Abstract
The clinical use of cellular immunotherapies is gaining momentum and the number of approved indications is steadily increasing. One class of cellular therapies—chimeric antigen receptor (CAR)-modified T cells—has achieved impressive results in distinct blood cancer indications. These existing cellular therapies treating blood cancers face significant relapse rates, and their application beyond hematology has been underwhelming, especially in solid oncology. Major reasons for resistance source largely in the tumor microenvironment (TME). The TME in fact functionally suppresses, restricts, and excludes adoptive immune cells, which limits the efficacy of cellular immunotherapies from the onset. Many promising efforts are ongoing to adapt cellular immunotherapies to address these obstacles, with the aim of reshaping the tumor microenvironment to ameliorate function and to achieve superior efficacy against both hematological and solid malignancies.
Keywords: Cellular immunotherapy, CAR-T cells, Tumor microenvironment, Inflammation, Cytokine, Stroma
Introduction
Cellular immunotherapies, encompassing the use of modified autologous or allogeneic immune cells as treatment against disease, have recently advanced to become part of the standard of care in a few types of relapsed and refractory hematological malignancies. Recent evidence suggests that they might even progress to earlier lines of treatment at least in some indications, highlighting the untapped potential of these approaches.
Overall, cellular immunotherapies have so far employed dendritic cell (DC), T cells, or natural killer cells (NK) into patients for treatment of disease. DC vaccines, which utilize mature dendritic cells or monocyte-derived dendritic cells derived from patient blood, harness the natural role of the dendritic cell in antigen presentation and T cell licensing to target cancer. Dendritic cells are pulsed with tumor-associated antigens and neoantigens that would then be presented to T cells in lymph nodes to induce cytotoxic lymphocyte priming and polarization to mount a specific immune response. Various DC vaccines have shown promising safety and efficacy in clinical trials against pediatric solid tumors and other forms of solid tumors [1–3]. These efforts in developing DC vaccine approaches culminated in FDA approval of Sipuleucel-T, a DC vaccine for the treatment of prostate cancer [4]. While Sipuleucel T did improve survival in prostate cancer patients, adoption of this therapy in clinics has been limited over questions of clinical efficacy and cost [5]. In fact, marketing authorization was even withdrawn in the European Union. While NK cell therapy to date has remained investigational only, most efforts and advances in cellular therapy have revolved around T cell-based therapeutics. These therapies can be subclassified into tumor infiltrating lymphocytes (TIL), chimeric antigen receptor (CAR)-T cells, and recombinant T cell receptor (TCR)-T cells. TILs are heterogeneous populations of immune cells, mostly composed of T cells, that are extracted from a patient’s own cancer as they are presumed to have high specificity against those tumors [6]. After expansion, the cells are reintroduced into the patient in a therapeutic intention. Such treatment was initially sought for in melanoma treatment, where high remission rates were observed [7]. The burden of generation and high variability of the product made up for an uneven comparison to checkpoint blockade and dismissed TIL therapy for a while. Recently, autologous TIL treatment after immune checkpoint blockade (ICB) failure demonstrated robust remission rates in a significant share of melanoma patients, bringing the concept back to clinical investigations [7].
Meanwhile, TCRs and CARs are receptors genetically engineered into T cells isolated from patient blood and reintroduced into the patient to target cancers. TCRs used are derived from natural sequences that bind the desired antigen and behave similarly to normal TCRs [8]. In contrast, CARs are fully synthetic receptors not naturally found and consist of an antibody-derived single chain variable fragment (scFv) combined with intracellular T cell receptor signaling domains only (so called 1st generation CAR) or with one or more intracellular domains of costimulatory molecules to further activate the T cell when antigen is bound (so called 2nd and 3rd generation CAR) [9]. The major difference is HLA restriction in the case of TCRs and lack thereof for CARs. Both strategies activate the T cell and induce cytotoxicity and proliferation when in contact with tumor cells [6, 9]. While TCRs have demonstrated only anecdotal evidence and a slow developmental pace [10], CAR usage has culminated into six FDA approvals for T cell-based therapies—idecabtagene vicleucel and ciltacabtagene autoleucel targeting B cell maturation antigen (BCMA) in multiple myeloma, and lisocabtagene maraleucel, tisagenlecleucel, brexucabtagene autoleucel, and axicabtagene ciloleucel targeting CD19 in a variety of B-cell lymphomas and leukemias [11]. There are also efforts also to adapt CAR engineering to NK cells, which would confer an antigen-specific tumor killing capacity to cells optimized for serial killing in efforts to induce efficient anti-tumor activity [12], and to γδ T cells, which have shown antigen cross-presentation capability and favorable persistence phenotypes [13, 14]. In hematological malignancies, CAR-T cells have achieved unparalleled clinical results as treatment for relapsed and refractory patients with otherwise poor prognosis [15]. Despite successes and high response rates, patients undergoing CAR-T cell therapy often experience relapse after only weeks or months, demonstrating that current forms of therapy must be improved [16]. At the same time, CAR-T cell therapies in their current form have shown modest or negligible efficacy in clinical trials for a range of solid tumors [17, 18]. Overall, ongoing issues of relapse in hematological malignancies and inefficacy in treating solid tumors can be routed back to a few main obstacles: antigen loss, low levels of immune cell infiltration, and the effects of an intensely immunosuppressive tumor microenvironment (TME) [17, 18]. As several of these aspects have been extensively reviewed by us and others in the past [19], this review will specifically focus on the obstacles presented by the TME. We will summarize current efforts in cellular immunotherapies to affect and change the TME to be more therapy permissive, with the aim of enabling increased therapeutic efficacy across indications.
The immunosuppressive tumor microenvironment
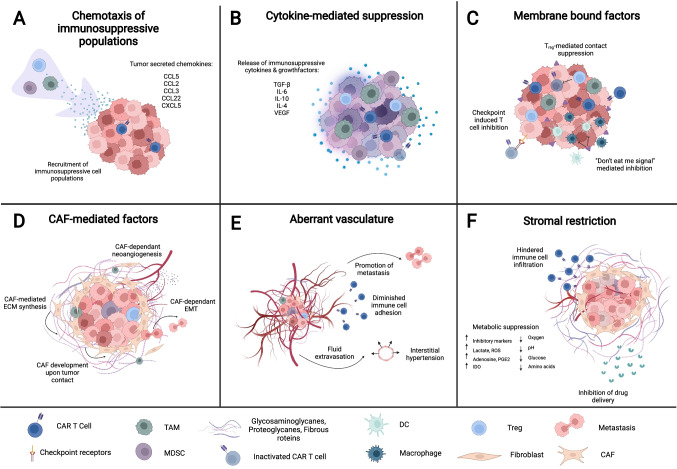
The tumor microenvironment—comprises malignant cells together with their immediate environment consisting of immune cells, fibroblasts, extracellular matrix (ECM), blood vessels, and secreted factors. The TME can support the survival and proliferation of tumor cells and tumor-associated cell populations and thus contributes a major share to cancer hallmarks (Fig. 1). Crucially, the TME suppresses the ability of cellular therapies to normally function through induction of inhibitory signals as well as forming physical barriers preventing contact with tumor cells in both solid tumors and hematological malignancies [20]. These immunosuppressive aspects relevant to cellular therapies are the focus of this section. Suppressive mechanisms embedded in the TME can be subdivided into chemokine-induced migration of immunosuppressive cells, cytokine-mediated suppression, membrane-bound and contact suppression, cancer-associated fibroblast-mediated factors, stromal restriction, and aberrant vasculature (Fig. 1).
