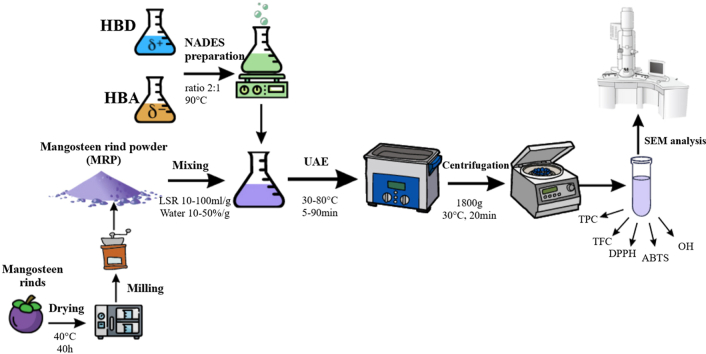
Abstract
This research combined ultrasonic-assisted extraction (UAE) and natural deep eutectic solvent (NADES) to recover phenolic and flavonoid components from mangosteen rind. The antioxidant activities were determined using DPPH, ABTS+, and hydroxyl assays. NADES prepared from lactic and 1,2–propanediol had the highest extraction efficiency based on the total flavonoid content (TFC) and phenolic contents (TPC). Single-factor experiments were employed to assess the influence of UAE conditions (liquid-to-solid ratio, temperature, water content in NADES, and time) on TFC, TPC, and antioxidant activities. NADES-based UAE conditions were optimized using response surface methodology with the Box-Behnken design model on five dependent responses (TPC, TFC, DPPH, ABTS, and OH). The optimal conditions for the lactic-1,2–Propanediol-based UAE process were 76.7 ml liquid/g solid with 30.3% of water content at 57.5 °C for 9.1 min. Scanning electron microscopy (SEM) was applied to examine the surface morphology of mangosteen rind before and after sonication. This study proposes an efficient, green, and practical approach for recovering phenolics and flavonoids from mangosteen rinds.
Keywords: Optimization, Natural deep eutectic solvent, Mangosteen rind, Ultrasonic-assisted extraction, Phenolic, Flavonoid
Graphical abstract
1. Introduction
Mangosteen (Garcinia mangostana L) is recognized as “the fruit queen” and is widely cultivated in tropical regions in Southeast Asian countries due to its delicious taste and nutritional value [1]. The mangosteen rinds, constituting approximately 60 wt% of the fruit, are usually disposed into landfills, which leads to environmental pollution and increased running costs [2]. However, they possess a diverse range of bioactive compounds, such as phenolic acids, flavonoids, xanthones, and anthocyanins, showing therapeutic potential in traditional medicine, including inflammation, fever, and skin infection treatment [2,3]. These biological substances also exhibit several pharmacological properties, such as antioxidant, anti-inflammatory, anti-tumor, antiviral, and anti-allergic effects [3]. The bioactivity of mangosteen rind extracts is determined by the solvents and extraction methods [4]; thus, a reliable way to extract bioactive compounds should be established.
Ultrasound-assisted extraction (UAE) has emerged as a promising green technology for extracting bioactive compounds from plants, gaining widespread attention in the scientific community [5]. This method can offer advantages over conventional methods, such as reducing extraction time, minimizing solvent usage, and improving yield [6]. The enhanced extraction efficiency of UAE is accounted for cavitation effect, which is produced when ultrasonic waves propagate through extractants. This phenomenon results in the formation of cavitation bubbles that implode after several compression and expansion cycles. The implosion of these bubbles creates intense turbulence, fragmentation, and surface erosion of cell walls, facilitating the mass transfer and the diffusion of solutes into solvents. Therefore, UAE is a powerful tool for enhancing the recovery of bioactive compounds from plants [7].
Among various solvents used for extraction, natural deep eutectic solvent (NADES) is an innovative class of ionic solvents composed of hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD) with specific molar ratios [8]. NADES have a lower melting point than their components due to the formation of extensive hydrogen bond networks [9]. The physicochemical properties of NADES can be tailored by adjusting the molar ratios of HBA and HBD or increasing the water content in NADES [10]. The NADES viscosity can be dropped by increasing water content; however, excessive water addition can disrupt its hydrogen bond networks. The advantageous points of NADES over conventional solvents are the ease of preparation, non-toxicity, low costs, biodegradability, and non-volatility [7]. Currently, NADES have been extensively studied for the extraction of bioactive compounds from plants and food processing waste, including foxtail millet bran [8], saffron processing waste [11], olive leaves [12], and olive pomace [13]. NADES and UAE have reciprocal interactions that enhance the recovery of bioactive components from a plant matrix [14]. Merichel Plaza et al., 2021 used NADES-based UAE to exploit the non-extractable phenolic matter from mangosteen peels. The authors reported that choline chloride-lactic acid with a molar ratio of 1:2 had the highest extraction efficiency of non-extractable phenolic compounds and proanthocyanidins [15]. Bailiang Zheng et al. used NADES-based UAE to extract phenolics from foxtail millet bran. The research found the optimized conditions and used scanning electron microscopy to demonstrate the more significant destruction of NADES-based UAE than conventional extraction [8]. However, previous studies have not examined the influence of various NADES-based UAE conditions on mangosteen rind extracts' phenolics, flavonoids, and antioxidant activity. Additionally, previous research has not found the optimal conditions to simultaneously reach the highest extraction yield of phenolics, flavonoids, and antioxidant activity; the differences in the surface morphology of mangosteen rinds with NADES-based and ethanolic-based UAE were not elucidated.
Therefore, the present study aimed to optimize the conditions of NADES-based UAE to achieve the highest extraction yield of phenolics, flavonoids, and antioxidant activities simultaneously. Furthermore, the study aimed to evaluate morphological changes in mangosteen rinds with and without sonication. The extraction performance of NADES-based UAE is compared to that of traditional solvents in extracting phenolics and flavonoids. Single-factor experiments were conducted to investigate the effect of liquid-to-solid ratio (LSR), the water content in NADES, temperature, and time on the extraction efficiency of phenolics and flavonoids from mangosteen rinds. The Box-Behnken Design (BBD) model was used to optimize the NADES-based UAE process. Scanning electron microscopy (SEM) was used to investigate changes in the surface morphology of mangosteen rind with and without sonication. By employing a systematic approach, this study can provide valuable insights into the optimal conditions of NADES-based UAE for the extraction of bioactive compounds from mangosteen rinds, which can have significant implications for reducing waste and improving the sustainability of the food industry.
