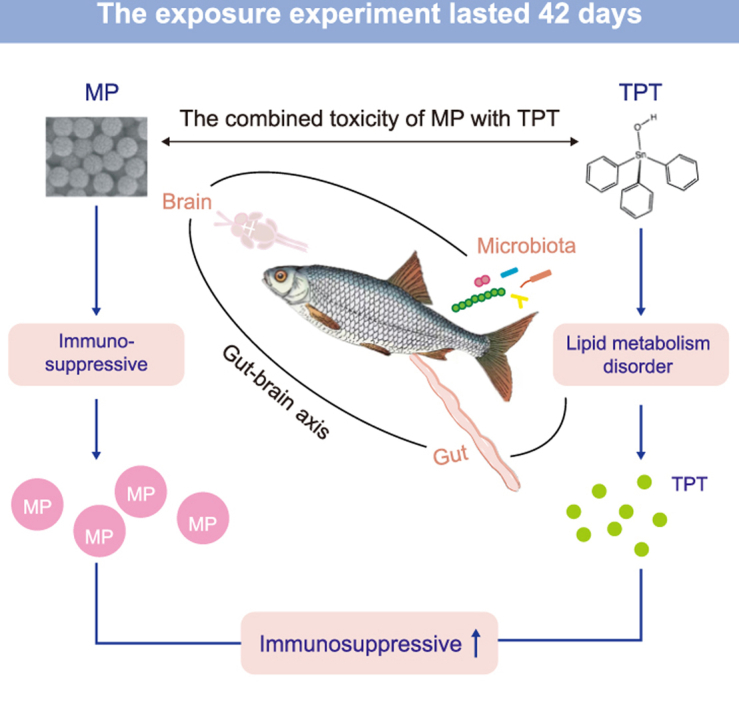
Abstract
Microplastics (MPs), an emerging group of pollutants, not only have direct toxic effects on aquatic organisms but also cause combined toxicity by absorbing other pollutants. Triphenyltin (TPT), one of the most widely used organotin compounds, has adverse effects on aquatic organisms. However, little is known about the combined toxicity of MPs and TPT to aquatic organisms. To investigate the individual and combined toxicity of MPs and TPT, we selected the common carp (Cyprinus carpio) for a 42-day exposure experiment. Based on the environmental concentrations in a heavily polluted area, the experimental concentrations of MPs and TPT were set at 0.5 mg L−1 and 1 μg L−1, respectively. The effects of MPs combined with TPT on the carp gut–brain axis were evaluated by detecting gut physiology and biochemical parameters, gut microbial 16S rRNA, and brain transcriptome sequencing. Our results suggest that a single TPT caused lipid metabolism disorder and a single MP induced immunosuppression in carp. When MPs were combined with TPT, the involvement of TPT amplified the immunotoxic effect induced by MPs. In this study, we also explored the gut–brain axis relationship of carp immunosuppression, providing new insights for assessing the combined toxicity of MPs and TPT. At the same time, our study provides a theoretical basis for evaluating the coexistence risk of MPs and TPT in the aquatic environment.
Keywords: Aquatic organism, Combined toxicity, Ecological risk, Environmental concentration
Graphical abstract
Highlights
-
•
Microplastics (MPs) and triphenyltin (TPT) coexist in water environment.
-
•
Explore the single/combined toxicity of MPs and TPT to carp.
-
•
Single MPs inhibit the immunity and TPT causes the lipid metabolism disorder.
-
•
The carp gut flora is correlated with immune and lipid metabolism genes in brain.
-
•
The combination of TPT amplifies MPs-induced immunosuppression.
1. Introduction
Plastic pollution has been declared by the United Nations Environment Programme as one of the most serious environmental problems of our time, and plastic debris has become a globally recognized emerging environmental problem [1]. Owing to unavoidable release, plastics, especially the microplastics (MPs; plastic particles smaller than 5 mm) [2], are detected in oceans, estuaries, fresh water, and sediments [3,4] and have even been found in remote regions of the Arctic and Antarctic [5,6]. Microplastics come in various shapes, including spherical, fibrous, fragmented, and thin-film shapes [7,8]. In addition to the breakdown of larger plastics, microplastics in the water come from “primary microplastics,” which are cosmetic beads, plastic pellets, or fibers specifically designed and produced for use in abrasive cleaning products such as face washes and toothpastes [9]. Spherical MPs have been found in Granada, Spain, the Northeast Atlantic Ocean, and Odisha Beach, India [10]. With the continuous breakdown of plastic waste and the increase in primary MP production, the residual amount of spherical MPs in the water is increasing rapidly. Therefore, spherical primary MP particles were selected as the research topic in this study.
Numerous studies have found that MPs can have a negative effect on aquatic biota. For example, MP exposure can affect the growth and reproduction of organisms [11,12], inhibit metabolism [13], induce immunotoxicity, lead to neurotoxicity [14], and disorder the endocrine [15]. The intestine is the main digestion, absorption, and immune organ, and plays an important role in maintaining the life activities of organisms [16]. Therefore, the intestine is a potential target organ of MPs and thus worthy of research. In recent years, many studies have proved that MPs can cause an imbalance of the intestinal flora and increase the proportion of harmful bacteria. Meanwhile, the intestinal barrier function will also be affected by MPs; that is, the structure of intestinal villi and mucus layer will change [17,18]. The gut–brain axis is the mutual information exchange system between the brain and gut, consisting of the neuroendocrine, vagal, and immune pathways, and plays a central role in host development, metabolism, and physiology [19]. Brain tissue, being an important link in the “gut–brain axis”, is the tissue in which environmental pollutants mainly accumulate [20,21], and MPs are likely to cause damage to biological brain tissue through the blood–brain barrier. As an emerging pollutant, MPs not only cause direct toxicity to organisms through the food chain [22], but owing to their hydrophobic surface and high surface area, they can also adsorb and interact with other environmental pollutants, leading to combined toxicity [23].
Organotin compounds (OTCs) are widely used in industrial, agricultural, and aquaculture areas owing to their excellent general properties, but this has resulted in severe water pollution [24,25]. In recent years, triphenyltin (TPT) pollution has become a topic of concern. For example, the highest concentration of TPT in the waters of Saudi Arabia and the port of Cape Town of South Africa reached 1.9 and 23008.0 ± 0.03 × 10−3 μg L−1, respectively [26]. Sewage wastewater is a major source of MPs and OTCs, so there is a high risk of coexistence with these two pollutants [27]. It was reported that MPs and nutritional status can mitigate TBT toxicity to the marine rotifer Brachionus koreanus during dietary exposure, but TBT-induced toxicity and its legacy effects are unavoidable [28]. Additionally, in a study of algae exposure to MPs combined with TPT, it was found that MPs damaged the structure of chlorella cells, thereby increasing the absorption of TPT by cells and amplifying the toxic effect [29]. In contrast, it is not only difficult for the MPs to destroy the dense silica shell of diatoms, but they can also adsorb TPT in water, reducing the TPT concentration in the water as well as the toxic effect on diatoms [30].
Currently, there are few reports on the combined toxicity of MPs and OTCs to aquatic fish despite the high risk of their coexistence. More importantly, MPs can be used as carriers of contaminants, such as polysulfates and metals, to reach the intestinal tract of fish through involuntary ingestion [[31], [32], [33]]. Therefore, it is necessary to study the combined effects of MPs and TPT on fish. The purpose of this study was to comprehensively explore the aquatic ecological risks of the coexistence of MPs and TPT by investigating the combined toxicity of MPs and TPT to common carp (Cyprinus carpio). Carp is often used as a biological indicator of polluted aquatic ecosystems, and it is also an important aquaculture species in many countries. In this study, the carp were exposed to TPT, MPs, and TPT combined with MPs for 42 days. Considering the possible adsorption effect of MPs on TPT, we hypothesized that their combined toxicity to carp might be lower than the individual toxicity of TPT, but greater than that of MPs alone.
To test this hypothesis, we investigated changes in intestinal flora, analyzed the results of brain tissue transcriptome sequencing, observed the intestinal glycoprotein secretion, and detected the biochemical parameters and the expression of genes involved in immunity and lipid metabolism. Additionally, we explored the effect of combined toxicity of MPs and TPT on the carp gut–brain axis in combination with the microbe–host relationship and provide a theoretical basis for evaluating the risk of combined toxicity caused by the coexistence of MPs and TPT.
2. Materials and methods
2.1. Chemicals and test fish
Polystyrene MP beads (diameter: 200 nm) were purchased from Wuxi Regal Biotechnology Co., Ltd. (Wuxi, China). The morphology of the beads was confirmed by scanning electron microscope TESCAN VEGA3 (Brno, Czech Republic) (Fig. S1). The final concentration of the MP-treated group was 0.5 mg L−1 (600 μL of the MP suspension at a concentration of 0.025 g mL−1 was added to 30 L of water). Considering the size-dependent toxicity of MPs and uncertainty as to whether plastic microspheres can penetrate various barriers in the organism, we chose submicron plastic microspheres with a particle size of 200 nm. The MP concentration of the MP exposure group was set with reference to previous studies [34,35].