Fig. 1.
Mechanisms of TME suppression of anti-tumor cellular therapies. (A) Recruitment of immunosuppressive TAMs, MDSCs, and T-regs by chemokine gradients secreted by tumor cells. (B) Immunosuppressive cytokine milieu secreted by tumor cells, immunosuppressive cells, and CAFs exhaust and inactivate infiltrated adoptive cells. (C) Membrane-bound mechanisms of TME suppression mediated by tumors and immunosuppressive cells. (D) Pro-tumorigenic and metastatic functions mediated by CAFs. (E) Inhibition of cellular therapies by the development of aberrant vasculature. (F) Stromal exclusion of immune infiltration and suppression of infiltrated cells
Chemokines released by tumor cells recruit and retain other populations of immunosuppressive cells into the tumor microenvironment (Fig. 1A). T-regulatory cells (T-reg) are recruited by tumor-secreted chemokines C–C motif ligand (CCL)5, CCL17, and CCL22 [21]. Chemokines such as CCL2, CCL5, and C-X-C motif chemokine (CXCL)5 recruit myeloid-derived suppressor cells (MDSC) [22], a heterogenous though poorly defined population of myeloid cells that are most clearly related by their myeloid lineage and suppressive capacity [22]. Tumor-associated macrophages (TAM), a suppressive type of macrophage closely related to M2 macrophages, derive from tumor-associated monocytes that migrate to tumors via CCL2 and CCL3 gradients [23]. Other varieties of recruited cells such as mast cells and neutrophils also contribute to suppression [24, 25] while cells such as bone-marrow-derived mesenchymal stem cells (MSC) additionally contribute to the development of the TME [26]. Tumor cells and recruited immunosuppressive cells release cytokines such as interleukin (IL)-10, transforming growth factor–beta (TGF-β), IL-4, and the enzyme indoleamine 2,3-dioxygenase 1 (IDO-1) that contribute a cytokine milieu that further suppresses infiltrated immune cells [20] (Fig. 1B). In addition to these soluble factors, tumors, MDSCs, TAMs, and T-regs directly suppress immune function by upregulating inhibitory signaling molecules on their membrane, including the well-known checkpoint molecules programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), among others [20, 27] (Fig. 1C). Tumor cells furthermore prominently express “don’t eat me” signals such as CD47 and CD24 to escape immunosurveillance by the innate immune system[28–30], and intratumoral T-regs can mediate antigen-dependent and -independent inhibition of immune cells [21]. Another intratumoral cell type, cancer-associated fibroblasts (CAF), are a highly heterogeneous population of activated fibroblasts that form around tumors and further shape the tumor microenvironment [31] (Fig. 1D). Though the mechanisms of CAF development are not fully understood, it is clear that these cells both shape and are shaped by the environment around tumors [32, 33]. Several intra-tumoral factors such as cell–cell contact with tumor cells, DNA damage, TGF-β secretion, and physiological stress have been shown to induce the transformation of normal tumor-adjacent fibroblasts and tumor-infiltrated MSCs into CAFs [26, 33]. CAFs then support tumor growth through the promotion of angiogenesis via the secretion of a variety of growth factors such as VEGF, PDGF, EGF, FGF2, FGF5, GDF15 and the secretion of immunosuppressive cytokines including TGF-β, IL-6, CXCL12, CCL2, LIF, and GAS6 [33, 34]. CAFs furthermore are crucial in the degradation and formation of the extracellular matrix (ECM) of the TME, a network of proteoglycans and glycoproteins that provides structural and mechanical support to cells and tissues. This CAF remodeling function has also been shown to be important for tumors undergoing endothelial-mesenchymal transitions (EMT) crucial for metastasis [35]. Altogether, CAFs and infiltrated immunosuppressive cells in turn constitute a protective layering of cells and ECM around the tumor known as the tumor stroma [36]. The stroma physically functions as a barrier excluding immune cells from accessing the tumor, while further metabolically suppressing immune cells by depleting vital acellular components such as amino acids, glucose, and oxygen from the TME [37, 38] (Fig. 1F). The stroma has also been shown to inhibit the delivery of anti-cancer drugs and therefore contributes to resistance against certain therapies [35, 39, 40]. Finally, aberrant vasculature needed by malignant cells to proliferate and metastasize poses an additional barrier confronting CAR-T cells upon entrance into the TME (Fig. 1E). This irregular vasculature is promoted by a combination of angiogenic signaling from tumor cells themselves and by tumor-associated cells mentioned previously. The vessels are not only characterized by their disorganized manner, but also through an upregulation of endothelial molecules and a highly permeable endothelial membrane, consequently limiting the trafficking of immune cells within the malignant tissue via interstitial hypertension and interference with T cell adhesion [41]. Along these lines, the TME profoundly regulates and suppresses immune responses and appears as the aspect to beat to enable cell therapy efficacy.
Cellular therapies to remodel the TME
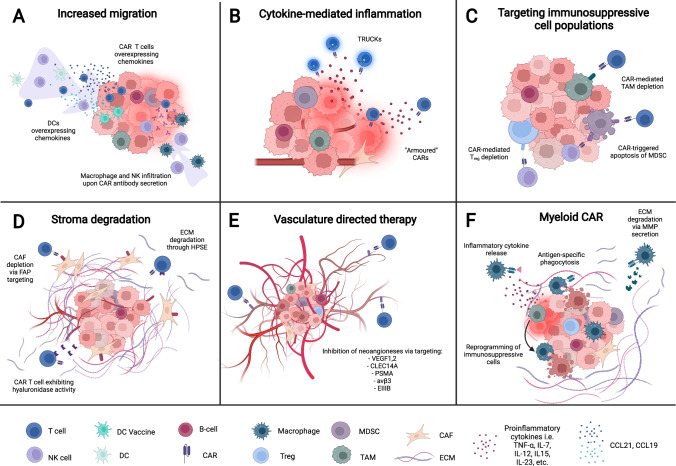
Many insights have already been gained into improving existing cellular therapy. For instance, application via intratumoral injection leads to increased efficacy of adoptive CAR-T cells in certain tumor indications by physically circumventing the issue of TME exclusion and adoptive cell infiltration [42, 43]. To further address the issues of cellular therapies in tumors, recent therapeutic concepts have incorporated elements designed to specifically counteract various aspects of the TME (Fig. 2), with some reaching the clinical trial stage (Table 1). Because the range of these approaches varies widely, the scope of the present review will focus on concepts that are specifically incorporated into the cellular therapy. Combinatorial approaches with application of secondary agents have already been extensively reviewed [44]. We emphasize novel approaches that have been shown to affect and change obstacles presented by the TME, covering topics such as increasing intratumoral immune cell infiltration, changing the cytokine environment within the tumor, elimination of suppressive immune cell populations, removal of physical barriers presented by the stroma, and targeting of the tumor vasculature (Fig. 2). Additionally, we focus on concepts that remodel the TME specifically rather than those enhancing cellular therapy function only. Such approaches have been shown to have higher potential of engaging concerted endogenous immune responses against the tumor and have been shown to pair well with standard of care ICB [45–48].