2. Materials and methods
2.1. Materials
Mangosteen rinds were purchased from Nam Viet company, Tan Binh, Di An, Binh Duong, Vietnam, and milled into small pieces. These pieces were dried at 45 °C for 45 h to achieve 8% moisture content and grounded to attain mangosteen rind powder (MRP). Absolute ethanol (purity ≥99.8%), acetone (purity ≥99.5%), potassium acetate (purity ≥99.5%), sodium carbonate (purity ≥99.5%), aluminum chloride hexahydrate (purity 99%), hydrochloric acid (purity ≥36.5%), potassium acetate (purity ≥99%), potassium chloride (purity ≥99%), sodium acetate (purity ≥99.5%), 2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, purity ≥98%), 1,1-diphenyl-2-picrylhydrazyl (DPPH, purity ≥97%), Whatman Filter Papers No.1 (WHA1001325), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, purity 98%), Folin–Ciocalteu reagent (concentration 1.9–2.1 N), gallic acid monohydrate (purity ≥98%), and rutin hydrate (purity ≥94%) were acquired from Sigma-Aldrich Chemical Co., Ltd, Singapore, Singapore. The chemicals used for NADES preparation were obtained from Xilong Scientific Co Ltd, Guangdong, China.
2.2. NADES preparation and the screening of solvents
In this study, six natural deep eutectic solvents were prepared using the heating method [16]. The HBAs and HBDs were blended in an appropriate molar ratio, heated at 90 °C, and stirred using a magnetic stirrer (model: C–MAG HS 7, IKA Industrie, Humboldtstraβe, Königswinter, Germany). This process was completed when a clear and homogeneous liquid formed. The chemicals, abbreviations, and molar ratios for NADES preparation are expressed in Table 1.
Table 1.
NADES used in this research.
| No. | Abbreviation | HBD | HBA | Molar ratio |
|---|---|---|---|---|
| 1 | So-Gly | Sodium acetate | Glycerin | 1:3 |
| 2 | Ci-Gly | Citric acid | Glycerin | 2:1 |
| 3 | Lac-Gly | Lactic acid | Glycerin | 2:1 |
| 4 | Lac-Pro | Lactic acid | 1,2 – Propanediol | 2:1 |
| 5 | Lac-Glu | Lactic acid | d-Glucose | 2:1 |
| 6 | Ci-Pro | Citric acid | 1,2 – Propanediol | 2:1 |
MRP weighed 1.25 g and was dispersed into 25 ml of the prepared NADES (20% water content, g/g) in 100 ml amber glass bottles. The mixtures were sonicated using an ultrasonic bath Elmasonic (S300H, Elma Schmidbauer, Gottlieb-Daimler-Straße, Hohentwiel, Germany) with a capacity of 28 L (37 kHz, ultrasonic power 300 W, total power 1200 W) at room temperature (30 °C) for 10 min. After sonication, the mixtures were centrifuged (DM0412, DLAB Scientific Co., Ltd, Shunyi, Beijing, China) at 4000 rpm for 20 min. Ethanol solution (50%), acetone solution (50%), and distilled water were used as control solvents. The phenolics and flavonoids of the extracts were quantified.
2.3. Single-factor experiments
MRP was sonicated at different LSRs (20–100 ml/g) with varying contents of the water in NADES (10, 20, 30, 40, 50% g/g) at various temperatures (30, 40, 50, 60, 70, 80 °C) for different extraction times (5, 10, 30, 50, 70, 90 min). After sonication, the mixtures were centrifugated at 4000 rpm for 20 min to obtain extracts. DPPH, ABTS, OH, total phenolic content (TPC), and total flavonoid content (TFC) of the extracts were measured using the methods in section 2.5.
2.4. Design of experiments
Optimizing the NADES-based UAE process was performed using the BBD model. The conditional ranges of the four variables used for the optimization section were selected from the results of single-factor experiments and three levels: high (+1), mid (0), and low (−1), presenting the higher, proper, and lower conditions, respectively. The highest extraction efficiency of each dependent response in section 2.3 was obtained at the proper conditions, while higher and lower conditions were boundary conditions. Four variables were LSR, the water content in NADES, temperature, and time; the dependent responses were TPC, TFC, DPPH, ABTS, and OH. The four variables at three levels (−1, 0, +1), designed with 29 experiments with five center points, were employed to quantify the dependent responses. The correlation between the variables and dependent responses was found using a second-order polynomial model using Equation (1):
| (1) |
K0, Ki, Kii, Kj, and Kij are the intercept, linear, quadratic, and interactive regression coefficients, and m is the number of variables (k = 4). The dependent variables (Xa and Xb) and their three levels were: LSR: 70, 80, and 90 ml/g (−1, 0, 1); water content in NADES: 20, 30, and 40% (−1, 0, 1); temperature: 50, 60, and 70 °C (−1, 0, 1); time: 5, 10, and 15 min (−1, 0, 1). Dependent responses (Y) were TPC (mg GAE/g dw), TFC (mg RE/g dw), DPPH (μM TE/g dw), and ABTS+ (μM TE/g dw). The prediction error (%) was used to illustrate the differences between predicted and experimental values and presented in Equation (2).
| (2) |
2.5. Determination of the total phenolic content, total flavonoid content, and antioxidant activity
The TPC was measured using the Folin-Ciocalteu reagent, and TFC was quantified using the colorimetric method [16]. TPC was presented as gallic acid equivalent milligrams per gram of dried basis weight (mg GAE/g dw), and TFC was shown as milligrams of rutin equivalent per gram of dried weight (mg RE/g dw).
DPPH free radical-scavenging activity (DPPH) was examined using an ethanolic-DPPH solution [17]. ABTS+ free radical-scavenging activity (ABTS) was quantified using ABTS+ working solution [17] and shown as millimole Trolox equivalent per gram of dried weight (mM TE/g dw). The OH radical-scavenging capacity (OH) was quantified by the Hongjie Yuan method [18]. DPPH was expressed as micromol Trolox equivalent per gram of dry basis (μM TE/g dw), and OH was shown as millimole Trolox equivalent per gram of dry basis (mM TE/g dw).
2.6. Surface morphology of the mangosteen rind powder
The surface morphology of MRP before and after sonication was investigated using SEM (model: Prisma E SEM, ThermoFisher Scientific, Waltham, Massachusetts, America). The procedure of sample preparation was founded on the Sujata S. Patil method [19]. Samples were placed on carbon tape (to prevent sample loss) and attached to the sample plate. Then, the samples were laminated with a thin layer of gold metal. The procedure was performed under vacuum; the samples were transferred and directly screened under SEM at different magnifications and 5 kV.
2.7. Statistical analysis
All experiments were repeated three times and expressed as the mean ± SD. Analysis of variance (ANOVA) with 95% of confidence and other statistical analysis was analyzed by Minitab 19 (Minitab, Inc, Pennsylvania, USA). The BBD model was analyzed utilizing Design-Expert v.13 software (Stat-Ease Inc., Minneapolis, Minnesota, USA). Graphics were constructed by Origin Pro (Origin Lab, Northampton, Massachusetts, USA).
