Abstract
Depressive patients suffer from a complex of symptoms of varying intensity compromising their mood, emotions, self-concept, neurocognition, and somatic function. Due to a mosaic of aetiologies involved in developing depression, such as somatic, neurobiological, (epi-)genetic factors, or adverse life events, patients often experience recurrent depressive episodes. About 20–30% of these patients develop difficult-to-treat depression. Here, we describe the design of the GEParD (Genetics and Epigenetics of Pharmaco- and Psychotherapy in acute and recurrent Depression) cohort and the DaCFail (Depression-associated Cardiac Failure) case–control protocol. Both protocols intended to investigate the incremental utility of multimodal biomarkers including cardiovascular and (epi-)genetic markers, functional brain and heart imaging when evaluating the response to antidepressive therapy using comprehensive psychometry. From 2012 to 2020, 346 depressed patients (mean age 45 years) were recruited to the prospective, observational GEParD cohort protocol. Between 2016 and 2020, the DaCFail case–control protocol was initiated integrating four study subgroups to focus on heart-brain interactions and stress systems in patients > 50 years with depression and heart failure, respectively. For DaCFail, 120 depressed patients (mean age 60 years, group 1 + 2), of which 115 also completed GEParD, and 95 non-depressed controls (mean age 66 years) were recruited. The latter comprised 47 patients with heart failure (group 3) and 48 healthy subjects (group 4) of a population-based control group derived from the Characteristics and Course of Heart Failure Stages A–B and Determinants of Progression (STAAB) cohort study. Our hypothesis-driven, exploratory study design may serve as an exemplary roadmap for a standardized, reproducible investigation of personalized antidepressant therapy in an inpatient setting with focus on heart comorbidities in future multicentre studies.
Keywords: Major depressive disorder, Affective disorders, Predictive markers, Biomarkers, Brain–heart interaction
Introduction
Depressive episodes in uni- and bipolar-affective disorders are multifactorial comprising (neuro-)biological and psychosocial factors. Such episodes can affect people during their entire life span compromising life quality and expectancy from early on (Otte et al. 2016; Vieta et al. 2018; Solmi et al. 2022). While the clinical phenomenology in depressed patients varies within a known framework of symptoms and may turn into a chronic, treatment-resistant condition in about 20–30% of the individuals (Fava and Davidson 1996), the neurobiological analogues of depression are heterogeneous and less well defined. Regarding personalized antidepressant therapy, the translation into clinical biomarkers for (deep) phenotyping of depressed patients has remained challenging.
The investigation of genetic heritability and gene–environment interactions (Karg and Sen 2012) based on candidate genes of neurotransmitter systems (Caspi et al. 2003; Baune et al. 2008) has contributed to the development of concepts on interacting risk and disease-modifying factors of depression. The read-out of epigenetic modifications ‘picturing’ a patient’s (adverse) life events has allowed to study pharmacoepigenetics patterns for the prediction of an impaired response to treatment, e.g. with selective serotonin reuptake inhibitors (SSRIs) (Schiele et al. 2021). (Epi-)genome-wide association studies (E-/GWAS) in depression, e.g. (Major Depressive Disorder Working Group of the Psychiatric Genomic Consortium et al. 2013; Menke et al. 2012a, b; Okbay et al. 2016; Story Jovanova et al. 2018) have upscaled single nucleotide polymorphisms (SNP) investigations to larger cohorts, thereby defining further genetic regions of interest and facilitating novel (predictive) measurements such as polygenic risk scores (Fanelli et al. 2022). Recent GWAS for depression have essentially emphasized the importance of genes in synaptic structure and function (Howard et al. 2019). Most of these studies, however, focused on categorical definitions of diseases not considering that depression is a heterogenous, dimensional, and systemic condition affecting multiple organs of the body (Sotelo and Nemeroff 2017).
To define prospective, reproducible biomarkers for depression subtypes based upon whole body disease concepts, a standardized organ and/or body system-tailored deep phenotyping for large cohorts of depressed patients is instrumental.
Essential stress-based pathophysiological mechanisms of depression comprise an impairment of the hypothalamus–pituitary–adrenal (HPA) axis, the adrenergic autonomic nervous system (ANS), and the immune system (Carney et al. 2005; Pariante and Lightman 2008; McEwen and Akil 2020; Beurel et al. 2020). In the pathogenesis of depression, a modulated heart–brain interaction may be responsible for the elevated risk of cardiovascular disease and cardiac mortality in individuals with major depression (Nemeroff and Goldschmidt-Clermont 2012; Hare et al. 2014; Nielsen et al. 2021). Previous studies showed that dysregulation of the HPA axis in depression is linked to an impaired sensitivity of glucocorticoid receptors (GR) (Pariante and Lightman 2008; Menke et al. 2012a, b). Depressed patients are at higher risk for cardiovascular diseases (CVD) including heart failure (Gustad et al. 2014), possibly due to a dysfunctional adrenergic ANS. This may result in increased heart rate, hypertension, and a reduced heart rate variability (HRV) (Koch et al. 2019), i.e. symptoms shared with patients suffering from heart failure (Parati and Esler 2012). Even though the insular cortex—a brain region associated with interoceptive attention (Wang et al. 2019) which is compromised in depressed patients (Eggart et al. 2019)—and its functional networks are considered the neuronal representations of ANS activity (Beissner et al. 2013), it remains unclear whether and how these neuronal substrates may affect the course of patients with depression and heart failure, respectively. Genotyping in patients with heart failure and comorbid depression revealed that genetic variants implicated in anxious behaviour (NPSR1) (Angermann et al. 2017) as well as inflammation (C-reactive protein (CRP), interleukin 6 (IL-6)) (Kittel-Schneider et al. 2018) modify the risk of progression and mortality. Patients with heart failure, on the other hand, not only face a higher risk for depression (Rutledge et al. 2006), but also an increased mortality caused by depression itself (Penninx et al. 2001; Zambrano et al. 2020). However, patients with heart failure and comorbid depression experience no prognostic benefit from antidepressive pharmacological treatment, indicating heterogeneity of the depression phenotype (Angermann et al. 2016).
Here, we introduce the design of the observational GEParD (Genetics and Epigenetics of Pharmaco- and Psychotherapy in acute and recurrent Depression) cohort and the DaCFail (Depression associated Cardiac Failure) protocol, the latter designed as a tailored protocol on the role of stress systems in heart–brain interaction. The main intention was to monitor a naturalistic antidepressant therapy response within an inpatient setting applying a broad repertoire of psychometric and neuropsychological testing, routine blood diagnostics including endocrine and inflammatory biomarkers, established genetic and epigenetic testing, and finally a comprehensive cardiac phenotyping including parameters of the ANS. We finally implemented our ideas in an exploratory approach to generate hypotheses for follow-up studies dealing among others with the following questions:
Which psychometric parameters and stress-related biomarkers (endocrine, inflammatory, and ANS) can be defined as biomarkers of depression symptoms and antidepressant treatment response?
Which (epi-)genetic biomarkers of depression symptoms and antidepressant treatment response can be defined in interaction with proximal and/or distal life events?
Which parameters of the heart–brain interaction differentiate depression with and without heart failure as compared to healthy controls and/or are relevant for antidepressant treatment response?
How does the function of the insula and its networks differ in depressed patients with and without heart failure as compared to healthy controls?
