Abstract
Hepatocellular carcinoma (HCC) is a major cause of death in many countries, including South Korea. To provide useful and sensible advice for clinical management of patients with HCC, the Korean Liver Cancer Association and National Cancer Center Korea Practice Guideline Revision Committee have recently revised the practice guidelines for HCC management. However, there are some differences between practice guidelines and real-life clinical practice. In this review, we describe some key recommendations of the 2022 version of practice guidelines and the real-life clinical situation in South Korea, together with discussion about efforts needed to reduce the difference between guidelines and real-life clinical practice.
Keywords: Clinical practice guideline, Hepatocellular carcinoma, Surveillance, Diagnosis, Treatment
INTRODUCTION
In South Korea, liver cancer has the second highest crude death rate and causes the largest economic burden among all types of cancer [1,2]. The Korean Liver Cancer Association (KLCA, formerly the Korean Liver Cancer Study Group [KLCSG]) and National Cancer Center (NCC) of Korea published the first practice guidelines for management of hepatocellular carcinoma (HCC) in 2003 [3] and revised them in 2009, 2014, and 2018 [4-6]. Since then, new research findings and therapies have accumulated. Accordingly, practice guidelines were revised again in 2022 by integrating the most up-to-date research findings, new therapies, and expert opinions [1]. Studies collected for evidence were analyzed through a systematic review, and levels of evidence were classified based on the revised Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) [7]. In recent years, systemic treatment of HCC has evolved dramatically. Atezolizumab plus bevacizumab has shown superior efficacy over sorafenib and is now considered as a preferred first-line option [1]. Second-line therapy is urgently needed for patients who have failed treatment with an immune checkpoint inhibitor-based regimen. However, there is little evidence to guide second-line therapy for these patients. Hence, for the first time, a D grade recommendation was described in the KLCA-NCC guidelines [1]. In this review, we summarize the 2022 KLCA-NCC Korea practice guidelines and real-life practice for HCC in South Korea
SURVEILLANCE
Key recommendations
The 2022 KLCA-NCC guidelines recommend HCC surveillance in high-risk groups (patients with chronic hepatitis B [A1], chronic hepatitis C [B1], and liver cirrhosis [A1]) with liver ultrasonography (US) plus serum alpha-fetoprotein (AFP) measurement every six months (A1). Guidelines also recommend dynamic contrast-enhanced computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) as an alternative when liver US cannot be performed adequately (C1).
Real-life situation and practice
In South Korea, most of HCC patients are diagnosed at an advanced stage. In an analysis of the Korean Primary Liver Cancer Registry between 2012 and 2014, which was a random sample consisting of 15% of newly diagnosed HCC patients in South Korea, about half were diagnosed at an advanced stage [8]. Timely diagnosis and treatment are suboptimal at the population level [9]. The Korean government initiated the National Liver Cancer Screening Program (NLCSP) in 2003 [10], which offers US and AFP tests for high-risk individuals [11]. According to a nationwide cohort study using the Korean National Health Insurance Service database, only 52.7% of high-risk individuals participated in the NLCSP [12]. To improve adherence to surveillance recommendations in Korea, additional efforts and strategies are needed. Initial presentation of HCC at an advanced stage in patients under regular HCC surveillance is another problem as the surveillance goal is to detect HCC at early stage. However, the sensitivity of US for detecting early-stage HCC is suboptimal [13], leading to surveillance failure in clinical practice [14,15]. Two Korean prospective studies have evaluated the usefulness of dynamiccontrast CT and MRI with liver-specific contrast for HCC surveillance. Both dynamic-contrast CT and MRI showed higher sensitivity and specificity than US-based surveillance [16,17]. However, alternative imaging methods for HCC surveillance are needed to overcome the limitations of CT and MRI. Hence, the guidelines recommend alternative screening tools only when liver US cannot be performed adequately.