3. Results and discussions
3.1. Assessing the extraction efficiency of NADES
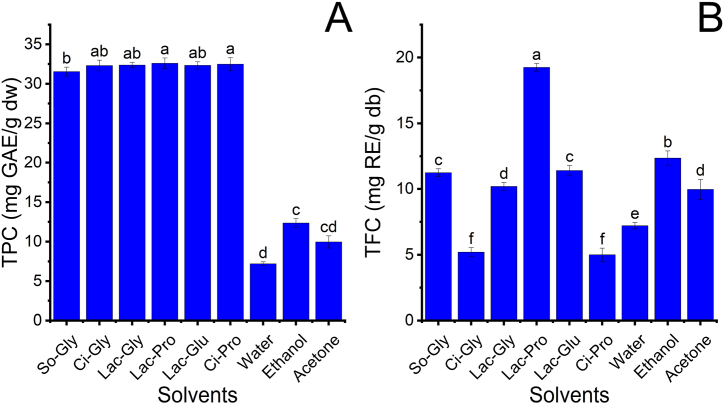
The extraction performance of NADES-based UAE was evaluated through TPC and TFC at 20 ml/g LSR, temperature 30 °C, and 20% water content for 10 min of retention time. Six NADES were synthesized by mixing hydrogen bond donors, mainly citric acid and lactic acid, with hydrogen bond acceptors glycerine, glucose, and 1,2-propanediol. The recovered TPC and TFC recovered were compared to those obtained by traditional solvents, and the results are presented in Fig. 1A–B. The performance of all NADES for extracting phenolics was approximately 33 mg GAE/g dw, which was higher than water, 50% ethanol, and 50% acetone at 7.2 ± 0.2, 12.4 ± 0.5, and 10 ± 0.8 mg GAE/g dw, respectively. Lac-Pro exhibited the highest extraction efficiency of flavonoids at 19.3 ± 0.3 mg RE/g dw, followed by 50% ethanol, So-Gly, and Lac-Glu. The highest performance of Lac-Pro can be attributed to similar polarity among Lac-Pro, phenolics, and flavonoids from MRP as well as low viscosity [7,20]. However, Ci-Pro and Ci-Gly showed the lowest TFC at 5.0 ± 0.5 and 5.2 ± 0.3 mg RE/g dw, respectively, whereas the reverse trend was true in the case of phenolics at 32.5 ± 0.8 and 32.3 ± 0.7 mg GAE/g dw in turn. Mangosteen rind flavonoids have lower polar components than other compounds due to the presence of numerous methoxyl groups [21]. The low extraction efficiency of Ci-Pro and Ci-Gly can result from their weak interaction with flavonoids because citric acid has higher polarity than lactic acid [[20], [21], [22]]. Therefore, Lac-Pro was chosen to use in the subsequent experimentation of this study.
Fig. 1.
The extraction efficiency of different solvents at 20 ml/g of LSR, 20% water content, 30 °C, and 10 min retention time; (A): TPC; (B): TFC. The same characters presented no significant statistical differences.
3.2. Effect of the liquid-to-solid ratio
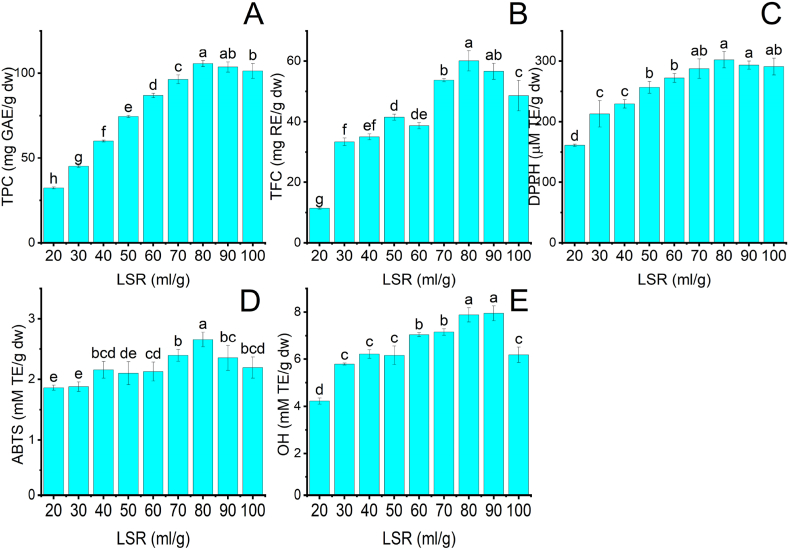
Fig. 2A–E presents the variation of TPC, TFC, and antioxidant activities in the NADES-based UAE process when LSR increased from 20 to 100 ml/g. TPC and TFC significantly increased by 3.3 and 5.3 times as the LSR rose from 20 to 80 ml/g (Fig. 2A–B). The increase in LSR can improve the contact area between Lac-Pro and MRP and decrease the viscosity of the extraction environment [8]. The reduction in viscosity enhances the cavitation effect by lowering the cavitation threshold [23]. The cavitation threshold is the minimum pressure required for initiating cavity growth in the extraction medium during the rarefaction cycles of ultrasonic waves [19]. An increase in the cavitation effect can produce more collapsing bubbles at higher intensity, generating more powerful shear force and interfacial turbulence. These phenomena can create more fragmentation and pore formation on the MRP surface, enhancing the diffusion of phenolics and flavonoids into Lac-Pro [7]. The combination of an increase in the contact area and the cavitation effect can improve the extraction efficiency of phenolics and flavonoids in MRP [24]. Nevertheless, the recovery of phenolics and flavonoids decreased to 101.3 ± 4.4 mg GAE/g dw and 48.6 ± 5.0 mg RE/g dw as LRS increased from 80 to 100 ml/g. It can be explained that the excessive cavitation effect degrades phenolics and flavonoids in MRP, decreasing extraction efficiency [25]. Rubiya Rashid et al. (2023) also experimented that when LSR increased to 30 ml/g in NADES-based UAE of phenolics from apple pomace, TPC and TFC increased to 5.8 mg GAE/g. However, with a continuous increment in LSR to 50 ml/g, a decreasing trend was observed in TPC obtained from apple pomace [7].
Fig. 2.
The influence of LSR on the extraction efficiency of phenolics and flavonoids and the antioxidant activity of MRP extracts at fixed conditions: 20% water content, 30 °C, and retention time 10 min. (A): TPC; (B): TFC; (C): DPPH; (D) ABTS; (E): OH. Different characters: a, b, c, d, e, f, g, and h presented significant statistical differences.
The antioxidant activities of MRP extracts were evaluated using DPPH, ABTS, and OH (Fig. 2C–E). When LSR grew from 20 to 80 ml/g, DPPH, ABTS, and OH increased by 1.9, 1.4, and 1.9 times. It can be justified that TPC and TFC experienced an increase, thus improving the antioxidant activity of MRP extracts. However, LSR continuously increased to 100 ml/g, ABTS and OH decreased by 1.2 and 1.3 times, respectively, compared to 80 ml/g of LSR, whereas DPPH remained unchanged. It can be attributed to decreasing TPC and TFC at higher LSR [16]. The relationship between TPC, TFC, and antioxidant activities was demonstrated by Oscar Zannou et al., which utilized the NADES-based UAE of blackberry to extract anthocyanin [26]. Therefore, the appropriate LSR was 80 ml/g to attain the highest extraction efficiency of phenolics and flavonoids from MRP.
