Background:
Inflammation is proposed to be involved in the pathogenesis of poststroke cognitive impairment. The aim of this study was to investigate associations between concentrations of systemic inflammatory biomarkers after ischemic stroke and poststroke cognitive impairment.
Methods:
The Nor-COAST study (Norwegian Cognitive Impairment After Stroke) is a prospective observational multicenter cohort study, including patients hospitalized with acute stroke between 2015 and 2017. Inflammatory biomarkers, including the TCC (terminal C5b-9 complement complex) and 20 cytokines, were analyzed in plasma, collected at baseline, 3-, and 18 months poststroke, using ELISA and a multiplex assay. Global cognitive outcome was assessed with the Montreal Cognitive Assessment (MoCA) scale. We investigated the associations between plasma inflammatory biomarkers at baseline and MoCA score at 3-, 18-, and 36-month follow-ups; the associations between inflammatory biomarkers at 3 months and MoCA score at 18- and 36-month follow-ups; and the association between these biomarkers at 18 months and MoCA score at 36-month follow-up. We used mixed linear regression adjusted for age and sex.
Results:
We included 455 survivors of ischemic stroke. Higher concentrations of 7 baseline biomarkers were significantly associated with lower MoCA score at 36 months; TCC, IL (interleukin)-6, and MIP (macrophage inflammatory protein)-1α were associated with MoCA at 3, 18, and 36 months (P<0.01). No biomarker at 3 months was significantly associated with MoCA score at either 18 or 36 months, whereas higher concentrations of 3 biomarkers at 18 months were associated with lower MoCA score at 36 months (P<0.01). TCC at baseline and IL-6 and MIP-1α measured both at baseline and 18 months were particularly strongly associated with MoCA (P<0.01).
Conclusions:
Higher concentrations of plasma inflammatory biomarkers were associated with lower MoCA scores up to 36 months poststroke. This was most pronounced for inflammatory biomarkers measured in the acute phase following stroke.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02650531.
Keywords: biomarkers, cognition, inflammation, interleukin, stroke
Cognitive impairment is reported in about 50% of stroke survivors.1,2 Although the risk is highest within the first-year poststroke, the risk of incident dementia remains increased several years after a stroke.3,4 One hypothesis is that acute ischemic stroke may trigger a degenerative process that causes dementia and is thought to involve neuroinflammation.5
Inflammation is a risk factor and a consequence of stroke.6–8 Acute stroke induces an inflammatory process, locally in the brain as well as systemically.6 Chronic low-grade inflammation is associated with stroke,7 risk factors of stroke (including atherosclerosis, age, and obesity), and with neurodegeneration.9–12 Potential strategies for attenuating inflammation to prevent cerebrovascular damage have been launched,7 and in animal models, anti-inflammatory approaches have reduced neuronal injury.6 If we can strengthen the hypothesis of inflammation involved in poststroke cognitive impairment (PSCI), there may be a potential for anti-inflammatory medications for cognitive preservation after stroke in the future.
Cytokines secreted from immune cells systemically and in the central nervous system act as inflammatory biomarkers. TNF (tumor necrosis factor), IL (interleukin)-1β, IL-6, and IFN (interferon)-ɣ are most studied in stroke.6,13 Additionally, the complement system reacts early to ischemia and upregulates the inflammatory response.14 Prior studies have investigated selections of cytokines and found associations between higher systemic concentrations of IL-1β, IL-6, IL-8, IL-10, and IL-12 and poorer cognitive outcomes on various tests.15–18 However, to the best of our knowledge, the complement cascade has not been studied in this context. Larger prospective studies exploring a more comprehensive set of inflammatory biomarkers at repeated time points and with longer follow-up times that can evaluate delayed PSCI are lacking. In the present substudy of the Nor-COAST study (Norwegian Cognitive Impairment After Stroke), we explored whether acute and chronic systemic inflammation, measured by complement activation and a broad spectrum of cytokines were associated with global cognitive impairment up to 36 months poststroke.
Methods
The study data are available from the corresponding author upon a reasonable request.
The Nor-COAST study is a prospective observational cohort study including patients hospitalized in 5 stroke units with acute stroke from May 2015 through March 2017. Inclusion criteria were hospitalization with acute ischemic or hemorrhagic stroke within 1 week of symptom onset, fluency in a Scandinavian language, age ≥18 years, and living in the catchment area for the recruiting hospitals.19 Exclusion criterion was expected survival <3 months.19 The participants in this substudy fulfilled 3 additional criteria: (1) having experienced ischemic stroke; (2) blood samples collected for biobank at baseline, 3, or 18 months; and (3) cognitive assessment at 3, 18, or 36 months. Participation was voluntary, and eligible patients signed an informed consent. Patients unable to write consented orally, and those unable to give their informed consent were included if their next of kin gave oral consent. The main study and this substudy are both approved by the Regional Committee for Medical and Health Research Ethics in Norway (2015/171/REK nord; 2021/242042/REK nord).