Fig. 2.
Strategies incorporated into cellular therapies to remodel the TME. (A) Increased migration of endogenous and adoptive cells via cellular therapies engineered to overexpress inflammatory chemokines and secreted antibodies. (B) Overexpression of inflammatory cytokines reprograms immunosuppressive populations, converts immune-restricted tumor to an immune-permissive tumor. (C) CAR-mediated depletion of MDSCs, TAMs, and T-regs via direct targeting. (D) Degradation of stroma components via enzymes engineered into cellular therapies or direct targeting by CARs. (E) CAR targeting of angiogenic markers inhibits tumor neoangiogenesis. (F) CAR-M- and CAR-P-mediated tumor phagocytosis, and the inflammatory and physical remodeling of the TME
Table 1.
Clinical trials
| Drug type | Mechanism | Target | Start | Phase | Status | Trial number |
|---|---|---|---|---|---|---|
| Increasing intratumoral immune infiltration | ||||||
| DC vaccine | Adenovirally transduced CCL21 DC vaccine | Melanoma | 2008 | I | Completed | NCT00798629 |
| DC vaccine | Adenovirally transduced CCL21 DC vaccine | NSCLC | 2011 | I | Completed | NCT01574222 |
| DC vaccine | Adenovirally transduced CCL21 DC vaccine and pembrolizumab | NSCLC | 2019 | I | Recruiting | NCT03546361 |
| CAR T cells | Anti-GPC3 or TGF- β CAR T cells overexpressing CCL19-IL7 | HCC | 2017 | I | Recruiting | NCT03198546 |
| CAR T cells | CAR T cells overexpressing CCL19-IL7 (single and dual targeting of: Integrin β7, BCMA, CS1, CD38 and CD138) | RRMM | 2018 | I | Recruiting | NCT03778346 |
| CAR T cells | Anti-Nectin4 + CAR T cells overexpressing -IL7 and IL-12 or CCL19 | Nectin-4 + malignant solid tumors | 2019 | I | Recruiting | NCT03932565 |
| CAR T cells | Anti-CD19 CAR T cells overexpressing CCL19-IL7 | B-cell lymphoma | 2019 | II | Unknown | NCT03929107 |
| CAR T cells | Anti-CD19 CAR T cells overexpressing CCL19-IL7 and PD1 monoclonal antibody | DLBCL | 2020 | I | Recruiting | NCT04381741 |
| CAR T cells | Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | EGFR + advanced solid tumors | 2017 | I,II | Unknown | NCT03182816 |
| Cytokine-mediated inflammation | ||||||
| CAR T cells | IL-12 armored anti-Nectin4 CAR T cells | Nectin-4 + malignant solid tumors | 2019 | I | Recruiting | NCT03932565 |
| CAR T cells | IL-12 armored anti-MUC16 CAR T cells | MUC16 + solid tumors | 2015 | I | Active, not recruiting | NCT02498912 |
| CAR T cells | IL-12 armored anti-EGFR CAR T cells | Metastatic CRC | 2018 | I | Unknown | NCT03542799 |
| CAR T cells | IL15 armored anti-GPC3 CAR T cells | GPC3 + solid tumors | 2021 | I | Recruiting | NCT04377932 |
| CAR T cells | IL15, IL21 armored anti-GPC3 CAR T cells | GPC3 + solid tumors | 2023 | I | Not yet recruiting | NCT04715191 |
| CAR T cells | IL15 armored anti-GPC3 CAR T cells | HCC | 2021 | I | Recruiting | NCT05103631 |
| CAR T cells | IL15 armored anti-GD2 CAR T cells | Neuroblastoma, osteosarcoma | 2019 | I | Recruiting | NCT03721068 |
| CAR T cells | IL18 armored anti-CD19 CAR T cells | NHL, CLL | 2021 | I | Recruiting | NCT04684563 |
| Targeting immunosuppressive axes | ||||||
| CAR T cells | Anti- CD123 CAR T cells | Refractory AML | 2015 | I | Terminated | NCT02623582 |
| CAR T cells | Anti- CD123 CAR T cells | AML | 2019 | I | Withdrawn | NCT04106076 |
| CAR T cells | Anti- CD123 CAR T cells | BPDCN | 2017 | I | Terminated | NCT03203369 |
| CAR T cells | Anti- CD123 CAR T cells | AL, AML | 2019 | I | Terminated | NCT03672851 |
| CAR T cells | Anti- CD123 CAR T cells | Refractory AML | 2018 | Terminated | NCT03473457 | |
| CAR NK cells | Anti-NKG2D CAR NK cells | Solid tumors | 2018 | I | Unknown | NCT03415100 |
| CAR NK cells | Anti-NKG2D CAR NK cells | Refractory metastatic CRC | 2022 | I | Recruiting | NCT05213195 |
| CAR NK cells | Anti-NKG2D CAR NK cells | Relapsed or refractory AML | 2022 | I | Recruiting | NCT05247957 |
| Physical barriers | ||||||
| CAR T cells | Anti-FAP CAR T cells | MPM | 2015 | I | Completed | NCT01722149 |
| CAR T cells | FAP/Nectin-4 CAR T cell | Nectin-4 + malignant solid tumors | 2019 | I | Recruiting | NCT03932565 |
| CAR T cells | Anti-VEGFR-2 CAR T cell + cyclophosphamide, aldesleukin, fludarabine | Metastatic cancer, metastatic melanoma, renal cancer | 2010 | I,II | Terminated | NCT01218867 |
| Myeloid CARs | ||||||
| CAR macrophage | Anti-HER2 + CAR macrophages | Breast cancer | 2021 | I | Not yet recruiting | NCT05007379 |
| CAR macrophage | Anti-HER2 + CAR macrophages | HER2 + solid tumors | 2021 | I | Recruiting | NCT04660929 |
Increasing intratumoral immune infiltration
Chemokines are mediators signaling for directed migration towards the source they originate from (a concept also known as chemotaxis). Chemokines orchestrate the localization of cells within the body and accordingly play a crucial role in the trafficking of immunosuppressive cells to the TME [49, 50]. However, they are also involved in intratumoral migration of proinflammatory and anti-tumoral immune cells such as CD1c + dendritic cells, T cells, and NK cells. In such situations, infiltration of these populations within the TME has been strongly correlated with better cancer patient prognosis [51–53]. We previously reviewed the role and therapeutic utilization of chemokines in cancer immunotherapy [50]. Based on such observations, infiltration of therapeutic immune cells can either be increased by engineering therapeutic cells for desired chemokine expression or by introducing chemokine receptors matching intratumoral chemokine gradients. The later does not impact the TME directly and thus is not part of this review, but a current summary can be found here [50].