3.3. Effect of the water content in NADES
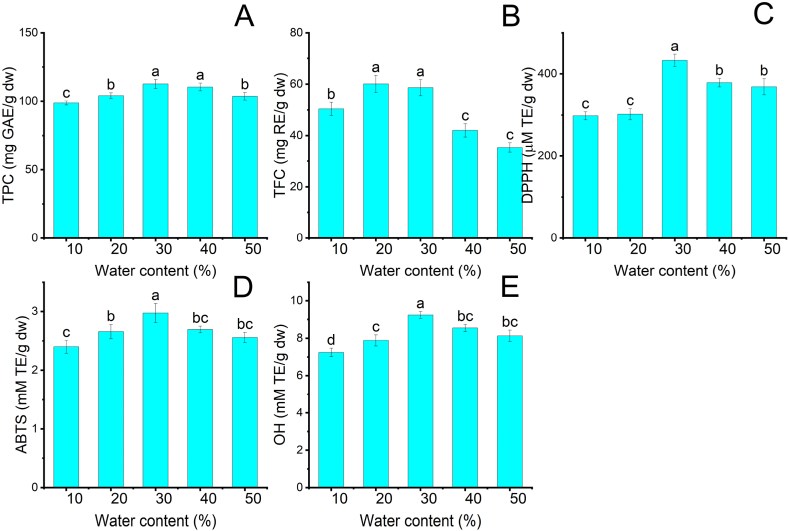
The influence of the water content in Lac-Pro from 10 to 50% on TPC, TFC, and antioxidant activities was analyzed under ultrasonic impact, and the results are presented in Fig. 3A–E. As shown in Fig. 3A–B, TPC escalated by 1.14 and 1.16 times when the water content increased from 10 to 30%.
Fig. 3.
The influence of water content in NADES on the extraction efficiency of phenolics and flavonoids and the antioxidant activity of MRP extracts at fixed conditions: 80 ml/g LSR, 30 °C, and retention time 10 min. (A): TPC; (B): TFC; (C): DPPH; (D) ABTS; (E): OH. Different characters: a, b, and c presented significant statistical differences.
Water addition drops Lac-Pro viscosity and density, accelerating the mass transfer rate from the MRP matrix to extractants. Adding water to Lac-Pro can increase surface tension by raising the number of hydrogen bonds. The increased hydrogen bonds can strengthen an attractive force against an external one, leading to strong mutual attraction on the NADES surface [27]. The increased surface tension can encourage mutual attraction between Lac-Pro and MRP matrix, facilitating the establishment of hydrogen bonds among Lac-Pro, phenolics, and flavonoids. This phenomenon can improve the solubility of phenolics and flavonoids in Lac-Pro, promoting extraction efficiency [28]. On the other hand, TPC and TFC decreased by 1.1 and 2.06 times when the water content increased from 30 to 50%. Excessive water addition can disintegrate the hydrogen-bonding networks and make significant differences in polarity among Lac-Pro, phenolics, and flavonoids, thus decreasing extraction efficiency [29]. This result agrees with Zeng et al. (2019) that used NADES-based UAE to extract phenolics from Chinese wild rice [30].
As water content increased from 10 to 30%, DPPH, ABTS, and OH increased by 1.4, 1.2, and 1.3 times. However, when LSR continuously rose to 50%, DPPH, ABTS, and OH shrank by 1.2, 1.2, and 1.1 times, respectively, compared to 30% water content. Therefore, the appropriate water content in Lac-Pro was 30% to obtain the highest extraction efficiency of phenolics and flavonoids from MRP.
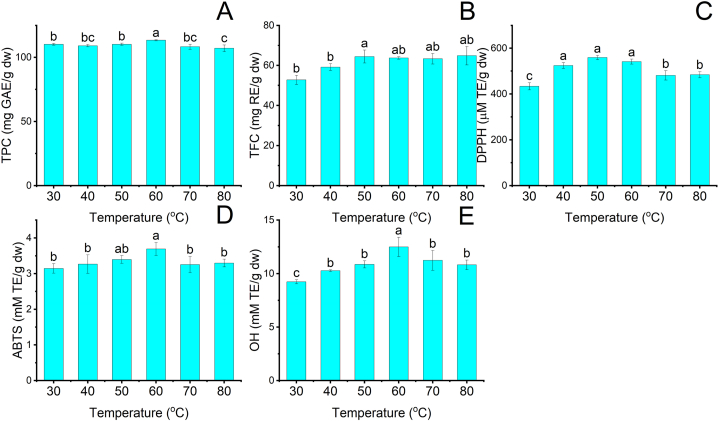
3.4. Effect of temperature
Temperature contributes to extraction efficiency due to varying solubility and viscosity of solvents [8]. The effect of temperature on the NADES-based UAE of phenolics and flavonoids was investigated in the range of 30–80 °C, and the results are demonstrated in Fig. 4A–E. As shown in Fig. 4A–B, the recovery of TPC and TFC displayed a slight increase of 1.03 and 1.21 times as temperature increased from 30 to 60 °C. An increase in temperature can decrease the viscosity of Lac-Pro, thereby increasing the diffusivity of the solvent into the tissue matrix. An increased diffusivity can improve mass transfer rates, enhancing the desorption property, which improves the extraction efficiency of phenolics and flavonoids in MRP [25]. However, TPC decreased by 1.06 times, whereas TFC remained unchanged when temperature increased from 60 °C to 80 °C. This decrease can be attributed to the thermal destruction of phenolics. Additionally, the high temperature can have an inverse influence on the cavitation effects, which can cause less damage to MRP cell walls. The combination of these phenomena can contribute to a decrease in extraction efficiency [24,25]. A similar influence was seen by Soumen Dey and Virendra K. Rathod et al. (2013) that used UAE to recover β-carotene from spirulina platensis [31].
Fig. 4.
The influence of temperature on the extraction efficiency of phenolics and flavonoids and the antioxidant activity of MRP extracts at fixed conditions: 80 ml/g LSR, 30% water content, and retention time 10 min. (A): TPC; (B): TFC; (C): DPPH; (D) ABTS; (E): OH. Different characters: a, b, and c presented significant statistical differences.
As depicted in Fig. 4C–E., the antioxidant activities of MRP extracts are measured by DPPH, ABTS, and OH. When the temperature increased from 30 °C to 60 °C, DPPH, ABTS, and OH increased by 1.2, 1.2, and 1.4 times [24]. However, DPPH, ABTS, and OH decreased by 1.1, 1.1, and 1.2 times as temperature continuously grew from 60 to 80 °C. Therefore, the temperature to obtain the highest extraction efficiency of phenolics and flavonoids from MRP was 60 °C.