DIAGNOSIS
Key recommendations
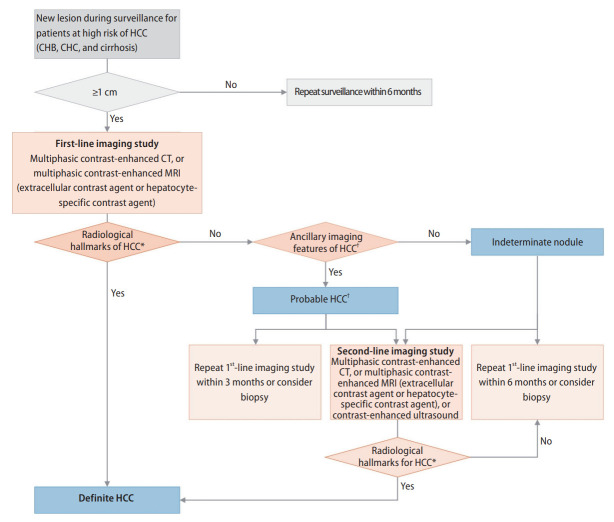
The diagnosis of HCC can be based on pathology or typical hallmarks of HCC obtained by non-invasive imaging for high-risk groups (chronic hepatitis B [A1], chronic hepatitis C [B1], or cirrhosis [A1]). For a new liver nodule ≥1 cm detected by surveillance tests in high-risk patients, multiphasic CT or multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents) should be performed as a first-line imaging study for diagnosis of HCC (A1). If a first-line imaging study is inconclusive for diagnosis of HCC, second-line imaging tests including multiphasic CT, multiphasic MRI, and contrast-enhanced US (blood-pool contrast agents or Kupffer cell-specific contrast agents) can be applied (B1). Imaging diagnosis of “definite” HCC can be based on a nodule ≥1 cm in high-risk patients in the presence of the hallmark arterial phase hyperenhancement with washout appearance (A1 for multiphasic CT or MRI with extracellular contrast agent; B1 for MRI with liver-specific contrast and contrast-enhanced US). A diagnosis of “probable” HCC can be based on ancillary imaging features of HCC (B1). The guidelines include a diagnostic algorithm (Fig. 1).
Figure 1.
Diagnostic algorithm of HCC. HCC, hepatocellular carcinoma; CHB, chronic hepatitis B; CHC, chronic hepatitis C; CT, computed tomography; MRI, magnetic resonance imaging; APHE, arterial phase hyperenhancement; US, ultrasonography. *The radiological hallmarks for diagnosing “definite” HCC on multiphasic contrast-enhanced CT or MRI are APHE with washout appearance in the portal venous, delayed, or hepatobiliary phase. These criteria should be applied only to a lesion that does not show either marked T2 hyperintensity or targetoid appearance on diffusion-weighted images or contrast-enhanced images. For a second-line imaging modality, contrast-enhanced US (blood-pool contrast agent or Kupffer cell-specific contrast agent) for a “definite” diagnosis of HCC is APHE with mild and late (≥60 seconds) washout. These criteria should be applied only to a lesion that does not show either rim or peripheral globular enhancement in the arterial phase. †For diagnosis of “probable” HCC, ancillary imaging features are applied as follows. There are two categories of ancillary imaging features, those favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, threshold growth) and those favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, fat or blood products in the mass). For nodules without APHE, “probable” HCC can be assigned only when the lesion fulfills at least one item from each of the two categories of ancillary imaging features. For nodules with APHE but without washout appearance, “probable” HCC can be assigned when the lesion fulfills at least one of the aforementioned ancillary imaging features. Adopted from 2022 KLCA-NCC HCC guidelines [1].
Real-life situation and practice
There are similarities and differences among guidelines for non-invasive diagnosis [18]. Of note, the 2022 KLCA-NCC guidelines contain a specific definition for washout appearance when using MRI with liver-specific contrast. They allow washout appearance in the portal venous, delayed, or hepatobiliary phase when a lesion does not show either T2 hyperintensity or targetoid appearance on diffusion-weighted images or contrast-enhanced images [1]. The allowance of washout appearance in hepatobiliary phases during diagnosis of HCC was a major change of radiological hallmarks in the 2018 KLCA-NCC guidelines [6,19]. Since then, several studies have compared the diagnostic performance of 2018 KLCA-NCC practice guidelines to that of the Liver Imaging-Reporting and Data System (LI-RADS) and found higher sensitivity without a reduction of specificity when using MRI with liver-specific contrast [20-22]. Hence, the updated 2022 KLCA-NCC guidelines retained the non-invasive diagnostic criteria of the 2018 KLCA-NCC guidelines when using MRI with liver-specific contrast. In the 2014 KLCSG-NCC guidelines, a lesion smaller than 1 cm could be non-invasively diagnosed as HCC [5]. However, for patients who HCC developed, histologically confirmed subcentimenter-sized HCC did not fulfill the non-invasive diagnostic criteria in real-life data [23]. The updated 2022 KLCA-NCC guidelines allow non-invasive imaging diagnosis for a nodule ≥1 cm in patients who HCC developed, In contrast, for patients with prior HCC history, the progression rate was high in patients with subcentimeter nodules showing imaging findings of HCC [24], allowing imaging diagnosis of recurrent HCC regardless of size.