Chemokine engineering into therapeutic immune cells has been incorporated into cellular therapies with the goal of increasing the migration of both endogenous inflammatory cells and also adoptively transferred cells into the tumor (Fig. 2A). The chemokine CCL21 was adenovirally engineered to be secreted from an anti-tumor DC vaccine to elicit signaling through CCR7 expressed on endogenous dendritic and T cells. As the DC vaccine is injected intratumorally, this establishes a gradient of CCL21 that promotes infiltration of those endogenous cells into the tumor, which has been shown to increase synergistic anti-tumor effects between adoptive and endogenous cells [54]. This approach has led to clinical trials in melanoma (NCT00798629), and in non-small cell lung cancer (NCT01574222) both as monotherapy and in combination with pembrolizumab (NCT03546361). The first results from these studies demonstrated increased immune infiltration and hints towards a modest increase in survival [50, 55]. Along the same lines, CAR-T cells have also been adapted to exploit chemokine signaling axes. While CAR-T cells are injected intravenously, the extracellular domain of the CAR construct confers an antigen-specific binding capacity that anchors the CAR-T cell within antigen-positive tumors [9]. Preclinically, CAR-T cells against CLDN18.2 were enhanced to overexpress CCL21 and IL-7 [56], with CCL21 here serving the same purpose of enhancing intratumoral migration of DC and T cells [50, 56]. In another approach, CAR-T cells have been enhanced by dual overexpression of CCL19 and IL-7, with CCL19 here being another ligand of CCR7 which leads to increased migration of endogenous immune cells into the tumor. CCL19-IL7 overexpression has been utilized in multiple CAR-T cells in numerous clinical trials: a phase I trial utilizing anti-GPC3 CARs against hepatocellular carcinoma (NCT03198546), a phase I trial utilizing multiple CARs against relapsed-refractory multiple myeloma (NCT03778346), a phase I trial utilizing anti-Nectin-4 CAR against Nectin-4-positive advanced-stage solid tumors (NCT03932565), a phase II trial utilizing CD19 CARs against B cell lymphomas (NCT03929107), and a phase I trial utilizing CD19 CARs in combination with tislelizumab against B cell lymphomas (NCT04381741) [50, 57, 58]. For the trials having reported data, safety of the approach was demonstrated along with early signs of activity [50].
Another related strategy to increase immune infiltration is endogenous activation and proliferation, ultimately leading to higher anti-tumor effector cell numbers at the tumor site. In this sense, T cells can be genetically engineered to express immune checkpoint inhibitors, with anti-PD-1 or anti-PD-L1 antibodies being the most utilized avenue [59–61]. Anti-PD-1 antibodies typically lead to an increased activation and expansion of CAR and endogenous T cells, thereby mediate an enhanced cytolytic activity [59]. Along these lines, CAR-T cells secreting anti-PD-L1 Ig1 isotype antibodies capable of mediating ADCC simultaneously increased the amount of tumor-infiltrating NK cells [61]. Similarly, CAR-T cells secreting nanobodies targeting the prominent “don’t eat me” signal CD47 induced enhanced infiltration and activation of macrophages in the tumor with limited systemic toxicity [62]. So far, only anti-CTLA-4- and anti-PD-1-secreting EGFR-specific CARs were tested in a phases I and II clinical trial in patients with EGFR + advanced solid tumors (NCT03182816). In total, strategies to equip cellular therapies to additionally increase migration of beneficial immune cells into the TME significantly increase their efficacy. While promising, other suppressive mechanisms of the TME remains relatively functional, necessitating further strategies to address them to further enable effective therapy.
Cytokine-mediated inflammation of the TME
The TME is typically rich in cytokines such as IL-1, favoring expansion and polarization of immune suppressive cell populations of myeloid and lymphoid origin [63]. In contrast, proinflammatory or T cell supporting cytokines are either absent or scavenged by immune suppressive populations. Several previously mentioned cellular therapy concepts that mediate migration through chemokine modulation also synergistically employ cytokine co-expression, such as the CCL19-IL7 and CCL21-IL7 CAR where IL-7 promotes proliferation and survival of T cells [57, 58]. These represent only a fraction of the cellular therapies that aim to modulate levels of cytokines in the tumor environment, thereby either enhancing the function of the cellular therapies themselves or creating a more permissive TME for their efficacy (Fig. 2B). Many concepts aim to directly increase levels of cytokines that enhance T cell function. T cells redirected for antigen-unrestricted cytokine-initiated killing (TRUCK) are engineered to express cytokines under the control of an NFAT or similar promoter which are activated upon CAR/TCR engagement [64]. “Armored” CARs in contrast constitutively express these cytokines for permanent enhanced cellular function [65, 66]. Though not exhaustively listed due to the magnitude of research, examples of cytokines targeted for co-expression on these cellular therapies include IL-7 [67], IL-12 [66, 68–70], IL-15 [71–73], IL-18 [74–76], IL-21 [77, 78], IL-23 [79], IL-24 [80], IL-33 [81], and IL-36 g [82]. These cytokines have a range of effects that include signaling T-cell survival, improving T-cell proliferation, and promoting their differentiation into further subtypes. CAR-T cells overexpressing these cytokines thus demonstrate a range of augmented functions including enhanced tumor killing, enhanced secretion of secondary cytokines, improved survival and proliferation, and resistance to immune suppression. In addition, effects of the cytokine modulation on the TME include downregulation of T-regs, reprogramming of TAMs and MDSCs from immune suppression to tumor engagement, and activation of endogenous immune cells [68, 76, 83]. In terms of development, several cytokine-overexpressing constructs have progressed to clinical trials. IL-12 engineering in anti-Nectin-4 CAR-T cells has progressed to a phase I clinical trial against Nectin-4 + solid tumors (NCT03932565), as well as with anti-MUC16ecto CAR-T cells against ovarian cancers (NCT02498912), and with anti-EGFR CAR-T cells against metastatic colorectal cancer (NCT03542799), all ongoing. Furthermore, IL-15 and IL-21 armored anti-GPC3 CAR-T cells have progressed to several ongoing phase I clinical trials against GPC3 + pediatric solid tumors (NCT04377932, NCT04715191), hepatocellular carcinoma (NCT05103631), neuroblastoma (NCT03721068), and osteosarcoma (NCT03721068). Moreover, IL-18 overexpressing anti-CD19 CAR-T cells are currently utilized in a phase I trial against non-Hodgkin lymphoma and chronic lymphocytic leukemia (NCT04684563). Overall, cellular therapy concepts engineered to induce cytokine-mediated inflammation via overexpression can affect broad ranging TME shifts and demonstrate encouraging improvements over their current clinical form. However, cytokines alone may not suffice to overcome immune suppression in the TME, which might require dedicated targeting.
