Summary
Background
Omega-3 fatty acids are critical for neuropsychological functioning. Adolescence is increasingly believed to entail brain vulnerability to dietary intake. The potential benefit on adolescent neurodevelopment of consuming walnuts, a source of omega-3 alpha-linolenic acid (ALA), remains unclear.
Methods
We conducted a 6-month multi-school-based randomised controlled nutrition intervention trial to assess whether walnut consumption has beneficial effects on the neuropsychological and behavioural development of adolescents. The study took place between 04/01/2016 and 06/30/2017 in twelve different high schools in Barcelona, Spain (ClinicalTrials.gov Identifier: NCT02590848). A total of 771 healthy teenagers aged 11–16 years were randomised into two equal groups (intervention or control). The intervention group received 30 g/day of raw walnut kernels to be incorporated into their diet for 6 months. Multiple primary endpoints concerning neuropsychological (working memory, attention, fluid intelligence, and executive function) and behavioural (socio-emotional and attention deficit hyperactivity disorder [ADHD] symptoms) development were assessed at baseline and after intervention. Red blood cell (RBC) ALA status was determined at baseline and 6 months as a measure of compliance. Main analyses were based on intention-to-treat using a linear mixed-effects model. A per-protocol effect of the intervention was analysed using inverse-probability weighting to account for post-randomisation prognostic factors (including adherence) using generalised estimating equations.
Findings
In intention-to-treat analyses, at 6 months there were no statistically significant changes between the intervention and control groups for all primary endpoints. RBC ALA (%) significantly increased only in the intervention group, coefficient = 0.04 (95% Confidence Interval (CI) = 0.03, 0.06; p < 0.0001). The per-protocol (adherence-adjusted) effect on improvement in attention score (hit reaction time variability) was −11.26 ms (95% CI = −19.92, −2.60; p = 0.011) for the intervention group as compared to the control group, improvement in fluid intelligence score was 1.78 (95% CI = 0.90, 2.67; p < 0.0001), and reduction of ADHD symptom score was −2.18 (95% CI = −3.70, −0.67; p = 0.0050).
Interpretation
Our study suggested that being prescribed eating walnuts for 6 months did not improve the neuropsychological function of healthy adolescents. However, improved sustained attention, fluid intelligence, and ADHD symptoms were observed in participants who better complied with the walnut intervention. This study provides a foundation for further clinical and epidemiological research on the effect of walnuts and ALA on neurodevelopment in adolescents.
Funding
This study was supported by Instituto de Salud Carlos III through the projects ‘CP14/00108, PI16/00261, PI21/00266’ (co-funded by European Union Regional Development Fund ‘A way to make Europe’). The California Walnut Commission (CWC) has given support by supplying the walnuts for free for the Walnuts Smart Snack Dietary Intervention Trial.
Keywords: Adolescent health, Cognitive function, Neuropsychology, Randomised nutritional intervention, Public health, Walnut intake
Research in context.
Evidence before this study
We searched PubMed on June 1, 2022 using the terms “walnuts” OR "alfa linolenic acid" AND “cognition” OR "neurodevelopment" OR "cognitive function" and reviewed all publications of animal and clinical studies. Only one observational study among children and adolescents, and another small intervention study among young adults have examined the relation between nut consumption and cognitive function in a target population similar to ours. To date, no randomised controlled trial study has focused on the effect of walnut consumption on adolescent neuropsychological function.
Added value of this study
The role of walnut consumption on adolescent neurodevelopment has barely been explored. Although we did not find that eating walnuts for 6 months improved neuropsychological function in healthy adolescents in this dietary intervention, we did find that participants who adhered to the intervention the most had improved sustained attention, fluid intelligence, and ADHD symptoms.
Implications of all the available evidence
While an intervention such as the one proposed here is unlikely to work in real life because it requires a strong commitment and few people will comply, compliance may change once the general public learns about the positive findings in compliers. Thus, this study provides valuable insights and a basis for future scientific research on the effect of walnuts on adolescent brain development.
Introduction
Childhood has long been considered a critical period for brain development, and much public health research has focused on this time period. Recently, more attention has been paid to the adolescence period.1 One reason for an increased interest in the adolescent brain is that the prefrontal cortex, which mediates key cognitive functions such as logical thinking, executive function and superior working memory, does not reach maturity until the early-twenties.2 Additionally, adolescence is known to be a period of refinement of brain connectivity and complex behaviours. Thus, not being fully mature, the adolescent brain is still sensitive to a number of environmental and lifestyle factors, including exposure to certain foods and nutrients.3 Considering that the brain requires a large amount of energy and nutrients, especially during its development, a lack of essential nutrients can interfere with optimal maturation.4,5 Several studies suggest that a healthy and balanced diet providing essential nutrients may have long-term beneficial functional consequences.6
Experimental and clinical studies have supported the important role of polyunsaturated fatty acids (PUFAs) in central nervous system architecture and function during neural development.4,7 Three specific PUFAs play an essential developmental role in the brain: long-chain omega-3 (docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids), and omega-6 (arachidonic acid (AA)). In mammals, DHA and EPA must be obtained through diet (mainly from seafood) because de novo synthesis is extremely inefficient.8
Walnuts are among the richest sources of the plant-derived omega-3 fatty acid alpha-linolenic acid (ALA), the precursor for longer-chain EPA and DHA.9,10 Although ALA is poorly converted to EPA and DHA,11 animal and clinical studies have shown that ALA by itself has positive effects on brain function and plasticity.12, 13, 14, 15 Walnuts have a high content of fibre, antioxidant vitamins, non-sodium minerals, phytosterols, polyphenols, and other bioactive compounds capable of improving brain health.16,17 Indeed, it has been suggested that walnut constituents, particularly polyphenols, may act synergistically with ALA to foster brain health.17 Studies in walnut-fed rats showed improvements in working memory,9 and a randomised controlled trial carried out among 447 older adults (mean age, 67 years), found that a Mediterranean diet supplemented with 30 g/day of mixed nuts (including 15 g of raw walnut kernels) improved memory and delayed cognitive decline after a median follow-up of 4.1 years.18
One observational study among children and adolescents have examined the relation between nut consumption and cognitive function and another small intervention study among young adults investigated the effect of walnut consumption.19,20 Positive associations with cognitive function have been suggested in both of them. To date, no randomised controlled trial study has focused on the effect of walnut consumption on adolescent neuropsychological function.21
We conducted a 6-month multi-school-based randomised controlled nutrition intervention trial entitled WALNUTs Smart-Snack (WSS)22 to assess whether walnut consumption would enhance neuropsychological and behavioural (socio-emotional) development among healthy adolescents from twelve high schools evenly distributed in Barcelona city districts.
