NIS represents an individual hospitalization with a primary diagnosis and up to 39 secondary diagnoses. All discharge diagnoses and procedures are coded using the International Classification of Disease, 10th revision, clinical modification (ICD-10-CM) codes. The patient identifiers are removed, and thereby patient confidentiality is protected.
As this study uses the publicly available database, according to the Wayne State University Institutional Review Board (IRB) policies, it is exempt from IRB review.
Study Population
The NIS was queried to identify all adult (>18 years) hospitalizations with a primary diagnosis of HF from 2017-2020 to capture hospitalizations that happened primarily for HF (Table 1 ). The hospitalization rates were calculated using the U.S. population for that corresponding year as the denominator. Monthly HF hospitalizations were calculated and trended over 4 years. The ICD-10-CM diagnosis code for COVID-19: U07.1, was recorded into NIS data beginning April 1, 2020. Using this code, HF hospitalizations with concomitant COVID-19 infections were identified. Subsequently, we classified HF hospitalizations between April 2020 and December 2020 (HF-2020) into HF hospitalizations with and without concomitant COVID-19 infection, ie, HF-COVID+ve and HF-COVID−ve, respectively. Furthermore, HF hospitalizations from April 2019 to December 2019 (HF-2019) were separately derived to compare the profiles of patients admitted during the prepandemic phase with patients admitted during the COVID-19 pandemic. Clinical characteristics were studied using ICD-10-CM diagnosis and procedure codes outlined in supplementary table S1.
Table 1.
Number of primary heart failure hospitalizations, as recorded by ICD-10 codes, nationally between 2017 and 2020 from the National Inpatient Sample
| Year | United States population | Number of primary HF admissions | Hospitalization rate per 100,000 population |
|---|---|---|---|
| 2017 | 324,985,539 | 1,193,385 | 367.2 |
| 2018 | 326,687,501 | 1,250,375 | 382.7 |
| 2019 | 328,239,523 | 1,296,815 | 395.1 |
| 2020 | 331,753,003 | 1,111,550 | 335.1 |
Baseline Characteristic
Various baseline characteristics were studied in the above groups, ie, between HF-COVID+ve vs HF-COVID−ve and between HF-2020 vs HF-2019. These included demographic factors like age, gender, race, insurance status, and patient location. Socioeconomic status was studied via a surrogate marker, ie, median household income in the patient's zip code classified as quartiles. Hospital-level characteristics included hospital bed size, teaching status, and region of the hospital. Multiple comorbidities, including hypertension (HTN), diabetes mellitus (DM), dyslipidemia (DL), smoking, obesity, known coronary artery disease (CAD), previous myocardial infarction (MI), previous percutaneous coronary intervention (PCI), previous coronary artery bypass graft surgery (CABG), atrial fibrillation, valvular heart disease, and Charlson comorbidity index were studied. Also, in-hospital events, including acute myocardial infarction (MI), complete heart block (CHB), cerebrovascular accident (CVA), pulmonary embolism (PE), acute respiratory failure, acute kidney injury (AKI), cardiogenic shock (CS), septic shock, gastrointestinal bleeding, and any bleeding were studied. Various procedures performed during the hospitalization were studied, including intra-aortic balloon pump (IABP), peripheral ventricular assist device (PVAD), extracorporeal membrane oxygenation (ECMO), left ventricular assist device (LVAD), CABG, PCI, noninvasive ventilation (NIV), and invasive mechanical ventilation (IMV). The outcomes studied are in-hospital mortality, Length of Stay (LOS), total hospitalization charges, and total hospitalization cost. Total charges reflect the amount billed by the hospital to the insurance, while total costs represent the expenses incurred in delivering the hospital services. Total hospital charges were multiplied by the cost-to-charge ratio provided by Healthcare Cost and Utilization Project (HCUP) to calculate hospital costs.
Statistical Analysis
We used the methods described by HCUP for the analysis. Discharge weights were used to produce national estimates. Above mentioned baseline characteristics were compared between HF-COVID+ve and HF-COVID−ve groups and between HF-2019 and HF-2020 groups. Pearson's chi-square test was used for categorical variables, and linear regression was used for continuous variables. Categorical variables were reported as percentages per 1000 or 10,000, and continuous variables were reported as a mean.
To study the hospitalization trends over time, we compared the annual rate of HF hospitalizations per 100,000 persons. We also compared the monthly rate of HF hospitalizations using the incidence rate ratio (IRR) estimated by obtaining the monthly hospitalization rate per 100,000 persons of each month in the year 2020 divided by the monthly rate of hospitalization per 100,000 persons for the corresponding month in the year 2019.
To study the impact of concurrent COVID-19 status on hospitalizations for HF, a multivariate analysis to account for confounding variables was performed that included multivariate logistic regression for inpatient mortality and multivariate linear regression for the LOS. Also, the predictors of inpatient mortality were studied in HF-COVID+ve hospitalizations using multivariate logistic regression analysis. We performed univariate analysis on demographic and clinical characteristics. Multivariate regression models using the backward selection method were made. The results were reported as an adjusted odds ratio (aOR) for inpatient mortality, mean difference for LOS, P-values, and 95% confidence intervals. All analyses were performed using Stata software version 15.1 (Stata Corporation, College Station, TX).