Triphenyltin chloride (purity >96%, CAS: 639-58-7) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). In accordance with a previous study [36], a TPT chloride stock solution was prepared to a concentration of 300 ng μL−1 in DMSO, and the final concentration of DMSO in the exposure group was less than 0.001%. The final concentration of TPT in the treatment group was set to 1 μg L−1, based on the highest actual environmental concentration of TPT in the waters of Saudi Arabia and Cape Town of South Africa, which can reach up to μg L−1 [26,37].
The juvenile carp (Cyprinus carpio) (16.70 ± 4.72 g, 11.67 ± 1.20 cm) came from Xinda Fish Farm (Tianjin, China). The relative fatness K (K = W(g)/L(cm)³ × 100) is shown in Table S1. Prior to the exposure experiments, carp were temporarily adapted in the laboratory for one week. The fish were randomly divided into four groups (three tanks per group), and six healthy carp were stocked in each glass tank with 30 L of water. During this period, they were fed commercial bait (Xinda, China) twice a day (9:00 a.m., 15:00 p.m.). The water was changed every two days to remove residual bait and metabolic waste. The temporary holding aquarium was a temperature-controlled circulatory system (pH 7.6 ± 0.2, 23 ± 1 °C), and several aerated stones were set to ensure sufficient oxygen in the water. All experimental operations on carp in this study were approved by the Local Animal Ethics Committee.
2.2. Experimental design and sampling
The exposure experiment was carried out after the carp were temporarily adapted for a week. The control, 1 μg L−1 TPT, 0.5 mg L−1 MPs, and 1 μg L−1 TPT + 0.5 mg L−1 MPs (TPT_MP) treatment groups were set up based on the actual environmental concentration of the pollutants, considering the most severe cases of pollution. The exposure group was set with the conditions of the respite period. During the exposure, the water quality parameters and feeding were consistent with the adaptation period. We replaced 2/3 of the water and added the pollutant stock solution to ensure a stable concentration every two days. All solution samples were analyzed, and the measured concentration of TPT (0.93 ± 0.10 μg L−1, corresponding to 1.0 μg L−1) was within 20% of the nominal concentration, which meets the OECD guidelines (OECD Guideline for Testing of Chemicals No. 204, “Fish, Prolonged Toxicity Test”).
After 42 days of continuous exposure, six fish (two fish from each tank in each group) were randomly selected from each group for sampling. The carp were not fed for 24 h prior to sampling. After the carp were anesthetized with MS222 (20 μg L−1, tricaine mesylate), their body weight and body length were measured. The abdomen of the carp was cut open, the liver was quickly separated, and the intestinal tissue was taken out. Then, the carp cranium was cut open, and the brain tissue was carefully removed with forceps. Some intestinal tissues were fixed in 10% formalin solution for observation of tissue structure and the remaining intestinal tissues and brain tissues were snap-frozen in liquid nitrogen and stored at −80 °C for the detection of biochemical indicators, RT-qPCR, and brain transcriptome sequencing. Additionally, six fish were randomly selected from each group (two fish from each tank in each group) for intestinal content sampling. Intestinal contents were removed after feeding for 6 h for 16S rRNA sequencing analysis of intestinal flora.
2.3. Histopathological examination
After a series of treatments, intestinal tissue fixed in 10% formalin was embedded in paraffin, cut into 4 μm-thick sections, and then stained with periodic acid–Schiff (PAS). The total area and density of glycoproteins in the carp intestinal tissue were detected and analyzed using Image Pro Plus 5.1 (MEDIA CYBERNETICS, Rockville, MD, USA).
2.4. Gut microbiota analysis
Three stool samples were randomly selected in each group to detect the intestinal bacterial community. According to the manufacturer's instructions, DNA was extracted from stool samples using a QIAamp DNA Stool Mini Kit (Qiagen 51504). The hypervariable V3–V4 region of the 16S-rDNA gene was amplified by PCR using primers. According to the standard protocol, the purified amplicons were pooled in the form of equimolar and paired-end sequencing (2 × 250) on the Illumina platform by Gene Denovo Biotechnology Co. (Guangzhou, China). Details steps and reagents used are presented in the supporting information (S1).
2.5. Biochemical parameter measurement
Intestinal tissues were taken out on a cold ice plate and then homogenized in ice-cold saline by sonication. Physiological saline was added in proportion (weight (g):volume (mL) = 1:9). The homogenate was centrifuged at 4 °C and 2000 g for 10 min, and the supernatant was taken and stored at −80 °C for analysis. Enzymes related to intestinal immunity include lysozyme (LZM), complement C3 and immunoglobulin (IgM), malondialdehyde (MDA), and digestive parameters of the intestine including total cholesterol (TCHO) and triglycerides (TG) were detected in carp. ELISA kits for detecting C3 (ELISA, CD90026) and IgM (ELISA, CD90081) were produced by Wuhan Chundu Biotechnology Co., Ltd. (Wuhan, China). The assay kits of LZM (turbidimetry, A050-1-1), MDA (TBA method, A003-1-2), TCHO (single-reagent GPO-PAP method, A111-1-1), TG (single-reagent GPO-PAP method, A110-1-1), and total protein (TP) (Coomassie brilliant blue method, A045-2-2) were purchased from Nanjing Jiancheng Bioengineering Institute and used according to the manufacturer's protocols (S2). All biochemical indexes were detected by Tecan enzyme marker (Männedorf, Switzerland).
2.6. Brain transcriptome analysis
To extract the total RNA of the sample, rRNA was removed using a conventional kit, and oligo (dT) magnetic beads were used for mRNA enrichment. We further reverse-transcribed the enriched mRNA to form a double-stranded cDNA, repaired both ends of the cDNA, added linkers, and amplified by PCR to construct a computerized library. To ensure the quality of sequencing, the purity of RNA was detected by agarose gel electrophoresis and a NanoPhotometer spectrophotometer (Hangzhou, China). The sequencing was completed using the Illumina Novaseq6000 of Gene Denovo Biotechnology Co. (Guangzhou, China). The detailed steps and reagents used are shown in the supplementary information (S3). The methods of RNA extraction and RT-qPCR were the same as those in previous studies [38]. The detailed steps are included in the supporting information (S4).
2.7. Statistical analysis
SPSS version 25 (SPSS Inc, Chicago, IL, USA) was used for all statistical analysis. The data are expressed as mean ± SD. All data were checked for normality of distribution and variance homogeneity using the Kolmogorov–Smirnoff test and Levene's test. Statistical significance was determined using the one-way ANOVA and post hoc Tukey–Kramer test. When the normality of variance was not apparent, equivalent non-parametric equivalent tests were used (Wilcoxon, post hoc Steel–Dwass test). For all analyses, there was a significant difference when p < 0.05. The Pearson correlation analysis was used to assess the correlation between variables.
Correlation analysis of gut biochemical parameters with genes in the brain was performed. Then, using O2PLS analysis and Spearman correlation analysis, the degree of correlation between microorganisms and transcriptome data was determined based on the correlation coefficient “r” and significance “p” (|r| > 0.6; p < 0.05).
3. Results
3.1. Changes in intestinal tissue glycoproteins
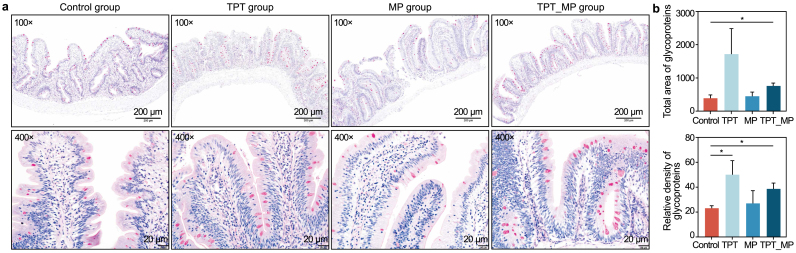
Mucus can surround bacteria and screen for metabolites or toxins to restrict their entry into epithelial tissues, and there are many molecules in mucus that can play an immunomodulatory role. Glycoproteins are the main component of intestinal mucus secreted by goblet cells. As shown in Fig. 1, the changes in the total area of glycoproteins and the relative density of glycoproteins were studied in the intestine of all test groups. Compared with the control group, the total area of glycoproteins in the TPT_MP group increased significantly (p < 0.05), and the relative density of glycoproteins in the TPT and TPT_MP treatment groups increased significantly (p < 0.05).
Fig. 1.