Information regarding stroke characteristics, acute treatment, and infection as a complication was collected during the index hospital admission. Demographic data were based on medical records and interviews with patients or caregivers. Prestroke cognitive impairment was assessed by study nurses through interviews with caregivers using the Global Deterioration Scale.20 The National Institutes of Health Stroke Scale (NIHSS) was used to measure stroke severity21 and modified Rankin Scale (mRS) for global function.22 Cognitive assessment, a 30-minute neuropsychological test battery based on the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network Harmonization Standards,23 was performed at the outpatient clinics after 3, 18, and 36 months. Participants unable to attend in person were assessed by telephone interview. Montreal Cognitive Assessment (MoCA) score was used as a measure of global cognitive function.24–26 In additional analyses, cognitive outcome was classified as normal cognition, mild, and major neurocognitive disorder (NCD), in accordance with the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders, as described elsewhere.2
Blood samples were collected during the acute hospital stay and at 3- and 18-month follow-ups. Among 737 participants with ischemic stroke, 197 lacked biobanking, due mainly to logistical challenges. Blood samples at baseline were obtained at median 4 days (interquartile range, 3–6 days) after onset of symptoms. A detailed description of the sampling and preparation of the blood samples until analyzed in the laboratory is given in the Supplemental Material.27 EDTA plasma samples were analyzed using a multiplex cytokine assay (Bio-Plex Human Cytokine 27-Plex Panel; Bio-Rad Laboratories, Inc, Hercules, CA), according to the manufacturer’s instructions and reported in pg/mL. Biomarkers were excluded if ≥20% of the values were not detectable. After excluding 7 biomarkers, 20 biomarkers from the multiplex assay were included in further analyses: IL-1β, IL-1Ra (IL-1 receptor antagonist), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-17a, eotaxin, bFGF (basic fibroblast growth factor), G-CSF (granulocyte colony-stimulating factor), IP (interferon-gamma inducible protein)-10, MCP (monocyte chemotactic protein)-1, MIP (macrophage inflammatory protein)-1α, MIP-1β, and TNF. Complement activation was measured by the TCC (terminal C5b-9 complement complex) using ELISA, based on a monoclonal antibody (aE11, RRID: AB_1119839) reacting with C9 only when incorporated in the TCC, as described in detail previously.28 The results were reported in complement activation arbitrary units.
Statistics
Acute inflammation was assessed by inflammatory biomarkers at baseline, whereas chronic inflammation was assessed by inflammatory biomarkers 3 and 18 months poststroke. The associations between inflammatory biomarkers and MoCA scores were investigated in 3 separate analysis models: (1) baseline biomarkers and MoCA score at 3, 18, and 36 months; (2) 3-month biomarkers and MoCA score at 18 and 36 months; and (3) 18-month biomarkers and MoCA score at 36 months. We applied mixed linear regression with MoCA sum as a dependent variable for each inflammatory biomarker. The covariates were the inflammatory biomarkers, time, the interaction between time and inflammatory biomarkers, and hospital. The participant was included as a random effect. For models 1 and 2, we included 3 categories of time; for model 3, we included 2 categories of time. In all models, we performed analyses unadjusted and adjusted for age and sex.
We performed sensitivity analyses adjusted for (1) age, sex, and NIHSS at day 1; (2) age, sex, and prestroke mRS; (3) age, sex, and education; (4) age, sex, and TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification (cause); and (5) age, sex and Oxfordshire Community Stroke Project classification (location). As comorbidities may affect both inflammatory biomarkers and cognition,8,12 we performed 4 sets of subgroup analyses where we excluded the following participants: (1) with prestroke cognitive impairment defined as Global Deterioration Scale score ≥320; (2) participants treated with antibiotics during the index hospital stay (model 1); (3) participants given antibiotics during the index hospital stay or CRP (C-reactive protein)>10 at day 1 (model 1); and (4) participants reporting any of the following comorbidities at baseline: prestroke cognitive impairment, current depression, rheumatic or inflammatory diseases, antibiotic treatment during the index hospital stay, kidney disease, cancer, alcohol abuse, or liver disease (model 1). In additional analyses, we used the 3 levels of NCD as outcome variables in mixed ordinal logistic regression with the same covariates as the other models, adjusted for age and sex. Due to multiple analyses, 2-sided P<0.01 were regarded as representing statistical significance. All analyses and graphical plots were performed using STATA 17.0 (RRID: SCR_012763).
Complementary information regarding definitions; the cognitive test battery2,19,29; plasma samples; and statistics are described in the Supplemental Material.