Results
Trends in HF Hospitalizations From 2017 to 2020
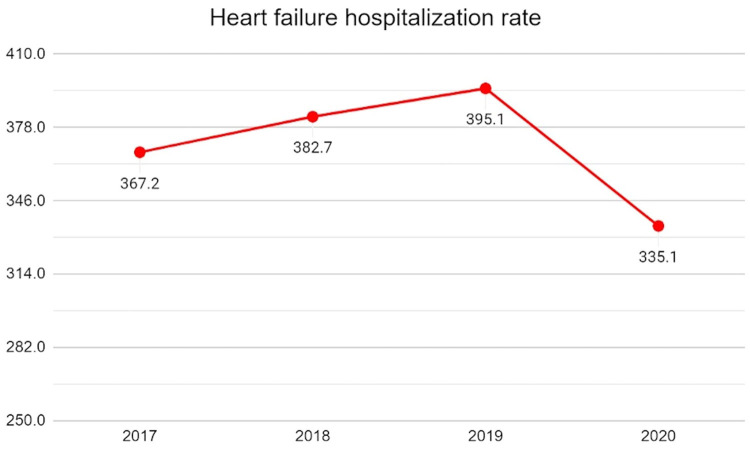
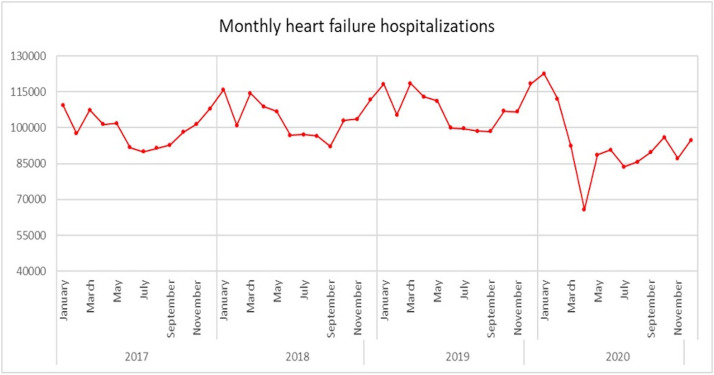
From 2017 to 2020, there has been a notable progressive increase in the number of HF hospitalizations across the United States. The onset of the COVID-19 pandemic resulted in a sharp decline in the HF hospitalization rate, dropping from a peak of 395.1/100,000 in 2019 to 335.1/100,000 in 2020, being a 15.2% reduction (Fig 1 , Table 1). Notably, the highest monthly HF hospitalization numbers were observed in January 2020 at 122,540, while the lowest in the 4 years was in April 2020 at 65,760, ie, a 46.3% decline—coinciding with the same month as the number of COVID-19 cases spiked in the United States (Fig 2 ).
FIG 1.
The annual rate of heart failure hospitalizations per 100,000 population from 2017 to 2020.
FIG 2.
The trend of monthly heart failure hospitalizations in the United States from 2017 to 2020.
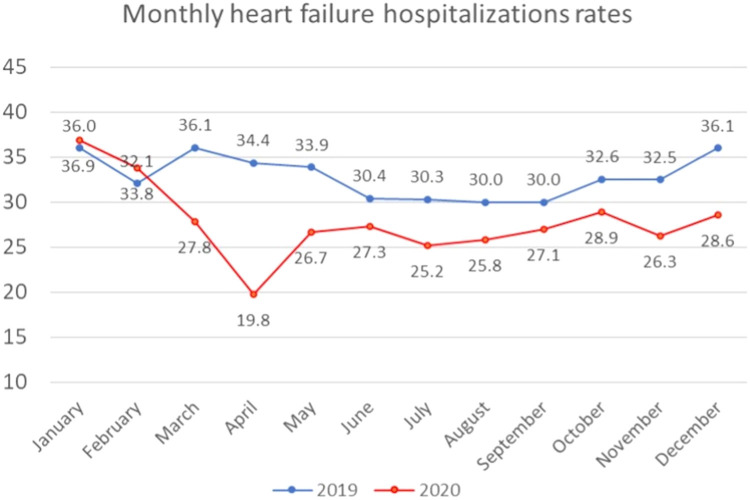
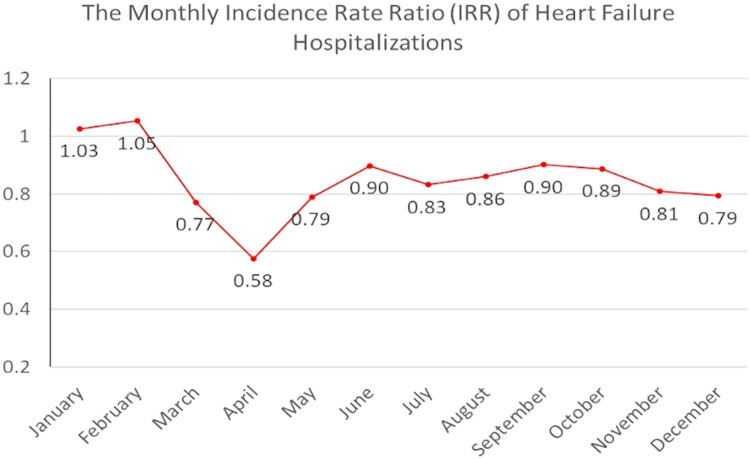
Monthly HF hospitalizations followed the national trend in the preceding year. They experienced a relative increase in the incidence of hospitalizations during the prepandemic months of January and February 2020, with IRRs of 1.03 in January 2020 and 1.05 in February 2020 (FIG 3, FIG 4 ). Beginning in March 2020, the incidence rate of hospitalization among HF patients exhibited a moderate decrease compared to baseline, with an IRR of 0.78 compared to March 2019 and a 17.6% reduction in hospitalizations compared to February 2020. The greatest relative declines occurred during April 2020, with an IRR of 0.58 compared to April 2019. The incidence of hospitalizations for HF was lower than in 2019 for all months following April 2020. There was a relative increase in the IRR of HF hospitalizations in the June-October 2020 period, being between 0.83 and 0.90. Nevertheless, another moderate decrease in the relative rate of HF hospitalizations between November 2020 and December 2020, with an IRR of 0.80, was noted. This finding coincided with the second peak of the COVID-19 pandemic that occurred from November 2020 to April 2021.