Methods
The protocols
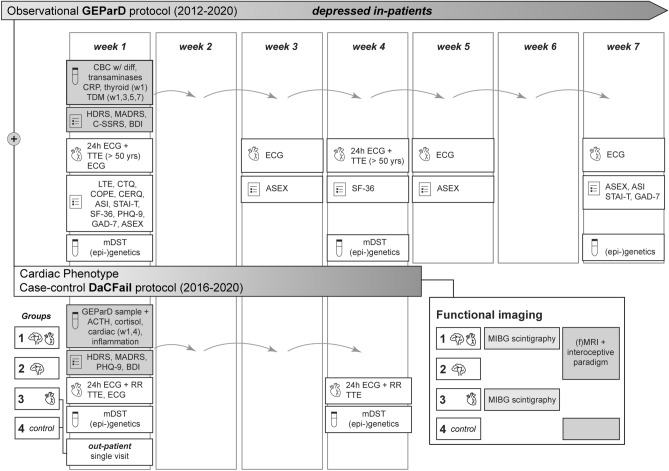
The GEParD protocol was initiated in 2012 and carried out as a prospective observational cohort study of hospitalized, depressed uni- and bipolar patients with weekly measurements for up to 7 weeks (Fig. 1). In a second phase (2016–2020), the DaCFail protocol was initiated complementing the existing study concept by parameters focusing on the stress and the cardiac system (Fig. 1). The DaCFail protocol was designed as a refined case–control protocol for 200 patients and methodologically based on GEParD. For DaCFail, patients were enclosed in four study groups: Group 1, patients with depression and heart failure; Group 2, depressed patients; Group 3, patients with heart failure; Group 4, healthy subjects (control group, Table 1). Measurements were carried out weekly for a maximum of four weeks duration (Fig. 1). Both functional magnetic resonance imaging (fMRI) of the insular cortex and 123I-meta-iodobenzylguanidine (MIBG) scintigraphy were integrated as quantitative imaging modalities within the DaCFail study protocol (Fig. 1). The procedures were approved by the local ethics committee of the University Hospital of Würzburg (GEParD: vote no 104/12 and 128/15, DaCFail: vote no. 285/14) and were carried out in accordance with the ethical standards of the Declaration of Helsinki and its later amendments (Williams 2013).
Fig. 1.
Study designs. After study enrolment, patients were scheduled for weekly blood sample collection in both study protocols. Psychometry was carried out within the clinical routine, with exemptions. In selected study weeks, patients were scheduled for cardiac assessment, modified dexamethasone-suppression test (mDST), and blood sample collection for (epi-)genetic analyses. Patients of DaCFail group 1 and 3 underwent MIBG scintigraphy. Abbreviations: diff = differential, MIBG = 123I-meta-iodobenzylguanidine, RR = blood pressure, TDM = therapeutic drug monitoring, TTE = transthoracic echocardiography, w = week, w/ = with. For psychometry and multimodal biomarkers including functional imaging in detail, please see Tables 2 and 3
Table 1.
Inclusion and exclusion criteria for the GEParD and DaCFail protocols
| (i) Inclusion criteria | |
|
GEParD Age of 18–80 yrs Clinically phenotypical depressive disorder (DSM-IV) |
DaCFail Age ≥ 50 yrs Group 1: depressive episode (DSM-IV, HDRS ≥ 14) + LVEF < 52% ♂ / 54% ♀ Group 2: depressive episode (DSM-IV, HDRS ≥ 14) + normal LVEF Group 3: no depression, LVEF < 52% ♂ / 54% ♀ Group 4: no depression + normal LVEF (healthy control probands from the STAAB cohort) |
| (ii) Exclusion criteria | |
|
GEParD Inability to give written informed consent, presence of a depressive disorder caused by substance use disorder, severe neurological condition, e.g. Parkinson’s disease, dementia, or stroke, malignant tumours, diagnosis of schizophrenia/psychosis, systemic medication with glucocorticoids |
DaCFail Inability to give written informed consent, presence of a depressive disorder caused by substance use disorder, severe neurological condition, e.g. Parkinson’s disease, dementia, or stroke, malignant tumours, diagnosis of schizophrenia/psychosis, systemic medication with glucocorticoids Group 3 and 4: Current or past depressive episode (PHQ-9, SCID-I), substance use disorder |
DSM-IV Diagnostic and Statistical Manual Of Mental Disorders-IV, HDRS Hamilton Depression Scale, LVEF left ventricular ejection fraction, PHQ-9 Patient Health Questionnaire, SCID-I Structured Clinical Interview for DSM-IV axis I disorders, STAAB characteristics and course of heart failure stages A–B and determinants of progression (Wagner et al. 2017)
Participant selection criteria
The GEParD protocol recruited patients aged 18–80 years (Table 1) presenting with a unipolar and bipolar depressive disorder diagnosed by a validated, standardized interview according to DSM-IV criteria (Structured Clinical Interview for DSM, SCID-I (First and Gibbon 2004)). Antidepressant treatment was carried out according to the treating physician’s choice within an inpatient setting using psychopharmacology, psychotherapy, and electroconvulsive therapy (ECT).
Within the DaCFail protocol, subjects beyond an age of 50 years were eligible for one of the following study arms applying the selection criteria detailed in Table 1. Healthy controls without medical and mental illness precondition were asked for participation from the population-based “Characteristics and Course of Heart Failure Stages A–B and Determinants of Progression (STAAB)” cohort study, comprising a representative age-stratified sample of about 5000 Würzburg residents aged 30–79 years at baseline assessment (Morbach et al. 2021; Wagner et al. 2017). Healthy subjects were matched in age (≥ 50 years) and gender to the other study groups. For both the GEParD and/or DaCFail study protocol, the exclusion criteria listed in Table 1 were applied.
Recruitment strategy
For both study protocols, hospitalized depressed patients were recruited on-site by specialized staff within the inpatient setting of the Department of Psychiatry, Psychotherapy and Psychosomatic Medicine, University Hospital Würzburg, Germany. Here, four wards with a therapy focus on affective disorders and intensive psychotherapy as well as one neuropsychiatric day-care unit specialized for geriatric patients offer a naturalistic study environment (Fig. 2). Newly admitted patients fulfilling the inclusion criteria of the study protocols were informed about a possible participation by their attending physician. Detailed information sheets of the study protocols were provided with the aim of informing about the study course, study criteria, and applied procedures and measurements. For the patients diagnosed with heart failure (DaCFail Group 3) and the healthy controls (DaCFail Group 4), the recruitment was performed in cooperation with the Department of Internal Medicine I and the Comprehensive Heart Failure Center Würzburg (CHFC) (Fig. 2). For both groups, study assessments were carried out at the Center of Mental Health.
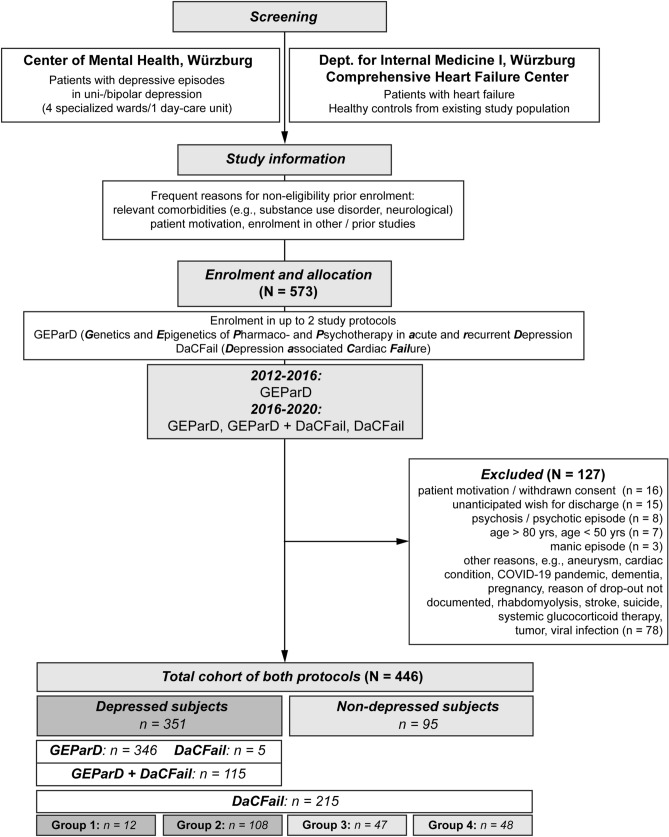
Fig. 2.
Consort chart for the GEParD and DaCFail protocols (2012–2020). Overview of screening and enrolment process in the GEParD and DaCFail study protocols
Minimal recruitment number
The GEParD study protocol as a prospective observational study aimed to recruit 500 depressed patients. For the DaCFail study protocol, the minimum recruitment number of participants was defined by a power analysis for expected HRV differences between groups as the original primary outcome of the case–control design. To test on HRV differences in depressed patients and controls with an analysis power of 0.8 on a significance level of α = 0.05 and an effect size of Cohen’s d = 1, a minimum recruitment number of N = 24 participants per group was calculated. It was assumed that investigated effects differ in the range of one standard deviation (SD) in the cohort of depressed patients versus controls. Given the four study groups, variances were doubled leading to a minimal recruitment number of N = 48 participants for each of the four DaCFail study groups under the defined test conditions.