Results
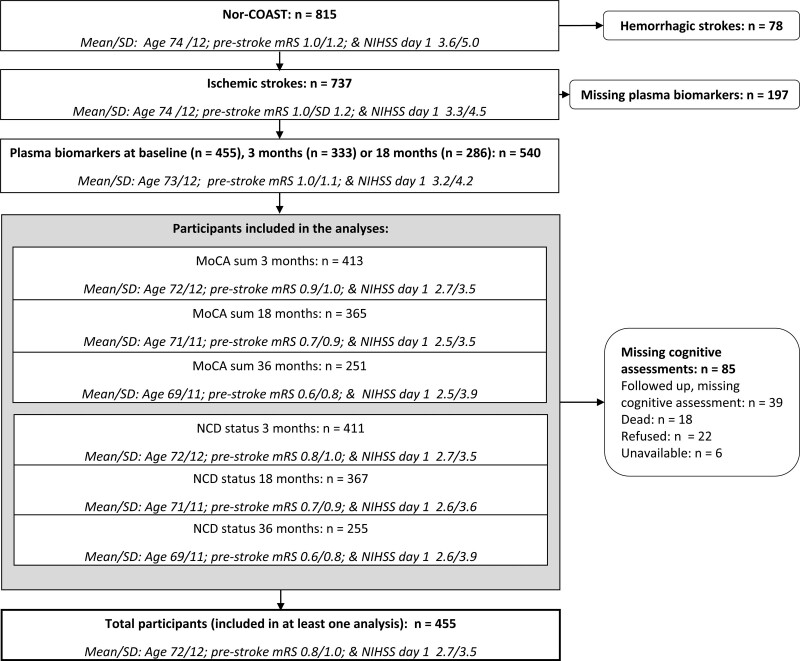
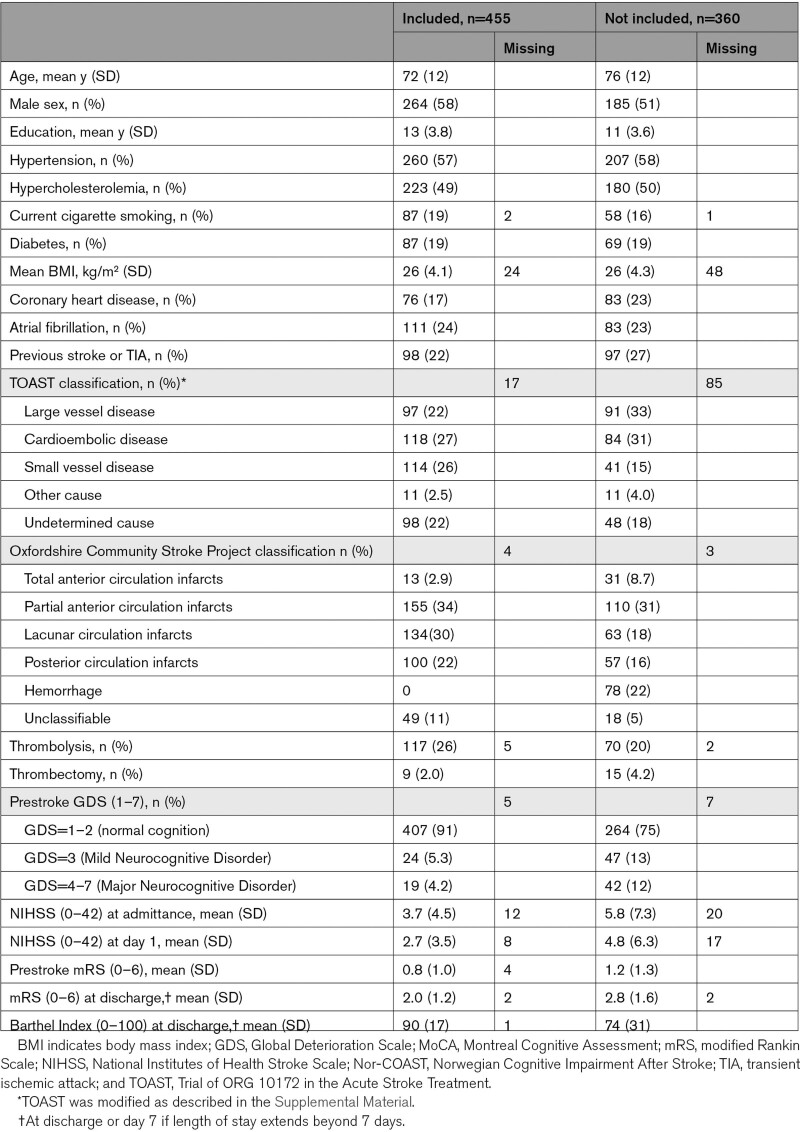
The analyses included 455 participants (Figure 1). Mean age/SD 72/12 years, 58% men, mean admission NIHSS score/SD 3.7/4.5, and prestroke mRS score/SD 0.8/1.0 (Table 1). Reasons for participants lost to follow-up are described in detail in Figure S1. Participants not included in this substudy and participants lost to follow-up were older and had higher admittance NIHSS, prestroke mRS, and discharge mRS scores (Table 1; Figure 1). Mean 3-, 18-, and 36-month MoCA scores/SD were 24.2/4.7, 24.6/4.6, and 25.4/4.5, respectively. Descriptive results and a correlation matrix of the inflammatory biomarkers at baseline are presented in Table S1 and Figure S2.
Figure 1.
Inclusion of participants. MoCA indicates Montreal Cognitive Assessment; mRS, modified Rankin Scale; NCD, Neurocognitive disorder; NIHSS, National Institutes of Health Stroke Scale; and Nor-COAST, the Norwegian Cognitive Impairment After Stroke.
Table 1.