FIG 3.
Monthly incidence rate (per 100,000 population) of heart failure hospitalizations in 2019 and 2020.
FIG 4.
The monthly incidence rate ratio of heart failure hospitalizations in 2020 and 2019.
Demographic and Hospital-Level Characteristics
Between April 2020 and December 2020, there were 783,015 hospitalizations for HF, out of which 775,225 were HF-COVID−ve, and 7790 were HF-COVID+ve (Table 2 ). HF-COVID+ve hospitalizations were relatively younger (mean age of 68.4 years vs 70.2 years, P < 0.005). While constituting the majority in both groups, males represented a higher proportion in the HF-COVID+ve group at 58% compared to the HF-COVID−ve group at 54% (P < 0.005).
Table 2.
Baseline demographic and hospitalization characteristics from the National Inpatient Sample among HF-2019 vs HF-2020 and HF-COVID+ve vs HF-COVID−ve groups
| Baseline demographic and hospital characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Category | Variable | HF-2019 (N = 953,405) | HF-2020 (N = 783,015) | P-value | HF-2020 (N = 783,015) |
||
| HF-COVID−ve (N = 775,225) | HF-COVID+ve (N = 7,790) | P-value | |||||
| Age (years) | 71.28 | 70.18 | <0.005 | 70.2 | 68.37 | <0.005 | |
| Age category (years) | <60 | 21% | 23% | <0.005 | 23% | 27% | <0.005 |
| 60-84 | 58% | 59% | 59% | 58% | |||
| >84 | 21% | 18% | 18% | 15% | |||
| Female | 46% | 48% | <0.005 | 46% | 42% | <0.005 | |
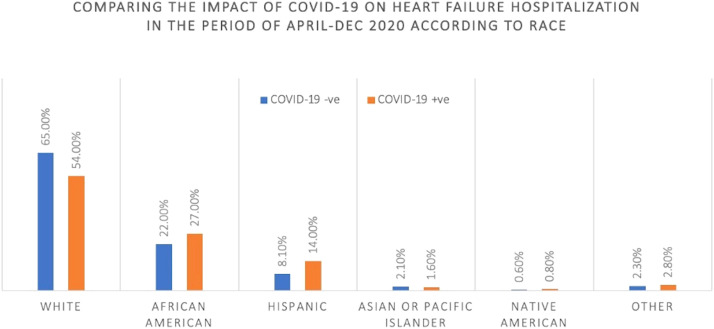
| Race | White | 65% | 65% | 0.9544 | 65% | 54% | <0.005 |
| Black | 22% | 22% | 22% | 27% | |||
| Hispanic | 8.1% | 8.2% | 8.1% | 14% | |||
| Asian or Pacific Islander | 2.2% | 2.1% | 2.1% | 1.6% | |||
| Native American | 0.6% | 0.6% | 0.6% | 0.8% | |||
| Other | 2.3% | 2.3% | 2.3% | 2.8% | |||
| Primary payer | Medicare | 74% | 71% | <0.005 | 71% | 67% | <0.005 |
| Medicaid | 11% | 13% | 13% | 16% | |||
| Private insurance | 11% | 12% | 12% | 14% | |||
| Self-pay | 3.1% | 3.3% | 3.3% | 3.4% | |||
| No charge | 0.2% | 0.2% | 0.2% | 0.1% | |||
| Zip income quartile | 1 | 34% | 34% | 0.0176 | 34% | 38% | 0.0146 |
| 2 | 26% | 28% | 28% | 26% | |||
| 3 | 23% | 21% | 21% | 22% | |||
| 4 | 17% | 16% | 16% | 15% | |||
| Hospital bed size | Small | 24% | 24% | 0.7617 | 24% | 22% | 0.1104 |
| Medium | 30% | 29% | 29% | 28% | |||
| Large | 47% | 47% | 47% | 50% | |||
| Patient location | “Central” counties of metro areas >1 million population | 29% | 29% | 0.9831 | 29% | 36% | <0.005 |
| “Fring” counties of metro area >1 million population | 24% | 14% | 24% | 23% | |||
| Counties in metro areas of 250,000-999,999 population | 21% | 21% | 21% | 18% | |||
| Counties in metro areas of 50,000-249,999 | 9.5% | 9.7% | 9.8% | 8.1% | |||
| Micropolitan Counties | 9.6% | 9.7% | 9.7% | 7.4% | |||
| Not metropolitan or micropolitan counties | 7.3% | 7.4% | 7.4% | 7.8% | |||
| Hospital location-teaching | Rural | 10% | 10% | 0.8829 | 10.2% | 8.2% | <0.005 |
| Urban nonteaching | 19% | 19% | 19% | 14% | |||
| Urban teaching | 70% | 71% | 71% | 77% | |||
| Region of hospital | Northeast | 18% | 18% | 0.9433 | 18% | 23% | <0.005 |
| Midwest | 23% | 20% | 23% | 23% | |||
| South | 42% | 41% | 42% | 38% | |||
| West | 17% | 42% | 17% | 16% | |||
While most HF hospitalizations with and without COVID-19 were among White patients (65% vs 54%), the proportion of hospitalizations with Black patients within the HF COVID+ve group compared to the HF COVID−ve group had more than a 5% difference (27% vs 22%). A similar difference pattern was observed among the Hispanic population (8.1% vs 14%). These differences are further outlined in Figure 5 .