STAGING
Key recommendations
The KLCA-NCC guidelines adopt the modified Union for International Cancer Control (mUICC) stages as the primary system, with the Barcelona Clinic Liver Cancer (BCLC) staging system and the American Joint Committee on Cancer/UICC TNM staging system serving as complements (B1).
Real-life situation and practice
Cancer staging plays a pivotal role in predicting prognosis, selecting treatment modality, and facilitating exchange of information. The 2022 KLCA-NCC guidelines adopted the 2003 5th version of the mUICC staging system as a primary system for HCC [1]. For consistent analysis of registry data, the guideline committee suggested continued use of this staging system. However, the mUICC staging system has limitations, such as difficulty in exchanging information internationally. Hence, the guidelines recommended the use of other staging systems as complements.
TREATMENT
Key recommendations
Multi-disciplinary treatment has been shown to improve HCC outcome [25]. However, the benefit, optimal frequency, format, and necessity are unknown and require further evaluation as a multi-disciplinary approach. In this situation, practice guidelines need to provide specific and practical information to clinicians when planning treatment. The KLCA-NCC 2014 guidelines began to provide best and alternative options according to mUICC stage for patients with HCC, Child-Pugh class A, no portal hypertension, and Eastern Cooperative Oncology Group (ECOG) performance status 0–1 [5]. One of the major changes to the 2022 updated guidelines addressed the quality of evidence for the best option. This will allow readers to make decisions based on increased evidence. The best option and alternative option according to mUICC stage are shown in Figure 2.
Figure 2.
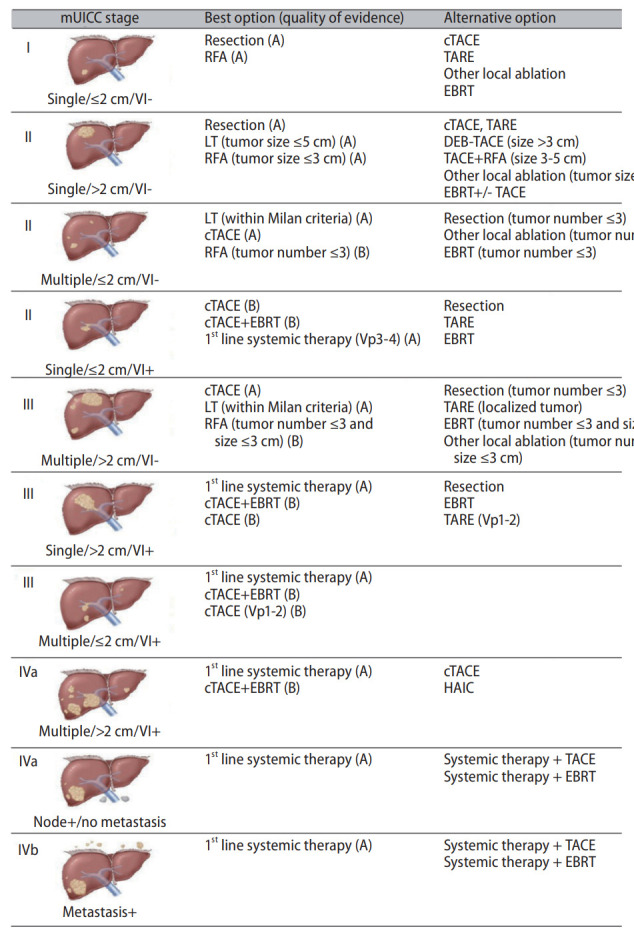
Best and alternative first-line treatment options in 2022 KLCA-NCC Korea guidelines for patients with HCC, Child-Pugh class A, no portal hypertension, and Eastern Cooperative Oncology Group performance status 0–1. KLCA-NCC, Korean Liver Cancer Association and National Cancer Center; HCC, hepatocellular carcinoma; mUICC, modified Union for International Cancer Control; VI, vascular or bile duct invasion; RFA, radiofrequency ablation; cTACE, conventional transarterial chemoembolization; TARE, transarterial radioembolization; Other local ablation included percutaneous ethanol injection, microwave ablation, and cryoablation; Vp, portal vein invasion; LT, liver transplantation; DEB-TACE, drug eluting bead-TACE; TACE included cTACE and DEB-TACE; HAIC, hepatic arterial infusion chemotherapy. Adopted from 2022 KLCA-NCC HCC guidelines [1].