Baseline Characteristics for Participants Included and Not Included in the Current Substudy of the Nor-COAST Study
Inflammatory Biomarkers at Baseline
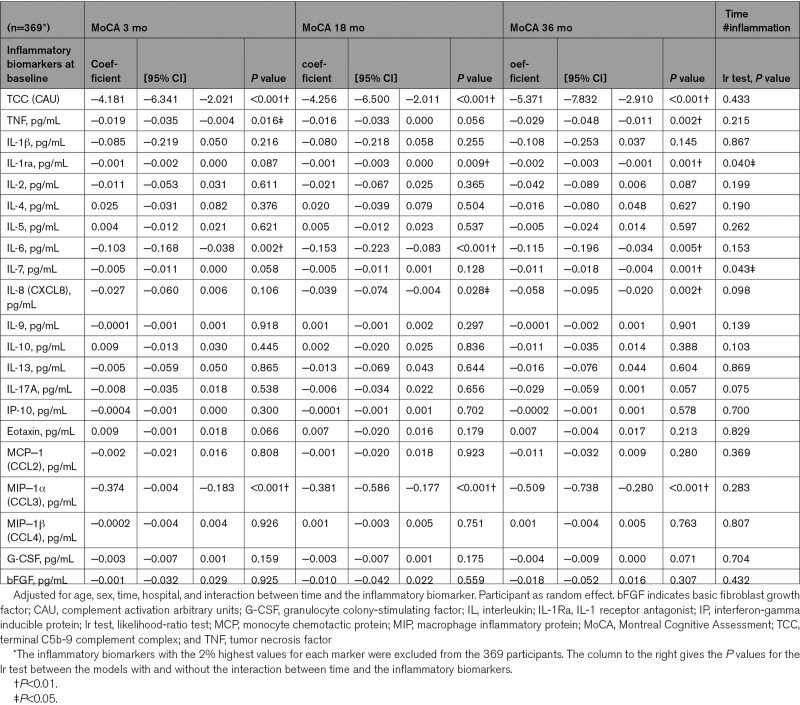
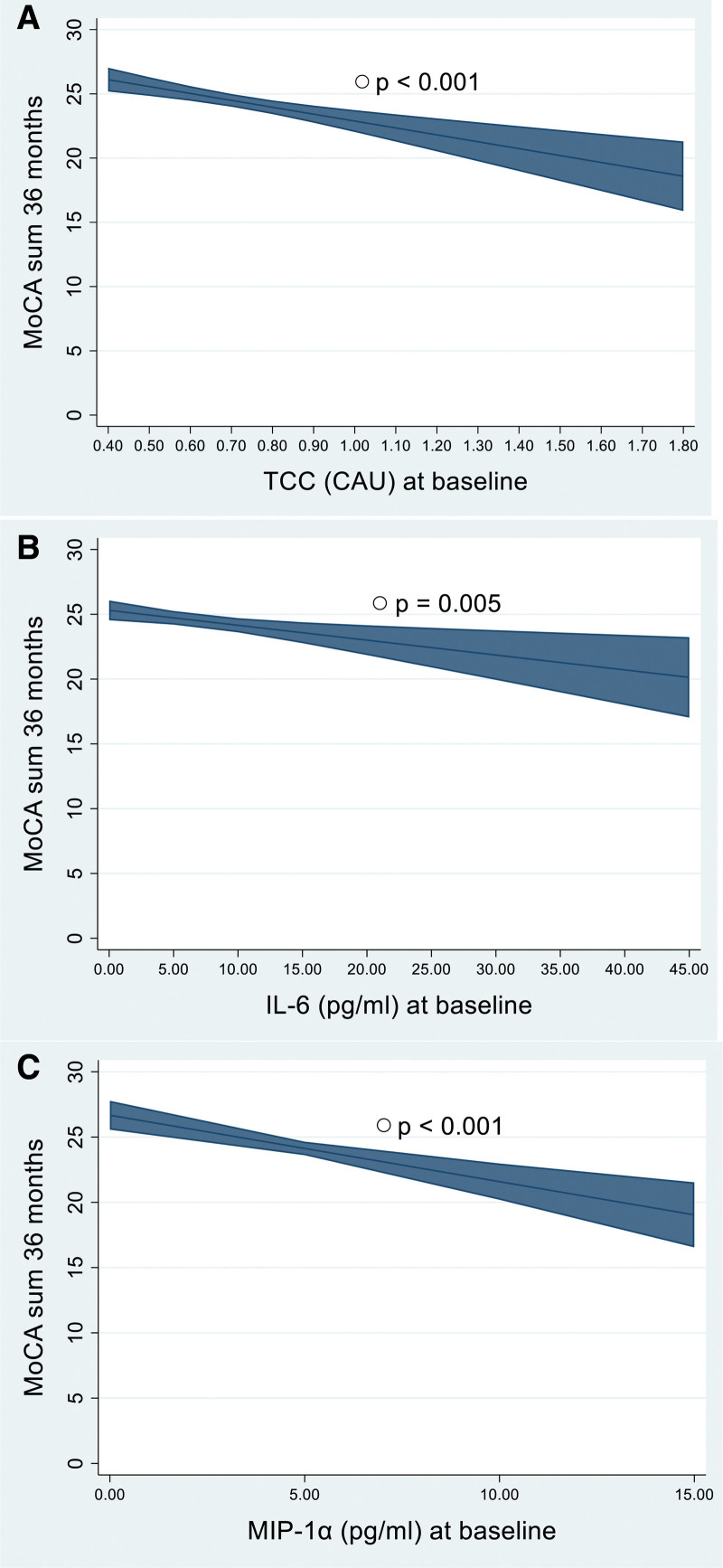
Adjusted for age and sex, higher concentrations of 7 baseline biomarkers were significantly associated with lower MoCA scores (P<0.01): TCC, IL-6, and MIP-1α at 3, 18, and 36 months; IL-1Ra at 18 and 36 months; and TNF, IL-7, and IL-8 at 36 months (Table 2). The predictive effects of baseline TCC, IL-6, and MIP-1α show differences of 5 to 7 points on MoCA scores at 36 months poststroke for those with the lowest compared with those with the highest concentrations (Figure 2). When testing the models with and without the interaction between the inflammatory biomarkers and time, the models with the interaction terms were not significantly better for any of the biomarkers (Table 2). In sensitivity analyses and subgroup analyses, the results were essentially the same as those in the main model adjusted for age and sex (Tables S2 through S10). No clear direction was found for the effect of acute inflammation as a whole on NCD (Table S11). However, increased concentrations of TCC were significantly associated with worse categories of NCD, which was true for all time categories of NCD (P=0.004, P<0.001, and P=0.009).