Periodic acid–Schiff (PAS) staining analysis of carp intestine tissue after TPT, MP, and TPT_MP exposure. a, Magnification is 100 × and 400 × . b, The total area and relative density of glycoproteins in the carp intestine are also presented. The presented values are the means ± SEM (n = 6).
3.2. Imbalance of intestinal microbiota
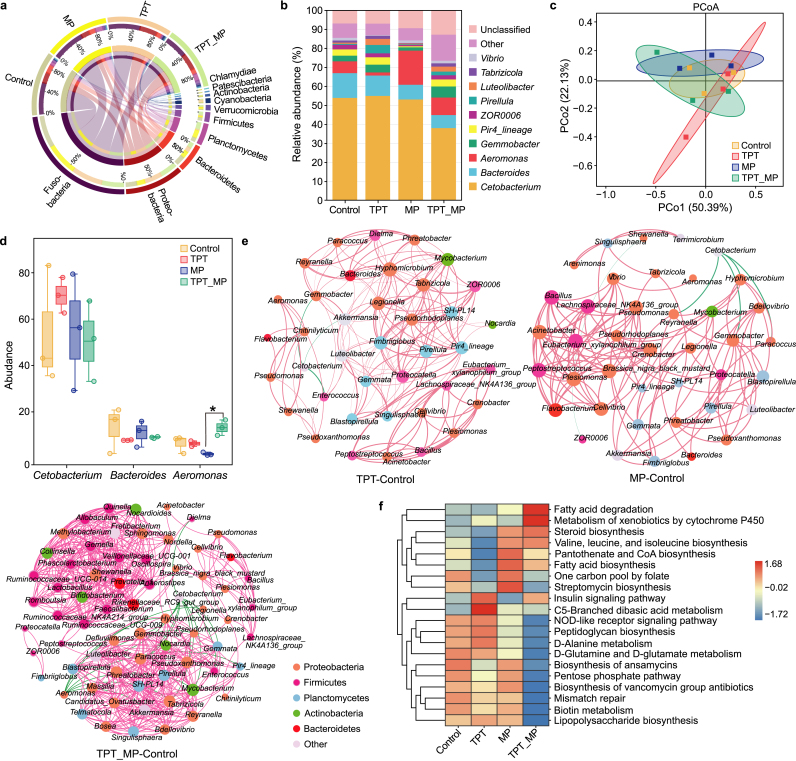
The top three phyla were Fusobacteria, Proteobacteria, and Bacteroidetes. For the genus level, the top three genera, Cetobacterium, Bacteroides, and Aeromonas, were found in the intestinal flora of carp (Fig. 2a and b). PCoA (principal co-ordinates analysis) showed differences in the structure of carp intestinal flora in different treatment groups (Fig. 2c). The analysis of the indicator species revealed there were no significant differences at the phylum level.
Fig. 2.
The species composition and indicator species analysis of carp gut microbes after exposure to TPT, MP, and TPT_MP. a, The composition of microbial species at the phylum level. b, The composition of microbial species at the genus level. c, Principal component (PCoA) analysis of OUT (Operational Taxonomic Unit) level. d, Indicator species at the genus level. ∗p < 0.05 indicates significant difference. e, Co-occurring network of the gut bacterial community based on correlation analysis. f, KEGG level three functional abundance cluster analysis.
At the genus level, the abundance of Aeromonas flora in MP was significantly downregulated compared with the TPT_MP group. There were no significant differences between the other treatment groups and the control group (Fig. 2d). The KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways enriched in all groups are classified into six categories at the KEGG level 1: “Metabolism”, “Genetic Information Processing”, “Cellular Processing”, “Environmental Information Processing”, “Organismal System”, and “Human Diseases” (Fig. S2). Additionally, the network complexity of the gut bacterial community, the number of vertices, the number of edges, the average degree, and the average clustering coefficient were different in different exposure groups. Moreover, the combined exposure TPT_MP had the highest network complexity, which is likely because of the combined action of the two pollutants (Fig. 2e). In KEGG level 3, the functional abundance of lipid metabolism of the flora in the TPT_MP group such as “Fatty acid degradation”, “Steroid biosynthesis”, and “Fatty acid biosynthesis” was higher than that of the control group, while the immune function abundance “NOD-like receptor signaling pathway” was lower than that of the control (Fig. 2f).
3.3. Intestinal biochemical parameters and immune genes
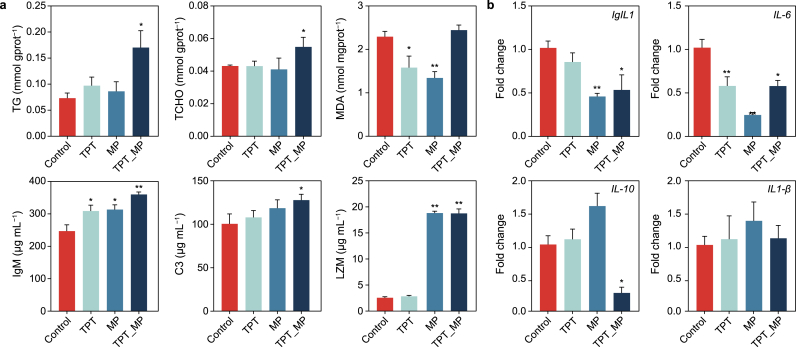
As shown in Fig. 3a and Table 1, after 42 days of exposure, the levels of TG and TCHO in the TPT_MP group (p < 0.05) were significantly higher than the control group. The level of MDA was significantly lower in the MP group (p < 0.05) compared with the control group, and the level in TPT (p < 0.05) was significantly higher than that in the control. Compared with the control, the IgM level in the TPT group, the IgM and LZM levels in the MP group, and the levels of three immune parameters (IgM, LZM, and C3) in the TPT_MP group were significantly increased (p < 0.05).
Fig. 3.
Analysis of physiological parameters and immune genes of carp intestine tissue exposed to TPT, MP, and TPT_MP. a, Intestinal biochemical parameters. b, Real Time -qPCR results of gut immune genes. The presented values are the means ± SEM (n = 6). ∗p < 0.05, ∗∗p < 0.01, indicate significant differences from the control.
Table 1.
One-way ANOVA of variance for the effects of TPT, MP, and TPT_MP on parameters measured in fish after chronic exposure.
| Source | Control |
F | TPT |
MP |
TPT_MP |
|||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | Mean ± SD | p | Mean ± SD | p | ||
| TG | 0.0733 ± 0.0170 | 3.705 | 0.0971 ± 0.0286 | 0.9334 | 0.08633 ± 0.0318 | 0.9950 | 0.1703 ± 0.0559 | 0.0259 |
| TCHO | 0.04318 ± 0.0010 | 1.484 | 0.0431 ± 0.0053+4 | 0.9999 | 0.0411 ± 0.0120 | 0.9994 | 0.0549 ± 0.0101 | 0.0406 |
| MDA | 2.2926 ± 0.2156 | 11.71 | 1.5816 ± 0.4588 | 0.0268 | 1.3434 ± 0.2530 | 0.0039 | 2.4449 ± 0.2019 | 0.2252 |
| IgM | 247.1250 ± 33.4833 | 4.882 | 309.2925 ± 30.8460 | 0.0386 | 31304545 ± 25.2121 | 0.0259 | 360.3239 ± 13.1050 | 0.0058 |
| C3 | 100.5405 ± 19.6611 | 2.096 | 107.8831 ± 13.8420 | 0.9851 | 118.5372 ± 16.7225 | 0.6280 | 127.8055 ± 11.7382 | 0.03518 |
| LZM | 2.5694 ± 0.3182 | 266.7 | 2.8472 ± 0.3182 | 0.9980 | 18.8194 ± 0.6365 | <0.0001 | 18.7500 ± 1.5023 | <0.0001 |
| IgIL1 | 1.0175 ± 0.1948 | 5.699 | 0.8562 ± 0.2557 | 0.7340 | 0.4598 ± 0.0863 | 0.0097 | 0.5338 ± 0.4280 | 0.0272 |
| IL-6 | 1.0206 ± 0.2340 | 15.45 | 0.5800 ± 0.2367 | 0.0078 | 0.2475 ± 0.0642 | <0.0001 | 0.5798 ± 0.1115 | 0.0230 |
| IL-10 | 1.0438 ± 0.3232 | 10.13 | 1.1232 ± 0.3721 | 0.9815 | 1.6213 ± 0.4741 | 0.0613 | 0.3172 ± 0.1734 | 0.0309 |
| IL1-β | 1.0382 ± 0.3180 | 0.3798 | 1.1235 ± 0.8571 | 0.9951 | 1.3940 ± 0.6911 | 0.7549 | 1.1376 ± 0.4312 | 0.9933 |
The RT-qPCR results of carp intestinal immunity-related genes are shown in Fig. 3b and Table 1. Compared with the control group, IL-6 in the TPT group was significantly decreased (p < 0.01), and IL-1 and IL-6 in the MP group were significantly decreased (p < 0.01). In the TPT_MP group, IL-6 and IL-10 were significantly decreased (p < 0.05).