Psychometric and neuropsychological testing
In the process of and after study enrolment, psychometric evaluation of patients was carried out in scheduled study rounds within the inpatient setting or single appointments at the outpatient clinic (DaCFail Group 3, 4) (Fig. 1, Table 2).
Table 2.
Psychometry, life events, and coping style
| (i) Screening tools for depression, anxiety, suicidal ideation, and life quality |
|
Depression module of the Patient Health Questionnaire (PHQ-9) (Kroenke et al. 2001) 7-Item anxiety scale for generalized anxiety disorder (GAD-7) (Spitzer et al. 2006) Short-Form-36 Health Survey (SF-36) (Bullinger et al. 1995) The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First and Gibbon 2004) |
| (ii) Dimension of depressive episodes |
|
Anxiety Sensitivity Index (ASI) (Reiss et al. 1986) Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2011) Beck Depression Inventory (BDI) (Beck et al. 1961) Hamilton Depression Scale (HDRS) (Hamilton 1960) Montgomery Ǻsberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) State-Trait Anxiety Inventory (STAI-T) |
| (iii) Neurocognition |
|
Cognitive Emotion Regulation Questionnaire (CERQ) (Garnefski et al. 2001) Montreal Cognitive Assessment (MOCA) (Nasreddine et al. 2005) |
| (iv) Life events and coping strategies |
|
Distal and proximal life events Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 2003) List of Threatening Experiences (LTE) (Brugha et al. 1985) Stress system Coping Orientation to Problems Experienced Inventory (COPE) (Carver et al. 1989) Life quality Arizona Sexual Experience Scale (ASEX) (McGahuey et al. 2000) |
As screening tool for depressive and anxious symptoms, the SCID-I, the depression module of Patient Health Questionnaire-9 (PHQ-9) and the 7-item anxiety scale for generalized anxiety disorder (GAD-7) (Spitzer et al. 2006) were used (Table 2).
Depression severity and dimension were assessed by the Beck Depression Inventory (BDI-II) (Beck et al. 1961), Hamilton Depression Rating Scale (HDRS21, 21 items in total, 17 of 21 items were used for evaluation) (Hamilton 1960), and the Montgomery Ǻsberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979), further, particularly focusing on the entity of anxious depression, the Anxiety Sensitivity Index (ASI) (Reiss et al. 1986) and the State-Trait Anxiety Inventory (STAI-T) were used. Emotional regulation in patients was evaluated by the Cognitive Emotion Regulation Questionnaire (CERQ) (Garnefski et al. 2001). The Montreal Cognitive Assessment (MOCA) (Nasreddine et al. 2005) was used to assess possible neurocognitive impairments.
To contextualize gene–environment interactions, distal and proximal life events of patients were evaluated by the Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 2003) and the List of Threatening Experiences (LTE) (Brugha et al. 1985). The patient’s coping strategies were assessed by the Coping Orientation to Problems Experienced Inventory (COPE) (Carver et al. 1989). The Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2011) was used to evaluate suicidal ideation (Menke et al. 2012a, b). The Short-Form-36 Health Survey (SF-36) (Bullinger et al. 1995) assessed the life quality of patients with the Arizona Sexual Experience Scale (ASEX) (McGahuey et al. 2000) monitoring sexual dysfunction.
Physical activity
To monitor (altered) locomotor activity in depressed patients (Wuthrich et al. 2022) and to investigate possible interactions with the HPA axis (Menke et al. 2014), step counts were quantified in a sub-cohort of depressed patients using actigraphy (ActiGraph GT9X Link, ActiGraph LLC, Pensacola, US).
Blood sampling
All enrolled patients were scheduled for blood sampling at defined time points to perform routine blood analysis including established therapeutic drug monitoring (TDM) of psychopharmaceutical medication, analysis of endocrine and inflammation markers, as well as genetic and epigenetic analysis (Fig. 1, Table 3). Blood sample collection was carried out by trained staff.
Table 3.
Laboratory, cardiological phenotype, and imaging
| Biomaterial asservation | Method | Parameter |
|---|---|---|
| (i) Laboratory | ||
| SST, EDTA | Standard haematology assay | CBC w/ differential, GOT, GPT, γ-GT, CRP |
| SST | Standard haematology assay | NTproBNP, trop T/I (cardiac) |
| SST | Enzyme-linked immunosorbent assay | IL-6, IL-1, TNF-α (inflammation) |
| SST | TSH, fT3, fT4 (thyroid) | |
| SST | mDST | ACTH, cortisol |
| SST | TDM using high-performance liquid chromatography | Psychopharmaceutical drug concentration |
| EDTA | Sequencing | DNA methylation, genotyping |
| PAXgene™ blood tube | RNA extraction, quantitative real-time polymerase chain reaction | mRNA expression |
| (ii) Quantitative cardiological assessment | ||
| 24-h ECG | bpm, HRV (SDNN, SDANN, SDRR, pNN50, RMSSD), VES, SVES | |
| 24-h blood pressure | RRsys, RRdia | |
| Echocardiography | LVEF, IVS, LVPWd/s, LVEDD | |
| Actigraphy | Step count | |
| MIBG scintigraphy | HMR | |
| (iii) Neuronal morphometry and interoception | ||
| MRI | High-resolution T1-weighted imaging (of the whole brain including the insular cortex) | |
| mod. Schandry task | Score considering counted and recorded heartbeats | |
| rs-fMRI | Functional connectivity analysis via resting-state fMRI | |
IVST interventricular septum thickness, LVPWd/s left ventricular posterior wall end diastole and end systole, LVEDD left ventricular end-diastolic diameter, pNN50 percentage of successive RR intervals that differ by more than 50 ms, RMSSD root mean square of successive RR interval differences, rs resting state, SDNN standard deviation (SD) of NN intervals, SDANN SD of the 5 min average NN intervals, SDRR SD of RR intervals, SST serum separator tube, SVES supraventricular extrasystole, VES ventricular extrasystole, w/ = with. Other abbreviations are stated in the text
On a weekly basis until study week 4 (DaCFail) and study week 7 (GEParD), respectively, a routine blood sample of each patient was collected for analysis of the complete blood count (CBC) with differential blood cell count, transaminases (glutamic oxaloacetic transaminase (GOT), glutamate-pyruvate transaminase (GPT), γ-glutamyltransferase (γ-GT)), and CRP. In study week 1, thyroid markers (thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), and free thyroxine (fT4)) were collected. In study week 1 and 4 (DaCFail), blood samples to investigate the inflammatory parameters IL-6, interleukin 1 (IL-1), and tumour necrosis factor alpha (TNF-α) were additionally collected. For analyses of (epi-)genetic parameters and the HPA axis, please see the respective sections.
(Epi-)genetic analyses
In study week 1, 4 and 7 as well as parallel to the modified dexamethasone-suppression test (mDST), PAXgene™ blood test tubes (Qiagen, Hilden, Germany) for analysis of mRNA expression and ethylene diamine tetra-acetic acid (EDTA) tubes for analyses of DNA methylation and analysis of genetic variants were taken.
Modified dexamethasone-suppression test (mDST)
An mDST, as previously described (Menke et al. 2021; Leistner and Menke 2018), was applied at week 1 and 4 in inpatients and once in the above described outpatient setting (Fig. 1, Table 3). Before oral administration of 1.5 mg dexamethasone, blood was drawn by trained staff at 6 p.m. for the analysis of a CBC with differential blood cell count, cortisol, and adrenocorticotropic hormone (ACTH). PAXgene™ blood test tubes (Qiagen, Hilden, Germany) for analysis of mRNA expression, and EDTA tubes for analysis of DNA methylation and genetic variants were also used. Three hours post-medication, a second blood sample was collected for analysis of the above-mentioned parameters.
Assessment of cardiovascular parameters
Comprehensive cardiological diagnostics were performed for all patients enclosed in the DaCFail study protocol. This included 24 h electrocardiogram (ECG), 24 h blood pressure measurement (Riva-Rocci, RR), and transthoracic echocardiography (TTE) for patients ≥ 50 yrs (Table 3). In study week 1 and 4 of the DaCFail study protocol (Fig. 1), amino-terminal pro-hormone brain natriuretic peptide (NTproBNP), and troponin T/I (trop T/I) were collected as cardiac blood biomarkers.