The KLCA-NCC Korea guidelines also have specific recommendations by treatment modality. Some key recommendations are shown below.
Hepatic resection
Hepatic resection is the primary treatment modality for single HCC limited to the liver in Child-Pugh class A patients without portal hypertension or hyperbilirubinemia (A1). Limited hepatic resection can be selectively performed for ChildPugh class A or B7 single HCC with mild portal hypertension or hyperbilirubinemia (C1). Laparoscopic liver resection can be selectively performed for HCC located in the left lateral section and anterolateral segments (B2).
Liver transplantation (LT)
LT is the primary treatment modality for patients with HCC unsuitable for resection but within the Milan criteria (A1). If HCC stage is downgraded by loco-regional therapies in patients initially exceeding the Milan criteria, LT shows better outcomes than other treatments (B1). Salvage transplantation can be indicated for recurrent HCC after resection according to the same criteria used for first-line transplantation (B1).
Local ablation therapies
Radiofrequency ablation (RFA) has an equivalent survival rate, a higher local tumor progression rate, and a lower complication rate compared to hepatic resection in patients with a single nodular HCC ≤3 cm in diameter (A1). Combined therapies with transarterial chemoembolization (TACE) and RFA or microwave ablation (MWA) can increase the survival rate in patients with 3–5 cm HCCs that are not amenable to hepatic resection compared to RFA or MWA alone (A2). MWA and cryoablation are expected to improve rates of survival, recurrence, and complications comparable to those of RFA (B2). Contrast-enhanced US and fusion imaging can improve the detection rate and technical success rate of local ablation therapy for HCCs ≤2 cm (B1).
TACE and radioembolization
Conventional TACE (cTACE) is recommended for HCC patients with a good performance status without major vascular invasion or extrahepatic spread who are ineligible for hepatic resection, LT, or local ablation therapies (A1). cTACE should be performed through tumor-feeding arteries in a superselective manner (B1). Drug-eluting bead TACE can be considered as an alternative treatment to cTACE in HCCs ≥3 cm (A2). TACE refractoriness is defined as absence of objective response (complete response or partial response), new vascular invasion, or new extrahepatic metastasis after two consecutive TACE sessions within six months; a new treatment modality should be considered in such cases (C1). cTACE alone (B2) or cTACE combined with external beam radiation therapy (EBRT) (B1) can be considered for HCC with portal vein invasion when tumors are localized within the liver and liver function is well preserved. 90Y transarterial radioembolization (TARE) can be considered an alternative treatment to cTACE when the remnant liver function is expected to be sufficient after TARE (B2).
EBRT
EBRT is recommended for patients with HCC unsuitable for hepatic resection, transplantation, local ablation treatments, or TACE (C1). EBRT is performed when the liver function is Child-Pugh class A or B7 and when the volume to be irradiated with ≤30 Gy is ≥40% of the total liver volume in the computerized treatment plan (B1). EBRT can be combined for HCC expected to have an incomplete response after TACE (B2) or HCC with portal vein invasion (B2). EBRT is recommended for palliating symptoms of HCC (B1). Proton beam therapy (PBT) showed similar survival and toxicity rates when treating recurrent or residual HCCs ≤3 cm in size (A2).
Systemic therapies
First-line therapies: atezolizumab plus bevacizumab or durvalumab plus tremelimumab are recommended for systemic treatment-naïve patients with locally advanced unresectable or metastatic HCC not amenable to curative or loco-regional therapy who have Child-Pugh class A with ECOG performance status 0–1 (A1). If these two combination therapies cannot be applied, sorafenib or lenvatinib is recommended (A1). Sorafenib is considered for patients with HCC who have Child-Pugh class B7 (B1) or B8–9 (B2).