FIG 5.
Racial distribution among heart failure hospitalizations from May to December 2020.
Although most HF admissions were comprised of the lowest income quartiles, the proportion of admissions from the lowest income quartile was higher in HF-COVID+ve group compared to HF-COVID−ve group (38.0% vs 34.0%, P < 0.01, Fig 6 ). Medicare comprised the majority of all primary payer sources in both groups, ie, at 71% for HF-COVID−ve and 67% for HF-COVID+ve, respectively (P < 0.005).
FIG 6.
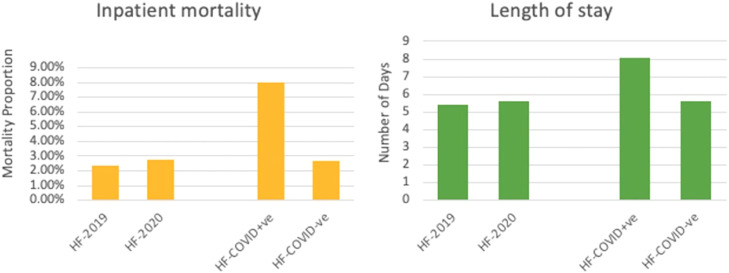
Inpatient mortality and length of stay in HF-2019 vs HF-2020 and HF-COVID+ve vs HF-COVID−ve.
When comparing HF-2020 with HF-2019, there was no significant difference in demographic characteristics except for a slightly younger cohort comprising the HF-2020 (70.2 vs 71.3, P < 0.005) when compared to HF-2019 with a slightly higher representation of males in the HF-2020 group (54% vs 52%, P < 0.005). Complete baseline characteristics are outlined in Table 2.
Most hospitalizations occurred in large hospital settings. Notably, a larger percentage of HF-COVID+ve hospitalizations were in central counties, denoted as metro areas of >1 million in population, in contrast to the HF-COVID−ve group (36% vs 29%, P < 0.005). Furthermore, the proportion of hospitalizations in urban teaching institutions was far greater in the HF-COVID+ve group (77% vs 71%, P < 0.005). Rural regions had a lower percentage of hospitalizations in the same group compared to the HF-COVID−ve proportions (8.2% vs 10%, P < 0.005).
Clinical Characteristics
There were no major differences in comorbidity prevalence when comparing HF-COVID+ve and HF-COVID−ve hospitalizations in the 2020 group, with specifics outlined in Table 3 . In comparison with HF-2019, there was a higher prevalence of DL (57.0% vs 52.0%, P < 0.005), smoking (16.0% vs 14.0%, P < 0.005), and obesity (30.0% vs 27.0%, P < 0.005) in HF-2020.
Table 3.
Clinical characteristics from the National Inpatient Sample among HF-2019 vs HF-2020 and HF-COVID+ve vs HF-COVID−ve groups
| Clinical characteristics | ||||||
|---|---|---|---|---|---|---|
| HF-2019 (N = 953,405) | HF-2020 (N = 783,015) | P-value | HF-2020 (N = 783,015) |
|||
| HF-COVID−ve (N = 775,225) | HF-COVID+ve (N = 7790) | P-value | ||||
| Comorbidities | ||||||
| Hypertension | 93% | 93% | 0.7775 | 93% | 94% | 0.511 |
| Diabetes mellitus | 49% | 50% | 0.0428 | 50% | 53% | 0.0031 |
| Dyslipidemia | 52% | 57% | <0.005 | 57% | 55% | 0.992 |
| Smoking | 14% | 16% | <0.005 | 16% | 11% | <0.005 |
| Obesity | 27% | 30% | <0.005 | 30% | 28% | 0.1002 |
| Known coronary artery disease | 53% | 53% | 0.3899 | 53% | 50% | 0.0681 |
| Previous myocardial infarction | 17% | 17% | <0.005 | 17% | 16% | 0.1742 |
| Previous percutaneous coronary intervention | 14% | 13% | <0.005 | 13% | 14% | 0.5224 |
| Previous coronary artery bypass graft | 14% | 13% | <0.005 | 12% | 13% | 0.5076 |
| Atrial fibrillation | 32% | 29% | <0.005 | 29% | 31% | 0.156 |
| Valvular heart disease | 29% | 29% | <0.005 | 29% | 25% | <0.005 |
| In-hospital events | ||||||
| Acute myocardial infarction | 6.6% | 8.9% | <0.005 | 8.9% | 9.7% | 0.2568 |
| Complete heart block | 0.9% | 1.1% | <0.005 | 1.1% | 1.3% | 0.4426 |
| Cerebrovascular accidents (per 10,000) | 37 | 47 | <0.005 | 47 | 71 | 0.1707 |