Table 2.
Results From the Mixed Linear Regression Models With MoCA as Dependent Variable and Inflammatory Biomarkers at Baseline as Independent Variables
Figure 2.
Estimated Montreal Cognitive Assessment (MoCA) score at 36 mo. Marginal means are estimated by using mixed linear regression, as described in Table 2 and illustrated for participants with mean values of age, sex, and hospital with 95% CIs. A, TCC (terminal C5b-9 complement complex). B, IL-6 (interleukin-6). C, MIP-1α (macrophage inflammatory protein-1α). The inflammatory biomarkers are presented from the lowest to the highest concentrations among the participants included in the analyses after excluding the 2% highest values, as described in the Supplemental Material. CAU indicates complement activation arbitrary units.
Inflammatory Biomarkers at 3 Months Poststroke
No biomarker measured at 3 months was associated with MoCA score or NCD at 18 or 36 months poststroke when adjusted for age and sex (Tables S12 and S13). When excluding participants with cognitive impairments prestroke (Global Deterioration Scale score ≥3), IL-1Ra was negatively associated with MoCA scores at 18 and 36 months (P=0.009; P=0.003; Table S14).
Inflammatory Biomarkers at 18 Months Poststroke
For inflammatory biomarkers measured at 18 months, higher concentrations of IL-1β, IL-6, and MIP-1α were significantly associated with lower MoCA scores when adjusted for age and sex (P<0.01; Table S15). When excluding participants with Global Deterioration Scale score ≥3, only IL-1β (P=0.002) and MIP-1α (P=0.001) remained significant (Table S16). No biomarkers were significantly associated with NCD (Table S17).
In unadjusted analyses, more biomarkers were inversely associated with MoCA scores than in analyses adjusted for age and sex (Tables S18 through S20).
Discussion
In this prospective, longitudinal exploratory study on the association between inflammation and PSCI over 36 months, we found that 7 of 21 biomarkers at baseline were negatively associated with global cognitive function, measured by MoCA score and adjusted for age and sex. Despite a weak trend that more baseline biomarkers were associated with lower MoCA scores at 36 months than at 3 months, we cannot conclude that acute inflammation is more strongly associated with delayed PSCI than with early PSCI. However, these results suggest that inflammation is associated with PSCI over a longer period. We found no consistent associations between systemic inflammation 3 months poststroke and cognition, whereas higher concentrations of 3 inflammatory biomarkers at 18 months were associated with cognitive impairment at the 36-month follow-up. The results were robust to adjustments for stroke severity (NIHSS), TOAST subtype, stroke location (Oxfordshire Community Stroke Project), education, and prestroke global function (mRS). They were minimally affected by exclusion of comorbidities. We emphasized results with P<0.01. However, additional results with P values ranging from 0.01 to 0.05, support our main finding of a negative association between concentrations of inflammatory biomarkers and MoCA score. As illustrated in Figure 2, baseline TCC, IL-6, and MIP-1α had an impact of 5 to 7 points on 36-month MoCA scores, considered a clinically meaningful effect.30 The study population represents patients with minor strokes compared with the general Norwegian stroke population31 and the more fit patients among the Nor-COAST participants (Table 1; Figure 1). However, this makes these results of particular interest as we expect good cognitive outcomes among these patients.
Our results indicate that chronic inflammation is less involved in cognitive outcome poststroke than acute inflammation. Cytokines and complement products are associated with neuronal damage.12,14 However, they also orchestrate the repair of damaged tissue and promote neurogenesis,13,14 and these processes may offset the harmful effects of inflammation when we measured the biomarkers 3 months poststroke. However, leakage across the damaged blood-brain barrier is most pronounced the first weeks to months after stroke32; later, their concentrations in plasma may reflect levels in the central nervous system to a lesser extent. Therefore, biomarkers in spinal fluid rather than in plasma would have provided a clearer picture of the processes in the central nervous system. Although we did a large set of sensitivity analyses, we cannot rule out that acute inflammation may be affected by other features of the stroke, and that chronic inflammation might have been present prestroke and may represent comorbidities that may not be properly adjusted for in our analyses.33,34 However, individual differences in the inflammatory response may be present in both phases and be relevant in PSCI.
This study highlights 3 important elements of the inflammatory system linked to poststroke cognition: the complement cascade; the IL-1β-IL-6-CRP axis; and the chemokines, as discussed in the following paragraphs.
As the end product of the complement cascade, our results of the TCC at baseline, indicate that the cascade is involved in poststroke cognitive outcome. However, other complement activation products may be the most important.35 Upstream of TCC, the C3 (complement component 3) has been associated with a worse prognosis after ischemic stroke in humans.14 By contrast, it also seems to play a part in synaptic plasticity and neurogenesis.14 In experimental studies, complement inhibition has been shown to attenuate neurological deficits when administered in the acute phase poststroke, while in the recovery period, it might be harmful.14,35 This is interesting as our results imply that the association between TCC and cognition is most evident at baseline and not in the chronic phases. As TCC is correlated with infarct size,34 another possibility is that the association between TCC and acute inflammation represents the lesion volume not properly captured by adjusting for the NIHSS.