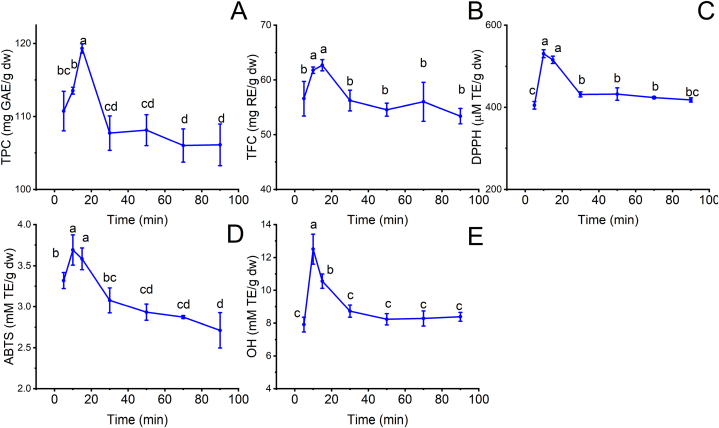
3.5. Effect of time
Extraction time can influence extraction efficiency and antioxidant of extracts as well as decide the cost of the extraction process [8]. The kinetic trend observed for phenolics, flavonoids, and antioxidant activity of MRP extracts upon simultaneous variation of extraction time from 5 to 90 min is depicted in Fig. 5A–E. TPC and TFC substantially increased by 1.08 and 1.10 times as extraction time varied from 5 to 15 min (Fig. 5A–B). At the initial increase in sonication time, pore formation, hydration, swelling, and fragmentation in the cellular tissue of the plant matrix can be enhanced by the cavitation effect of the ultrasound [25]. These phenomena can cooperate with the significant concentration gradient of phenolics and flavonoids between MRP tissues and Lac-Pro. The combined effects can increase mass transfer rates and solvent diffusivity into the matrix, producing more phenolics and flavonoids [24]. When time increased from 15 to 90 min, TPC and TFC decreased to 106.1 ± 2.9 mg RE/g dw and 53.4 ± 1.4 mg RE/g dw. The prolonged cavitation effect may cause the degradation of extracted compounds, reducing extraction efficiency [24]. Daniella Pingret et al. (2013) demonstrated the same trend, which used UAE to extract phenolics from apple pomace [21,31]. The decrease in TPC and TFC can result in a drop in DPPH, ABTS, and OH. Therefore, to ensure the antioxidant activities and flavonoids of MRP extracts, 10 min was the appropriate extraction time to obtain the highest extraction efficiency of phenolics and flavonoids.
Fig. 5.
The influence of time on the extraction efficiency of phenolics and flavonoids and the antioxidant activity of MRP extracts at fixed conditions: 80 ml/g LSR, 30% water content, and 60 °C. (A): TPC; (B): TFC; (C): DPPH; (D) ABTS; (E): OH. Different characters: a, b, c, and d presented significant statistical differences.
3.6. Optimization of the NADES-based UAE process
3.6.1. Regression models
The variables and dependent responses of RSM with BBD models are presented in Table 2, and the coefficients of regression are shown in Table 3. After analyzing the multiple regression of experimental results, a second-order polynomial model was employed to present the suggested model. The second-order polynomial models (Equations (3), (4), (5), (6), (7)) expressed a correlation between the variables and the dependent responses shown as follows:
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Table 2.
The results of experimental designs.
| Factor | TPC | TFC | DPPH | ABTS | OH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | X4 | Experimental Values | Forecast Values | Experimental Values | Forecast Values | Experimental Values | Forecast Values | Experimental Values | Forecast Values | Experimental Values | Forecast Values |
| 1 | 1 | 0 | −1 | 0 | 121.6 ± 8.2 | 119.3 | 43.9 ± 5.7 | 39.2 | 422 ± 21 | 421 | 2.2 ± 0.1 | 2.2 | 6.4 ± 0.8 | 6.3 |
| 2 | 0 | 1 | −1 | 0 | 116.3 ± 1.0 | 118.8 | 14.7 ± 0.8 | 24.86 | 434 ± 19 | 436 | 2.4 ± 0.1 | 2.5 | 5.1 ± 0.3 | 4.9 |
| 3 | 0 | −1 | −1 | 0 | 117.6 ± 6.1 | 118.9 | 50.5 ± 4.9 | 48.08 | 441 ± 04 | 435 | 2.4 ± 0.0 | 2.5 | 6.5 ± 0.5 | 6.3 |
| 4 | 0 | 0 | −1 | −1 | 118.1 ± 3.4 | 119.1 | 37.6 ± 4.1 | 38.09 | 418 ± 1 | 423 | 2.6 ± 0.1 | 2.6 | 6.5 ± 0.6 | 6.5 |
| 5 | −1 | 0 | −1 | 0 | 124.0 ± 0.5 | 121.6 | 47.5 ± 5.3 | 43.98 | 444 ± 9 | 451 | 2.8 ± 0.0 | 2.8 | 5.6 ± 0.2 | 6.2 |
| 6 | 0 | 0 | −1 | 1 | 120.0 ± 2.6 | 120 | 39.9 ± 5.2 | 39.97 | 444 ± 23 | 435 | 2.4 ± 0.1 | 2.3 | 6.4 ± 0.9 | 6.1 |
| 7 | −1 | −1 | 0 | 0 | 115.0 ± 4.4 | 116.9 | 28.5 ± 4.6 | 32.21 | 314 ± 3 | 317 | 2.9 ± 0.1 | 3.1 | 12.0 ± 0.6 | 11 |
| 8 | 0 | 1 | 0 | −1 | 122.2 ± 1.0 | 121.2 | 21.0 ± 1.8 | 16.87 | 296 ± 14 | 294 | 2.6 ± 0.1 | 2.6 | 10.5 ± 0.3 | 10 |