| Pulmonary embolism (per 10,000) | 69 | 93 | <0.005 | 93 | 103 | 0.6973 |
| Acute respiratory failure | 33% | 36% | <0.005 | 36% | 37% | 0.2928 |
| Acute kidney injury | 34% | 37% | <0.005 | 37% | 40% | 0.0059 |
| Septic shock (per 10,000) | 41 | 54 | <0.005 | 53 | 154 | <0.005 |
| Cardiogenic shock | 3.4% | 4.1% | <0.005 | 3.4% | 4.1% | 0.1495 |
| Gastrointestinal bleed | 1.9% | 2.2% | <0.005 | 2.2% | 2.7% | 0.1818 |
| All bleeds | 2.4% | 2.8% | <0.005 | 2.8% | 3.5% | 0.2928 |
| Procedures | ||||||
| Intra-aortic balloon pump (per 10,000) | 32 | 38 | 0.0968 | 38 | 71 | 0.0763 |
| Peripheral ventricular assist device (per 10,000) | 32 | 38 | 0.0911 | 38 | 45 | 0.6386 |
| Extracorporeal membrane oxygenation (per 10,000) | 6.7 | 8.9 | 0.1188 | 8 | 13 | 0.5618 |
| Left ventricular assist device (per 10,000) | 18 | 20 | 0.5852 | 20 | 26 | 0.5832 |
| Coronary artery bypass graft (per 10,000) | 19 | 23 | 0.0135 | 23 | 19 | 0.7346 |
| Percutaneous coronary intervention (per 10,000) | 120 | 130 | <0.005 | 134 | 51 | 0.046 |
| Noninvasive ventilation | 9.7% | 9.6% | 0.758 | 9.6% | 8.4% | 0.1338 |
| Invasive mechanical ventilation | 2.0% | 2.5% | <0.005 | 2.5% | 5.2% | <0.005 |
When comparing HF-COVID+ve hospitalizations with HF-COVID−ve, there were noticeable differences in in-hospital events. There was a significant increase in the incidence of AKI (40.0% vs 37.0%, P = 0.005) and septic shock (1.5% vs 0.5%, P < 0.005) among HF-COVID+ve compared to HF-COVID−ve hospitalizations. There was a higher utilization of IMV (5.2% vs 2.5%, P < 0.005) among HF-COVID+ve hospitalizations compared to HF-COVID−ve.
When comparing in-hospital events between HF-2020 and HF-2019 groups (Table 3), there was a significant increase in the incidence of acute myocardial infarction (AMI) (8.9% vs 6.6%, P < 0.005), CS (4,1% vs 3.4%, P < 0.005), and CHB (1.1% vs 0.9%, P < 0.005). Notably, there was an increase in the incidence of both PE (9.3% vs 6.9%, P < 0.005) and AKI (37.0% vs 34.0%) in HF-2020 compared to HF-2019. There was no major difference in the requirement for invasive cardiovascular interventions, including IABP, PVAD, and LVAD.
Outcomes and Predictors
Inpatient mortality for the HF-COVID+ve group was markedly higher compared to the HF-COVID−ve group, ie, 8.0% vs 2.7% (P < 0.005) with 2.86 times higher aOR of inpatient mortality (95% CI 2.27-2.62, P < 0.005) (Table 4 ). We observed that cardiac arrest (aOR, 19.302; P < 0.01), IABP (aOR, 9.758; P < 0.01), septic shock (aOR, 8.85; P < 0.01) and advanced age (> = 85 vs < 60 years; aOR 3.74, P < 0.01; 60-84 vs < 60 years; aOR 1.3, P < 0.01) were significantly associated with in-hospital mortality in HF-COVID+ve patients (Table 5 ).
Table 4.
Outcomes among HF-2019 vs HF-2020 and HF-COVID+ve vs HF-COVID−ve groups
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| Outcome | HF-2019 (N = 953,405) | HF-2020 (N = 783,015) | P-value | HF-2020 (N = 783,015) |
||
| HF-COVID−ve (N= 775,225) | HF-COVID+ve (N= 7790) | P-value | ||||
| In-hospital mortality | 2.4% | 2.8% | <0.005 | 2.7% | 8.0% | <0.005 |
| Length of stay | 5.4 days | 5.62 days | <0.005 | 8.08 days | 5.29 days | <0.005 |
| Total charge | $57,850 | $63,936 | <0.005 | $63,787 | $78,709 | <0.005 |
| Total cost | $13,525 | $15,160 | <0.005 | $15,124 | $18,671 | <0.005 |
Table 5.