123I-meta-iodobenzylguanidine scintigraphy
MIBG scintigraphy (Chirumamilla and Travin 2011) was used to monitor synaptic activity of cardiac sympathetic neurons in HF patients (DaCFail Group 1, 3) and carried out at the Department for Nuclear Medicine at University Hospital of Würzburg (Werner et al. 2018). Increased uptake of the tracer 123I-MIBG in synaptic vesicles was used as a marker of synaptic transmission and plasticity in sympathetic neurons and quantified via the heart mediastinum ratio (HMR). The imaging protocol set two time points of scanning (early scan 15 min post-injection, delayed scan 4 h post-injection). For single- and three-dimensional imaging, a planar scintigraphy protocol as well as single-photon emission computed tomography (SPECT) was used.
Functional MRI and interoceptive paradigm
For an investigation of the insular cortex–heart axis in depression (Fig. 1, Table 3), functional MRI measurements in combination with interoceptive paradigms were applied. The insular cortices are considered as neuronal substrates for the perception of internal sensory information (interoception), which is discussed to be compromised in patients with depression (Eggart et al. 2019), as well as of ANS activity (Beissner et al. 2013). Patients of DaCFail groups suffering from depression (groups 1 + 2) and controls (group 4), respectively, underwent the following measurements in one single session:
-
(i)
Based on the concept of interoceptive accuracy (Garfinkel et al. 2015), a modified version of the Schandry task (Schandry 1981) as heartbeat perception task was used to objectively measure perception of internal sensory information.
-
(ii)
HRV recordings were established to evaluate possible confounder phenomena and analysed for a defined time (300 s) using the RMSSD (Root Mean Sum of Squared Distance, Unit: ms).
-
(iii)
For morphometry of defined regions of interest in the insular cortex of both hemispheres (ventral and dorsal anterior insula, posterior insula) and for functional connectivity analyses, MRI data were acquired on a 3 Tesla scanner (MAGNETOM Skyra, Siemens, Erlangen, Germany). A 64-channel head coil was used. Structural images were acquired with a high-resolution T1-weighted magnetization prepared rapid gradient echo (MPRAGE). Resting-state recordings were acquired for a total duration of 10 min using T2*-weighted blood oxygen level-dependent (BOLD) images as echo-planar imaging (EPI) sequence. Participants were instructed to stay awake and keep their eyes open.
Study end point
For both study protocols, at the study end point after 7 weeks or after 4 weeks of antidepressant therapy in the inpatient setting, the difference of the individual HDRS scores was measured (Fig. 1). This therapy outcome parameter in both study protocols was identical because the second case-controlled study protocol (DaCFail) had been designed on the initial prospective, observational GEParD cohort protocol. Demographic characterization, psychometric parameters, somatic, laboratory, and (epi-)genetic biomarkers were used for evaluation of and/or correlation with therapy response. In the DaCFail study protocol, patients in addition were evaluated by HRV analyses (24 h ECG) using the RMSSD as the original primary outcome parameter and HMR analyses using MIBG scintigraphy in correlation with psychometric measurements of depression.
Statistics
All statistical calculations were and will be carried out with the IBM SPSS software package 26 (SPSS Inc., Chicago, USA) and SigmaPlot 14 (Systat Software, Düsseldorf, Germany). Further calculations may implement custom-written programming scripts for individual deep phenotyping based on (un-)supervised machine leaning algorithms.
Results
Recruitment
In total, N = 573 patients were recruited to the GEParD study protocol between October 2012 and December 2020 (N = 458 patients) and to the DaCFail study protocol between March 2016 and December 2020 (N = 268 patients). For the parallel inclusion in both protocols, N = 153 patients were initially evaluated. In the process of enrolment in one or both protocols, N = 127 patients had to be excluded due to somatic conditions or unforeseen disease courses (N = 94), changes in the patients’ personal motivation for study participation (N = 16), and unanticipated wishes for discharge (N = 15). In most cases, somatic or mental exclusion criteria (manic or psychotic episodes) had been unknown and/or unfolded within the first study week based upon the detailed diagnostic pipeline. Of the 120 participating depressed patients in the DacFail protocol, N = 115 completed the GEParD study protocol in parallel. In the process of and after enrolment, five depressed patients decided to solely participate in the DaCFail protocol. The number of patients completing one or both protocols thus consisted of 446 probands, of which 351 patients suffered from depression and 95 probands, recruited for the DaCFail protocol, were considered as non-depressed controls (patients with heart failure, healthy probands). Sizes of study groups are further detailed in Fig. 2 and Tables 4 and 5.
Table 4.
Basic demographic data and baseline depressive phenotype for the entire cohort of depressed patients (N = 351)
| Parameter | Reference | Depressed patients | |||
|---|---|---|---|---|---|
| Total (N = 351) | GEParD (N = 346) | ||||
| Baseline demographic data | |||||
| Mean ± SD (range) | Mean ± SD (range) | ||||
| Age [yrs] | 18–80 | 45.8 ± 15.3 (18–80) | 45.6 ± 15.3 (18–80) | ||
| N | % | N | % | ||
| Gender | m/f | 149/202 | 42.5/57.5 | 146/200 | 42.2/57.8 |
| Marital status | Married | 163 | 46.4 | 162 | 46.8 |
| Single | 93 | 26.5 | 93 | 26.9 | |
| Relationship | 37 | 10.5 | 37 | 10.7 | |
| Separated/divorced | 40 | 11.4 | 39 | 11.3 | |
| Widowed | 16 | 4.6 | 15 | 4.3 | |
| Missing | 2 | 0.6 | - | - | |
| Education | Apprenticeship/training | 225 | 64.1 | 222 | 64.2 |
| College/university | 57 | 16.2 | 57 | 16.5 | |
| None | 64 | 18.2 | 64 | 18.5 | |
| Missing | 5 | 1.4 | 3 | 0.9 | |
| Smoking | y/n/missing | 123/119/109 | 35.0/33.9/31.1 | 123/115/108 | 35.5/33.2/31.2 |
| Alcohol | y/n/missing | 49/133/169 | 14.0/37.9/48.1 | 46/131/169 | 13.3/37.9/48.8 |
| Baseline depressive phenotype | |||||
| Depressive episode w/o psychotic symptoms | First depressive episode | 49 | 14.0 | 48 | 13.9 |
| Recurrent depressive disorder | 259 | 73.8 | 255 | 73.7 | |
| Bipolar disorder | 43 | 12.3 | 43 | 12.4 | |
| N of depressive episodes (incl. current) | 1 | 52 | 14.8 | 52 | 15.0 |
| 2–3 | 92 | 26.2 | 92 | 26.6 | |
| 4–5 | 49 | 14.0 | 49 | 14.2 | |
| > 5 | 71 | 20.2 | 71 | 20.5 | |
| Not clearly definable | 70 | 20.0 | 70 | 20.2 | |
| Not stated | 17 | 4.8 | 12 | 3.5 | |
| Age of first onset [yrs] | < 18 | 75 | 21.4 | 75 | 21.7 |
| 18–29 | 95 | 27.1 | 95 | 27.5 | |
| 30–39 | 54 | 15.4 | 54 | 15.6 | |
| 40–49 | 73 | 20.8 | 73 | 21.1 | |
| 50–59 | 34 | 9.7 | 34 | 9.8 | |
| > 60 | 8 | 2.3 | 8 | 2.3 | |
| Not stated | 12 | 3.4 | 7 | 2.0 | |
|
Family history for depression |
y/n/missing | 222/123/6 | 63.2/35.0/1.7 | 222/123/1 | 64.2/35.5/0.3 |
| Previous treatment with AD | y/n/missing | 286/53/12 | 81.5/15.1/3.4 | 286/53/7 | 82.7/15.3/2.0 |
| History of suicide attempt(s) | y/n/missing | 84/259/8 | 23.9/73.8/2.3 | 84/259/3 | 24.3/74.9/0.9 |
| Sleep disturbances | y/n/missing | 251/100/0 | 71.5/28.5/0 | 247/99/0 | 71.4/28.6/0 |
| Baseline questionnaires for depression (admission, w1) | |||||
| Mean ± SD/median (25th–75th percentile) | Mean ± SD/median (25th–75th percentile) | ||||
| HDRS | 21.9 ± 6.6 | 20.3 ± 6.0 | |||
| BDI-II | 24 (17–32.5), N = 344 | 24.0 (17–33), N = 339 | |||
| MADRS | 32 (27–36), N = 252 | 32 (27–36), N = 247 | |||
| GAD-7 | 12 (8–15), N = 248 | 12 (8–15), N = 243 | |||
AD antidepressant, BDI-II Beck Depression Inventory II, GAD-7 7-item anxiety scale for generalized anxiety disorder, HDRS Hamilton Depression Scale, N/n sample number, MADRS montgomery Ǻsberg depression rating scale, PHQ-9 Patient Health Questionnaire-9, SD standard deviation, w1 study week 1, yrs years
Table 5.