Targeting immunosuppressive axes in the TME
The aforementioned strategies remodeling the cytokine environment in the TME indirectly affect TAMs, MDSCs, and T-regs by antagonizing their suppressiveness or reversing their suppression program. However, other cellular therapy approaches aim to directly target these immunosuppressive populations for elimination or remodeling (Fig. 2C). For example, CAR-T cells against CD123, target both Hodgkin lymphoma (HL) cells as well as TAM that prominently present in the tumor microenvironment of that indication, as similar expression is found on both cell populations [84]. These CAR-T cells recognize and kill both HL cells and TAMs, which leads to resistance against suppression, and sustained clearance in in vivo models [84]. Targeting of CD123 thus advanced to phase I clinical trials. Interestingly, many trials seem to have been stopped for non-clinical reasons with unclear results (NCT02623582, NCT04106076, NCT03203369), while one did not achieve expected therapeutic effects (NCT03473457). Another trial was terminated for adverse effects (NCT03672851). Further approaches feature T cells and NK-92 cells (an immortalized natural killer cell line that can be employed for therapy) engineered with a CAR against colony-stimulating factor 1 receptor (CSF1R), a receptor expressed on both TAMs in the tumor microenvironment and M2 macrophages. Albeit promising, the study remains in the early proof-of-concept phase [85]. A similar approach utilizing CAR-T cells targets folate receptor β (FRβ) on TAMs of ovarian cancer. These CAR-T cells eliminate TAMs in the TME and led to an increase in endogenous immune cells and prolonged survival of mice [86]. Of particular interest, these cells enabled the enhancement of a secondary tumor targeting anti-MSLN CAR-T cells in further studies. Clinical translation of the concept is pending as well. MDSCs, another immunosuppressive population in the TME, can be also targeted by cellular immunotherapy. Tumor necrosis factor-related apoptosis induced ligand-receptor 2 (TR2) expression on MDSCs can trigger apoptosis when bound to its ligand TRAIL. To exploit this axis, a chimeric costimulatory receptor consisting extracellularly of the scFv of a TR2 agonist antibody and intracellular 4-1BB was co-expressed on anti-Muc1 CAR-T cells. There, it converts normal suppressive CAR-T cell interactions with MDSCs into apoptotic signals for the MDSCs and a costimulatory signal for CAR-T cells [87]. When utilized against an in vivo breast tumor model with additional exogenous MDSCs, the construct enhanced Muc1 CAR anti-tumor activity despite the presence of immunosuppressive MDSCs [87]. Similarly, a novel NKG2D-CD3z chimeric activating receptor was engineered into NK cells, which then targeted NKG2D on MDSCs [88]. In MDSC-negative models, the construct delayed tumor growth only modestly. However, the NKG2D-based construct induced significant anti-tumor activity and displayed promising synergy with CAR-T cells in models with exogenous MDSCs [88]. While most of the aforementioned MDSC-targeting concepts have not yet advanced to the clinical trial stage, NKG2D-targeting NK cells have advanced to clinical trials targeting metastatic solid tumors (NCT03415100), metastatic, refractory colorectal cancer (NCT05213195), and relapsed, refractory AML (NCT05247957), all currently in the recruitment phase. It should be noted however, that these mentioned NKG2D-targeting NK trials were initiated for NKG2D expression on target tumor cells, though effective function on intratumoral MDSCs may very well influence trial results. Finally, T-regs can also be targeted by cellular immunotherapies in efforts to reduce the immunosuppressiveness of the TME. An anti-CD25 CAR was employed in NK-92 cells to target intratumoral T-regs in preclinical studies [89]. As CD25 is highly expressed on endogenous T-regs and activated T cells, this concept instead incorporated NK cells to reduce predicted on-target, off-tumor CAR-T cell-induced toxicity [89]. Though the anti-tumor function of these CAR-NK cells was promising, no conclusive result on increased safety and reduced toxicity was determined. Ultimately, targeting intratumoral suppressive cells has shown to be an efficacious approach, though special care needs to be taken to achieve effective targeting without inducing harmful off-tumor adverse effects.
Removing physical barriers to cellular immunotherapy
Significant obstacles of cellular immunotherapy in solid tumors do not only arise from immunosuppressive cell populations and a lack of immune cell activation within the TME but are additionally characterized by the establishment of CAF-mediated tumor stroma as a physical barrier, diminishing cellular therapy effectiveness. Along these lines, therapy can be enhanced if either the cellular components creating stroma are depleted or acellular stromal components are digested (Fig. 2D). Fibroblast activation protein (FAP), a transmembrane serine protease/type 2 dipeptidyl peptidase, has been shown to be one of the preponderantly expressed surface markers of most CAF populations and correlates with poor clinical outcome in various carcinomas [90, 91]. With this rationale, anti-FAP CAR-T cells were developed to target CAFs, with multiple concepts aiming to treat malignant pleural mesothelioma (MPM) in human and murine models [92, 93]. These efforts advanced into a now-completed phase I clinical trial against MPM, demonstrating good safety data and encouraging efficacy (NCT01722149) [94]. Similarly, pre-clinical studies on dual therapy with anti-FAP CAR targeting CAFs and anti-Ephedrin A2 (EphA2) targeting human lung cancer demonstrated synergy otherwise not achieved with either therapy alone [93]. Dual therapy is also being attempted in an ongoing clinical trial with CARs targeting both FAP and Nectin-4 in Nectin-4-positive malignant solid tumors (NCT03932565). It should be noted that FAP targeting does show discordant results in terms of toxicity with reports of severe cachexia and bone marrow hypoplasia in mice upon FAP-directed CAR-T cell administration, thus urging caution in clinical trials [95].
A different strategy to surmount the desmoplastic stroma of the TME and enable superior immune cell infiltration is the direct targeting of fibrous proteins, glycosaminoglycans, and proteoglycans composing the ECM via matrix-degrading components. Heparanase (HPSE) is an enzyme that decomposes heparan sulfate proteoglycans and has been found to be insufficiently expressed in CAR-T cells, thereby limiting their capacity to infiltrate stroma-rich tumors [96]. Equipping anti-GD2 CAR-T cells with HPSE enabled superior ability to degrade the ECM, resulting in increased T cell infiltration and antitumor efficacy in human neuroblastoma and melanoma models [96]. Another concept engineered anti-mesothelin CAR-T cells to secrete the hyaluronidase PH20. PH20-expressing CAR-T cells degraded hyaluronic acid within the ECM and resulted in enhanced transmigratory efficacy in vitro and diminished tumor growth in two different xenograft gastric cancer models in mice [97]. Despite possible hints at toxicity with specifically targeting FAP, targeting the CAF-mediated stroma as a whole remains a viable approach being tested preclinically and clinically both as standalone therapy and in combination with other tumor-targeting therapies.