Methods
Study design and participants
WSS is a multi-school, parallel (two-arm), controlled, 6-month superiority randomised intervention trial conducted from 04/01/2016 to 06/30/2017 in a large population-based sample (n = 771) of healthy adolescents from twelve high schools in Barcelona, Catalonia, Spain (Figure S1). All study individuals were instructed to follow general healthy eating recommendations and were randomly assigned 1:1 to one of two groups immediately following baseline assessment: the intervention group (usual diet and provided with walnuts daily, n = 386) or the control group (usual diet and with no specific recommendation, n = 385). Participants were assessed at baseline and after the 6-month intervention by using several validated neuropsychological tests and behavioural rating scales. The eligibility criteria were adolescents aged between 11 and 16 years attending high schools in Barcelona, Spain. Exclusion criteria included those consuming regularly supplements of omega-3 PUFAs; already eating walnuts on a daily basis, and/or self-reported allergy to walnuts and/or gluten. Individuals were also excluded if they reported lactose intolerance or allergy to cereals, dried fruits, peanuts, soy, sesame, or sulphites, since there may be traces in walnut packages. The CEIC Parc Salut Mar approved the study protocol (available at the following link: https://www.frontiersin.org/articles/10.3389/fped.2021.593847/full), and the study was conducted in accordance with the guidelines of the Declaration of Helsinki.22
Ethics statement
This study was reviewed and approved by CEIC Parc Salut Mar (approval number: 2015/6026/I). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Procedures
We obtained permission from the boards of participating schools to inform them of the trial. Then, we provided the schools and families with a recruitment leaflet to assess the willingness to collaborate and permission to contact families by telephone. Signed informed consent was obtained from each participant and one of their parents. During enrolment (2016), fieldworkers verified the eligibility criteria of each adolescent. After the baseline assessment, participants were assigned to one of the two study groups (walnut intervention or control).
Randomisation and masking
We performed the whole randomisation procedure at school level. We defined the blocking variable according to age (at the date of randomisation, in years) and sex of the participant, and the education level of the mother (or main tutor). All individuals who agreed to participate in the intervention were asked about their age, sex and maternal education in a short telephone questionnaire prior to randomisation, but some of these participants were not eventually accessible, so there were some missing values in these variables (sex = 1; age = 37; maternal education = 34). Specifically, for each of the 12 schools we proceeded as follows.
First, we imputed the missing data for sex, age and parental education (all considered as factors) using multivariate imputation by chained equations. For each of the three variables, we set the other two as predictors (i.e., each of the three variables was imputed using the information available on the other two). Imputation methods were logistic regression (for sex) and multinomial regression (for age and parental education). A single imputation procedure was performed using the R package mice.
Second, we created the blocking variable as a stratum variable. The strata consisted of all possible combinations of the factors age, sex, and parental education. Within each stratum, we randomly assigned each participant to one of the two groups (intervention or control). The randomisation algorithm automatically repeated randomisation until all the following conditions were met within each stratum: 1) Children from the same household were assigned to the same group (i.e., the algorithm randomised only one of the children and then forced the other ones into the same group); 2) Both groups (intervention or control) had equal sizes (or a difference of one unit when the stratum size was odd); 3) The frequency of each of the three variables (sex, age, and parental education) differed by at most two units, when comparing the intervention and control groups. Once all conditions met, we kept the optimal seed (for reproducibility of results) and checked the quality control of optimal randomisation. Eligible candidates who were siblings of participating adolescents and were allocated to the same group, later on, in the main analyses, they were analysed as clusters.
The randomisation process was conducted by a statistician with RStudio statistical software package (R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Investigators and teachers assessing outcomes, fieldworkers who administered the questionnaires, nurses and psychologists who assessed neuropsychological and clinical data and obtained blood samples, the statistician who performed the randomisation process, and investigators that analysed the data were blinded to intervention assignment. Given the nature of the study (nutritional intervention), it was not possible to mask participants and their families.
Intervention
Participants randomised to the intervention group received sachets containing 30 g of raw walnut kernels to incorporate into their daily diet. The type of walnut was the Californian walnut, which is estimated to contain about 9 g of ALA per 100 g.17,23 It is ethical and feasible to recommend and facilitate walnut intake over a long period of time (e.g., 6 months).24 Californian walnut Kernel (30 g per day) would meet recommendations to consume about 2.5 g of omega-3 PUFAs per day.23,25 All families received a guide (seasonal fruit calendar) to follow and were asked to eat, at least, a piece of seasonal fruit every day in order to ensure implementation and adherence. Families in the intervention group received additional instructions on how to encourage their adolescents to eat their daily amount of walnuts. Parents were asked to supervise the intervention by monitoring the adolescents’ adherence to walnut consumption. To evaluate adherence to the intervention, parents were asked to report weekly the daily walnut consumption of their child using a web-based platform indicating the days they had eaten or not eaten the allocated amounts of walnuts. For participants assigned to the intervention group, adherence was defined as ≥100 days during 6 months of reporting eating walnuts.22 We measured changes in omega-3 PUFA RBC in a randomly selected sub-set of participants (n = 170 for the intervention group and n = 162 for the control group) as an objective biomarker of adherence to the intervention. Furthermore, adolescents in the intervention group were contacted at the mid-point of follow-up to provide them with more recipes of walnut-based dishes.