3.4. Brain transcriptome sequencing
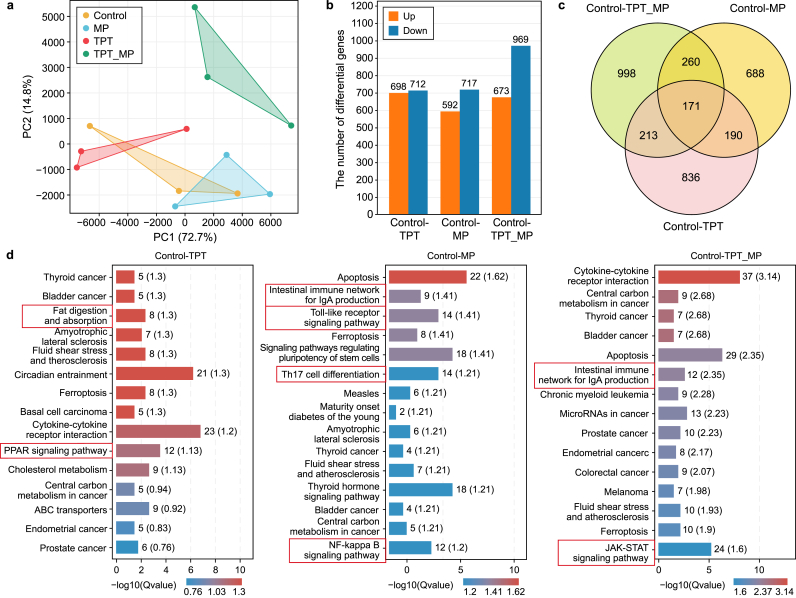
To further study the single/combined toxicity of TPT and MPs to carp brain tissue, we performed transcriptome analysis based on the FPKM (fragments per kilobase per million mapped reads) value of the detected gene. According to the PCA results, the carp brain transcriptome samples are highly correlated (Fig. 4a), and the correlations of repeated samples within the same treatment group were all higher than 0.99 (Fig. S5). The upregulated and downregulated genes of each group are shown in Fig. 4b (fold change ≥1.5 or ≤ −1.5, and p-value ≤0.05). Compared with the control, the differentially expressed genes (DEGs) in the TPT group (upregulated: 698; downregulated: 712), MP group (upregulated: 592; downregulated: 717), and TPT_MP group (upregulated: 673; downregulated: 969) are presented (Fig. 4b). In total, 171 DEGs were found across the three groups (Fig. 4c). Subsequently, to further determine the function of DEGs and distinguish the differences among the treatment groups, KEGG analysis was performed, and the 15 top pathways in each group are shown in Fig. 4d. Clearly, TPT has a significant effect on “Fat digestion and absorption” and the “PPAR signaling pathway”. Moreover, MP affects immune-related pathways. “Intestinal immune network for IgA production”, “Toll-like receptor signaling pathway”, “Th17 cell differentiation”, “NF-kappa B signaling pathway” are significantly enriched. Some pathways related to immunity such as the “Intestinal immune network for IgA production”, “Cytokine–cytokine receptor interaction” and other pathways, and the “JAK-STAT signaling pathway” in the TPT_MP group are also significantly enriched.
Fig. 4.
Transcriptome analysis of brain tissue of carp exposed to TPT, MP, and TPT_MP. a, PCA analysis. b, Histogram of differentially expressed genes (DEGs). c, Venn diagram of differentially expressed genes (DEGs). d, The KEGG pathways that were significantly enriched in DEGs in carp brain tissue were exposed to TPT, MP, and TPT_MP (p < 0.05).
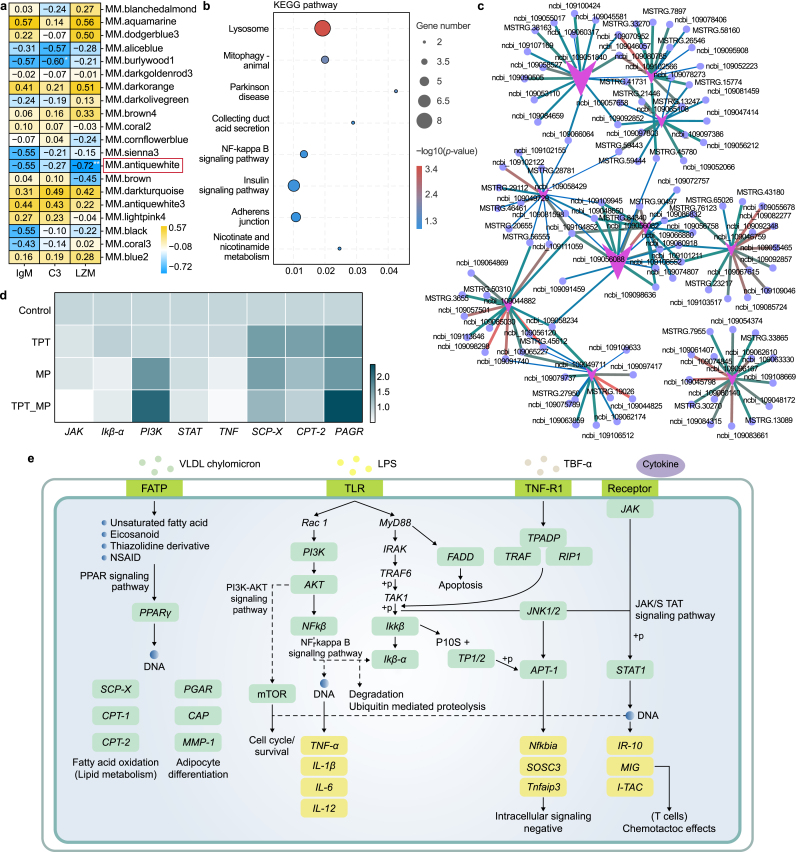
To explore the toxic effects of TPT, MP, and TPT_MP on carp, a weighted co-expression network analysis (WGCNA) considering gut immune parameter traits was studied. Results were validated by RT-qPCR and by exploring gene relationships in pathways. The results of WGCNA showed that genes with FPKM values > 5 were divided into 21 modules. According to the correlation analysis between intestinal immune parameters and the top 20 modules, LZM was significantly negatively correlated with module antique white (r = −0.72, p < 0.01) (Fig. 5a). The KEGG result of genes in the module antique white is involved in the immune-related NF-kappa B signaling pathway and insulin signaling pathway (Fig. 5b). The gene regulatory network map shows the association of the top ten pairs of transcription factors in the module antique white (Fig. 5c). To verify the KEGG pathway, we selected immune-related genes (TNF-α, PI3K, STAT, JAK, IKβ-α) and genes related to fat metabolism (SCP-X, PAGR, and CPT-2) and used RT-qPCR to check their relative expression. The trend of RT-qPCR results for eight genes is consistent with the transcriptome, confirming the reliability of the transcriptome results (Fig. S3 and Fig. 5d). The gene relationship between KEGG-enriched immune pathways and lipid metabolism pathways is shown in Fig. 5e. In terms of changes in DEGs, TPT induced an increase in the levels of lipid metabolism-related genes, and MPs induced a decrease in the levels of immune-related genes. Additionally, the differential gene expression changes were significant in the TPT_MP group. Thus, the co-exposure of TPT with MPs amplified the single toxic effects of pollutants.
Fig. 5.
Weighted correlation network analysis of brain transcriptome and expression of genes related to immunity and lipid metabolism. a, Correlation analysis between intestinal immune function and each module. b, The KEGG pathways (p < 0.05) in module antique white. c, Network regulation of genes in module antique white. d, mRNA levels of immune and lipid metabolism genes in brain transcriptome. e, Immune and lipid metabolism pathways.