Multivariate logistics regression analysis for predictors of inpatient mortality among HF-COVID+ve patients
| Variable | Odds ratio | P-value | [95% conf. interval] | |
|---|---|---|---|---|
| Age category (years) | ||||
| 60-84 vs <60 | 3.321 | 0.002 | 1.533 | 7.189 |
| > = 85 vs <60 | 8.205 | <0.001 | 3.354 | 20.072 |
| Interventions or events | ||||
| Intra-aortic balloon pump | 9.758 | 0.001 | 2.585 | 36.835 |
| Invasive mechanical ventilation | 7.835 | <0.001 | 3.863 | 15.89 |
| Acute respiratory failure | 5.672 | <0.001 | 3.245 | 9.917 |
| Septic shock | 8.845 | 0.002 | 2.293 | 34.119 |
| Cardiac arrest | 19.302 | <0.001 | 5.727 | 65.061 |
| Acute kidney injury | 2.699 | <0.001 | 1.605 | 4.539 |
The LOS in the HF-COVID+ve group was 8.08 days, significantly higher than the 5.59 days (P < 0.005) found in the HF-COVID−ve group. Multivariate linear regression analysis demonstrated that the adjusted mean difference in the LOS between HF-COVID+ve and HF-COVID−ve was 2.16 days (95% CI 1.65-2.67, P < 0.005).
Hospitalization charges and costs were also significantly elevated for the HF-COVID+ve group than HF-COVID−ve group ($78,709 vs $63,787, P < 0.005 and $18,671 vs $15,124, P < 0.005, respectively). HF-2020 had higher total cost and total charge compared to HF-2019 ($63,936 vs $57,850, P < 0.005 and $15,160 vs $13,525, P < 0.005, respectively).
Unadjusted outcomes comparatively are depicted visually in Figure 6. Univariate and multivariate regression analyses for in-hospital mortality among the HF-2020 group alongside univariate logistic regression analysis for inpatient mortality among the HF-COVID+ve group are depicted in Supplementary Tables S2-S4.
Discussion
The onset of the COVID-19 pandemic has profoundly impacted healthcare delivery in the United States. To the best of our knowledge, this is the first nationwide study to assess the impact of the COVID-19 pandemic on HF hospitalizations.
HF Trends From 2017 to 2020
Studies using the NIS database reported that from 2013 to 2018, there had been a steady increase in the number of HF hospitalizations across different age groups and sexes.10 Our study showed an initial increase in HF hospitalizations in the prepandemic years, ie, 2017-2019, as reported in previous studies. This uptrend was followed by a sharp “dip” in 2020, with annual hospitalizations for all HF patients per 100,000 falling from 395.1/100,000 hospitalizations in 2019 (the highest they have been compared to the prior 3 years) to 335.1/100,000 in 2020. This trend is consistent with results from other countries showing similar downtrends without recovery to a prepandemic baseline of hospitalizations, noting that milder acute HF patients may avoid coming to the hospital.11 During the first wave of the pandemic, significant resources were diverted toward caring for patients with COVID-19 and its related health complications while supplementing with technological methods to provide case. The consequence of the reconfiguration of healthcare delivery that followed resulted in a significant reduction in the hospitalizations of several non-COVID conditions, including acute HF.
Unlike other noncardiac conditions, which recovered to prepandemic levels after the first “dip,” the HF hospitalizations did not return to the prepandemic level during the study period.12 Multiple reasons can explain this incomplete recovery. First, telemedicine resources were rapidly mobilized, after the initial surge, to manage a proportion of HF patients remotely.13 , 14 Second, there was perceived anxiety and enhanced hesitancy among patients in accessing healthcare infrastructure and routine medical care. Although strategies were employed to help the health delivery system cope with the pandemic's peak, most protocols were developed hastily and with limited evidence. During the second pandemic peak, HF hospitalization rates dropped to their lowest, with an IRR of 0.79, highlighting the ineffective pandemic response to cope with cardiovascular health demands. Timely preparedness on the part of the health system, with a more robust utilization of telemedicine for this group of patients who are always at risk of sudden worsening of their underlying condition, can potentially aid in better management in the future.
Demographic Characteristics
Men with HF are found to possess an increased concentration of ACE-2 receptor in conjunction with a decreased immune response, evidencing that the male sex is an antecedent for worsened severity of a COVID-19 infection's on their cardiovascular health.15 Our study showed that Black and Hispanic individuals bore a disproportionate rate of COVID-19 infections and HF hospitalizations during the early months of the pandemic when compared to White individuals. These data are consistent with global reports, uncovering possible key health disparities and socioeconomic determinants of health. The reasons for these disparities are complex, including the disproportionate level of poverty and the prevalence of underlying comorbidities among minority communities. Furthermore, the pervasive effects of structural racism have emerged a lack of healthcare access in multigenerational households predisposing to environments that increase the risk of transmission of COVID-19.16, 17, 18, 19
Interestingly, the proportion of rural hospitalizations that were HF-COVID+ve was lower than those not infected, while hospitalizations at urban teaching hospitals increased. This difference was not identified in the HF-2019 vs HF-2020 data. Still, subanalysis among the HF-2020 presented a significant proportion of HF-COVID+ve hospitalizations occurred in central counties and metropolitan areas with >1 million in population. There may be a preference for urban centers to take care of HF-COVID+ve cases due to the greater complexity while also denoting that the increased density of metropolitan areas likely contributed to a higher number of infections. Furthermore, nationwide hospital closures in rural regions grew before 2020 and, continued to accelerate during the pandemic.20 This may have influenced rural patients to seek care at urban institutions, but more data is required to characterize these observations.