Sociodemographic and lifestyle data
At baseline, two questionnaires of sociodemographic and lifestyle habits, such as physical activity (dichotomic, ≤twice a week/≥3 times a week), sleep duration (average number of hours the participant sleeps on weekdays), and parental education (dichotomic, up to high school studies/university studies), were obtained. A mood score subscale was based on several inquiries related to mood during the last month prior to the questionnaire using the KIDSCREEN-27 quality of life measure for children and adolescents.26 In addition, to assess their general diet, all participants completed a food frequency questionnaire (FFQ), validated for the Spanish population27 and adapted to the adolescent range at baseline and at the end of the intervention (after 6 months). Adherence to the Mediterranean diet was also assessed by creating a score based on the consumption of fruits, vegetables, legumes, seafood, cereals, nuts, dairy, and olive oil (KIDMED Index).22,28
Neuropsychological and behavioural testing for primary endpoints
Several primary endpoints concerning the neuropsychological (attention, working memory, fluid intelligence and executive function) and behavioural (socio-emotional and attention deficit hyperactivity disorder [ADHD] symptoms) development of adolescents were assessed at baseline (pre-intervention) and after 6 months (post-intervention).29 The administration of all neuropsychological tests was carried out at the school by one trained psychologist and two fieldwork technicians. The Attention Network Test (ANT) was used to assess attention, where lower scores indicate better attention performance (measured as hit reaction time variability in milliseconds).30 The N-back task was used to assess working memory, where a higher score indicates a more accurate performance.31 To assess fluid intelligence, we used the inductive reasoning subtest of the Tests of Primary Mental Abilities (PMA-R), where the total score is the number of correct item responses, and thus, a higher score indicates higher abilities.32 The fourth measurement was The Roulettes Task which assesses risky decision-making (executive function).33 The scores here are measured as total risk adjustment and the closer to 0 the risk adjustment index is, the more risk insensitivity. Fifth, a self-reported version of the Strengths and Difficulties Questionnaire (SDQ) provided a total score of problem behavior.34 A higher score indicates a more problematic behaviour. Finally, ADHD symptoms were assessed by school teachers filling up the attention deficit hyperactivity disorder DSM-IV form list. An extended description of these outcomes can be found in the Supplement and in the published protocol paper.22
Secondary endpoints
In addition to the neuropsychological and behavioural endpoints, secondary outcomes measured were changes in height, weight, waist circumference and body mass index (BMI), as well as RBC proportions of omega-3 fatty acids (DHA, EPA, and ALA) expressed as relative amounts (percent of total fatty acids) at baseline and after 6 months of intervention.22 An extended description of the measurement of these outcomes can be found in the Supplement.
Statistical analysis
All main analyses were based on an intention-to-treat (ITT) method, that is, we analysed all participants according to their original group assignment regardless of their adherence to the intervention. Descriptive statistics were used to show the baseline characteristics of each group. First, a simple comparison of population means was performed to assess baseline and 6-month changes in primary and secondary endpoints within (paired t-test) and between (independent two-sample t-test) intervention groups. To assess the effect of the intervention on neurodevelopment, the main analyses were conducted using linear mixed-effects models with follow-up scores as the outcome variable, the intervention arm as the explanatory variable of interest, and using school and household as random effects. Although only two measures are involved, we decided to use a mixed-model method to gain more statistical power, but especially to account for the correlation between individuals from the same school and/or household. Models were adjusted for sex, age and maternal education. Models were fit separately for each outcome. Missing data from outcome variables were excluded (the participant was excluded from the analyses of that outcome). Similar models were used to assess the effect of the intervention on RBC ALA status as a secondary outcome variable. In sensitivity analyses, we repeated the models adjusting for baseline outcomes, as well as analysing the intervention effect on secondary outcomes.
A secondary analysis was also performed to estimate the per-protocol (PP) effect of the intervention group compared to the control group. This analysis estimated the effect if all participants had sufficiently adhered to their assigned intervention during the 6-month intervention period. We censored data for participants who reported eating walnuts for less than 100 days during the 6-month intervention trial (<55% of the total recommended time period, which corresponds to less than 3 servings per week),22 and for those who were lost to follow-up or left the intervention. We then estimated inverse-probability of censoring weights to adjust for selection. Variables used to calculate the weights included the following post-randomisation prognostic factors: baseline neuropsychological test results, body weight at baseline and after the intervention, adherence to a Mediterranean diet, physical activity frequency, daily sleeping time, and mood at baseline. The weighted analysis estimated again the intervention effect (excluding non-compliers) for all primary endpoints and RBC ALA status using Generalized Estimating Equations (GEE) with school as a panel variable.
Using multiple primary endpoints, the study was considered to have a beneficial effect if the results of either one of the primary endpoints was statistically significant in favour of the experimental intervention and/or treatment adherence. The nominal statistical significance was set at the p < 0.05 level (two-sided) for the outcomes. Calculations were corrected for multiplicity using the Benjamin-Hochberg method. Since no universal false discovery rate (FDR) significance threshold has been defined, a cut-point of 0.05 was used. Statistical analyses were performed using STATA 15 statistical software package (StataCorp, 2017, College Station, TX: StataCorp LLC). Statistical power calculations can be found in the Supplement.