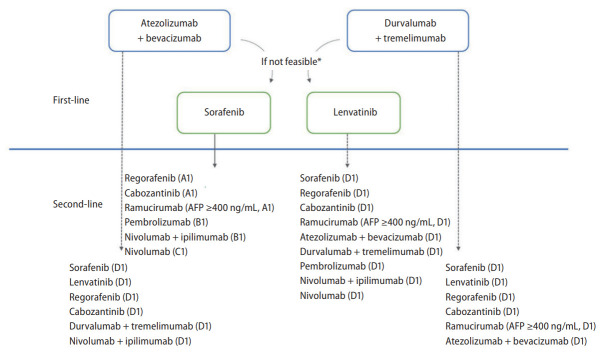
Second-line therapies: the following second-line therapies can be considered or tried in patients with Child-Pugh class A and ECOG performance status 0–1 (Fig. 3).
Figure 3.
Treatment algorithm of systemic therapies for hepatocellular carcinoma. AFP, alpha-fetoprotein. *If patients have absolute or relative contraindications for immune-checkpoint inhibitors or bevacizumab, multiple tyrosine kinase inhibitors such as sorafenib or lenvatinib should be recommended. Adopted from 2022 KLCA-NCC HCC guidelines.
Adjuvant therapy
Adjuvant immunotherapy with CIK cells can be considered after curative treatment in patients with HCC ≤2 cm without lymph node or distant metastasis (A2).
Real-life situation and practice
Analysis of the Korean Primary Liver Cancer Registry between 2012 and 2014 showed that various treatments were applied for the same BCLC stage [8]. A prospective cohort study has assessed treatment patterns and outcomes of HCC patients with portal vein invasion in South Korea and found that treatment patterns are very heterogeneous without a dominant treatment modality [26]. Many patients are being treated outside recommendations in real-life clinical practice [27-29]. Major reasons for such difference between guidelines and real-life practice can be summarized as follows. First, study results with a high quality of evidence are not yet available for some clinical questions regarding management of HCC. Second, some tests, drugs, and treatments cannot be strongly recommended due to high cost or resource consumption. Third, significant disparities remain between guideline recommendation and reimbursement policy of the National Health Insurance system. South Korea has a public and single-payer system for healthcare services based on fee-for-service payments [30]. The National Health Insurance reimbursement claim codes are used by all healthcare providers for reimbursement for their healthcare services. For patients with liver cancer, 95% of costs are reimbursed. However, not all medical services are covered by the National Health Insurance. For some medical services, the National Health Insurance provides partial reimbursement (e.g., 50% of TARE costs are reimbursed). For some medical services (e.g., second-line treatment after atezolizumab-bevacizumab or lenvatinib in year 2022), costs are not covered by the National Health Insurance. The health insurance coverage for anti-cancer treatment has an impact on real-life practice patterns in South Korea [31,32]. Fourth, there is a serious shortage of deceased donor organs. Thus, living donor liver transplantation accounts for the majority of liver transplant candidates [33]. Living donor liver transplantation depends entirely on the discretion of the transplant team and the donor. Hence, many transplant centers consider liver transplantation even for patients with advanced HCC if a recipient has no other effective treatment options and a well-informed donor wishes to willingly participate [33]. Fifth, substantial differences in resources or expertise for management of HCC exist according to individual medical institutions. Last, diagnostic and therapeutic methods for HCC management are among the most complicated and rapidly changing medical fields.
DISCUSSION
There are several important characteristics of the 2022 KLCA-NCC Korea guidelines. First, the guidelines adopt evidence-based recommendations by incorporating the most recent clinical data and real-world clinical practice in South Korea. Second, its recommendation regarding HCC treatment is composed of a description of individual treatment options rather than algorithm-based recommendations. Third, this guideline is a multi-disciplinary one as it is reviewed by experts in various fields of HCC management who provide various treatment modalities, including combination therapies. Fourth, the 2022 KLCA-NCC Korea guidelines suggest the best and alternative first-line treatment options for patients with HCC, Child-Pugh class A, no portal hypertension, and ECOG status 0–1. Last, since the rapidly evolving field of systemic therapy with newly approved drugs lacks robust information, expert opinion graded as level D is used to determine the optimal sequential systemic treatment in patients with advanced HCC. Although such recommendations have been reviewed by the Delphi panel of experts, they require further improvement.
The following efforts are needed to reduce the gap between guidelines and practice for HCC management. First, studies are needed to obtain results with a high quality of evidence to answer various unresolved issues in the field of HCC management. Second, research studies should evaluate cost-benefit of tests, drugs, or treatment in South Korea. Such studies can provide a strong basis not only for clinicians, but also for policy authorities. Third, multi-disciplinary and multi-institutional studies are needed to suggest solutions for various unanswered questions. Last, we have a plan for updating guidelines when new test methods, drugs, and treatments regarding HCC are developed and new significant research findings are published. This will ultimately lead to better outcomes for HCC patients.