Basic demographic data and baseline depressive phenotype for the DaCFail study groups (N = 215)
| Parameter | Reference | Depressed patients | Non-depressed patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DaCFail Gr 1 N = 12 |
DaCFail Gr 2 N = 108 |
DaCFail Gr 3 N = 47 |
DaCFail Gr 4 N = 48 |
||||||
| Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | ||||||
| Baseline demographic data | |||||||||
| Age [yrs] | ≥ 50 | 62.1 ± 10.1 (51–79) | 59.5 ± 7.7 (50–80) | 69.2 ± 8.3 (52–84) | 63.1 ± 6.3 (51–81) | ||||
| N | % | N | % | N | % | N | % | ||
| Gender | m/f | 6/6 | 50/50 | 40/68 | 37/63 | 41/6 | 87.2/12.8 | 25/23 | 52.1/47.9 |
| Marital status | Married | 5 | 41.7 | 63 | 58.3 | 34 | 72.3 | 34 | 70.8 |
| Single | 0 | 0 | 8 | 7.4 | 3 | 6.4 | 0 | 0 | |
| Relationship | 1 | 8.3 | 9 | 8.3 | 0 | 0 | 4 | 8.3 | |
| Separated/divorced | 1 | 8.3 | 19 | 17.6 | 0 | 0 | 6 | 12.5 | |
| Widowed | 3 | 25.0 | 9 | 8.3 | 0 | 0 | 4 | 8.3 | |
| Missing | 2 | 16.7 | 0 | 0 | 10 | 21.3 | 0 | 0 | |
| Education |
Apprenticeship/ training |
10 | 83.3 | 71 | 65.7 | 32 | 68.1 | 22 | 45.8 |
|
College/ university |
0 | 0 | 17 | 15.7 | 5 | 10.6 | 23 | 47.9 | |
| None | 0 | 0 | 18 | 16.7 | 0 | 0 | 1 | 2.1 | |
| Missing | 2 | 16.7 | 2 | 1.9 | 10 | 21.3 | 2 | 4.2 | |
| Smoking | y/n/missing | 2/8/2 | 16.7/66.7/16.7 | 29/66/13 | 26.9/61.1/12.0 | 2/44/1 | 4.3/93.6/2.1 | 6/42/0 | 12.5/87.5/0 |
| Alcohol | y/n/missing | 5/6/1 | 41.7/50/8.3 | 24/65/19 | 22.2/60.2/17.6 | 20/24/3 | 42.6/51.1/6.4 | 26/4/18 | 54.2/8.3/37.5 |
| Baseline depressive phenotype | |||||||||
| Depressive episode w/o psychotic symptoms | First depressive episode | 1 | 8.3 | 12 | 11.1 | n/a | |||
| Recurrent depressive disorder | 10 | 83.3 | 81 | 75.0 | |||||
| Bipolar disorder | 1 | 8.3 | 15 | 13.9 | |||||
| N of depressive episodes (incl. current) | 1 | 0 | 0 | 9 | 8.3 | n/a | |||
| 2–3 | 2 | 16.7 | 28 | 25.9 | |||||
| 4–5 | 2 | 16.7 | 16 | 14.8 | |||||
| > 5 | 3 | 25.0 | 40 | 37.0 | |||||
| Not clearly definable | 0 | 0 | 15 | 13.9 | |||||
| Not stated | 5 | 41.7 | 0 | 0 | |||||
| Age of first onset [yrs] | < 18 | 1 | 8.3 | 13 | 12.0 | n/a | |||
| 18–29 | 1 | 8.3 | 18 | 16.7 | |||||
| 30–39 | 2 | 16.7 | 17 | 15.7 | |||||
| 40–49 | 1 | 8.3 | 32 | 29.6 | |||||
| 50–59 | 1 | 8.3 | 21 | 19.4 | |||||
| > 60 | 1 | 8.3 | 2 | 1.9 | |||||
| Not stated | 5 | 41.7 | 5 | 4.6 | |||||
| Family history for depression | y/n/missing | 2/5/5 | 16.7/41.7/41.7 | 69/39/0 | 63.9/36.1/0 | n/a | |||
| Previous treatment with ADs | y/n/missing | 4/3/5 | 33.3/25.0/41.7 | 96/12/0 | 88.9/11.1/0 | n/a | |||
| History for suicide attempt(s) | y/n/missing | 0/7/5 | 0/58.3/41.7 | 24/82/2 | 22.2/75.9/1.9 | n/a | |||
| Sleep disturbances | y/n/missing | 9/3/0 | 75/25/0 | 79/29/0 | 73.1/26.9/0 | 12/34/1 | 25.5/72.3/2.1 | 11/37/0 | 22.9/77.1/0 |
| Baseline questionnaires for depression / screening tools (admission, w1) | |||||||||
| Median (25th–75th percentile) | Median (25th–75th percentile) | Median (25th–75th percentile) | Median (25th–75th percentile) | ||||||
| HDRS | 18.5 (9.5–29.25) | 22.5 (18–27) | 2 (1–4) | 1 (0–2.75) | |||||
| BDI-II | 17 (11–28.25) | 21 (14–30), N = 107 | 6 (4–9) | 6 (5–8) | |||||
| MADRS | 25.5 (12.5–35.75) | 30 (26–35) | 2 (0–3) | 0 (0–1) | |||||
| GAD-7 | 6.5 (4.25–16) | 11 (7–15), N = 107 | 1 (0–4) | 1 (0–1.75), N = 39 | |||||
| PHQ-9 | 11 (7–20.5), N = 9 | 14 (10–19), N = 107 | 2 (0–3.25), N = 46 | 2 (0.25–3), N = 39 | |||||
AD antidepressant, BDI-II Beck Depression Inventory II, GAD-7 7-item anxiety scale for generalized anxiety disorder, HDRS Hamilton Depression Scale, MADRS Montgomery Ǻsberg Depression Rating Scale, N/n sample number, PHQ-9 Patient Health Questionnaire-9, SD standard deviation, w1 study week 1, yrs years
Demographics
The mean age of all 351 depressed subjects was 45.8 years (standard deviation (SD) 15.3, range 18–80), N = 202 depressed participants (57.5%) were male. N = 200 depressed patients (57.0%) were married or engaged in a relationship. The control cohort of 95 non-depressed subjects had a mean age of 66.1 years (SD 8.0, range 51–84), of which 66 participants (69.5%) were male. N = 72 control patients (75.8%) were married or engaged in a relationship. Approximately, one-third of the depressive participants (N = 123, 35.0%) were active smokers. Basic demographic data of the different study groups including the DaCFail subgroups are further detailed in Tables 4 and 5.