Role of the funding source
The funders had no role in the study design, collection, management, analysis and interpretation of data, writing of the report or decision to submit it for publication. APM, FG and JJ had access to dataset and decision to submit for publication. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
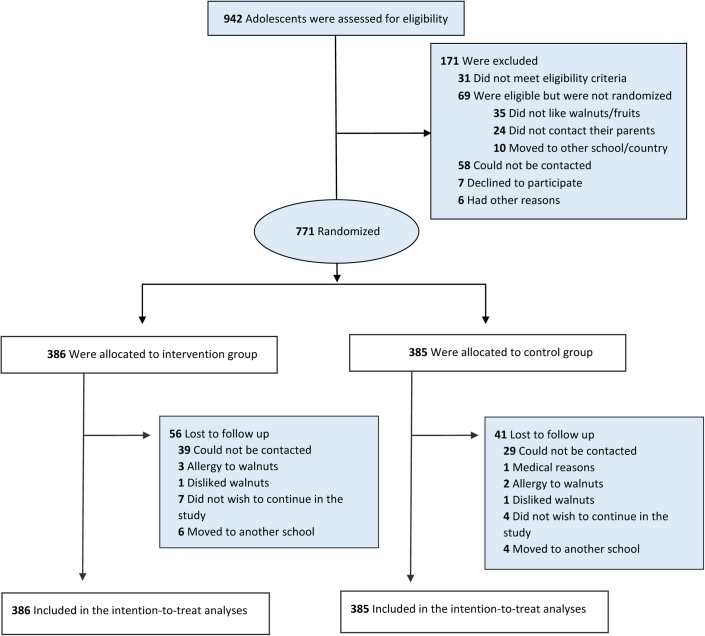
A total of 942 adolescents were eligible for the study trial (Fig. 1). After exclusion, a total of 771 adolescents were randomised. Study retention was high through 6 months (87%, n = 674). Baseline characteristics of participants included in the ITT analysis by group allocation are shown in Table 1. The average age (standard deviation, SD) of study individuals was 13.9 (0.9) and 13.8 (0.9) years for the walnut and control groups, respectively. Most participants were born in Spain (n = 312 (92%) in the walnut group and n = 299 (88%) in the control group) and had a medium or high adherence to the Mediterranean diet. There were no meaningful differences between the intervention and control groups in baseline or lifestyle characteristics. In addition, participants had similar mean scores in all primary endpoints in the walnut and control groups at baseline (Table 1). Adolescents in the intervention group reported consuming walnuts (30 g) on average (SD) 70 (60) days, and a total of 133 of 386 (34%) participants adhered to more than half the time period of the intervention (threshold = 100 days out of 180 days of the intervention).
Fig. 1.
Flowchart of the WALNUTs Smart-Snack study.
Table 1.
Baseline characteristics of the intention-to-treat population by intervention group (n = 771).
| Characteristicsa | n | Walnut group (n = 386) | n | Control group (n = 385) |
|---|---|---|---|---|
| Sex, female | 385 | 209 (54.3) | 385 | 207 (53.8) |
| Age, mean (SD) y | 374 | 13.9 (0.9) | 377 | 13.8 (0.9) |
| BMI, mean (SD) kg/m2 | 367 | 20.5 (3.0) | 374 | 20.7 (3.7) |
| Physical activity frequency | ||||
| Twice a week or less | – | 138 (42.3) | – | 153 (46.0) |
| Three times a week or more | – | 188 (57.7) | – | 180 (54.1) |
| Daily sleeping time, mean (SD) h | 323 | 8.0 (0.9) | 326 | 8.0 (1.0) |
| Mood score, mean (SD)b | 348 | 28.5 (4.2) | 340 | 28.0 (4.3) |
| Adherence to Mediterranean dietc | ||||
| Low | – | 22 (6.9) | – | 22 (6.8) |
| Medium | – | 173 (54.1) | – | 171 (52.5) |
| High | – | 125 (39.1) | – | 133 (40.8) |
| Maternal | ||||
| Maternal education | ||||
| High school or lower | – | 154 (40.9) | – | 155 (40.8) |
| At least some university studies | – | 223 (59.2) | – | 225 (59.2) |
| Baseline primary endpoints | ||||
| Attention score (ms), mean (SD)d | 358 | 149.1 (78.9) | 358 | 148.2 (78.3) |
| Working memory score, mean (SD)e | 350 | 1.75 (1.15) | 357 | 1.82 (1.18) |
| Fluid intelligence score, mean (SD)e | 360 | 16.6 (5.5) | 356 | 16.5 (5.8) |
| Risky decision-making score, mean (SD)e | 358 | 8.3 (4.3) | 356 | 7.9 (4.4) |
| Behavioural problem score, mean (SD)d | 321 | 10.4 (4.8) | 323 | 10.6 (4.7) |
| ADHD general score, mean (SD)d | 316 | 7.6 (10.1) | 319 | 7.6 (9.7) |
ADHD, Attention deficit hyperactivity disorder.
Unless otherwise indicated, data are expressed as number (percentage) of participants. Percentages have been rounded and may not total 100.
The higher the score, the better the mood, with a maximum score of 35.
Data were obtained from a short true/false 16-item questionnaire from the FFQ modified to a relative score, with a final total score of 12. A score of 3 or less equals low adherence, a score from 4 to 7 equals medium adherence and a score of 8 or greater equals high adherence.
Lower scores indicate better performance/less problematic behaviour.
Higher scores indicate better performance.
Table 2 presents baseline and 6-month differences in primary and secondary endpoints within and between intervention groups. The successful rate of retesting at 6 months was of 644 out of 771 (83.5%). Within groups, there was a significant increase in attention function, working memory, fluid intelligence and risky decision-making scores. RBC ALA status increased by 0.03% in the intervention group and by 0.01% in the control group (significant mean difference p < 0.0001) (Table 2 and Figure S2). There were no statistically significant differences in primary outcomes between the two groups.
Table 2.
Changes in outcome variables from baseline to end of intervention, in intention-to-treat analyses.