Targeting aberrant tumor vasculature
Various strategies have also been developed to address the secondary issue of physical tumor-exclusion—aberrant and dysfunctional vasculature that leads to poor T cell infiltration (Fig. 2E). One strategy conceived to address this are CAR-T cells engineered to target VEGFR-1, a receptor expressed on both tumor cells and tumor vasculature. These cells achieved both slowed tumor progression and inhibition of neo-angiogenesis in human models of metastatic lung cancer [98]. Another concept transducing T cells to co-express both an anti-VEGFR-2 CAR and inducible IL-12 augmented the tumor clearance in multiple in vivo models by targeting tumor vasculature as well as decreasing VEGFR-2-expressing MDSC subsets [99]. Despite promising preclinical data, the first clinical trials with anti-VEGFR-2 CAR-T cells in various metastatic cancers have yet to show robust efficacy, with only one patient out of 24 experiencing a partial response (NCT01218867). Additional targets, such as TEM8 have been utilized to further evaluate vasculature-directed CAR-T cell therapy in patient-derived xenograft and pulmonary metastatic triple-negative breast cancer (TNBC) cell line-derived xenograft models, resulting not only in ECM clearance and inhibition of neovascularization, but also suppression of associated breast cancer stem cells [100]. The selection of CLEC14A in models of pancreatic and lung carcinomas, as well as the human prostate-specific membrane antigen (PSMA), expressed on malignant vasculature of various tumors or the neoangiogenesis-associated αvβ3 integrin, demonstrated further promising antigen options for future CAR T-cell constructs [101–103]. Lastly, the use of nanobody-based CAR-T cell therapy with the variable domain of the heavy chain-only antibody directed against EIIIB, a fibronectin splice variant found in tumors and during angiogenesis, has proven to inhibit tumor growth in melanoma- and colon carcinoma-bearing mice [104]. These studies point to the promising future of targeting tumor vasculature, though clinical efficacy of these approaches has yet to be fully demonstrated.
Myeloid CAR for broad tumor and TME targeting
Recent efforts to remodel the tumor microenvironment with cellular therapies have advanced with the advent of engineering CARs or CAR-like receptors on myeloid lineage cells, notably on macrophages and in some instances dendritic cells (Fig. 2F). Traditionally associated with the innate immune system, these macrophages and dendritic cells are known to be prominent antigen presenting cells (APC) at the forefront of infection and cancer surveillance [105–107]. During the course of infection and other aberrant stimuli, these cells sense pathogen-derived proteins and molecules, and their resulting activation leads to the mobilization of an immune response, the release of inflammatory cytokines including type I interferons (IFN), and presentation of foreign antigen [108]. In the cancer setting, the TME of many cancer types features this type I IFN signature from APCs [109]. Typically, however, tumors adversely suppress this immune response via expression of “don’t eat me” signals, immunosuppressive cytokines, and in the case of TAMs, reprogramming of this population of cells towards pro-tumorigenic function, leading to escape from tumor immune surveillance [28, 110]. To hijack this tumor suppression function, chimeric antigen receptors for phagocytosis (CAR-P) have been proposed. These CAR-like receptors encompass antigen-specific scFv extracellularly and either the multiple EGF-like-domains 10 (Megf10) intracellular domain or the common ɣ subunit of Fc receptors (FcRɣ). CAR-Ps can be engineered onto human monocyte-derived macrophages (MDM) for antigen-specific phagocytosis of target cells and inflammation [111]. Another concept engineering MDMs with a first-generation CAR targeting HER-2 has similar potent phagocytotic anti-tumor activity against breast cancer. These CAR-macrophages (CAR-M) maintain an inflammatory M1 phenotype intratumorally, and further demonstrate therapy-induced remodeling of the TME with increased expression of pro-inflammatory markers on intratumoral immune populations in humanized murine models [112]. CAR-Ms have proceeded to two ongoing clinical trials in patients suffering from HER2 + breast cancer (NCT05007379) and HER2 + solid tumors (NCT04660929). Both CAR-Ps and CAR-Ms demonstrated the capacity to cross-present antigen on MHC-I, adding synergistic potential for these cells to further mobilize an endogenous CD8 T cell-mediated immune response against tumors [111, 112]. CAR engineering in macrophages can be further augmented by addition of matrix metalloproteinases (MMP) for increased ECM degradation. Though macrophages are endogenously a significant source of MMPs, these engineered macrophages demonstrated enhanced inhibition of tumor growth and led to elevated T cell infiltration in human breast cancer models [113]. While myeloid CAR has demonstrated promising results, questions remain over intratumoral persistence as well as the stability of the M1 phenotype, the loss of which may instead cause the therapy to promote tumor growth [114].
Conclusions and future perspectives
While cellular therapies have come a long way to become an established method of cancer treatment, their future potential for increased efficacy in hematological malignancies and even meaningful efficacy in solid tumor indications largely relies on their ability to address the obstacles posed by the TME. This review highlights the current considerable efforts that have been made to improve various aspects of cellular therapies to reshape the TME to become more permissible for treatment: novel concepts that target immunosuppressive cell populations, inflame an immune-restricted tumor, and address chemotactic signaling and physical barriers excluding immune infiltration. The improvements made on these therapies have been shown to lead to superior anti-tumor efficacy compared to more standard cellular therapies. Moreover, many cellular therapies covered here that target the TME as an entity have been combined with other treatments that primarily target the tumor, forming a dual-pronged targeting approach that in many cases shows synergistic efficacy. Additionally, concepts incorporating combinatorial improvements that address multiple TME obstructions show further enhanced function, demonstrating additionally the promising and synergistic nature of reshaping the tumor microenvironment, and hints at robust effects for combinatorial treatments with existing therapies such as ICB. Promising pre-clinical and clinical results of many of the therapies listed bode well for the application of cellular immunotherapies for the treatment of both hematological and solid tumors in the future.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the Marie-Sklodowska-Curie Program Training Network for Optimizing Adoptive T Cell Therapy of Cancer funded by the H2020 Program of the European Union (Grant 955575, to S.K.); by the Hector Foundation (to S.K.); by the International Doctoral Program i-Target: Immunotargeting of Cancer funded by the Elite Network of Bavaria (to S.K. and S.E.); by Melanoma Research Alliance Grants 409510 (to S.K.); by the Else Kröner-Fresenius-Stiftung (to S.K.); by the German Cancer Aid (to S.K.); by the Ernst-Jung-Stiftung (to S.K.); by the LMU Munich’s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (to S.E. and S.K.); by the Go-Bio initiative (S.K.); by the Bundesministerium für Bildung und Forschung (S.K.); by the European Research Council Grant 756017, ARMOR-T (to S.K.); by the German Research Foundation (DFG) (to S.K.); by the SFB-TRR 338/1 2021–452881907 (to S.K.); by the Fritz-Bender Foundation (to S.K.); by the Wilhelm-Sander-Stiftung (to S.K.); and by the Deutsche José-Carreras Leukämie Stiftung (to S.K.). Figures were created with BioRender.com.