Clinical Characteristics
From the co-morbidity proportions recorded in the NIS, it was evident that a significant proportion of HF-COVID+ve hospitalizations had tobacco use disorder and obesity. When comparing HF-2019 to HF-2020 hospitalizations, the relative proportions of these co-morbidities in HF hospitalizations were also elevated in HF-2020 by significant margins. The CDC describes obesity as having an almost 3 times greater risk for hospitalization from a COVID-19 infection.21
The incidence of AKI was higher in HF-COVID+ve hospitalizations than in those not infected. While likely multifactorial in cause, studies have suggested a concomitant COVID-19 infection may propagate decreased cardiac output by impairing cardiac activity or causing primary kidney injury through viral infection.22
Cardiovascular complications, including AMI, CHB, and CS, were also significantly elevated in HF-2020 hospitalizations compared to HF-2019 hospitalizations. While COVID-19 infections have been associated with elevated incidence and in-hospital mortality from AMI in a multicenter registry study,23 there are only sparse case reports on the infection's attribution with CHB24 , 25 and CS.26 , 27 Additionally, there was a far greater incidence of PE (9.3% vs 6.9%, P < 0.005) in the HF-2020 group compared to HF-2019. This finding was in line with the incidence of PE reported in other studies.28
Our results also reflect that a sicker cohort of HF patients, with a greater co-morbidity burden, were admitted in 2020 compared to 2019. There can be multiple reasons to explain this finding, including patients with mild degrees of illness avoiding healthcare exposure due to perceived anxiety and a proportion of HF patients being managed remotely through telemedicine. It is to be noted that this cohort of patients, although minor degree compared to patients who were admitted, still represent patients that are triaged as high acuity, with potential for sudden adverse outcomes. Proactive utilization of telemedicine health tools can potentially help in the management of many of these patients.
Outcomes and Predictors of In-Hospital Mortality
Mortality was also markedly increased in the HF-COVID+ve group, with both the incidence of cardiac arrest (aOR, 19.3; P < 0.01) and IABP implementation (aOR, 9.76; P < 0.01) being the first and second most likely predictors for mortality in our cohort respectively. This is consistent with other reports, presenting similar differences in mortality ranging from a 2- to a 4-fold increase.29, 30, 31 This is most likely attributed to the physiological burden of an active COVID-19 infection. Active infections can increase both cardiac stress and the likelihood of primary cardiac injury which can diminish the vitality of functioning myocardium. HF patients already have a baseline reduced cardiac capacity, and infectious demand may exhaust what remains.
While most of the studies reporting on LOS are single-center, this national database study has shown a significant increase in LOS by around 2 days in HF-COVID+ve infections nationally. Increased clinical severity of HF, indicated by New York Heart Association (NYHA) class, is well documented to increase LOS.32 These links suggest that a more significant proportion of HF hospitalizations with concomitant COVID-19 infections had a higher severity of pre-existing HF and subsequent illness during hospitalization.
A COVID-19 infection has also been found to manifest a primary septic shock event in upwards of 70.8% of total septic episodes in a cohort by Shappell et al.,33 thereby underscoring the need for vigilance in infected patients. Subsequently, a con-current COVID-19 infection almost doubles the incidence of mortality among those experiencing septic shock, regardless of cause.34 We report that there is a substantial risk of mortality in patients admitted with HF who have a concurrent COVID-19 infection (aOR, 8.85; P < 0.01). While we were unable to discern the cause of septic shock within our cohort, our findings suggest patients infected with COVID-19 with a HF admission should be routinely evaluated for septic features due to these associations.
Limitations
Though our study has the essential strengths of a larger size and real-world data, the NIS relies on claims data, which can incur inaccurate billing and underestimation of covariates of interest, thus leading to coding bias. We acknowledge that during the initial surge of COVID-19 cases, the health system was overwhelmed, and this would have at least partially impacted the accuracy of coding and the quality of data, especially for secondary diagnosis and procedure codes. More granular clinical information, such as the results of diagnostic tests and medication use, is unavailable in the database and, thereby could not be accounted for in the analysis. We could not evaluate the causes that could have contributed to this difference. Especially since they are not patient-related but related to the hospital (eg, staffing differences).
Conclusion
The COVID-19 pandemic has dramatically impacted U.S. healthcare delivery in several ways. There was a nationwide sharp decrease in hospital admissions for HF, following which the HF hospitalization numbers plateaued but did not reach the prepandemic levels. The HF hospitalizations during the COVID-19 pandemic constituted patients with higher illness severity than prepandemic hospitalizations. And for HF hospitalizations during the pandemic, a concurrent COVID-19 infection was associated with worse outcomes, including an increase in in-hospital mortality and length of stay. This information can be helpful in the future to proactive prepare the health system to deal with a challenge of this magnitude, which ensure that the quality of care to this vulnerable population is not compromised.
Funding
The authors of this article certify that no funding sources were obtained during the creation of this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cpcardiol.2023.101749.