The data for IL-1β, IL-1Ra, and IL-6 in our analyses may represent the IL-1β-IL-6-CRP axis, an important proinflammatory pathway.10 A strong relation to the proinflammatory cytokines, as IL-1Ra blocks IL-1β, can explain the effect of the anti-inflammatory cytokine IL-1Ra. Our baseline results of IL-6 are consistent with prior studies that have found an inverse association between IL-6 within the first weeks poststroke and cognitive outcome.15,17,18 The negative associations between IL-1β and IL-6 measured at 18 months and MoCA scores at 36 months poststroke indicate the involvement of chronic elevated IL-1β and IL-6 in PSCI. Modulating IL-6 directly or through therapy directed against IL-1β has been shown to be associated with increased myocardial salvage and preventing cardiovascular events in patients with previous myocardial infarction,36,37 whereas anti-IL-6 treatment to prevent PSCI remains to be investigated.
Two chemokines in the acute phase, MIP-1α and IL-8, were negatively associated with MoCA score; the latter finding was supported by a study by Narasimhalu et al.16 The increase in MIP-1α and IL-8 could lead to the infiltration of leukocytes, which may play a significant role in PSCI,38,39 although these chemokines may also exert neuronal effects and promote angiogenesis.40,41 Drugs targeting chemokine receptors are already on the market, and although some have been shown to improve recovery poststroke in animals,42 their role in PSCI needs to be explored.
The strengths of the present study are its large sample size, the broad spectrum of inflammatory biomarkers including both the complement system and the cytokine network, and measured at 3 time points, allowing us to compare acute and chronic inflammation. We could examine both early and delayed cognitive impairment due to the long follow-up time. We used standardized procedures for sampling and plasma analyses as cytokines are more stable in plasma than in serum.43 Instead of ELISA, as used by several studies,15,17,18 we applied a multiplex assay that enabled us to analyze more cytokines at once without bias due to multiple ELISA kits. Finally, the complement activation product was measured by ELISA using the most-reliable activation product, TCC.28,44
This study has several limitations. The time between symptom onset and blood sampling coincides well with the increase in IL-6 and TCC,34,45 but we may have missed the peaks of other cytokines (ie, TNF and IL-2) as their temporal profiles differ.45 IL-12 and IFN-ɣ were not assessed in this study, although prior studies indicate that they play a role in PSCI.46 Due to the observational study design without intervention and the possibility of unmeasured confounders, we cannot establish a causal relationship. Furthermore, participants lost to follow-up led to lower sample size at later time points. A consequence of this is wider CIs as more time has passed since baseline (Table 2). Finally, the multiple analyses limit the interpretation of biomarkers significant only in a single analysis. However, the close correlation between the biomarkers included substantially limits statistical errors due to multiple testing.
Conclusions
This study supports the proposed association between inflammation and PSCI and outline several pathways of the inflammatory systems involved in PSCI. Inflammation appears to have different impact on cognitive outcome depending on when the inflammation is measured in the poststroke period. This may be important for anti-inflammatory treatment. Other inflammatory pathways (ie, the IFN-ɣ pathway), which trajectories of the inflammatory response that are the most harmful to cognition, and how inflammation can be linked to brain pathology require further investigation. Finally, due to the exploratory design, new studies are needed to confirm the results.
Article Information
Acknowledgments
The authors thank all participants and their relatives, the Nor-COAST (Norwegian Cognitive Impairment After Stroke) research group, the staff at St. Olavs Hospital, Haukeland University Hospital, Ålesund Hospital, Vestre Viken Hospital Trust, Oslo University Hospital, and Biobank1 for their contributions. Additional thanks go to Dr Daniel Soule for feedback on the first article and Ingrid Reime for design of the Graphical Abstract.
Sources of Funding
The Nor-COAST study (Norwegian Cognitive Impairment After Stroke) was funded by the Norwegian Health Association and the Norwegian University of Science and Technology (NTNU). Dr Mollnes was founded by ERA-NET NEURON JTC 2019, STATEMENT, project code NEURON-082, and the Norwegian Research Council. Dr Sandvig is funded by the Liaison Committee for Education, Research and Innovation in Central Norway.
Disclosures
Dr Brita Knapskog has worked on clinical trials for Roche (BN29553), Boehringer-Ingelheim (1346.0023), and Novo Nordisk (NN6535-4730). The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Figures S1–S2
Tables S1–S20
STROBE Checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- bFGF
- basic fibroblast growth factor
- C3
- complement component 3
- G-CSF
- granulocyte colony-stimulating factor
- IFN
- interferon
- IL
- interleukin
- IP-10
- interferon-gamma inducible protein 10
- MCP-1
- monocyte chemotactic protein 1
- MIP
- macrophage inflammatory protein
- MoCA
- Montreal Cognitive Assessment
- mRS
- modified Rankin Scale
- NCD
- neurocognitive disorder
- NIHSS
- National Institutes of Health Stroke Scale
- Nor-COAST
- Norwegian Cognitive Impairment After Stroke
- PSCI
- poststroke cognitive impairment
- TCC
- terminal C5b-9 complement complex
- TNF
- tumor necrosis factor
- TOAST
- Trial of ORG 10172 in Acute Stroke Treatment
For Sources of Funding and Disclosures, see page 1310.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.041965.