| Outcome variables (mean, (SD)) | Walnut group |
Control group |
pb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | After 6 months | Change | pa | N | Baseline | After 6 months | Change | pa | ||
| Primary endpoints | |||||||||||
| Attention score (ms) | 315 | 146.38 (76.52) | 124.55 (65.58) | −21.83 | <0.0001 | 329 | 146.71 (76.09) | 124.96 (65.43) | −21.75 | <0.0001 | 0.99 |
| Working memory score | 308 | 1.73 (1.17) | 1.99 (1.15) | 0.25 | 0.0014 | 323 | 1.86 (1.18) | 2.04 (1.28) | 0.18 | 0.047 | 0.54 |
| Fluid intelligence score | 315 | 16.72 (5.48) | 19.44 (5.54) | 2.72 | <0.0001 | 327 | 16.71 (5.66) | 19.54 (5.48) | 2.83 | <0.0001 | 0.70 |
| Risky decision-making score | 316 | 8.35 (4.33) | 9.62 (4.15) | 1.27 | <0.0001 | 328 | 8.01 (4.31) | 9.59 (4.04) | 1.58 | <0.0001 | 0.36 |
| Behavioural problem score | 158 | 9.83 (5.04) | 10.06 (4.86) | 0.23 | 0.42 | 172 | 10.02 (4.29) | 9.54 (4.45) | −0.48 | 0.092 | 0.079 |
| ADHD general score | 248 | 6.64 (8.84) | 7.74 (9.46) | 1.10 | 0.016 | 262 | 6.70 (8.99) | 7.63 (9.56) | 0.93 | 0.057 | 0.79 |
| Secondary endpoints | |||||||||||
| Height, m | 316 | 1.62 (0.08) | 1.64 (0.08) | 0.02 | <0.0001 | 327 | 1.62 (0.09) | 1.64 (0.09) | 0.02 | <0.0001 | 0.63 |
| Weight, kg | 316 | 54.04 (10.37) | 56.49 (10.41) | 2.45 | <0.0001 | 327 | 53.78 (11.46) | 56.40 (11.73) | 2.63 | <0.0001 | 0.47 |
| Waist circumference, cm | 143 | 65.99 (7.01) | 67.36 (7.23) | 1.37 | <0.0001 | 134 | 65.89 (8.08) | 67.15 (8.61) | 1.26 | <0.0001 | 0.76 |
| BMI, kg/m2 | 316 | 20.45 (2.94) | 20.84 (2.95) | 0.34 | <0.0001 | 327 | 20.41 (3.33) | 20.84 (3.37) | 0.43 | <0.0001 | 0.65 |
| ALA, % | 136 | 0.10 (0.05) | 0.13 (0.11) | 0.03 | 0.0002 | 129 | 0.10 (0.07) | 0.09 (0.04) | −0.01 | 0.042 | <0.0001 |
| EPA, % | 136 | 0.31 (0.14) | 0.33 (0.14) | 0.02 | 0.069 | 129 | 0.31 (0.15) | 0.31 (0.14) | 0.00 | 0.76 | 0.12 |
| DHA, % | 136 | 4.03 (0.78) | 3.94 (0.79) | −0.09 | 0.052 | 129 | 3.96 (0.87) | 3.99 (0.89) | 0.03 | 0.52 | 0.073 |
ADHD, attention deficit hyperactivity disorder; BMI, body mass index; ALA, alpha-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; N, number of observations for the analyses.
p-values were calculated using the paired t-test (difference after intervention within groups).
p-values were calculated using the independent two-sample t-test (mean change differences between two groups).
There were no significant differences in changes detected after six months between the intervention and control group for all primary endpoints after adjusting for confounders (Table 3). Differences in secondary outcomes were also small and non-significant, except for RBC ALA status (Table 3 and Table S1). We found a significant 0.04% (95% confidence interval (CI) (0.03%, 0.06%)) higher mean of RBC ALA in the walnut group compared to the control group (Table 3). Similar results were obtained after adjusting for baseline outcomes values (Table S2 and Table S3).
Table 3.
Comparison of primary endpoints and ALA between intervention and control group at 6 months using intention-to-treat analysis.
| Outcomes after 6-month intervention | N | β Coef.a | 95% CI | p |
|---|---|---|---|---|
| Attention score (ms) | 669 | 1.74 | (−7.97, 11.46) | 0.73 |
| Working memory score | 662 | −0.03 | (−0.21, 0.15) | 0.76 |
| Fluid intelligence score | 667 | −0.21 | (−1.02, 0.59) | 0.60 |
| Risky decision-making score | 671 | 0.01 | (−0.60, 0.61) | 0.98 |
| Behavioural problem score | 343 | 0.63 | (−0.35, 1.62) | 0.21 |
| ADHD general score | 566 | 0.19 | (−1.27, 1.66) | 0.80 |
| ALA (%) | 270 | 0.04 | (0.03, 0.06) | <0.0001 |
ADHD, attention deficit hyperactivity disorder; ALA, alpha-linolenic acid; N, number of observations for the models.
Data was calculated using a linear mixed model with school and household as random effects and adjusted for sex, age and maternal education.
Some statistically significant differences in changes were detected in the PP analysis between the intervention (compliers) and control groups (Table 4). The PP (adherence-adjusted) estimated effect for an improvement on attention score was −11.26 ms (95% CI = −19.92, −2.60; p = 0.011) for the intervention group as compared to the control group, the fluid intelligence score was of 1.78 (95% CI = 0.90, 2.67; p < 0.0001), and a reduction in ADHD symptom score was −2.18 (95% CI = −3.70, −0.67; p = 0.0050) for the intervention group as compared to the control group. Test significance did not change after correcting p-values for multiple testing using the Benjamini-Hochberg false discovery rate (Table S4).
Table 4.
Comparison of primary endpoints and ALA between intervention and control groups at 6 months, with estimates based on per-protocol analysis.
| Outcomes after 6-month intervention | n/Na | β Coef.b | 95% CI | p |
|---|---|---|---|---|
| Attention score (ms) | 454/460 | −11.26 | (−19.92, −2.60) | 0.011 |
| Working memory score | 448/460 | 0.04 | (−0.31, 0.38) | 0.84 |
| Fluid intelligence score | 454/460 | 1.78 | (0.90, 2.67) | <0.0001 |
| Risky decision-making score | 455/460 | 0.34 | (−0.42, 1.09) | 0.38 |
| Behavioural problem score | 247/460 | −0.38 | (−1.74, 0.99) | 0.59 |
| ADHD general score | 367/460 | −2.18 | (−3.70, −0.67) | <0.0050 |
| ALA (%) | 185/210 | 0.08 | (0.05, 0.10) | <0.0001 |
ADHD, attention deficit hyperactivity disorder; ALA, alpha-linolenic acid.
Number of observations/total number of participants (excluding non-compliers). NTotal = 771; NALA = 332.
Data was calculated using a generalized linear regression (GEE) model with school as panel variable. Inverse-probability weights were estimated to adjust for non-compliance, based on post-randomization prognostic factors. Models were adjusted for sex, age, maternal education, weight before and after the intervention, adherence to Mediterranean diet at baseline, physical activity frequency, sleeping time, mood, and the neuropsychological outcome at baseline.