Acknowledgments
This paper was supported by a grant (grant number: S-2019-2739-000) of the Research & Business Foundation of Sungkyunkwan University, Korea.
Abbreviations
- HCC
hepatocellular carcinoma
- KLCA-NCC
Korean Liver Cancer Association and National Cancer Center
- GRADE
Grading of Recommendations
- US
ultrasonography
- AFP
alphafetoprotein
- CT
computed tomography
- MRI
magnetic resonance imaging
- NLCSP
National Liver Cancer Screening Program
- LI-RADS
Liver Imaging-Reporting and Data System
- mUICC
modified Union for International Cancer Control
- BCLC
Barcelona Clinic Liver Cancer
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- MWA
microwave ablation
- EBRT
external beam radiation therapy
- TARE
transarterial radioembolization
- ECOG
Eastern Cooperative Oncology Group
Footnotes
Authors’ contribution
Drafting of the manuscript (M.J.Goh, D.H.Sinn,M.S.Choi); Critical revision of the manuscript (all authors); Obtained funding (M.S.Choi); Study supervision (M.S.Choi); Approval of the final version of the manuscript (all authors).
Conflicts of Interest
The authors have no conflicts to disclose.
REFERENCES
- 1.Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:583–705. doi: 10.3350/cmh.2022.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chon YE, Jeong SW, Jun DW. Hepatocellular carcinoma statistics in South Korea. Clin Mol Hepatol. 2021;27:512–514. doi: 10.3350/cmh.2021.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JW, Korean Liver Cancer Study Group and National Cancer Center [Practice guideline for diagnosis and treatment of hepatocellular carcinoma] Korean J Hepatol. 2004;10:88–98. Korean. [PubMed] [Google Scholar]
- 4.Korean Liver Cancer Study Group and National Cancer Center, Korea [Practice guidelines for management of hepatocellular carcinoma 2009] Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. Korean. [DOI] [PubMed] [Google Scholar]
- 5.Korean Liver Cancer Study Group (KLCSG) National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korean Liver Cancer Association. National Cancer Center 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. GRADE Working Group Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ 2008;336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean Nationwide Cancer Registry. J Liver Cancer. 2020;20:135–147. doi: 10.17998/jlc.20.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinn DH, Kang D, Kang M, Paik SW, Guallar E, Cho J, et al. Late presentation of hepatitis B among patients with newly diagnosed hepatocellular carcinoma: a national cohort study. BMC Cancer. 2019;19:286. doi: 10.1186/s12885-019-5508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725–730. [PubMed] [Google Scholar]
- 11.Sohn W, Lee Y-S, Lee JG, An J, Jang ES, Lee DH, et al. A survey of liver cancer specialists’ views on the national liver cancer screening program in Korea. J Liver Cancer. 2020;20:53–59. doi: 10.17998/jlc.20.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn W, Kang D, Kang M, Guallar E, Cho J, Paik YH. Impact of nationwide hepatocellular carcinoma surveillance on the prognosis in patients with chronic liver disease. Clin Mol Hepatol. 2022;28:851–863. doi: 10.3350/cmh.2022.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH, Hong SB, Choi SH, Kim SY, Shim JH, Lee JS, et al. Surveillance failure in ultrasound for hepatocellular carcinoma: a systematic review and meta-analysis. Gut. 2022;71:212–213. doi: 10.1136/gutjnl-2020-323615. [DOI] [PubMed] [Google Scholar]
- 14.Kim YY, An C, Kim DY, Aljoqiman KS, Choi JY, Kim MJ. Failure of hepatocellular carcinoma surveillance: inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrasonography. 2019;38:311–320. doi: 10.14366/usg.18051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinn DH, Yi J, Choi MS, Choi D, Gwak GY, Paik YH, et al. Incidence and risk factors for surveillance failure in patients with regular hepatocellular carcinoma surveillance. Hepatol Int. 2013;7:1010–1018. doi: 10.1007/s12072-013-9462-z. [DOI] [PubMed] [Google Scholar]