Contributor Information
Stina Aam, Email: stina.aam@stolav.no.
Katinka Nordheim Alme, Email: katinka.alme@gmail.com.
Torunn Askim, Email: torunn.askim@ntnu.no.
Mona K. Beyer, Email: monbey@ous-hf.no.
Hanne Ellekjær, Email: hanne.ellekjer@stolav.no.
Hege Ihle-Hansen, Email: hmihle@ous-hf.no.
Stian Lydersen, Email: stian.lydersen@ntnu.no.
Tom Eirik Mollnes, Email: t.e.mollnes@medisin.uio.no.
Ragnhild Munthe-Kaas, Email: Ragnhild.Munthe-Kaas@vestreviken.no.
Halvor Næss, Email: halvor.ness@helse-bergen.no.
Ingvild Saltvedt, Email: ingvild.saltvedt@ntnu.no.
Yngve Müller Seljeseth, Email: Yngve.Muller.Seljeseth@helse-mr.no.
Pernille Thingstad, Email: pernille.thingstad@ntnu.no.
Torgeir Wethal, Email: torgeir.wethal@stolav.no.
Anne-Brita Knapskog, Email: anne-brita@knapskog.net.
References
- 1.Barbay M, Diouf M, Roussel M, Godefroy O, group G. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement Geriatr Cogn Disord. 2018;46:322–334. doi: 10.1159/000492920 [DOI] [PubMed] [Google Scholar]
- 2.Munthe-Kaas R, Aam S, Ihle-Hansen H, Lydersen S, Knapskog AB, Wyller TB, Fure B, Thingstad P, Askim T, Beyer MK, et al. Impact of different methods defining post-stroke neurocognitive disorder: the nor-COAST study. Alzheimers Dement (N Y). 2020;6:e12000. doi: 10.1002/trc2.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendlebury ST, Rothwell PM, Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford vascular study. Lancet Neurol. 2019;18:248–258. doi: 10.1016/S1474-4422(18)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok VC, Lam BY, Wong A, Ko H, Markus HS, Wong LK. Early-onset and delayed-onset poststroke dementia - revisiting the mechanisms. Nat Rev Neurol. 2017;13:148–159. doi: 10.1038/nrneurol.2017.16 [DOI] [PubMed] [Google Scholar]
- 5.Thiel A, Cechetto DF, Heiss WD, Hachinski V, Whitehead SN. Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke. 2014;45:2825–2829. doi: 10.1161/STROKEAHA.114.004285 [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Li Y, Zhang Y, He WY, Jin WN. Role of immune and inflammatory mechanisms in stroke: a review of current advances. Neuroimmunomodulation. 2022;29:255–268. doi: 10.1159/000524951 [DOI] [PubMed] [Google Scholar]
- 7.Mun KT, Hinman JD. Inflammation and the link to vascular brain health: timing is brain. Stroke. 2022;53:427–436. doi: 10.1161/strokeaha.121.032613 [DOI] [PubMed] [Google Scholar]
- 8.Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51:3156–3168. doi: 10.1161/STROKEAHA.120.030429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solas M, Milagro FI, Ramirez MJ, Martinez JA. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92. doi: 10.1016/j.coph.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jian B, Hu M, Cai W, Zhang B, Lu Z. Update of immunosenescence in cerebral small vessel disease. Front Immunol. 2020;11:585655. doi: 10.3389/fimmu.2020.585655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–236. doi: 10.1038/ni.3102 [DOI] [PubMed] [Google Scholar]
- 13.Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. 2020;21:6454. doi: 10.3390/ijms21186454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Liu Y, Zhang Z, Yang GY. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 2019;10:429–462. doi: 10.14336/AD.2019.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, Gladstone DJ, Ween J, Snaiderman A, Lanctôt KL. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:199–205. doi: 10.1177/0891988710373598 [DOI] [PubMed] [Google Scholar]
- 16.Narasimhalu K, Lee J, Leong YL, Ma L, De Silva DA, Wong MC, Chang HM, Chen C. Inflammatory markers and their association with post stroke cognitive decline. Int J Stroke. 2015;10:513–518. doi: 10.1111/ijs.12001 [DOI] [PubMed] [Google Scholar]
- 17.Kulesh A, Drobakha V, Kuklina E, Nekrasova I, Shestakov V. Cytokine response, tract-specific fractional anisotropy, and brain morphometry in post-stroke cognitive impairment. J Stroke Cerebrovasc Dis. 2018;27:1752–1759. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li J, Pan Y, Wang M, Lin J, Meng X, Liao X, Wang Y. Interleukin-6 as predictor of one-year cognitive function after ischemic stroke or TIA. Neuropsychiatr Dis Treat. 2022;18:391–399. doi: 10.2147/NDT.S348409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thingstad P, Askim T, Beyer MK, Brathen G, Ellekjaer H, Ihle-Hansen H, Knapskog AB, Lydersen S, Munthe-Kaas R, Naess H, et al. The Norwegian Cognitive impairment after stroke study (Nor-COAST): study protocol of a multicentre, prospective cohort study. BMC Neurol. 2018;18:193. doi: 10.1186/s12883-018-1198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136 [DOI] [PubMed] [Google Scholar]
- 21.Lyden PD, Lu M, Levine SR, Brott TG, Broderick J; NINDS rtPA Stroke Study Group. A modified national institutes of health stroke scale for use in stroke clinical trials: preliminary reliability and validity. Stroke. 2001;32:1310–1317. doi: 10.1161/01.str.32.6.1310 [DOI] [PubMed] [Google Scholar]
- 22.Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke. 1991;22:1242–1244. doi: 10.1161/01.str.22.10.1242 [DOI] [PubMed] [Google Scholar]