Discussion
To the best of our knowledge, WSS is the first randomised intervention trial to assess the effect of walnut consumption on cognitive and behavioural development in adolescents. We found that this intervention did not result in significant changes in adolescent neurodevelopment when analysing all participants, regardless of their adherence to the intervention (ITT analysis). Notwithstanding the absence of differences in cognitive and behavioural functions, participants in the active intervention group disclosed increases in RBC ALA compared to those in the control group. However, after accounting for non-adherence (PP analysis), significant benefits were found for attention, fluid intelligence, and ADHD symptoms, meaning that consuming 30 g of walnuts daily for 100 days, or more, improved these neuropsychological outcomes.
Currently, data regarding the beneficial effects of walnut consumption on cognitive and behavioural development in adolescents are limited. In this regard, our findings for the ITT analysis are contrary to a previous cohort study that reported a positive association of nut consumption on visual attention and processing in adolescents (mean age of 11.8 (3.3) years).19 Additionally, one double-blind, randomised, placebo-controlled cross-over trial (8-week intervention and 6-week washout) with walnuts conducted in college students aged 18–25 years showed improvements in inferential verbal reasoning. However this trial had a small sample size, 64 participants, and we cannot consider them adolescents.20
There are, however, possible explanations for our findings. First, a 6-month period for a nutritional intervention is considered relatively long for detecting physiological changes but perhaps less effective for detecting changes from brain function using cognitive and behavioural tests. Including brain magnetic resonance imaging (MRI) and functional magnetic resonance imaging (fMRI) could help detect small brain activity and expression of functional brain networks.35 Indeed, it has been suggested that, for healthy students, initial cognitive functioning may be too good for nutrient supplementation to be effective, i.e., further improvement may be difficult to achieve.20 Further, regarding the non-significant differences found in our six neuropsychological endpoints, the fact that only less than half of participants in the intervention group adhered to eating walnuts daily for 6 months might have impacted the data accuracy. Second, the mechanisms by which walnuts may influence cognitive performance and behavioural development in the medium term are not completely understood.20 Though we did not find significant results in our primary outcomes, we observed that RBC ALA status was slightly higher in the walnut group after 6 months. In fact, ALA is one of the components of walnuts hypothesised to benefit brain function. However, it remains unclear how much ALA exerts its effects alone (i.e., independent of its precursor role of EPA and DHA) or in synergy with other antioxidant and anti-inflammatory components of walnuts.16 Studies have suggested that ALA alone is neuroprotective,36,37 could reduce anxiety symptoms (e.g., mood and stress levels)38 and that, in combination with iron, it appeared to be more effective in enhancing the conversion of ALA into DHA, thus positively affecting cognitive performance.39 Furthermore, another study showed that supplementation of 2 g/day of oil containing 1 g ALA for 8 weeks had no effect on symptoms in children with ADHD.40 Overall, there remains a wide gap in our knowledge regarding the effects of ALA, the plant-derived omega-3 fatty acid, in neurodevelopment, particularly in adolescents.
On the other hand, when we reanalysed the data using methods that estimate the effect as if all participants adhered to their assigned intervention, the results showed an improvement in attention performance and fluid intelligence and a reduction in ADHD symptoms for the intervention group compared with the control group. Although results of a PP analysis usually provide a lower level of evidence since we cannot be sure that participants in the two arms still have comparable characteristics, it better reflects the effects of the intervention as prescribed (with better adherence to the protocol).41 From a public health point of view, these findings are important since they illustrate that consuming walnuts on a daily basis can positively affect neuropsychological development in adolescents, particularly in attention and fluid intelligence, as well as reducing ADHD-related symptoms. As a matter of fact, ADHD symptoms include impairment of attention development in relation to structural and functional brain changes, specifically deficits in sustained attention.42,43 Although the role of dietary PUFA in ADHD is still controversial, many studies have found lower PUFA plasma fractions in children with ADHD (specifically AA, DHA, and EPA). Further, several trials have shown that few weeks of PUFA supplementation can easily normalise PUFA blood levels and that ADHD symptoms are responsive to and can be improved by PUFA supplementation.44,45 Thus, the current study's findings may not only help to better shape basic dietary recommendations for the adolescent population in order to ensure optimal omega-3 PUFA intake for healthy brain development, but also show that regular consumption of omega-3s (in this case, ALA) may help in the improvement of ADHD symptomatology.44 However, these findings should be interpreted with caution since they are statistically significant when only those participants who adhered to the intervention the most are considered (PP analysis), but they are no longer statistically significant in the ITT analysis, meaning that only if eating walnuts regularly (>3 servings per week) may result in an improvement on cognitive function.
The strengths of the study were its randomised controlled design; the choice of healthy adolescents (rather than clinical populations as often targeted); the use of several validated computer-based neuropsychological tests that directly assessed the adolescents’ cognitive functioning; and the use of both, the ITT and the PP approach, both valid but with different roles in the analysis of clinical studies; and the inclusion of objective biomarkers of adherence to the intervention. Nevertheless, the study also faced some limitations. First, the inability to perform a double-blind dietary trial, the gold standard of clinical trials, is a limitation in feeding studies of whole foods.46,47 For example, no placebo can be used in the control group, and participants cannot be blinded to the forms of whole foods, i.e., they are aware of their treatment assignment. This can lead to significant expectation bias in both the intervention and control groups (i.e., the expectation of benefit may lead to a more favourable outcome in those receiving the dietary intervention vs. the expectation of lack of benefit that could lead to less favourable outcome in those not receiving the intervention). Second, because participants from the same school were randomised to different arms, there is a possibility of contamination in the control group, thus there is a slight chance that people in the control group learned about the intervention diet and adopted it themselves. Third, ensuring compliance of adolescents with daily walnuts for a period of 6 months was difficult and the less-than-optimal adherence in our study may have affected data accuracy, as the PP analysis showed in a more definite group of compliers. Fourth, the moderate successful rate for re-testing (83.5%) leads us not to completely discard a certain risk of reducing the internal validity of the findings. Finally, a minor limitation of the study was that some variables (child sex, child age, and maternal education) had to be imputed due to missing data before randomisation, although only a small number of values were involved. However, most of the missing values were obtained during follow-up.