Baseline depressive phenotype
The cohort of depressed patients (N = 351) consisted of 305 unipolar depressed patients, of which 49 patients (14.0%) suffered from their first depressive episode and 259 patients (73.8%) from a recurrent depressive episode (basic characteristics of all depressed patients are stated in Table 4). N = 43 patients (12.3%) had the diagnosis of a depressive episode within a bipolar-affective disorder. Regarding the disease load of depressive episodes, N = 120 patients (34.2%) stated an experience of more than three depressive episodes before study enrolment. In the total cohort, two peaks of first depression onset could be differentiated in patients retrospectively based on the previous medical history at the time point of study enrolment: 1. youth and adolescence (< 18 years) and young adulthood (19–29 years) in 170 patients (48.4%); 2. fifth decade of life (40–49 years) in 73 patients (20.8%). Before admission, 286 patients (81.5%) had received treatment with antidepressants. Nearly 25% of all depressed patients had a history of suicide attempts. Most depressed patients (71.5%) had suffered from various sleep disturbances. Regarding the initial quantitative psychometry in the first week of study, depressed patients were assessed by researchers with a mean of 22 (SD 6.6) points in the HDRS (17 of 21 items used for evaluation) and a median of 32 (27–36) points in the MADRS. Using the BDI-II, patients self-rated the severity of their depressive episode with a median of 24 points (16–32). 70.7% of the depressed patients suffered from anxious symptoms and rated a median of 12 points (SD 8–15) on the GAD-7. Further data on the basic depressive phenotype of the DaCFail groups are detailed in Table 5.
Regarding their baseline somatic phenotype, the majority (N = 212, 60.4%) of depressive participants were classified as overweight or obese (body mass index (BMI) ≥ 25). In the 24 h RR analyses, nearly a third of all depressed patients suffered from either a stage of prehypertension with elevated measurements in total (RRsys 130–139 mmHg and/or RRdia 85–89) or from arterial hypertension at a minimum stage of 1 (stage 1: RRsys 140–159 mmHg and/or RRdia 90–99 mmHg, stage 2: RRsys 160–179 mmHg and/or RRdia 100–109 mmHg).
Discussion
The GEParD cohort study as prospective observational study protocol and the DaCFail study as a case–control study protocol are an example for deep phenotyping of depressed patients in an inpatient setting, particularly emphasizing the stress system and the heart–brain axis. The present repertoire of psychometry, tailored quantification of somatic and cardiac parameters, as well as laboratory parameters including (epi-)genetic analyses allows to evaluate response or resistance to antidepressant therapy during hospitalization using biological and psychological markers.
A recent GWAS study for major depressive disorder has accentuated the need of standardized quantification of disease parameters to impede biased views on genetic architecture and underlying pathogenesis by minimal phenotyping (Cai et al. 2020). Study protocols such as the Biological Classification of Mental Disorders (BeCOME) study (Bruckl et al. 2020) focus on in depth phenotyping of patients suffering from affective, anxiety and stress-related mental disorders focusing on mental phenotypes. The present protocol complements this approach by adding somatic phenotypes under the concept of depression as a systemic disorder with a focus on cardiac phenotypes.
Preliminary analyses based on selected phenotypes in subcohorts of the GEParD and DaCFail protocols have facilitated further understanding of the disease course and risk factors in depressive episodes which will be used for comprehensive analyses of the overall study cohort and additional subgroup analyses (e.g. regarding different age groups, onset of disease manifestations):
-
(i)
Anxious depression and traumatic events in the childhood of patients are associated with increased sensitivity of the HPA axis and the immune system using FKBP5 mRNA expression and the CTQ as a phenotypic marker (Menke et al. 2018).
-
(ii)
Severe life events occurring prior to depressive episodes may impair psychopharmaceutical treatment assessed by FKBP5, SGK1, and NR3C1 mRNA-expression levels (Menke et al. 2021).
-
(iii)
Covariation bias, an overestimation of the relationship between fear-relevant stimuli and aversive consequences, has been revealed as possible characterization of non-responders for antidepressant treatment. It may serve as a possible neurocognitive marker for emotional information processing in depressive episodes (Stonawski et al. 2019).
-
(iv)
Psychological paradigms show that fear acquisition and extinction may be impaired in patients suffering from severe depressive episodes. This may underpin the importance of future studies addressing extinction learning elements in antidepressant treatment (Wurst et al. 2021).
The present two-level study design faces limitations of which the foremost are: the naturalistic and observational inpatient setting does not allow for controlled interventions with randomized and matched treatment and control samples. Comprehensive diagnostics results in a high number of single procedures. This may challenge depressed patients who are treated in an inpatient setting because of severe depression and cause a relevant dropout of patients due to motivation, distress, and wishes for discharge (Figs. 1 and 2, Tables 3, 4 and 5). In addition, this contributes to a fragmentary mosaic of quantified biomarkers with missing data, which counteracts the effort for standardized endo-phenotyping of depressive episodes in a cohort representative for the inpatient population. The Department of Psychiatry, Psychotherapy and Psychosomatic Medicine of the University Hospital of Würzburg provides specialized and acute psychiatric care for Würzburg, a medium-sized German university town, which in a monocentric approach limits the recruitment of rare, however, severely ill patient groups, such as DaCFail study group 1 (depression and HF, Fig. 2).
Overall, the described concept for deep phenotyping of depressive episodes illustrates a promising approach to unravel predictive measurements for the onset, course, and treatment response of depressive episodes with a focus on heart comorbidity using a broad repertoire of established psychometric, somatic, and laboratory including (epi-)genetic markers. Experiences with these mainly naturalistic protocols will contribute to successful multicentre studies investigating personalized antidepressant therapies.
Acknowledgements
We wish to thank the laboratory of the Department of Psychiatry, Psychotherapy and Psychosomatic Medicine of the University Hospital Würzburg for technical assistance in handling biological samples. We thank all patients who participated in the study.
Author contributions
Conceptualization, methodology, and resources: AM, KD, SK-S, JD; investigation: all authors; formal analysis: KL, CK, SK-S; validation, data interpretation: KL, CK, AM, KD, SK-S, JD; funding acquisition: LH, SS, GG, PUH, TH, MP, AM, JD; data curation: KL, CK, AM, KD, SK-S, JD; visualization, writing—original draft: KL, SK-S, JD; writing—review and editing: KL, JD with contributions from all authors. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Federal Ministry of Education and Research (BMBF) within the framework of the Comprehensive Heart Failure Center (grant nr. 01EO1504 to S.Stö., G.G., P.U.H., T.H., M.P., A.M., and J.D.) and the Interdisciplinary Center for Clinical Research at the University Hospital of Würzburg (grant nr. N-355 to T.H. and A.M. and grant nr. N-258 to L.H. and J.D.).
Data availability
The datasets generated during the study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
J.D. was a co-recipient of a grant of the Bavarian State Government to BioVariance and an investigator in a European grant to P1Vital. K.D. is a member of the Steering Committee Neurosciences, Janssen Inc. A.M. has given talks for the health insurance company AOK, the Bavarian General Practitioner Group, Neuraxpharm and Medice Arzneimittel Pütter GmbH & Co KG. S.K.-S. has received author’s and speaker’s honoraria from Medice Arzneimittel Pütter GmbH & Co KG and Takeda. All other authors declare no conflicts of interest regarding this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Andreas Menke, Katharina Domschke, Sarah Kittel-Schneider, and Jürgen Deckert contributed equally to this work.