Appendix. Supplementary materials
References
- 1.Jain U. Effect of COVID-19 on the organs. Cureus. 2020;12:e9540. doi: 10.7759/cureus.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu-Ray I, Almaddah NK, Adeboye A, Soos MP. StatPearls Publishing; Treasure Island, FL: 2022. Cardiac Manifestations of Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 6.Bromage DI, Cannatà A, Rind IA, et al. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22:978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colivicchi F, Di Fusco SA, Magnanti M, Cipriani M, Imperoli G. The impact of the Coronavirus disease-2019 pandemic and Italian lockdown measures on clinical presentation and management of acute heart failure. J Card Fail. 2020;26:464–465. doi: 10.1016/j.cardfail.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox ZL, Lai P, Lindenfeld J. Decreases in acute heart failure hospitalizations during COVID-19. Eur J Heart Fail. 2020;22:1045–1046. doi: 10.1002/ejhf.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salah HM, Fudim M, O'Neil ST, Manna A, Chute CG, Caughey MC. Post-recovery COVID-19 and incident heart failure in the national COVID cohort collaborative (N3C) study. Nat Commun. 2022;13:4117. doi: 10.1038/s41467-022-31834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salah HM, Minhas AMK, Khan MS, et al. Trends and characteristics of hospitalizations for heart failure in the United States from 2004 to 2018. ESC Heart Fail. 2022;9:947–952. doi: 10.1002/ehf2.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassell K, Zipfel CM, Bansal S, Weinberger DM. Trends in non-COVID-19 hospitalizations prior to and during the COVID-19 pandemic period, United States, 2017–2021. Nat Commun. 2022;13:5930. doi: 10.1038/s41467-022-33686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budd J, Miller BS, Manning EM, et al. Digital technologies in the public health response to COVID-19. Nat Med. 2020;26:1183–1192. doi: 10.1038/s41591-020-1011-4. [DOI] [PubMed] [Google Scholar]
- 13.Afonso Nogueira M, Ferreira F, Raposo AF, et al. Impact of telemedicine on the management of heart failure patients during the Coronavirus disease 2019 pandemic. ESC Heart Fail. 2021;8:1150–1155. doi: 10.1002/ehf2.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clay SL, Woodson MJ, Mazurek K, Antonio B. Racial disparities and COVID-19: exploring the relationship between race/ethnicity, personal factors, health access/affordability, and conditions associated with an increased severity of COVID-19. Race Soc Probl. 2021;13:279–291. doi: 10.1007/s12552-021-09320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman N, Pebley AR, Lee K, Andrasfay T, Pratt B. Racial and ethnic differentials in COVID-19-related job exposures by occupational standing in the US. medRxiv [Preprint] medRxiv PLoS One. 2021;16(9) doi: 10.1101/2020.11.13.20231431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 cardiovascular disease registry. Circulation. 2021;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of patients with Coronavirus disease 2019 (COVID-19) presenting to the emergency department. Clin Infect Dis. 2022;74:387–394. doi: 10.1093/cid/ciab290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saghafian S, Song LD, Raja AS. Towards a more efficient healthcare system: opportunities and challenges caused by hospital closures amid the COVID-19 pandemic. Health Care Manag Sci. 2022;25:187–190. doi: 10.1007/s10729-022-09591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2022. Obesity, Race/Ethnicity, and COVID-19. Vol 2022. [Google Scholar]
- 21.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11:4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddadin FI, Mahdawi TE, Hattar L, Beydoun H, Fram F, Homoud M. A case of complete heart block in a COVID-19 infected patient. J Cardiol Cases. 2021;23:27–30. doi: 10.1016/j.jccase.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini Z, Ghodsi S, Hejazi SF. Persistent Complete Heart Block in a Patient with COVID-19 Infection: a Case Report. SN Compr Clin Med. 2021;3(1):259–262. doi: 10.1007/s42399-020-00712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomila-Grange A, Espasa M, Moglia E. Cardiogenic shock caused by SARS-CoV-2 in a patient with serial negative nucleic acid amplification tests: case report. SN Compr Clin Med. 2020;2:1903–1905. doi: 10.1007/s42399-020-00496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurin MI, Lin YJ, Bernard S, et al. Cardiogenic shock complicating multisystem inflammatory syndrome following COVID-19 infection: a case report. BMC Cardiovasc Disord. 2021;21:522. doi: 10.1186/s12872-021-02304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watchmaker JM, Goldman DT, Lee JY, et al. Increased incidence of acute pulmonary embolism in emergency department patients during the COVID-19 pandemic. Acad Emerg Med. 2020;27:1340–1343. doi: 10.1111/acem.14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey JR, Caro-Codón J, Rosillo SO, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhopalwala H, Akbar A, Dewaswala N, et al. Outcomes of heart failure in COVID-19 patients: an Appalachian experience. Cardiol Res. 2022;13:162–171. doi: 10.14740/cr1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standl E, Schnell O. Heart failure outcomes and Covid-19. Diabetes Res Clin Pract. 2021;175 doi: 10.1016/j.diabres.2021.108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Citu IM, Citu C, Gorun F, et al. Using the NYHA classification as forecasting tool for hospital readmission and mortality in heart failure patients with COVID-19. J Clin Med. 2022;11:1382. doi: 10.3390/jcm11051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMartino JK, Swallow E, Goldschmidt D, et al. Direct health care costs associated with COVID-19 in the United States. J Manag Care Spec Pharm. 2022;28:936–947. doi: 10.18553/jmcp.2022.22050. [DOI] [PubMed] [Google Scholar]
- 33.Shappell CN, Klompas M, Kanjilal S, Chan C, Rhee C. Prevalence, clinical characteristics, and outcomes of sepsis caused by severe acute respiratory syndrome Coronavirus 2 versus other pathogens in hospitalized patients with COVID-19. Crit Care Explor. 2022;4:e0703. doi: 10.1097/CCE.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heubner L, Hattenhauer S, Güldner A, et al. Characteristics and outcomes of sepsis patients with and without COVID-19. J Infect Public Health. 2022;15:670–676. doi: 10.1016/j.jiph.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.