Based on our findings, we can reach two main conclusions. First, a dietary intervention that prescribed eating walnuts for 6 months had no significant effects on neurodevelopment in healthy adolescents, indicating that the intervention did not work overall. However, RBC ALA status was slightly higher in adolescents following a walnut diet compared to those in the control group. Second, our study suggests that there can be neuropsychological benefits if one complies with the dietary intervention, meaning that eating walnuts regularly (more than 3 servings per week) may result in an improvement in sustained attention, fluid intelligence, and ADHD symptoms. Thus, an intervention such as the one proposed here is unlikely to work in real life, because it requires a strong commitment and few people will comply. Nonetheless, compliance may change once the general public learns about the positive findings in compliers. Thus, this study provides valuable insights and a basis for further clinical and epidemiological research on the effect of walnuts on brain development in adolescents. Future studies should consider including brain imaging and a longer intervention period.
Contributors
Author contributions included experimental and study design (JS, JJ), data collection (CP, AD, JT, JG, AS-V, IL, EdR), data analyses and/or interpretation (FG, APM, XB, JJ, JB), and drafting and writing of the manuscript (FG, APM, JJ). APM, FG and JJ verified the underlying data. All authors reviewed and commented on versions of the manuscript and read and approved the final manuscript. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
The original contributions presented in the study are included in the article/supplement content and the study protocol,22 further inquiries can be directed to the corresponding author.
Declaration of interests
APM holds a pre-doctoral research training (PFIS) contract (grant FI22/00119) awarded by the Instituto de Salud Carlos III. JJ holds a Miguel Servet-II contract (grant CPII19/00015) awarded by the Instituto de Salud Carlos III (Co-funded by European Union Social Fund “Investing in your future”). ER reports research grants through his institution, personal fees, non-financial support and other from the California Walnut Commission; grants, personal fees, non-financial support and other from Alexion; personal fees and other from Amarin, outside the submitted work. JS-S is partially supported by ICREA under the ICREA Academia programme. JS-S reports serving on the board of the International Nut and Dried Fruit Council (nonpaid member of the scientific committee) and receiving grant support from this entity through his institution. He also reports serving on the Executive Committee of the Instituto Danone Spain. He has also received research funding (almonds and pistachios for the PREDIMED-Plus pilot study participants) from the Almond Board of California, and Pistachio Growers of California, respectively. MCT is funded by a Ramón y Cajal fellowship (RYC-2017-01892) from the Spanish Ministry of Science, Innovation and Universities and co-funded by the European Social Fund. OTR is funded by a Sara Borrell grant from the Instituto de Salud Carlos III (CD19/00110). ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. ASV has received payments from California Walnut Commission to him and his institution, but with no role in the interpretation of the findings. Other authors have nothing to disclose.
Acknowledgments
This study was supported by Instituto de Salud Carlos III through the projects ‘CP14/00108, PI16/00261, PI21/00266’ (co-funded by European Union Regional Development Fund ‘A way to make Europe’). The California Walnut Commission (CWC) has given support by supplying the walnuts for free for the Walnuts Smart Snack Dietary Intervention Trial.
We thank all the study participants, families and schools (Escola Padre Damián; Escola Proa; Escola Sant Miquel; Escola Solc; IES Ernest Lluch; IES Front Marítim; IES Galileo Galilei; IES Joan Boscà; IES La Sedeta; IES Montserrat; IES Príncep de Viana; IES Verdaguer) that accepted to participate and gave support to the development of the Walnuts Smart Snack Dietary Intervention Trial, as well as all the project investigators involved.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101954.
Appendix A. Supplementary data
References
- 1.Patton G.C., Sawyer S.M., Santelli J.S., et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet (London, England) 2016;387:2423–2478. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvan A., Hare T.A., Parra C.E., et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arain M., Haque M., Johal L., et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darcey V.L., McQuaid G.A., Fishbein D.H., VanMeter J.W. Dietary long-chain omega-3 fatty acids are related to impulse control and anterior cingulate function in adolescents. Front Neurosci. 2019;12 doi: 10.3389/FNINS.2018.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen C.I.F., Kiliaan A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallahan B., Garland M.R. Essential fatty acids and mental health. Br J Psychiatry. 2005;186:275–277. doi: 10.1192/bjp.186.4.275. [DOI] [PubMed] [Google Scholar]
- 8.Tapiero H., Nguyen Ba G., Couvreur P., Tew K. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 9.Poulose S.M., Miller M.G., Shukitt-Hale B. Role of walnuts in maintaining brain health with age. J Nutr. 2014;144 doi: 10.3945/JN.113.184838. [DOI] [PubMed] [Google Scholar]
- 10.Domenichiello A.F., Kitson A.P., Bazinet R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog Lipid Res. 2015;59:54–66. doi: 10.1016/j.plipres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Barceló-Coblijn G., Murphy E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Blondeau N., Nguemeni C., Debruyne D.N., et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology. 2009;34:2548–2559. doi: 10.1038/npp.2009.84. [DOI] [PubMed] [Google Scholar]
- 13.Sakayori N., Tokuda H., Yoshizaki K., et al. Maternal nutritional imbalance between linoleic acid and alpha-linolenic acid increases offspring's anxious behavior with a sex-dependent manner in mice. Tohoku J Exp Med. 2016;240:31–37. doi: 10.1620/tjem.240.31. [DOI] [PubMed] [Google Scholar]