| 9 | 1 | 0 | 0 | 1 | 117.2 ± 0.3 | 119.4 | 19.4 ± 2.6 | 22.86 | 285 ± 11 | 286 | 2.7 ± 0.1 | 2.7 | 10.8 ± 0.6 | 10.7 |
| 10 | −1 | 0 | 0 | −1 | 126.2 ± 2.0 | 126.4 | 30.2 ± 5.1 | 30.32 | 307 ± 7 | 301 | 3.2 ± 0.4 | 3.1 | 10.3 ± 0.2 | 10.4 |
| 11 | −1 | 1 | 0 | 0 | 125.7 ± 3.1 | 125.5 | 19.5 ± 1.6 | 16.47 | 307 ± 3 | 301 | 2.8 ± 0.0 | 3 | 9.6 ± 0.2 | 9.4 |
| 12 | 1 | 1 | 0 | 0 | 117.6 ± 3.1 | 116.1 | 24.5 ± 2.8 | 18.99 | 297 ± 31 | 290 | 2.6 ± 0.2 | 2.6 | 8.8 ± 2.2 | 10 |
| 13 | −1 | 0 | 0 | 1 | 116.4 ± 2.7 | 117.4 | 23.6 ± 0.8 | 23.48 | 310 ± 11 | 303 | 2.9 ± 0.4 | 2.9 | 10.8 ± 0.3 | 11.4 |
| 14 | 0 | −1 | 0 | 1 | 116.8 ± 5.9 | 115.1 | 20.3 ± 0.6 | 22.63 | 296 ± 09 | 307 | 2.8 ± 0.1 | 2.7 | 10.9 ± 0.2 | 11.3 |
| 15 | 1 | 0 | 0 | −1 | 110.3 ± 1.8 | 111.7 | 17.8 ± 0.8 | 21.5 | 276 ± 7 | 277 | 2.5 ± 0.0 | 2.4 | 12.4 ± 2.5 | 11.9 |
| 16 | 1 | −1 | 0 | 0 | 113.1 ± 3.2 | 113.7 | 19.0 ± 1.0 | 20.25 | 284 ± 10 | 287 | 2.6 ± 0.1 | 2.7 | 10.9 ± 0.2 | 11.2 |
| 17 | 0 | −1 | 0 | −1 | 115.6 ± 0.9 | 113.7 | 22.9 ± 3.8 | 24.71 | 287 ± 7 | 283 | 3.1 ± 0.4 | 3 | 10.6 ± 0.6 | 11 |
| 18 | 0 | 1 | 0 | 1 | 119.4 ± 1.4 | 118.6 | 17.0 ± 1.0 | 13.47 | 269 ± 12 | 282 | 2.9 ± 0.1 | 2.9 | 10.0 ± 0.4 | 9.5 |
| 19 | 1 | 0 | 1 | 0 | 104.8 ± 0.9 | 104.4 | 20.8 ± 2.3 | 22.62 | 366 ± 18 | 367 | 2.4 ± 0.3 | 2.3 | 4.7 ± 0.4 | 4 |
| 20 | 0 | −1 | 1 | 0 | 102.5 ± 0.5 | 102.4 | 23.3 ± 1.2 | 16.72 | 387 ± 27 | 379 | 2.8 ± 0.4 | 2.5 | 3.7 ± 0.1 | 3.8 |
| 21 | −1 | 0 | 1 | 0 | 115.1 ± 0.8 | 114.7 | 24.3 ± 1.6 | 27.28 | 368 ± 17 | 378 | 2.6 ± 0.1 | 2.5 | 3.3 ± 0.2 | 3.3 |
| 22 | 0 | 0 | 1 | 1 | 108.2 ± 1.6 | 107.6 | 21.0 ± 0.4 | 18.71 | 374 ± 13 | 365 | 2.2 ± 0.1 | 2.5 | 3.8 ± 0.5 | 3.9 |
| 23 | 0 | 0 | 1 | −1 | 109.2 ± 0.7 | 109.7 | 28.0 ± 2.0 | 26.07 | 361 ± 9 | 366 | 1.8 ± 1.0 | 2.2 | 3.2 ± 0.3 | 3.7 |
| 24 | 0 | 1 | 1 | 0 | 112.5 ± 1.4 | 113.6 | 16.9 ± 0.6 | 22.94 | 364 ± 11 | 365 | 2.6 ± 0.1 | 2.3 | 2.3 ± 0.4 | 2.4 |
| 25 | 0 | 0 | 0 | 0 | 113.5 ± 0.5 | 113.5 | 61.8 ± 0.6 | 61.78 | 531 ± 10 | 531 | 3.7 ± 0.2 | 3.7 | 12.5 ± 0.9 | 12.5 |
| 26 | 0 | 0 | 0 | 0 | 113.5 ± 0.5 | 113.5 | 61.8 ± 0.6 | 61.78 | 531 ± 10 | 531 | 3.7 ± 0.2 | 3.7 | 12.5 ± 0.9 | 12.5 |
| 27 | 0 | 0 | 0 | 0 | 113.5 ± 0.5 | 113.5 | 61.8 ± 0.6 | 61.78 | 531 ± 10 | 531 | 3.7 ± 0.2 | 3.7 | 12.5 ± 0.9 | 12.5 |
| 28 | 0 | 0 | 0 | 0 | 113.5 ± 0.5 | 113.5 | 61.8 ± 0.6 | 61.78 | 531 ± 10 | 531 | 3.7 ± 0.2 | 3.7 | 12.5 ± 0.9 | 12.5 |
| 29 | 0 | 0 | 0 | 0 | 113.5 ± 0.5 | 113.5 | 61.8 ± 0.6 | 61.78 | 531 ± 10 | 531 | 3.7 ± 0.2 | 3.7 | 12.5 ± 0.9 | 12.5 |
Table 3.
The regression coefficients of second-order polynomial equations.
| Coefficients | TPC | TFC | DPPH | ABTS | OH | |
|---|---|---|---|---|---|---|
| Intercept | K0 | 113.5** | 61.78** | 530.7** | 3.69** | 12.50** |
| Linear | K1 | −3.2** | −2.36 | −10.1 | −0.20** | 0.19 |
| K2 | 2.8** | −4.25* | −3.5** | −0.07 | −0.69** | |
| K3 | −5.4** | −8.32* | −31.9 | −0.03 | −1.28** | |
| K4 | −0.3 | −1.37 | 2.8 | −0.01 | −0.06 | |
| Interaction | K12 | −1.56 | 3.62 | 4.7 | 0.004 | 0.09 |
| K13 | −2.0* | 0.03 | 5.0 | 0.11 | 0.15 | |
| K14 | 4.2** | 2.05 | 1.7 | 0.13 | −0.54 | |
| K23 | 2.8** | 7.36* | −3.8 | −0.09 | −0.01 | |
| K24 | −1.0 | −0.33 | −9.2* | 0.14 | −0.19 | |
| K34 | −0.7 | −2.31 | −3.2 | 0.09 | 0.17 | |
| Quadratic | K11 | 3.1** | −17.34** | −115.8** | −0.42** | −0.75** |
| K22 | 1.5 | −22.46** | −116.3** | −0.43** | −1.36** | |
| K33 | −1.6** | −11.17** | −10.6** | −0.79** | −6.79** | |
| K44 | 2.1* | −19.90** | −122.9** | −0.47** | −0.67* | |
| Degree of freedom | 14 | 14 | 14 | 14 | 14 | |
| F-values | 58.84 | 19.86 | 237.5 | 22.13 | 23.62 | |
| p-values | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| R2 | 0.9481 | 0.9521 | 0.9958 | 0.9568 | 0.9847 | |
| Adjusted R2 | 0.8963 | 0.9041 | 0.9916 | 0.9135 | 0.9695 |
Notes: * significant statistical differences (p < 0.05), ** highly significant statistical differences (p < 0.01).