3.5. Correlation analysis between intestine and brain in carp
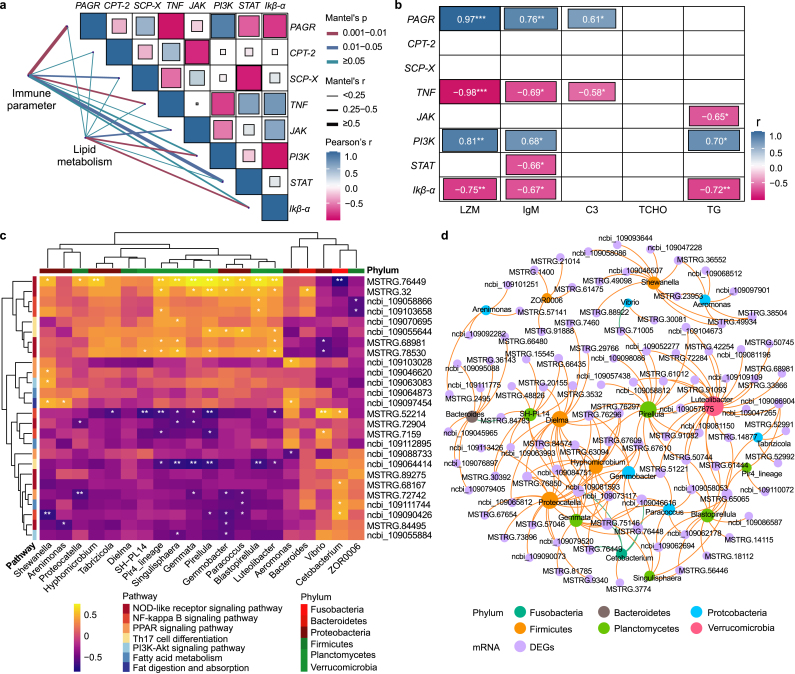
In this study, biochemical parameters of gut immunity and lipid metabolism were correlated with brain tissue immunity and lipid metabolism genes (Fig. 6a and b), with the aim of investigating the effects of TPT, MP, and TPT_MP exposure on the gut–brain axis. Gut immune parameters (LZM, IgM, and C3) were significantly negatively correlated with immune genes (TNF, STAT and Ikβ-α) in brain tissue. To study the relationship between the abundance of carp intestinal flora and the expression of brain immunity and lipid metabolism genes, a cluster heat map was presented using Spearman correlation analysis. In our results, the level of bacterial genus abundance has little correlation with lipid metabolism in the brain. However, the correlation between immune-related pathways in the brain and the intestinal flora is significant, mainly at the genera Pir4_lineage, Singulisphaera, Gemmata, Pirellula, and Blastopirellula. These genera belong to the phylum Planctomycetes. The genes in the “NOD-like receptor signaling pathway” were mainly positively correlated with the above five bacterial genera of the phylum Phytophthora. Some genes of the “NF-kappa B signaling pathway” and “Th17 cell differentiation” were positively correlated with intestinal flora, whereas some genes were negatively correlated (Fig. 6c). The network interaction diagram of brain genes and the top 20 intestinal flora at the genus abundance are shown in Fig. 6d. These genes were also enriched in immune-related pathways through KEGG (Fig. S4). In conclusion, the abundance of bacteria in the intestine of carp was significantly correlated with genes related to brain immunity.
Fig. 6.
Correlation analysis between intestine and brain tissue. a, Correlation heat map of immune and metabolic functions with differential genes. b, Correlation analysis between differential genes and biochemical parameters. c, Clustering heat map of genes related to immunity and lipid metabolism in brain tissues and the top 20 intestinal flora at the genus abundance. The color change from light yellow to dark blue indicates that the correlation changes from positive to negative. d, The interaction network between the top 20 intestinal flora at the genus abundance and brain DEGs. The gray nodes represent the DEGs of the brain, each node in the remaining colors represents a bacterial genus, and the node color represents the bacterial phyla. The orange line indicates that the correlation is positive, and the green line is negative.
4. Discussion
4.1. Effect on the intestinal barrier
Intestinal health has an important effect on the growth of fish [39,40]. The intestinal barrier occupies an irreplaceable position in life and health. It responds to various stimuli and can effectively prevent the body from being harmed by endogenous microorganisms and their toxins [41]. Mucus is the first physical defense in the barrier. The structure of intestinal flora is affected by various factors [42,43], and its structural changes may mediate many diseases [44,45]. Mucus can surround bacteria and screen for metabolites or toxins to limit their entry into epithelial tissues. Moreover, there are many molecules in mucus that can have immunomodulatory effects [47]. Glycoprotein is the main component of intestinal mucus secreted by goblet cells. Recent studies have demonstrated that MPs, metals, and their combinations can increase the expression of the intestinal mucin gene in common carp [48], which is consistent with the results of this study. We found that mucus glycoprotein secreted by the intestine in the combined exposure group increased significantly. It may be that TPT_MP was toxic to the gut and protected against foreign TPT_MP by stimulating and regulating the secretion of mucus glycoproteins to promote intestinal barrier function. Based on changes in glycoprotein density and area, it was further demonstrated that TPT played an important role in the combined exposure of TPT_MP.
To further determine the toxicity of TPT combined with MPs, 16S rRNA sequencing of the carp intestinal flora was performed. At the genus level, in previous studies, MPs greatly altered the intestinal flora of aquatic organisms [49,50]. In our study, there was no significant difference between the treatment group with MPs and the control group (Fig. 2d). This may be because of the inconsistency of the plastic, exposure time, and the species of the tested animals, which contribute differently to the imbalance of intestinal flora [51]. Intestinal function is predicted by PICRUST (Fig. 2f). In KEGG level 3, the changes in the TPT_MP group were the most obvious, and the immune function abundance of intestinal flora decreased compared with the control group. It may be that the combination of TPT and MPs amplifies MP-induced immunotoxicity.
In this study, MP and TPT_MP affected intestinal lipid metabolism and glycolipid digestion. Indeed, according to previous studies, MPs inhibit the amylase activity of Mytilus galloprovincialis [52]. In the TPT_MP group, TCHO increased significantly, and TG showed an upward trend, indicating that the combination of TPT and MPs would increase the accumulation of intestinal lipids in carp. This may be because of the disturbance in the intestinal circulation of bile acids, which affects the digestion and absorption of lipids, resulting in lipid metabolism disorders [53]. Moreover, the results of previous studies are consistent with the results of this study. Triphenyltin has the effect of inducing fat accumulation in fish, amphibians, and mammals [54,55]. Microplastics can also induce glucose and lipid metabolism disorders in organisms [56]. Currently, it is difficult to clarify the biochemical mechanism of biological oxidative stress induced by MP exposure, but MPs can lead to the disturbance of oxidative stress parameters [57]. Huang et al. found that when guppies were exposed to MPs at concentrations of 100 and 1000 μg L−1 for 28 d, the increase in MDA content indicated that MPs did indeed cause oxidative stress and peroxidative damage [58]. However, in the present study, the results were reversed. Malondialdehyde is an indicator of lipid peroxidation. Levels of MDA depend on the presence of ROS and free radicals as well as substrates, unsaturated fatty acids [59]. Therefore, the decrease in MDA may reflect the decrease in highly unsaturated fatty acids (HUFAs) in intestinal fat. This further suggests that exposure to these toxic substances can affect lipid metabolism, possibly by altering the distribution of fatty acids and reducing the proportion of HUFAs, leading to a decrease in MDA. Additionally, fish can metabolize aldehydes, and the decrease in MDA may be related to the activation of hydrolytic enzymes [60].
Lysozyme, C3, and IgM play important immune roles in animals [[61], [62], [63], [64]]. In this study, compared with the control group, TPT significantly increased IgM levels, and MPs significantly increased LZM and IgM levels. This may be because the carp intestines secrete immune factors to increase immune barrier function after exposure to pollutants [65]. Triphenyltin combined with MPs will significantly increase the levels of three immune parameters. The MP group also significantly increased the levels of IgM and LZM. Moreover, the trend of the TPT combined exposure group is also higher than that of the other treatment groups. Interestingly, the immune parameters in the intestine are significantly increased after the combined exposure, and its immune function may be activated. Furthermore, we found that TPT_MP can inhibit the immune function of the intestine through PICRUST2 predicting the function of intestinal flora, causing it to be immunosuppressed. The reason for the conflicting results may be that the gene level is more sensitive to the corresponding pollutants than the biochemical physiological parameters, or that the damage caused by the inflammation leads to the decrease of the gene level. Additionally, at the gene level, MP and TPT_MP gradually suppressed the occurrence of immune responses. An anti-inflammatory cytokine, IL-10, inhibits the expression of pro-inflammatory factors and prevents the development of inflammation [66]. Based on the expression level of the anti-inflammatory factor IL-10 gene, the single toxic effect of MPs is further amplified when combined with TP, and the occurrence of anti-inflammatory factors is inhibited. In conclusion, long-term exposure to MPs will produce immunotoxicity in the carp intestine and inhibit the occurrence of immune function at the gene level.
Overall, long-term exposure to TPT_MP induced an increase in the intestinal TG and TCHO levels of carp, leading to disturbances in lipid metabolism. Additionally, TPT_MP led to the disturbance of intestinal immune function in carp. The TPT_MP treatment induced an increase in intestinal mucus and increased the level of intestinal immune biochemical parameters such as LZM, IgM, and C3, while inhibiting the occurrence of immune function at the gene level. Moreover, the combined exposure of TPT and MPs amplified the toxic effects of single pollutants on lipid metabolism and the MP-induced intestinal immune dysfunction.