- 14.Kim H., Kim H., Lee E., Kim Y., Ha E.H., Chang N. Association between maternal intake of n-6 to n-3 fatty acid ratio during pregnancy and infant neurodevelopment at 6 months of age: results of the MOCEH cohort study. Nutr J. 2017;16 doi: 10.1186/S12937-017-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala-Vila A., Fleming J., Kris-Etherton P., Ros E. Impact of α-linolenic acid, the vegetable ω-3 fatty acid, on cardiovascular disease and cognition. Adv Nutr. 2022;13 doi: 10.1093/ADVANCES/NMAC016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan A., Chauhan V. Beneficial effects of walnuts on cognition and brain health. Nutrition. 2020;12:550. doi: 10.3390/nu12020550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ros E., Izquierdo-Pulido M., Sala-Vila A. Beneficial effects of walnut consumption on human health: role of micronutrients. Curr Opin Clin Nutr Metab Care. 2018;21:498–504. doi: 10.1097/MCO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 18.Valls-Pedret C., Sala-Vila A., Serra-Mir M., et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. doi: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.Y., Kang S.W. Relationships between dietary intake and cognitive function in healthy Korean children and adolescents. J Lifestyle Med. 2017;7 doi: 10.15280/jlm.2017.7.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pribis P., Bailey R.N., Russell A.A., et al. Effects of walnut consumption on cognitive performance in young adults. Br J Nutr. 2012;107 doi: 10.1017/S0007114511004302. [DOI] [PubMed] [Google Scholar]
- 21.Nishi S.K., Sala-Vila A., Julvez J., Sabaté J., Ros E. Impact of nut consumption on cognition across the lifespan. Nutrition. 2023;15:1000. doi: 10.3390/nu15041000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julvez J., Gignac F., Fernández-Barrés S., et al. Walnuts, long-chain polyunsaturated fatty acids, and adolescent brain development: protocol for the walnuts smart snack dietary intervention trial. Front Pediatr. 2021;9:425. doi: 10.3389/fped.2021.593847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.USDA national nutrient database for standard reference, legacy release|Ag data commons. https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release
- 24.Marangoni F., Colombo C., Martiello A., Poli A., Paoletti R., Galli C. Levels of the n-3 fatty acid eicosapentaenoic acid in addition to those of alpha linolenic acid are significantly raised in blood lipids by the intake of four walnuts a day in humans. Nutr Metab Cardiovasc Dis. 2007;17:457–461. doi: 10.1016/j.numecd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Food and Nutrition Board|National Academies. https://www.nationalacademies.org/fnb/food-and-nutrition-board
- 26.Ravens-Sieberer U., Auquier P., Erhart M., et al. The KIDSCREEN-27 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Qual Life Res. 2007;16:1347–1356. doi: 10.1007/s11136-007-9240-2. [DOI] [PubMed] [Google Scholar]
- 27.Vioque J., Navarrete-Muñoz E.M., Gimenez-Monzó D., et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12 doi: 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rei M., Severo M., Rodrigues S. Reproducibility and validity of the Mediterranean diet quality index (KIDMED index) in a sample of Portuguese adolescents. Br J Nutr. 2021;126:1737–1748. doi: 10.1017/S0007114521000532. [DOI] [PubMed] [Google Scholar]
- 29.Stonehouse W. Does consumption of LC omega-3 PUFA enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials. Nutrients. 2014;6:2730–2758. doi: 10.3390/nu6072730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forns J., Esnaola M., López-Vicente M., et al. The n-back test and the attentional network task as measures of child neuropsychological development in epidemiological studies. Neuropsychology. 2014;28:519–529. doi: 10.1037/neu0000085. [DOI] [PubMed] [Google Scholar]
- 31.López-Vicente M., Forns J., Suades-González E., et al. Developmental trajectories in primary schoolchildren using n-back task. Front Psychol. 2016;7 doi: 10.3389/FPSYG.2016.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurstone T.G. The tests of primary mental abilities. Pers Guid J. 1957;35:569–576. [Google Scholar]
- 33.Levin I.P., Weller J.A., Pederson A.A., Harshman L.A. Age-related differences in adaptive decision making: sensitivity to expected value in risky choice. Judgm Decis Mak. 2007;2:225–233. [Google Scholar]
- 34.Ortuño-Sierra J., Fonseca-Pedrero E., Paino M., Sastre I Riba S., Muñiz J. Screening mental health problems during adolescence: psychometric properties of the Spanish version of the Strengths and Difficulties Questionnaire. J Adolesc. 2015;38:49–56. doi: 10.1016/j.adolescence.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Sala-Vila A., Valls-Pedret C., Rajaram S., et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts and Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. 2020;111:590–600. doi: 10.1093/ajcn/nqz328. [DOI] [PubMed] [Google Scholar]
- 36.Piermartiri T., Pan H., Figueiredo T.H., Marini A.M. α-Linolenic acid, a nutraceutical with pleiotropic properties that targets endogenous neuroprotective pathways to protect against organophosphate nerve agent-induced neuropathology. Molecules. 2015;20 doi: 10.3390/molecules201119698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H., Hu X.Z., Jacobowitz D.M., et al. Alpha-linolenic acid is a potent neuroprotective agent against soman-induced neuropathology. Neurotoxicology. 2012;33:1219–1229. doi: 10.1016/j.neuro.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Yehuda S., Rabinovitz S., Mostofsky D.I. Mixture of essential fatty acids lowers test anxiety. Nutr Neurosci. 2013;8:265–267. doi: 10.1080/10284150500445795. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner J., Smuts C.M., Zimmermann M.B. Providing male rats deficient in iron and n-3 fatty acids with iron and alpha-linolenic acid alone affects brain serotonin and cognition differently from combined provision. Lipids Health Dis. 2014;13:1–13. doi: 10.1186/1476-511X-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubnov-Raz G., Khoury Z., Wright I., Raz R., Berger I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: a randomized controlled double-blind study. Front Hum Neurosci. 2014;8:780. doi: 10.3389/fnhum.2014.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripepi G., Chesnaye N.C., Dekker F.W., Zoccali C., Jager K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology. 2020;25:513–517. doi: 10.1111/nep.13709. [DOI] [PubMed] [Google Scholar]
- 42.Suades-González E., Forns J., García-Esteban R., et al. A longitudinal study on attention development in primary school children with and without teacher-reported symptoms of ADHD. Front Psychol. 2017;8:655. doi: 10.3389/fpsyg.2017.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson K.A., Kelly S.P., Bellgrove M.A., et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Bloch M.H., Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:991. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raz R., Gabis L. Essential fatty acids and attention-deficit–hyperactivity disorder: a systematic review. Dev Med Child Neurol. 2009;51:580–592. doi: 10.1111/j.1469-8749.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 46.Weaver C.M., Miller J.W. Challenges in conducting clinical nutrition research. Nutr Rev. 2017;75:491–499. doi: 10.1093/nutrit/nux026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staudacher H.M., Irving P.M., Lomer M.C.E., Whelan K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203–212. doi: 10.1017/S0029665117000350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.