Declarations
Conflict of interest
S.K. has received honoraria from TCR2 Inc., Novartis, BMS, and GSK. S.K. and S.E. are inventors of several patents in the field of immuno-oncology. S.K. and S.E. received license fees from TCR2 Inc. and Carina Biotech. S.K. and S.E. received research support from TCR2 Inc., Arcus Bioscience, and Tabby Therapeutics for work unrelated to the manuscript. All remaining authors have no conflicts of interest to declare.
Footnotes
This article is a contribution to the special issue on Novel immunotherapeutic combinations moving forward: the modulation of the immunosuppressive microenvironment - Guest Editor: Mads Hald Andersen
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geiger JD, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression1. Can Res. 2001;61(23):8513–8519. [PubMed] [Google Scholar]
- 2.Dohnal AM, et al. Phase I study of tumor Ag-loaded IL-12 secreting semi-mature DC for the treatment of pediatric cancer. Cytotherapy. 2007;9(8):755–770. doi: 10.1080/14653240701589221. [DOI] [PubMed] [Google Scholar]
- 3.Lasky JL, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res. 2013;33(5):2047. [PMC free article] [PubMed] [Google Scholar]
- 4.Wooster AL, et al. Dendritic cell vaccine therapy for colorectal cancer. Pharmacol Res. 2021;164:105374. doi: 10.1016/j.phrs.2020.105374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland SIM et al (2021) Moving on from Sipuleucel-T: new dendritic cell vaccine strategies for prostate cancer. Front Immunol 12:641307. 10.3389/fimmu.2021.641307 [DOI] [PMC free article] [PubMed]
- 6.Zhao L, Cao YJ (2019) Engineered T cell therapy for cancer in the clinic. Front Immunol 10:2250. 10.3389/fimmu.2019.02250 [DOI] [PMC free article] [PubMed]
- 7.Rohaan MW, et al. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6(1):102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Wang L. The emerging world of TCR-T cell trials against cancer: a systematic review. Technol Cancer Res Treat. 2019;18:1533033819831068. doi: 10.1177/1533033819831068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, et al. Engineering CAR-T cells. Biomark Res. 2017;5(1):22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaissmaier L, Elshiaty M, Christopoulos P (2020) Breaking bottlenecks for the TCR therapy of cancer. Cells 9(9):2095. 10.3390/cells9092095 [DOI] [PMC free article] [PubMed]
- 11.CAR T Cells: Engineering patients’ immune cells to treat their cancers. 2022 March 10, 2022 [cited 2022 July 26, 2022]; Available from: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- 12.Xie G, et al. CAR-NK cells: a promising cellular immunotherapy for cancer. eBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capsomidis A, et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther. 2018;26(2):354–365. doi: 10.1016/j.ymthe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher J, Anderson J. engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol. 2018;9:1409. doi: 10.3389/fimmu.2018.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.June Carl H, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 16.Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol. 2019;37(S1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 17.D’Aloia MM, et al. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9(3):282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newick K, et al. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68(1):139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 19.Lesch S, et al. Determinants of response and resistance to CAR T cell therapy. Semin Cancer Biol. 2020;65:80–90. doi: 10.1016/j.semcancer.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhary B, Elkord E (2016) Regulatory T Cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines 4(3):28. 10.3390/vaccines4030028 [DOI] [PMC free article] [PubMed]
- 22.Kumar V, et al. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singhal S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 2019;11(479):eaat1500. doi: 10.1126/scitranslmed.aat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komi DEA, Redegeld FA. Role of mast cells in shaping the tumor microenvironment. Clin Rev Allergy Immunol. 2020;58(3):313–325. doi: 10.1007/s12016-019-08753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37(1):41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atiya H et al (2020) Mesenchymal stem cells in the tumor microenvironment. In: Birbrair A (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1234. Springer, Cham [DOI] [PubMed]
- 27.Landskron G, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee HN et al (2021) Efferocytosis and the story of “Find Me,” “Eat Me,” and “Don’t Eat Me” signaling in the tumor microenvironment. In: Birbrair A (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1329. Springer, Cham [DOI] [PubMed]
- 29.Takimoto CH, et al. The macrophage & #x2018;Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol. 2019;30(3):486–489. doi: 10.1093/annonc/mdz006. [DOI] [PubMed] [Google Scholar]
- 30.Bradley CA. CD24 — a novel ‘don’t eat me’ signal. Nat Rev Cancer. 2019;19(10):541–541. doi: 10.1038/s41568-019-0193-x. [DOI] [PubMed] [Google Scholar]
- 31.Ganguly D et al (2020) Cancer-associated fibroblasts: versatile players in the tumor microenvironment. Cancers 12(9):2652. 10.3390/cancers12092652 [DOI] [PMC free article] [PubMed]
- 32.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010;15(1):166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discovery. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 35.Fiori ME, et al. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santi A, Kugeratski FG, Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. 2018;18(5–6):e1700167. doi: 10.1002/pmic.201700167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101(4):805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 39.Sebens S, Schafer H. The tumor stroma as mediator of drug resistance - a potential target to improve cancer therapy? Curr Pharm Biotechnol. 2012;13(11):2259–2272. doi: 10.2174/138920112802501999. [DOI] [PubMed] [Google Scholar]
- 40.Chi J-Y, et al. Targeting chemotherapy-induced PTX3 in tumor stroma to prevent the progression of drug-resistant cancers. Oncotarget. 2015;6(27):23987–24001. doi: 10.18632/oncotarget.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 42.Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: lessons learned and future directions. Pharmacol Ther. 2020;205:107419. doi: 10.1016/j.pharmthera.2019.107419. [DOI] [PubMed] [Google Scholar]
- 43.Fu R, et al. Delivery techniques for enhancing CAR T cell therapy against solid tumors. Adv Func Mater. 2021;31(44):2009489. doi: 10.1002/adfm.202009489. [DOI] [Google Scholar]
- 44.Stock S et al (2022) Enhanced chimeric antigen receptor T cell therapy through co-application of synergistic combination partners. Biomedicines 10(2):307. 10.3390/biomedicines10020307 [DOI] [PMC free article] [PubMed]
- 45.Gkretsi V et al (2015) Remodeling components of the tumor microenvironment to enhance cancer therapy. Front Oncol 5:214. 10.3389/fonc.2015.00214 [DOI] [PMC free article] [PubMed]
- 46.Duan Q, et al. Turning cold into hot: firing up the tumor microenvironment. Trends in Cancer. 2020;6(7):605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Haanen JBAG. Converting cold into hot tumors by combining immunotherapies. Cell. 2017;170(6):1055–1056. doi: 10.1016/j.cell.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 48.Wargo JA, et al. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol. 2016;41:23–31. doi: 10.1016/j.coi.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohli K, Pillarisetty VG, Kim TS. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022;29(1):10–21. doi: 10.1038/s41417-021-00303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Märkl F, et al. Utilizing chemokines in cancer immunotherapy. Trends in Cancer. 2022 doi: 10.1016/j.trecan.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med. 2011;236(5):567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther. 2015;22(4):198–206. doi: 10.1038/cgt.2015.7. [DOI] [PubMed] [Google Scholar]
- 54.Yang S-C, et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10(8):2891–2901. doi: 10.1158/1078-0432.CCR-03-0380. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, et al. Phase I trial of intratumoral injection of CCL21 gene–modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8+ T-cell infiltration. Clin Cancer Res. 2017;23(16):4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo H, et al. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin Cancer Res. 2020;26(20):5494–5505. doi: 10.1158/1078-0432.CCR-20-0777. [DOI] [PubMed] [Google Scholar]