References
- Angermann CE, Gelbrich G, Stork S, Gunold H, Edelmann F, Wachter R, Schunkert H, Graf T, Kindermann I, Haass M, Blankenberg S, Pankuweit S, Prettin C, Gottwik M, Bohm M, Faller H, Deckert J, Ertl G, Mood-Hf Study Investigators, and Members Committee Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315:2683–2693. doi: 10.1001/jama.2016.7635. [DOI] [PubMed] [Google Scholar]
- Angermann CE, Kaspar M, Marx A, Kittel-Schneider S, Menhofer D, Stork S, Ertl G, Domschke K, Deckert J, Reif A. A functional variant of the neuropeptide S receptor-1 gene modulates clinical outcomes and healthcare utilization in patients with systolic heart failure: results from the Interdisciplinary Network Heart Failure (INH) Study. Eur J Heart Fail. 2017;19:314–323. doi: 10.1002/ejhf.706. [DOI] [PubMed] [Google Scholar]
- Baune BT, Hohoff C, Berger K, Neumann A, Mortensen S, Roehrs T, Deckert J, Arolt V, Domschke K. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33:924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckl TM, Spoormaker VI, Samann PG, Brem AK, Henco L, Czamara D, Elbau I, Grandi NC, Jollans L, Kuhnel A, Leuchs L, Pohlchen D, Schneider M, Tontsch A, Keck ME, Schilbach L, Czisch M, Lucae S, Erhardt A, Binder EB. The biological classification of mental disorders (BeCOME) study: a protocol for an observational deep-phenotyping study for the identification of biological subtypes. BMC Psychiatry. 2020;20:213. doi: 10.1186/s12888-020-02541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. 1985;15:189–194. doi: 10.1017/S003329170002105X. [DOI] [PubMed] [Google Scholar]
- Bullinger M, Kirchberger I, Ware J. Der deutsche SF-36 Health Survey Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. J Public Health. 1995;3:21–36. doi: 10.1007/BF02959944. [DOI] [Google Scholar]
- Cai N, Revez JA, Adams MJ, Andlauer TFM, Breen G, Byrne EM, Clarke TK, Forstner AJ, Grabe HJ, Hamilton SP, Levinson DF, Lewis CM, Lewis G, Martin NG, Milaneschi Y, Mors O, Muller-Myhsok B, Penninx B, Perlis RH, Pistis G, Potash JB, Preisig M, Shi J, Smoller JW, Streit F, Tiemeier H, Uher R, Van der Auwera S, Viktorin A, Weissman MM, M. D. D. Working Group of the Psychiatric Genomics Consortium, Kendler KS, Flint J (2020) Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet 52:437–447 [DOI] [PMC free article] [PubMed]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267–283. doi: 10.1037/0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chirumamilla A, Travin MI. Cardiac applications of 123I-mIBG imaging. Semin Nucl Med. 2011;41:374–387. doi: 10.1053/j.semnuclmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Eggart M, Lange A, Binser MJ, Queri S, Muller-Oerlinghausen B. Major depressive disorder is associated with impaired interoceptive accuracy: a systematic review. Brain Sci. 2019;9:131. doi: 10.3390/brainsci9060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli G, Domschke K, Minelli A, Gennarelli M, Martini P, Bortolomasi M, Maron E, Squassina A, Kasper S, Zohar J, Souery D, Montgomery S, Albani D, Forloni G, Ferentinos P, Rujescu D, Mendlewicz J, De Ronchi D, Baune BT, Pharmacogenomics European College of Neuropsychopharmacology, Group Transcriptomics Thematic Working. Serretti A, Fabbri C. A meta-analysis of polygenic risk scores for mood disorders, neuroticism, and schizophrenia in antidepressant response. Eur Neuropsychopharmacol. 2022;55:86–95. doi: 10.1016/j.euroneuro.2021.11.005. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/S0193-953X(05)70283-5. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M (2004) The structured clinical interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II)', comprehensive handbook of psychological assessment, Vol. 2: Personality assessment.: 134–43.
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Personality Individ Differ. 2001;30:1311–1327. doi: 10.1016/S0191-8869(00)00113-6. [DOI] [Google Scholar]
- Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of heart failure: the HUNT Study. Eur J Heart Fail. 2014;16:861–870. doi: 10.1002/ejhf.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, Hemani G, Berger K, Teismann H, Rawal R, Arolt V, Baune BT, Dannlowski U, Domschke K, Tian C, Hinds DA, Team andMe Research, Consortium Major Depressive Disorder Working Group of the Psychiatric Genomics. Trzaskowski M, Byrne EM, Ripke S, Smith DJ, Sullivan PF, Wray NR, Breen G, Lewis CM, McIntosh AM. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Sen S. Gene x environment interaction models in psychiatric genetics. Curr Top Behav Neurosci. 2012;12:441–462. doi: 10.1007/7854_2011_184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel-Schneider S, Kaspar M, Berliner D, Weber H, Deckert J, Ertl G, Stork S, Angermann C, Reif A. CRP genetic variants are associated with mortality and depressive symptoms in chronic heart failure patients. Brain Behav Immun. 2018;71:133–141. doi: 10.1016/j.bbi.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol Med. 2019;49:1948–1957. doi: 10.1017/S0033291719001351. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner C, Menke A. How to measure glucocorticoid receptor's sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology. 2018;91:235–260. doi: 10.1016/j.psyneuen.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Major Depressive Disorder Working Group of the Psychiatric, Gwas Consortium. Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G, Byrne EM, Blackwood DH, Boomsma DI, Cichon S, Heath AC, Holsboer F, Lucae S, Madden PA, Martin NG, McGuffin P, Muglia P, Noethen MM, Penninx BP, Pergadia ML, Potash JB, Rietschel M, Lin D, Muller-Myhsok B, Shi J, Steinberg S, Grabe HJ, Lichtenstein P, Magnusson P, Perlis RH, Preisig M, Smoller JW, Stefansson K, Uher R, Kutalik Z, Tansey KE, Teumer A, Viktorin A, Barnes MR, Bettecken T, Binder EB, Breuer R, Castro VM, Churchill SE, Coryell WH, Craddock N, Craig IW, Czamara D, De Geus EJ, Degenhardt F, Farmer AE, Fava M, Frank J, Gainer VS, Gallagher PJ, Gordon SD, Goryachev S, Gross M, Guipponi M, Henders AK, Herms S, Hickie IB, Hoefels S, Hoogendijk W, Hottenga JJ, Iosifescu DV, Ising M, Jones I, Jones L, Jung-Ying T, Knowles JA, Kohane IS, Kohli MA, Korszun A, Landen M, Lawson WB, Lewis G, Macintyre D, Maier W, Mattheisen M, McGrath PJ, McIntosh A, McLean A, Middeldorp CM, Middleton L, Montgomery GM, Murphy SN, Nauck M, Nolen WA, Nyholt DR, O'Donovan M, Oskarsson H, Pedersen N, Scheftner WA, Schulz A, Schulze TG, Shyn SI, Sigurdsson E, Slager SL, Smit JH, Stefansson H, Steffens M, Thorgeirsson T, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Volzke H, Weilburg JB, Willemsen G, Zitman FG, Neale B, Daly M, Levinson DF, Sullivan PF. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Akil H. Revisiting the Stress Concept: Implications for Affective Disorders. J Neurosci. 2020;40:12–21. doi: 10.1523/JNEUROSCI.0733-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, Manber R. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26:25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D, Gonik M, Rex-Haffner M, Rubel J, Uhr M, Lucae S, Deussing JM, Muller-Myhsok B, Holsboer F, Binder EB. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37:1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Domschke K, Czamara D, Klengel T, Hennings J, Lucae S, Baune BT, Arolt V, Muller-Myhsok B, Holsboer F, Binder EB. Genome-wide association study of antidepressant treatment-emergent suicidal ideation. Neuropsychopharmacology. 2012;37:797–807. doi: 10.1038/npp.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Arloth J, Gerber M, Rex-Haffner M, Uhr M, Holsboer F, Binder EB, Holsboer-Trachsler E, Beck J. Dexamethasone stimulated gene expression in peripheral blood indicates glucocorticoid-receptor hypersensitivity in job-related exhaustion. Psychoneuroendocrinology. 