4.2. Effect on the brain
High-throughput transcriptomics sequencing technology has become an effective tool to assess the potential toxicity mechanisms of environmental pollutants from the mRNA level [67]. In our results (Fig. 4), TPT led to disorders of lipid metabolism in carp. The digestion and absorption of fat and PPAR signaling pathway in the TPT group were significant. It has been reported that TPT induces lipid accumulation in rainbow trout [27]. Our research results also support this point. Many studies have reported that after MP exposure, the immune response of fish is significantly increased [68]. However, in the present experiment, the results were opposite, with MPs significantly suppressing immunity, which inhibits the toll-like receptor signaling pathway and NF-kappa B signaling pathway. Additionally, it was found that the absorption of MPs reduces the level of immune-functioning neutrophils in Oreochromis niloticus [69]. As foreign compounds, MPs could stimulate the immune response of fish or inhibit immune function by inducing immunotoxicity.
The expression of genes related to lipid metabolism and immunity was detected by RT-qPCR. The detected gene expression trend is consistent with the transcriptome trend, which proves the reliability of the transcriptome data. The immune system of fish is sensitive to chemicals, and changes in innate immunity can be used as an early indicator of the response to environmental stress factors [70,71]. Interestingly, the expression of related immune genes in the TPT_MP group was significantly lower than that in the control group; that is, immunosuppression occurred. However, most studies have shown that MPs induce the occurrence of immune function [72,73]. In our study, the expression of the immune factors TNF-α and IKβ-α and the upstream genes STAT and JAK in the MP group and TPT_MP treatment group decreased significantly. This may be because nanoscale plastic microspheres can enter the carp body through daily activities such as breathing and eating, and are likely to cross the blood–brain barrier, thereby causing damage to the brain tissue [68,74]. This brain damage is likely to lead to a decrease in the ability to produce the inflammatory factors TNF-α and IKβ-α, leading to immunosuppression. Moreover, owing to the adsorption of MPs [75,76], TPT can use MPs as carriers to enter the body of carp and combine with MPs to amplify the toxicity of TPT to carp brain tissue, but the mechanism of its compound toxicity needs to be explored in the future.
Generally, we found that TPT can lead to lipid accumulation in carp brain tissue and MPs can lead to immunosuppression in carp brain tissue. The exposure of TPT combined with MPs also amplified the immunosuppression of MPs on brain tissue and increased the disturbance of TPT on carp brain tissue metabolism. This may be because of the relatively large surface area of nanoscale MPs that can adsorb TPT, increasing the risk of these two pollutants entering brain tissue and damaging it.
4.3. Effect on the gut–brain axis
Various reports have shown that TPT and MPs can cause brain and intestinal organ damage [36,77,78]. The intestine is also known as the “the brain in gut” [79,80]. It interacts with the brain through the vagus nerve to form the “gut–brain axis” and has significant biological functions [81].
As shown in Fig. 6, after TPT_MP exposure, intestinal immune function was suppressed through function prediction. Although the levels of LZM, IgM, and C3 increased (see Fig. 3), immunosuppression occurred at the gene level based on the decrease in expression of immune genes (TNF, STAT, and Ikβ-α) (Fig. 6). When the expression level of immune factors such as TNF, IKβ-α, and other genes in the brain tissue is reduced, immunosuppression will also occur. Similarly, compared with the control, the genes related to lipid metabolism and accumulation (SCP-X, PAGR, and CPT-2) were also increased in the brain tissue. This may be attributed to the role of the gut–brain axis.
New insights were provided from the perspective of the “gut–brain axis”. That is, immune-related DEGs in carp brain tissue were significantly negatively correlated with gut immune parameters and significantly correlated with the dominant microbes in the gut microbiota. Flora of the genera Pir4_lineage, Singulisphaera, Gemmata, Pirellula, and Blastopirellula are associated with brain tissue immune dysfunction. For example, “NOD-like receptor signaling pathway”, “NF-kappa B signaling pathway”, and “Th17 cell differentiation pathway” were inhibited. Additionally, TCHO and TG levels were elevated in the gut in the TPT_MP group, and genes related to lipid metabolism and accumulation, such as SCP-X, PAGR, and CPT-2, were also increased in brain tissue compared with controls. This may be attributed to the role of the “gut–brain axis”. In conclusion, when TPT is combined with MPs for long-term exposure, the involvement of TPT amplified the MP-mediated immunosuppressive effect. However, the regulation mechanism of the gut–brain axis is not yet fully understood, and current studies have mainly focused on mammals such as mice. The regulation of the gut–brain axis of lower organisms like fish still needs to be further explored.
In this study, intestinal flora phyla of Fusobacteria, Bacteroidetes, Proteobacteria, Firmicutes, Planctomycetes, and Verrucomicrobia pass through the “NOD-like receptor signaling pathway”, “NF-kappa B signaling pathway”, “PPAR signaling pathway”, “Th17 cell differentiation”, “PI3K-AKT signaling pathway”, “Fatty acid metabolism”, “Fatty acid metabolism”, and “Fat digestion and absorption” feedback to brain immune and lipid metabolism changes. This resulted in lipid metabolism disorders and immune function disorders. The results of this study still require further verification and discussion.
5. Conclusion
Our results demonstrate that single exposure to TPT resulted in the disturbance of lipid metabolism in carp, while MPs suppressed immunity. Meanwhile, TPT_MP also amplified the immunosuppressive effect induced by a single MP. Additionally, the secretion of mucus granules in the intestinal tissue of carp, the detection results of immune parameters and immune genes, changes in the abundance of intestinal flora, and functional prediction results all proved that the combined exposure of TPT and MPs resulted in an immunosuppressive response. This study provides new insights from the perspective of the gut–brain axis and finds that immune-related DEGs are significantly associated with gut-dominant microbes and immune parameters, but the mechanisms require further investigation. Therefore, based on actual environmental conditions as well as a novel perspective, this study comprehensively evaluates the toxic effects of MPs and TPT and the existing ecological risks, while also identifying directions for future research.
CRediT author contribution statement
Siqi Zhang: Writing - Original draft preparation. Ping Li: Writing - Reviewing and Editing. Shuwen He: Indices Measurement. Shaoying Xing: Data analysis. Zhihan Cao: Fish Culture and sampling. Xueli Zhao: Methodology and Software. Cuici Sun: Data analysis. Zhihua Li: Conceptualization and Overall guidance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (42277269) and State Key Laboratory of Tropical Oceanography, South China Sea Institute of Oceanology, Chinese Academy of Sciences (Project No. LTO2119).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100266.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Gall S.C., Thompson R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015;92(1–2):170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M., Scherer C., Alvarez-Munoz D., Brennholt N., Bourrain X., Buchinger S., Fries E., Grosbois C., Klasmeier J., Marti T., Rodriguez-Mozaz S., Urbatzka R., Vethaak A.D., Winther-Nielsen M., Reifferscheid G. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ. Sci. Eur. 2014;26(1):12. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksen M., Mason S., Wilson S., Box C., Zellers A., Edwards W., Farley H., Amato S. Microplastic pollution in the surface waters of the laurentian great lakes. Mar. Pollut. Bull. 2013;77(1–2):177–182. doi: 10.1016/j.marpolbul.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sadri S.S., Thompson R.C. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014;81(1):55–60. doi: 10.1016/j.marpolbul.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Kanhai D.K., Gardfeldt K., Lyashevska O., Hassellov M., Thompson R.C., O'Connor I. Microplastics in sub-surface waters of the arctic central basin. Mar. Pollut. Bull. 2018;130:8–18. doi: 10.1016/j.marpolbul.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Waller C.L., Griffiths H.J., Waluda C.M., Thorpe S.E., Loaiza I., Moreno B., Pacherres C.O., Hughes K.A. Microplastics in the Antarctic marine system: an emerging area of research. Sci. Total Environ. 2017;598:220–227. doi: 10.1016/j.scitotenv.2017.03.283. [DOI] [PubMed] [Google Scholar]