- 57.Adachi K, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 58.Goto S, et al. Enhanced anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells in orthotopic and patient-derived xenograft tumor models. Cancer Immunol Immunother. 2021;70(9):2503–2515. doi: 10.1007/s00262-021-02853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafiq S, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, et al. Enhanced cancer immunotherapy by chimeric antigen receptor–modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res. 2017;23(22):6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 61.Suarez ER, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie YJ, et al. Improved antitumor efficacy of chimeric antigen receptor T cells that secrete single-domain antibody fragments. Cancer Immunol Res. 2020;8(4):518–529. doi: 10.1158/2326-6066.CIR-19-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briukhovetska D, et al. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21(8):481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 65.Hawkins ER, D’Souza RR, Klampatsa A. Armored CAR T-cells: the next chapter in T-cell cancer immunotherapy. Biologics. 2021;15:95–105. doi: 10.2147/BTT.S291768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeku OO, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7(1):10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golumba-Nagy V, et al. CD28-ζ CAR T cells resist TGF-β repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol Ther. 2018;26(9):2218–2230. doi: 10.1016/j.ymthe.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pegram HJ, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koneru M, et al. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koneru M, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13(1):102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, et al. Eradication of neuroblastoma by T cells redirected with an Optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res. 2019;25(9):2915–2924. doi: 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 72.Krenciute G, et al. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol Res. 2017;5(7):571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu B, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20(13):3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chmielewski M, Abken H. CAR T cells releasing IL-18 convert to T-Bet<sup>high</sup> FoxO1<sup>low</sup> effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 2017;21(11):3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 76.Avanzi MP, et al. Engineered tumor-targeted t cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 2018;23(7):2130–2141. doi: 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Štach M, et al. Inducible secretion of IL-21 augments anti-tumor activity of piggyBac-manufactured chimeric antigen receptor T cells. Cytotherapy. 2020;22(12):744–754. doi: 10.1016/j.jcyt.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Steffin DHM, et al. A phase I clinical trial using armored GPC3 CAR T cells for children with relapsed/refractory liver tumors. J Clin Oncol. 2019;37(15_suppl):TPS2647–TPS2647. doi: 10.1200/JCO.2019.37.15_suppl.TPS2647. [DOI] [Google Scholar]
- 79.Ma X, et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat Biotechnol. 2020;38(4):448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu Q, et al. IL-24 armored CAR19-T cells show enhanced antitumor activity and persistence. Signal Transduct Target Ther. 2021;6(1):14. doi: 10.1038/s41392-020-00380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y, Lu B. Guided delivery of the “alarming” cytokine IL-33 to tumor by chimeric antigen receptor T cells. J Immunol. 2017;198(1):204.23. doi: 10.4049/jimmunol.198.Supp.204.23. [DOI] [Google Scholar]
- 82.Li X, et al. Cytokine IL-36gamma improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia. 2021;35(2):506–521. doi: 10.1038/s41375-020-0874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chmielewski M, et al. IL-12 Release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Can Res. 2011;71(17):5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 84.Ruella M, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7(10):1154–1167. doi: 10.1158/2159-8290.CD-16-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang P, et al. Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy. 2018;10(11):935–949. doi: 10.2217/imt-2018-0012. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez-Garcia A, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12(1):877. doi: 10.1038/s41467-021-20893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nalawade SA, et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9(11):e003237. doi: 10.1136/jitc-2021-003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parihar R, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7(3):363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dehbashi M, et al. A novel CAR expressing NK cell targeting CD25 with the prospect of overcoming immune escape mechanism in cancers. Front Oncol. 2021;11:649710. doi: 10.3389/fonc.2021.649710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen SJ et al (2008) Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 37(2):154–158. 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed]
- 91.Lo A et al (2017) Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight 2(19):e92232. 10.1172/jci.insight.92232 [DOI] [PMC free article] [PubMed]
- 92.Schuberth PC, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med. 2013;11:187. doi: 10.1186/1479-5876-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kakarla S, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21(8):1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curioni A, et al. 1226P - A phase I clinical trial of malignant pleural mesothelioma treated with locally delivered autologous anti-FAP-targeted CAR T-cells. Ann Oncol. 2019;30:v501. doi: 10.1093/annonc/mdz253.052. [DOI] [Google Scholar]
- 95.Tran E, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210(6):1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caruana I, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao R et al (2021) Human hyaluronidase PH20 potentiates the antitumor activities of mesothelin-specific CAR-T cells against gastric cancer. Front Immunol 12:660488. 10.3389/fimmu.2021.660488 [DOI] [PMC free article] [PubMed]
- 98.Wang W, et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 2013;20(10):970–978. doi: 10.1038/gt.2013.19. [DOI] [PubMed] [Google Scholar]
- 99.Chinnasamy D, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Investig. 2010;120(11):3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Byrd TT, et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Can Res. 2018;78(2):489–500. doi: 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu X, et al. Genetically modified T cells targeting neovasculature efficiently destroy tumor blood vessels, shrink established solid tumors and increase nanoparticle delivery. Int J Cancer. 2013;133(10):2483–2492. doi: 10.1002/ijc.28269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santoro SP, et al. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res. 2015;3(1):68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhuang X et al (2020) CAR T cells targeting tumor endothelial marker CLEC14A inhibit tumor growth. JCI Insight 5(19):e138808. 10.1172/jci.insight.138808 [DOI] [PMC free article] [PubMed]
- 104.Xie Yushu J, et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci. 2019;116(16):7624–7631. doi: 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12(1):33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 106.Hegde S, et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell. 2020;37(3):289–307.e9. doi: 10.1016/j.ccell.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pike MC, Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a mechanism for abrogation of immune surveillance. J Immunol. 1976;117(4):1243. doi: 10.4049/jimmunol.117.4.1243. [DOI] [PubMed] [Google Scholar]
- 108.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Woo S-R, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33(1):445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 110.Kim R (2007) Chapter 2 - Cancer immunoediting: from immune surveillance to immune escape, in: Cancer immunotherapy, Prendergast GC, Jaffee EM (eds). Academic Press, Burlington, p 9–27. 10.1016/B978-012372551-6/50066-3
- 111.Morrissey MA, et al. Chimeric antigen receptors that trigger phagocytosis. eLife. 2018;7:e36688. doi: 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klichinsky M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang W, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–845. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan K, et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41(1):119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]