The ANOVA and regression models were constructed using Design Expert 13. The results of ANOVA can demonstrate the confidence of the second-order polynomial models. As illustrated in Table 3, it was clear that the models were highly significant (p < 0.01) for the measurement of TPC, TFC, DPPH, ABTS, and OH. Additionally, the coefficients of determination (R2, 0.9481, 0.9521, 0.9958, 0.9568, and 0.9847) were higher than 0.9, and the coefficients of adjusted determination (Adjusted R2. 0.8963, 0.9041, 0.9916, 0.9135, and 0.9695) for TPC, TFC, DPPH, ABTS, and OH, respectively were close to the values of determination coefficients, implying that the models were reliable to show the correlation between variables and dependent responses. All the results verified that the second-order polynomial models could match the experimental results and were appropriate for the anticipation of independent responses.
LSR, temperature, time, and water content had interactive effects on TPC (p < 0.05). In terms of linear effects, LSR and temperature had a negative effect on TPC, while water content had a positive effect. TFC was affected by temperature and time as well as positively impacted by the interactive effect of water content and temperature. Water content influenced DPPH, which was also affected by the interaction of water content and time. LSR profoundly impacted ABTS, while OH was affected by water content and temperature. There were no interactive effects of variables that significantly affected ABTS and OH.
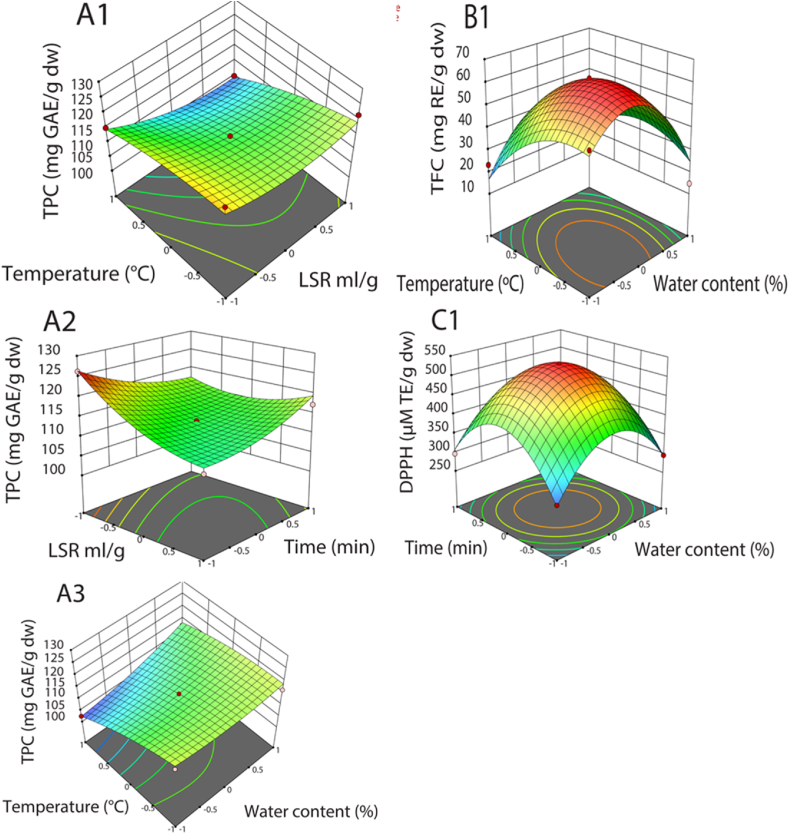
The 3D response surface graphics based on the regression models to imagine the interactive influence of variables on the responses and the mutual interaction that considerably impacted TPC, TFC, DPPH, ABTS, and OH are shown in Fig. 6A1-C1. When temperature and LSR increased, TPC experienced a slight decrease, while the reverse trend was confirmed in the case of temperature and water content. As temperature and water content grew, TFC witnessed a rise, followed by a modest reduction. It can be attributed to the combined effects of high temperature and water content in NADES, which decreased the viscosity of the extraction medium. The vicious reduction can enhance the cavitation effect, improving the recovery of flavonoids. However, excessively high temperatures and water content in NADES can destroy the structure of flavonoids and NADES, decreasing the extraction yield of flavonoids [7,24]. This result agreed with Ju-Zhao Liu et al. (2022), which used NADES to recover natural organic acid and flavonoids from Hibiscus manihot L. flower [32].
Fig. 6.
3D response surface graphics showing the significant mutual interaction of variables on TPC (A1-A3), TFC (B1), and DPPH (C1).
As time and water content rose, there was an increase in DPPH, followed by a slight drop. The slight drop in DPPH can be ascribed to a drop in anthocyanin contents, which play vital roles in DPPH scavenging activity. Excessive temperature can decompose anthocyanins. The destruction of the NADES structure can reduce the hydrogen bond between NADES and anthocyanins, reducing the recovery of anthocyanin [26]. On the other hand, an increase in DPPH can be attributed to a rise in TPC and TFC at the initial stage [26]. Based on the response surface, the optimal values of four variables for the simultaneous optimization of the five responses were: 76.7 ml/g LSR, 30.3% water content, and 57.5 °C for 9.1 min to acquire maximized values of TPC, TFC, DPPH, ABTS, and OH at 116.3 mg GAE/g dw, 61.8 mg RE/g dw, 527.5 μM TE/g dw, 3.68 mM TE/g dw, and 12.2 mM OH/g dw, respectively.
3.6.2. Model verification
The experiments were conducted at optimal Lac-Pro-based UAE conditions to verify the reliability of the regression models, and the measured dependent responses are presented in Table 4. According to the 3D response surface plots and the analysis results, the optimal Lac-Pro-based UAE conditions were generated at 76.7 ml/g LSR, 30.3% water content, and 57.5 °C for 9.1 min of extraction time to maximize values of TPC, TFC, DPPH, ABTS, and OH at 116.3 mg GAE/g dw, 61.8 mg RE/g dw, 527.5 μM TE/g dw, 3.68 mM TE/g dw, and 12.2 mM OH/g dw, respectively. It can be shown that experimental and anticipated values well matched with low prediction errors (<5%).
Table 4.
The experimental and predicted values of five responses at optimal conditions.
| The variables |
Dependent responses | |||||||
|---|---|---|---|---|---|---|---|---|
| LSR ml/g | Water content % | Temperature °C | Time min | Predicted values | Experimental values | Prediction error % | R2predicted | |
| 76.7 | 30.3 | 57.5 | 9.1 | TPC (mg GAE/g db) | 116.3 | 118.2 ± 3.8 | 1.60 | 0.7013 |
| TFC (mg RE/g db) | 61.8 | 59.3 ± 2.1 | 4.21 | 0.7238 | ||||
| DPPH (μM TE/g db) | 527.5 | 537 ± 19 | 1.86 | 0.9758 | ||||
| OH (mM TE/g db) | 3.68 | 3.87 ± 0.25 | 4.91 | 0.7510 | ||||
| ABTS (mM TE/g db) | 12.2 | 11.9 ± 1.4 | 2.52 | 0.9120 | ||||
3.7. The surface morphology of mangosteen rind powder
The variations in surface morphology of MRP before and after sonication were investigated using SEM; the results are presented in Fig. 7A–C. As shown in Fig. 7A, the surface of MRP had an unwrinkled appearance before ultrasonic treatment. However, the surface of MRP experienced erosion after sonication with 50% ethanolic solution and Lac-Pro. In the case of 50%-ethanolic solution-based UAE, small pores were observed on the surface of MRP, which could be ascribed to the acoustic cavitation effect [19]. Lac-Pro-based ultrasonic treatment devastated cell walls, which was indicated by large holes on the surface morphology of MRP. It can be attributed to the synergistic effect of the acoustic cavitation effect between ultrasound and the partial destruction of Lac-Pro on the cellulosic matrix of MRP cell walls. This synergistic effect can generate large holes on the surface of MRP that can considerably improve the permeation capacity of Lac-Pro into the internal structure of MRP, accelerating the extraction of phenolics and flavonoids more significantly than 50%-ethanol solution-based UAE [8,19]. This finding supports the development of Lac-Pro-based UAE as an eco-friendly and practical method for improving the extraction efficiency of bioactive components from industrial food processing wastes.