- 7.Free C.M., Jensen O.P., Mason S.A., Eriksen M., Williamson N.J., Boldgiv B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014;85(1):156–163. doi: 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Zhang Y., Kang S., Wang Z., Wu C. Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.141948. [DOI] [PubMed] [Google Scholar]
- 9.Yin J., Long Y., Xiao W., Liu D., Tian Q., Li Y., Liu C., Chen L., Pan Y. Ecotoxicology of microplastics in Daphnia: a review focusing on microplastic properties and multiscale attributes of Daphnia. Ecotoxicol. Environ. Saf. 2023;249 doi: 10.1016/j.ecoenv.2022.114433. [DOI] [PubMed] [Google Scholar]
- 10.Akanyange S.N., Zhang Y., Zhao X., Adom-Asamoah G., Ature A.-R.A., Anning C., Tianpeng C., Zhao H., Lyu X., Crittenden J.C. A holistic assessment of microplastic ubiquitousness: pathway for source identification in the environment. Sustain. Prod. Consum. 2022;33:113–145. [Google Scholar]
- 11.Li Y., Yang G., Wang J., Lu L., Li X., Zheng Y., Zhang Z., Ru S. Microplastics increase the accumulation of phenanthrene in the ovaries of marine medaka (Oryzias melastigma) and its transgenerational toxicity. J. Hazard Mater. 2021;424:127754. doi: 10.1016/j.jhazmat.2021.127754. [DOI] [PubMed] [Google Scholar]
- 12.Rist S., Baun A., Hartmann N.B. Ingestion of micro- and nanoplastics in Daphnia magna - quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017;228:398–407. doi: 10.1016/j.envpol.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Kratina P., Watts T.J., Green D.S., Kordas R.L., O'Gorman E.J. Interactive effects of warming and microplastics on metabolism but not feeding rates of a key freshwater detritivore. Environ. Pollut. 2019;255(Pt 2) doi: 10.1016/j.envpol.2019.113259. [DOI] [PubMed] [Google Scholar]
- 14.Chen H., Hua X., Li H., Wang C., Dang Y., Ding P., Yu Y. Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2021.129642. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Li X., Gao M., Li X., Zhao L., Ru S. Polystyrene microplastics increase estrogenic effects of 17alpha-ethynylestradiol on male marine medaka (Oryzias melastigma) Chemosphere. 2022;287(Pt 3) doi: 10.1016/j.chemosphere.2021.132312. [DOI] [PubMed] [Google Scholar]
- 16.You C., Chen B., Wang M., Wang S., Zhang M., Sun Z., Juventus A.J., Ma H., Li Y. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus) Fish Shellfish Immunol. 2019;89:187–197. doi: 10.1016/j.fsi.2019.03.060. [DOI] [PubMed] [Google Scholar]
- 17.Fackelmann G., Sommer S. Microplastics and the gut microbiome: how chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019;143:193–203. doi: 10.1016/j.marpolbul.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Suman T.Y., Jia P.P., Li W.G., Junaid M., Xin G.Y., Wang Y., Pei D.S. Acute and chronic effects of polystyrene microplastics on brine shrimp: first evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard Mater. 2020;400 doi: 10.1016/j.jhazmat.2020.123220. [DOI] [PubMed] [Google Scholar]
- 19.Sommer F., Backhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 20.Hoyo-Alvarez E., Arechavala-Lopez P., Jimenez-Garcia M., Solomando A., Alomar C., Sureda A., Moranta D., Deudero S. Effects of pollutants and microplastics ingestion on oxidative stress and monoaminergic activity of seabream brains. Aquat. Toxicol. 2022;242 doi: 10.1016/j.aquatox.2021.106048. [DOI] [PubMed] [Google Scholar]
- 21.Li Z.H., Li P., Shi Z.C. Chronic exposure to Tributyltin induces brain functional damage in juvenile common carp (Cyprinus carpio) PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrell P., Nelson K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.) Environ. Pollut. 2013;177:1–3. doi: 10.1016/j.envpol.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Anastopoulos I., Pashalidis I., Kayan B., Kalderis D. Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq. 2021;350:118182. [Google Scholar]
- 24.Gao J.M., Wu L., Chen Y.P., Zhou B., Guo J.S., Zhang K., Ouyang W.J. Spatiotemporal distribution and risk assessment of organotins in the surface water of the Three Gorges Reservoir Region, China. Chemosphere. 2017;171:405–414. doi: 10.1016/j.chemosphere.2016.12.089. [DOI] [PubMed] [Google Scholar]
- 25.Li P., Li Z.H. Environmental co-exposure to TBT and Cd caused neurotoxicity and thyroid endocrine disruption in zebrafish, a three-generation study in a simulated environment. Environ. Pollut. 2020;259 doi: 10.1016/j.envpol.2019.113868. [DOI] [PubMed] [Google Scholar]
- 26.Okoro H.K., Fatoki O.S., Adekola F.A., Ximba B.J., Snyman R.G. Spatio-temporal variation of organotin compounds in seawater and sediments from Cape Town harbour, South Africa using gas chromatography with flame photometric detector (GC-FPD) Arab. J. Chem. 2016;9(1):95–104. [Google Scholar]
- 27.Chen C., Chen L., Li Y., Fu W., Shi X., Duan J., Zhang W. Impacts of microplastics on organotins' photodegradation in aquatic environments. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115686. [DOI] [PubMed] [Google Scholar]
- 28.Yoon D.S., Lee Y., Park J.C., Lee M.C., Lee J.S. Alleviation of tributyltin-induced toxicity by diet and microplastics in the marine rotifer Brachionus koreanus. J. Hazard Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123739. [DOI] [PubMed] [Google Scholar]
- 29.Yi X., Chi T., Li Z., Wang J., Yu M., Wu M., Zhou H. Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. Int. 2019;26(15):15011–15018. doi: 10.1007/s11356-019-04865-0. [DOI] [PubMed] [Google Scholar]
- 30.Yi X., Wang J., Li Z., Zhang Z., Chi T., Guo M., Li W., Zhou H. The effect of polystyrene plastics on the toxicity of triphenyltin to the marine diatom Skeletonema costatum-influence of plastic particle size. Environ. Sci. Pollut. Res. Int. 2019;26(25):25445–25451. doi: 10.1007/s11356-019-05826-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Peng L.B., Wang D., Zhu Q.L., Zheng J.L. Combined effects of polystyrene microplastics and cadmium on oxidative stress, apoptosis, and GH/IGF axis in zebrafish early life stages. Sci. Total Environ. 2022;813 doi: 10.1016/j.scitotenv.2021.152514. [DOI] [PubMed] [Google Scholar]
- 32.Hoseini S.M., Khosraviani K., Hosseinpour Delavar F., Arghideh M., Zavvar F., Hoseinifar S.H., Van Doan H., Zabihi E., Reverter M. Hepatic transcriptomic and histopathological responses of common carp, Cyprinus carpio, to copper and microplastic exposure. Mar. Pollut. Bull. 2022;175 doi: 10.1016/j.marpolbul.2022.113401. [DOI] [PubMed] [Google Scholar]
- 33.Wen B., Jin S.R., Chen Z.Z., Gao J.Z., Liu Y.N., Liu J.H., Feng X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus) Environ. Pollut. 2018;243(Pt A):462–471. doi: 10.1016/j.envpol.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Feng C., Pang W., Tian C., Zhao Y. Nanoplastic-induced genotoxicity and intestinal damage in freshwater benthic clams (Corbicula fluminea): comparison with microplastics. ACS Nano. 2021;15(6):9469–9481. doi: 10.1021/acsnano.1c02407. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Wang Y., Wang Q., Lv M., Zhao X., Ji Y., Han X., Wang X., Chen L. The combined toxic effects of polyvinyl chloride microplastics and di(2-ethylhexyl) phthalate on the juvenile zebrafish (Danio rerio) J. Hazard Mater. 2022;440 doi: 10.1016/j.jhazmat.2022.129711. [DOI] [PubMed] [Google Scholar]
- 36.He S., Yu D., Li P., Zhang M., Xing S., Sun C., Li Z.H. Triphenyltin exposure causes changes in health-associated gut microbiome and metabolites in marine medaka. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117751. [DOI] [PubMed] [Google Scholar]
- 37.Al-shatri M.A., Nuhu A.A., Basheer C., Al-Arfaj A., A.-T B. Assessment of tributyltin and triphenyltin compounds and their main degradation products in Saudi coastal waters. Arabian J. Sci. Eng. 2015;40:2959–2967. [Google Scholar]
- 38.Zhang S.Q., Li P., Zhao X.L., He S.W., Xing S.Y., Cao Z.H., Zhang H.Q., Li Z.H. Hepatotoxicity in carp (Cyprinus carpio) exposed to environmental levels of norfloxacin (NOR): some latest evidences from transcriptomics analysis, biochemical parameters and histopathological changes. Chemosphere. 2021;283 doi: 10.1016/j.chemosphere.2021.131210. [DOI] [PubMed] [Google Scholar]