2014;44:35–46. doi: 10.1016/j.psyneuen.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Menke A, Lehrieder D, Fietz J, Leistner C, Wurst C, Stonawski S, Reitz J, Lechner K, Busch Y, Weber H, Deckert J, Domschke K. Childhood trauma dependent anxious depression sensitizes HPA axis function. Psychoneuroendocrinology. 2018;98:22–29. doi: 10.1016/j.psyneuen.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Menke A, Nitschke F, Hellmuth A, Helmel J, Wurst C, Stonawski S, Blickle M, Weiss C, Weber H, Hommers L, Domschke K, Deckert J. Stress impairs response to antidepressants via HPA axis and immune system activation. Brain Behav Immun. 2021;93:132–140. doi: 10.1016/j.bbi.2020.12.033. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morbach C, Gelbrich G, Tiffe T, Eichner FA, Christa M, Mattern R, Breunig M, Cejka V, Wagner M, Heuschmann PU, Stefan Störk S, Frantz C, Maack G, Ertl M, Fassnacht C, Wanner R, Leyh J, Volkmann J, Deckert HF, Jahns R. Prevalence and determinants of the precursor stages of heart failure: results from the population-based STAAB cohort study. Eur J Prev Cardiol. 2021;28:924–934. doi: 10.1177/2047487320922636. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak–the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–539. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. 2021;18:136–145. doi: 10.1038/s41569-020-00463-7. [DOI] [PubMed] [Google Scholar]
- Okbay, A., B. M. Baselmans, J. E. De Neve, P. Turley, M. G. Nivard, M. A. Fontana, S. F. Meddens, R. K. Linner, C. A. Rietveld, J. Derringer, J. Gratten, J. J. Lee, J. Z. Liu, R. de Vlaming, T. S. Ahluwalia, J. Buchwald, A. Cavadino, A. C. Frazier-Wood, N. A. Furlotte, V. Garfield, M. H. Geisel, J. R. Gonzalez, S. Haitjema, R. Karlsson, S. W. van der Laan, K. H. Ladwig, J. Lahti, S. J. van der Lee, P. A. Lind, T. Liu, L. Matteson, E. Mihailov, M. B. Miller, C. C. Minica, I. M. Nolte, D. Mook-Kanamori, P. J. van der Most, C. Oldmeadow, Y. Qian, O. Raitakari, R. Rawal, A. Realo, R. Rueedi, B. Schmidt, A. V. Smith, E. Stergiakouli, T. Tanaka, K. Taylor, G. Thorleifsson, J. Wedenoja, J. Wellmann, H. J. Westra, S. M. Willems, W. Zhao, Study LifeLines Cohort, N. Amin, A. Bakshi, S. Bergmann, G. Bjornsdottir, P. A. Boyle, S. Cherney, S. R. Cox, G. Davies, O. S. Davis, J. Ding, N. Direk, P. Eibich, R. T. Emeny, G. Fatemifar, J. D. Faul, L. Ferrucci, A. J. Forstner, C. Gieger, R. Gupta, T. B. Harris, J. M. Harris, E. G. Holliday, J. J. Hottenga, P. L. De Jager, M. A. Kaakinen, E. Kajantie, V. Karhunen, I. Kolcic, M. Kumari, L. J. Launer, L. Franke, R. Li-Gao, D. C. Liewald, M. Koini, A. Loukola, P. Marques-Vidal, G. W. Montgomery, M. A. Mosing, L. Paternoster, A. Pattie, K. E. Petrovic, L. Pulkki-Raback, L. Quaye, K. Raikkonen, I. Rudan, R. J. Scott, J. A. Smith, A. R. Sutin, M. Trzaskowski, A. E. Vinkhuyzen, L. Yu, D. Zabaneh, J. R. Attia, D. A. Bennett, K. Berger, L. Bertram, D. I. Boomsma, H. Snieder, S. C. Chang, F. Cucca, I. J. Deary, C. M. van Duijn, J. G. Eriksson, U. Bultmann, E. J. de Geus, P. J. Groenen, V. Gudnason, T. Hansen, C. A. Hartman, C. M. Haworth, C. Hayward, A. C. Heath, D. A. Hinds, E. Hypponen, W. G. Iacono, M. R. Jarvelin, K. H. Jockel, J. Kaprio, S. L. Kardia, L. Keltikangas-Jarvinen, P. Kraft, L. D. Kubzansky, T. Lehtimaki, P. K. Magnusson, N. G. Martin, M. McGue, A. Metspalu, M. Mills, R. de Mutsert, A. J. Oldehinkel, G. Pasterkamp, N. L. Pedersen, R. Plomin, O. Polasek, C. Power, S. S. Rich, F. R. Rosendaal, H. M. den Ruijter, D. Schlessinger, H. Schmidt, R. Svento, R. Schmidt, B. Z. Alizadeh, T. I. Sorensen, T. D. Spector, J. M. Starr, K. Stefansson, A. Steptoe, A. Terracciano, U. Thorsteinsdottir, A. R. Thurik, N. J. Timpson, H. Tiemeier, A. G. Uitterlinden, P. Vollenweider, G. G. Wagner, D. R. Weir, J. Yang, D. C. Conley, G. D. Smith, A. Hofman, M. Johannesson, D. I. Laibson, S. E. Medland, M. N. Meyer, J. K. Pickrell, T. Esko, R. F. Krueger, J. P. Beauchamp, P. D. Koellinger, D. J. Benjamin, M. Bartels, and D. Cesarini Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48:624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schiele MA, Zwanzger P, Schwarte K, Arolt V, Baune BT, Domschke K. Serotonin Transporter Gene Promoter Hypomethylation as a Predictor of Antidepressant Treatment Response in Major Depression: A Replication Study. Int J Neuropsychopharmacol. 2021;24:191–199. doi: 10.1093/ijnp/pyaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–295. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo JL, Nemeroff CB. Depression as a systemic disease. Personalized Medicine in Psychiatry. 2017;1–2:11–25. doi: 10.1016/j.pmip.2016.11.002. [DOI] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stonawski S, Wiemer J, Wurst C, Reitz J, Hommers L, Menke A, Domschke K, Schiele MA, Pauli P. Covariation bias in depression—a predictor of treatment response? J Neural Transm (vienna) 2019;126:1653–1665. doi: 10.1007/s00702-019-02091-z. [DOI] [PubMed] [Google Scholar]
- Story Jovanova O, Nedeljkovic I, Spieler D, Walker RM, Liu C, Luciano M, Bressler J, Brody J, Drake AJ, Evans KL, Gondalia R, Kunze S, Kuhnel B, Lahti J, Lemaitre RN, Marioni RE, Swenson B, Himali JJ, Wu H, Li Y, McRae AF, Russ TC, Stewart J, Wang Z, Zhang G, Ladwig KH, Uitterlinden AG, Guo X, Peters A, Raikkonen K, Starr JM, Waldenberger M, Wray NR, Whitsel EA, Sotoodehnia N, Seshadri S, Porteous DJ, van Meurs J, Mosley TH, McIntosh AM, Mendelson MM, Levy D, Hou L, Eriksson JG, Fornage M, Deary IJ, Baccarelli A, Tiemeier H, Amin N. DNA Methylation Signatures of Depressive Symptoms in Middle-aged and Elderly Persons: Meta-analysis of Multiethnic Epigenome-wide Studies. JAMA Psychiat. 2018;75:949–959. doi: 10.1001/jamapsychiatry.2018.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, Gao K, Miskowiak KW, Grande I. Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- Wagner M, Tiffe T, Morbach C, Gelbrich G, Stork S, Heuschmann PU. Characteristics and course of heart failure stages A-B and determinants of progression—design and rationale of the STAAB cohort study. Eur J Prev Cardiol. 2017;24:468–479. doi: 10.1177/2047487316680693. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu Q, Egan L, Gu X, Liu P, Gu H, Yang Y, Luo J, Wu Y, Gao Z, Fan J. Anterior insular cortex plays a critical role in interoceptive attention. Elife. 2019;8:e42265. doi: 10.7554/eLife.42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner RA, Kobayashi R, Javadi MS, Kock Z, Wakabayashi H, Unterecker S, Nakajima K, Lapa C, Menke A, Higuchi T. Impact of Novel Antidepressants on Cardiac (123)I-Metaiodobenzylguanidine Uptake: Experimental Studies on SK-N-SH Cells and Healthy Rabbits. J Nucl Med. 2018;59:1099–1103. doi: 10.2967/jnumed.117.206045. [DOI] [PubMed] [Google Scholar]
- Williams A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Wurst C, Schiele MA, Stonawski S, Weiss C, Nitschke F, Hommers L, Domschke K, Herrmann MJ, Pauli P, Deckert J, Menke A. Impaired fear learning and extinction, but not generalization, in anxious and non-anxious depression. J Psychiatr Res. 2021;135:294–301. doi: 10.1016/j.jpsychires.2021.01.034. [DOI] [PubMed] [Google Scholar]
- Wuthrich F, Nabb CB, Mittal VA, Shankman SA, Walther S. Actigraphically measured psychomotor slowing in depression: systematic review and meta-analysis. Psychol Med. 2022;52:1–14. doi: 10.1017/S0033291722000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano J, Celano CM, Januzzi JL, Massey CN, Chung WJ, Millstein RA, Huffman JC. Psychiatric and Psychological Interventions for Depression in Patients With Heart Disease: A Scoping Review. J Am Heart Assoc. 2020;9:e018686. doi: 10.1161/JAHA.120.018686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the study are available from the corresponding author on reasonable request.