Fig. 7.
The variation in surface morphology of MRP among 50%-ethanolic solution-based, Lac-Pro-based UAE, and MRP without sonication. (A): surface morphology of MRP without ultrasonic treatment; (B): surface morphology of MRP with 50%-ethanolic solution-based UAE; (C): surface morphology of MRP after Lac-Pro-based UAE.
4. Conclusion
In this work, Lac-Pro was the appropriate solvent to extract phenolics and flavonoids from MRP and had a higher extraction efficiency than that of water, ethanolic, and acetone solutions. The optimal Lac-Pro-based UAE conditions were at 76.7 ml/g LSR, 30.3% water content, and 57.5 °C for 9.1 min of extraction time to acquire 116.3 mg GAE/g dw of TPC, 61.8 mg RE/g dw of TFC, 527.5 μM TE/g dw of DPPH, 3.68 mM TE/g dw of ABTS, and 12.2 mM OH/g dw of OH. SEM images demonstrated more considerable destruction in the surface morphology of MRP after Lac-Pro-based UAE than 50%-ethanolic solution-based UAE and without ultrasonic treatment. The study suggested that Lac-Pro-based UAE is a green and effective method to extract bioactive compounds from MRP.
Author contribution statement
Tan Phat Vo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ngoc Duyen Pham, Thuy Vy Pham, Hoang Yen Nguyen, Thi Ngoc Huyen Tran, Tri Nguyen Tran: Performed the experiments.
Le Thao Vy Vo: Analyzed and interpreted the data.
Dinh Quan Nguyen: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
References
- 1.Wittenauer J., et al. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012;134(1):445–452. [Google Scholar]
- 2.Chong Y.M., et al. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015;12:18–25. [Google Scholar]
- 3.Obolskiy D., et al. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother Res. 2009;23(8):1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- 4.Suvarnakuta P., Chaweerungrat C., Devahastin S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011;125(1):240–247. [Google Scholar]
- 5.Palma A., et al. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrason. Sonochem. 2021;76:105654. doi: 10.1016/j.ultsonch.2021.105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J., et al. Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: optimization study. Ultrason. Sonochem. 2021;78:105739. doi: 10.1016/j.ultsonch.2021.105739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rashid R., et al. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: optimisation, comparison and bioactivity. Food Chem. 2023;398:133871. doi: 10.1016/j.foodchem.2022.133871. [DOI] [PubMed] [Google Scholar]
- 8.Zheng B., et al. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: optimization, comparison and bioactivities. LWT. 2022;154:112740. [Google Scholar]
- 9.Fourmentin S., Gomes M.C., Lichtfouse E. vol. 56. Springer; 2020. (Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances). [Google Scholar]
- 10.Zhang Q., et al. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012;41(21):7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- 11.Lakka A., et al. Saffron processing wastes as a bioresource of high-value added compounds: development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants. 2019;8(12) doi: 10.3390/antiox8120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Almeida Pontes P.V., et al. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: extraction optimization and solvent characterization. Food Chem. 2021;352:129346. doi: 10.1016/j.foodchem.2021.129346. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith C.D., et al. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT. 2018;89:284–290. [Google Scholar]
- 14.Xue H., et al. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods. 2020;9(10):1409. doi: 10.3390/foods9101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plaza M., et al. A sustainable approach for extracting non-extractable phenolic compounds from mangosteen peel using ultrasound-assisted extraction and natural deep eutectic solvents. Appl. Sci. 2021;11 doi: 10.3390/app11125625. [DOI] [Google Scholar]
- 16.Wu L., et al. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: optimization, comparison and antioxidant activity. Separ. Purif. Technol. 2020;247:117014. [Google Scholar]
- 17.Müller L., Fröhlich K., Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129(1):139–148. [Google Scholar]
- 18.Yuan H., et al. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis. Food Sci. Hum. Wellness. 2022;11(4):1010–1017. [Google Scholar]
- 19.Patil S.S., Pathak A., Rathod V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: a greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021;70:105267. doi: 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lactic acid. [cited 2022 October 26]; Available from: https://pubchem.ncbi.nlm.nih.gov/compound/612#section=MeSH-Entry-Terms.
- 21.Xu M., et al. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019;297:124970. doi: 10.1016/j.foodchem.2019.124970. [DOI] [PubMed] [Google Scholar]
- 22.Citric Acid. [cited 2022 October 26]; Available from: https://pubchem.ncbi.nlm.nih.gov/compound/311.
- 23.Santos H.M., Lodeiro C., Capelo-Martínez J.-L. Ultrasound in Chemistry. 2008. The power of ultrasound; pp. 1–16. [Google Scholar]
- 24.Rao M.V., et al. Ultrasonication - a green technology extraction technique for spices: a review. Trends Food Sci. Technol. 2021;116:975–991. [Google Scholar]
- 25.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. 2021;70:105325. doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannou O., Koca I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp) using natural deep eutectic solvents. LWT. 2022;158:113184. [Google Scholar]
- 27.Shafie M.H., Yusof R., Gan C.-Y. Synthesis of citric acid monohydrate-choline chloride based deep eutectic solvents (DES) and characterization of their physicochemical properties. J. Mol. Liq. 2019;288:111081. [Google Scholar]
- 28.Huang H., et al. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022;380:132216. doi: 10.1016/j.foodchem.2022.132216. [DOI] [PubMed] [Google Scholar]
- 29.Fu X., et al. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: composition, extraction mechanism and in vitro biological activities. Food Chem. 2022;370:131042. doi: 10.1016/j.foodchem.2021.131042. [DOI] [PubMed] [Google Scholar]
- 30.Zeng J., et al. Optimizing ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from Chinese wild rice. Molecules. 2019;24 doi: 10.3390/molecules24152718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dey S., Rathod V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013;20(1):271–276. doi: 10.1016/j.ultsonch.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Liu J.-Z., et al. Simultaneous extraction of natural organic acid and flavonoid antioxidants from Hibiscus manihot L. flower by tailor-made deep eutectic solvent. LWT. 2022;163:113533. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.