- 39.Su Y.N., Wu P., Feng L., Jiang W.D., Jiang J., Zhang Y.A., Figueiredo-Silva C., Zhou X.Q., Liu Y. The improved growth performance and enhanced immune function by DL-methionyl-DL-methionine are associated with NF-kappaB and TOR signalling in intestine of juvenile grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2018;74:101–118. doi: 10.1016/j.fsi.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 40.Li Z.H., Li P., Shi Z.C. Molecular responses in digestive tract of juvenile common carp after chronic exposure to sublethal tributyltin. Ecotoxicol. Environ. Saf. 2014;109:10–14. doi: 10.1016/j.ecoenv.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Zhong J.R., Feng L., Jiang W.D., Wu P., Liu Y., Jiang J., Kuang S.Y., Tang L., Zhou X.Q. Phytic acid disrupted intestinal immune status and suppressed growth performance in on-growing grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2019;92:536–551. doi: 10.1016/j.fsi.2019.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Chang X., Li H., Feng J., Chen Y., Nie G., Zhang J. Effects of cadmium exposure on the composition and diversity of the intestinal microbial community of common carp (Cyprinus carpio L.) Ecotoxicol. Environ. Saf. 2019;171:92–98. doi: 10.1016/j.ecoenv.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y.S., Kim J., Park S.J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe. 2015;33:1–7. doi: 10.1016/j.anaerobe.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Sartor R.B., Wu G.D. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152(2):327–339 e324. doi: 10.1053/j.gastro.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Watanabe K., Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017;8:1882. doi: 10.3389/fimmu.2017.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., Young V.B., Henrissat B., Wilmes P., Stappenbeck T.S., Nunez G., Martens E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353 e1321. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoseini S.M., Sinha R., Fazel A., Khosraviani K., Hosseinpour Delavar F., Arghideh M., Sedaghat M., Paolucci M., Hoseinifar S.H., Van Doan H. Histopathological damage and stress- and immune-related genes' expression in the intestine of common carp, Cyprinus carpio exposed to copper and polyvinyl chloride microparticle. J. Exp. Zool. A Ecol. Integr. Physiol. 2022;337(2):181–190. doi: 10.1002/jez.2555. [DOI] [PubMed] [Google Scholar]
- 49.Kang H.M., Byeon E., Jeong H., Kim M.S., Chen Q., Lee J.S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124207. [DOI] [PubMed] [Google Scholar]
- 50.Gu H., Wang S., Wang X., Yu X., Hu M., Huang W., Wang Y. Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122773. [DOI] [PubMed] [Google Scholar]
- 51.Qiao R., Deng Y., Zhang S., Wolosker M.B., Zhu Q., Ren H., Zhang Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.07.065. [DOI] [PubMed] [Google Scholar]
- 52.Trestrail C., Walpitagama M., Miranda A., Nugegoda D., Shimeta J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146418. [DOI] [PubMed] [Google Scholar]
- 53.Jin Y., Lu L., Tu W., Luo T., Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 54.Lutfi E., Riera-Heredia N., Cordoba M., Porte C., Gutierrez J., Capilla E., Navarro I. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquat. Toxicol. 2017;188:148–158. doi: 10.1016/j.aquatox.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Tinkov A.A., Ajsuvakova O.P., Skalnaya M.G., Skalny A.V., Aschner M., Suliburska J., Aaseth J. Organotins in obesity and associated metabolic disturbances. J. Inorg. Biochem. 2019;191:49–59. doi: 10.1016/j.jinorgbio.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H., Wang J., Wei X., Chang L., Liu S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.143085. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C., Wang J., Pan Z., Wang S., Zhang L., Wang Q., Ye Q., Zhou A., Xie S., Zeng F., Xu G., Zou J. A dosage-effect assessment of acute toxicology tests of microplastic exposure in filter-feeding fish. Fish Shellfish Immunol. 2021;113:154–161. doi: 10.1016/j.fsi.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Huang J.N., Wen B., Meng L.J., Li X.X., Wang M.H., Gao J.Z., Chen Z.Z. Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata) J. Hazard Mater. 2020;399 doi: 10.1016/j.jhazmat.2020.123044. [DOI] [PubMed] [Google Scholar]
- 59.Yu H., Gao Q., Dong S., Zhou J., Ye Z., Lan Y. Effects of dietary n-3 highly unsaturated fatty acids (HUFAs) on growth, fatty acid profiles, antioxidant capacity and immunity of sea cucumber Apostichopus japonicus (Selenka) Fish Shellfish Immunol. 2016;54:211–219. doi: 10.1016/j.fsi.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Garcia D., Lima D., da Silva D.G.H., de Almeida E.A. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf. 2020;190 doi: 10.1016/j.ecoenv.2019.110107. [DOI] [PubMed] [Google Scholar]
- 61.Wang J., Liang D., Yang Q., Tan B., Dong X., Chi S., Liu H., Zhang S. The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus female symbol x Epinephelus lanceolatus male symbol) Fish Shellfish Immunol. 2020;98:619–631. doi: 10.1016/j.fsi.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 62.Saurabh S., Sahoo P.K. Lysozyme: an important defence molecule of fish innate immune system. Aquacult. Res. 2008;39(3):223–239. [Google Scholar]
- 63.Awad E., Cerezuela R., Esteban M.A. Effects of fenugreek (Trigonella foenum graecum) on gilthead seabream (Sparus aurata L.) immune status and growth performance. Fish Shellfish Immunol. 2015;45(2):454–464. doi: 10.1016/j.fsi.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 64.PilstrÖM L., BengtÉN E.V.A. Immunoglobulin in fish—genes, expression and structure. Fish Shellfish Immunol. 1996;6(4):243–262. [Google Scholar]
- 65.Rauta P.R., Nayak B., Das S. Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol. Lett. 2012;148(1):23–33. doi: 10.1016/j.imlet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Xu Y., Han Q., Yao Y., Xing H., Immunosuppression X. Teng. Oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J. Hazard Mater. 2019;366:386–394. doi: 10.1016/j.jhazmat.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.H., Yu Y.B., Choi J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: a review. J. Hazard Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125423. [DOI] [PubMed] [Google Scholar]
- 69.Hamed M., Soliman H.A.M., Osman A.G.M., Sayed A.E.H. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere. 2019;228:345–350. doi: 10.1016/j.chemosphere.2019.04.153. [DOI] [PubMed] [Google Scholar]
- 70.Li X., Liu L., Zhang Y., Fang Q., Li Y., Li Y. Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio L.) Chemosphere. 2013;93(2):428–433. doi: 10.1016/j.chemosphere.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 71.Jiang W.D., Hu K., Liu Y., Jiang J., Wu P., Zhao J., Zhang Y.A., Zhou X.Q., Feng L. Dietary myo-inositol modulates immunity through antioxidant activity and the Nrf2 and E2F4/cyclin signalling factors in the head kidney and spleen following infection of juvenile fish with Aeromonas hydrophila. Fish Shellfish Immunol. 2016;49:374–386. doi: 10.1016/j.fsi.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z., Fan L., Wang J., Xie S., Zhang C., Zhou J., Zhang L., Xu G., Zou J. Insight into the immune and microbial response of the white-leg shrimp Litopenaeus vannamei to microplastics. Mar. Environ. Res. 2021;169 doi: 10.1016/j.marenvres.2021.105377. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Liu Z., Li M., Jiang Q., Wu D., Huang Y., Jiao Y., Zhang M., Zhao Y. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122990. [DOI] [PubMed] [Google Scholar]
- 74.Rahman A., Sarkar A., Yadav O.P., Achari G., Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- 75.Fu L., Li J., Wang G., Luan Y., Dai W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112207. [DOI] [PubMed] [Google Scholar]
- 76.Luo H., Liu C., He D., Xu J., Sun J., Li J., Pan X. Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard Mater. 2022;423(Pt A) doi: 10.1016/j.jhazmat.2021.126915. [DOI] [PubMed] [Google Scholar]
- 77.Li P., Li Z.H., Zhong L. Effects of low concentrations of triphenyltin on neurobehavior and the thyroid endocrine system in zebrafish. Ecotoxicol. Environ. Saf. 2019;186 doi: 10.1016/j.ecoenv.2019.109776. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X.L., Li P., Zhang S.Q., He S.W., Xing S.Y., Cao Z.H., Lu R., Li Z.H. Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ. Pollut. 2021;287 doi: 10.1016/j.envpol.2021.117612. [DOI] [PubMed] [Google Scholar]
- 79.Ezra-Nevo G., Henriques S.F., Ribeiro C. The diet-microbiome tango: how nutrients lead the gut brain axis. Curr. Opin. Neurobiol. 2020;62:122–132. doi: 10.1016/j.conb.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Li Z.H., Zlabek V., Velisek J., Grabic R., Machova J., Randak T. Enzymatic alterations and RNA/DNA ratio in intestine of rainbow trout, Oncorhynchus mykiss, induced by chronic exposure to carbamazepine. Ecotoxicology. 2010;19(5):872–878. doi: 10.1007/s10646-010-0468-1. [DOI] [PubMed] [Google Scholar]
- 81.Teichman E.M., O'Riordan K.J., Gahan C.G.M., Dinan T.G., Cryan J.F. When rhythms meet the blues: circadian interactions with the microbiota-gut-brain Axis. Cell Metabol. 2020;31(3):448–471. doi: 10.1016/j.cmet.2020.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.