- 23.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, et al. National institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47 [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 25.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone montreal cognitive assessment versus face-to-face montreal cognitive assessment and neuropsychological battery. Stroke. 2013;44:227–229. doi: 10.1161/STROKEAHA.112.673384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munthe-Kaas R, Aam S, Saltvedt I, Wyller TB, Pendlebury ST, Lydersen S, Ihle-Hansen H. Test Accuracy of the montreal cognitive assessment in screening for early poststroke neurocognitive disorder: the nor-COAST study. Stroke. 2021;52:317–320. doi: 10.1161/STROKEAHA.120.031030 [DOI] [PubMed] [Google Scholar]
- 27.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol. 1988;73:484–488. [PMC free article] [PubMed] [Google Scholar]
- 28.Bergseth G, Ludviksen JK, Kirschfink M, Giclas PC, Nilsson B, Mollnes TE. An international serum standard for application in assays to detect human complement activation products. Mol Immunol. 2013;56:232–239. doi: 10.1016/j.molimm.2013.05.221 [DOI] [PubMed] [Google Scholar]
- 29.Aam S, Gynnild MN, Munthe-Kaas R, Saltvedt I, Lydersen S, Knapskog AB, Ihle-Hansen H, Ellekjær H, Eldholm RS, Fure B. The impact of vascular risk factors on post-stroke cognitive impairment: the nor-COAST study. Front Neurol. 2021;12:678794. doi: 10.3389/fneur.2021.678794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CY, Hung SJ, Lin KC, Chen KH, Chen P, Tsay PK. Responsiveness, minimal clinically important difference, and validity of the MoCA in stroke rehabilitation. Occup Ther Int. 2019;2019:2517658. doi: 10.1155/2019/2517658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuvås KR, Saltvedt I, Aam S, Thingstad P, Ellekjær H, Askim T. The risk of selection bias in a clinical multi-center cohort study. results from the Norwegian Cognitive Impairment After Stroke (Nor-COAST) study. Clin Epidemiol. 2020;12:1327–1336. doi: 10.2147/CLEP.S276631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, Abo-Ramadan U, Tatlisumak T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 33.Stanne TM, Angerfors A, Andersson B, Brännmark C, Holmegaard L, Jern C. Longitudinal study reveals long-term proinflammatory proteomic signature after ischemic stroke across subtypes. Stroke. 2022;101161:strokeaha121038349. doi: 10.1161/strokeaha.121.038349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE. Systemic complement activation following human acute ischaemic stroke. Clin Exp Immunol. 2004;137:117–122. doi: 10.1111/j.1365-2249.2004.02489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke AR, Christophe BR, Khahera A, Sim JL, Connolly ES, Jr. Therapeutic modulation of the complement cascade in stroke. Front Immunol. 2019;10:1723. doi: 10.3389/fimmu.2019.01723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310 [DOI] [PubMed] [Google Scholar]
- 37.Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049 [DOI] [PubMed] [Google Scholar]
- 38.Mirabelli-Badenier M, Braunersreuther V, Viviani GL, Dallegri F, Quercioli A, Veneselli E, Mach F, Montecucco F. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011;105:409–420. doi: 10.1160/TH10-10-0662 [DOI] [PubMed] [Google Scholar]
- 39.Park KW, Ju H, Kim ID, Cave JW, Guo Y, Wang W, Wu Z, Cho S. Delayed infiltration of peripheral monocyte contributes to phagocytosis and transneuronal degeneration in chronic stroke. Stroke. 2022;101161:strokeaha122038701. doi: 10.1161/strokeaha.122.038701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marciniak E, Faivre E, Dutar P, Alves Pires C, Demeyer D, Caillierez R, Laloux C, Buée L, Blum D, Humez S. The Chemokine MIP-1α/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. doi: 10.1038/srep15862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joy MT, Ben Assayag E, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M, Abduljawad N, Kliper E, Korczyn AD, Thareja NS, et al. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell. 2019;176:1143–1157.e13. doi: 10.1016/j.cell.2019.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hennø LT, Storjord E, Christiansen D, Bergseth G, Ludviksen JK, Fure H, Barene S, Nielsen EW, Mollnes TE, Brekke OL. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines - consequences for defining reference values in healthy humans. Cytokine. 2017;97:86–95. doi: 10.1016/j.cyto.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 44.Harboe M, Thorgersen EB, Mollnes TE. Advances in assay of complement function and activation. Adv Drug Deliv Rev. 2011;63:976–987. doi: 10.1016/j.addr.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 45.Nayak AR, Kashyap RS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Time course of inflammatory cytokines in acute ischemic stroke patients and their relation to inter-alfa trypsin inhibitor heavy chain 4 and outcome. Ann Indian Acad Neurol. 2012;15:181–185. doi: 10.4103/0972-2327.99707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold AB, Herrmann N, Swardfager W, Black SE, Aviv RI, Tennen G, Kiss A, Lanctot KL. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflammation. 2011;8:17. doi: 10.1186/1742-2094-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]