- 16.Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, et al. A comparison of biannual two-phase low-dose liver CT and US for HCC surveillance in a group at high risk of HCC development. Liver Cancer. 2020;9:503–517. doi: 10.1159/000506834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3:456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25:245–263. doi: 10.3350/cmh.2018.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Lee JM, Kim TH, Yoon JH. Imaging diagnosis of hepatocellular carcinoma: Future directions with special emphasis on hepatobiliary magnetic resonance imaging and contrast-enhanced ultrasound. Clin Mol Hepatol. 2022;28:362–379. doi: 10.3350/cmh.2021.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH. Diagnostic performance of 2018 KLCA-NCC practice guideline for hepatocellular carcinoma on Gadoxetic acid-enhanced MRI in patients with chronic hepatitis B or cirrhosis: comparison with LI-RADS version 2018. Korean J Radiol. 2021;22:1066–1076. doi: 10.3348/kjr.2020.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Kim SS, Chang DR, Kim H, Kim MJ. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin Mol Hepatol. 2020;26:340–351. doi: 10.3350/cmh.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon SK, Lee JM, Joo I, Yoo J, Park JY. Comparison of guidelines for diagnosis of hepatocellular carcinoma using gadoxetic acid-enhanced MRI in transplantation candidates. Eur Radiol. 2020;30:4762–4771. doi: 10.1007/s00330-020-06881-y. [DOI] [PubMed] [Google Scholar]
- 23.Kim NJ, Shin DH, Kang W, Paik YH, Choi MS, Lee JH, et al. Noninvasive diagnostic criteria of the revised 2014 the Korean Liver Cancer Study Group and the National Cancer Center guideline for subcentimetersized hepatocellular carcinoma: is it too strict? J Liver Cancer. 2018;18:44–50. [Google Scholar]
- 24.Song KD, Kim SH, Lim HK, Jung SH, Sohn I, Kim HS. Subcentimeter hypervascular nodule with typical imaging findings of hepatocellular carcinoma in patients with history of hepatocellular carcinoma: natural course on serial gadoxetic acid-enhanced MRI and diffusion-weighted imaging. Eur Radiol. 2015;25:2789–2796. doi: 10.1007/s00330-015-3680-9. [DOI] [PubMed] [Google Scholar]
- 25.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14:e0210730. doi: 10.1371/journal.pone.0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinn DH, Lee HW, Paik YH, Kim DY, Kim YJ, Kim KM, et al. Patterns and outcomes in hepatocellular carcinoma patients with portal vein invasion: a multicenter prospective cohort study. Dig Dis Sci. 2021;66:315–324. doi: 10.1007/s10620-020-06134-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim KM, Sinn DH, Jung SH, Gwak GY, Paik YH, Choi MS, et al. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016;36:1490–1497. doi: 10.1111/liv.13107. [DOI] [PubMed] [Google Scholar]
- 28.Kim BK, Kim DY, Han KH, Seong J. Changes in real-life practice for hepatocellular carcinoma patients in the Republic of Korea over a 12-year period: A nationwide random sample study. PLoS One. 2019;14:e0223678. doi: 10.1371/journal.pone.0223678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Kim BK, Kim SU, Park JY, Ahn SH, Seong JS, et al. A survey on transarterial chemoembolization refractoriness and a real-world treatment pattern for hepatocellular carcinoma in Korea. Clin Mol Hepatol. 2020;26:24–32. doi: 10.3350/cmh.2018.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang H, Park HA. Mapping Korean National Health Insurance reimbursement claim codes for therapeutic and surgical procedures to SNOMED-CT to facilitate data reuse. Stud Health Technol Inform. 2022;290:101–105. doi: 10.3233/SHTI220040. [DOI] [PubMed] [Google Scholar]
- 31.Cho DY, Park J, Kim DS. The impact of expanding health insurance coverage for anti-cancer drugs on cancer survival in Korea. Cancer Med. 2021;10:4555–4563. doi: 10.1002/cam4.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayzah M, Ryu JM, Lee JH, Nam SJ, Kim SW, Lee SK, et al. Changes in Korean National Healthcare Insurance policy and breast cancer surgery trend in Korea. J Korean Med Sci. 2021;36:e194. doi: 10.3346/jkms.2021.36.e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HW, Suh KS. Advancements of liver transplantation for hepatocellular carcinoma in Korea. Jpn J Clin Oncol. 2017;47:93–100. doi: 10.1093/jjco/hyw168. [DOI] [PubMed] [Google Scholar]